Abstract
Selpercatinib is a first-in-class, highly selective and potent, central nervous system-active RET kinase inhibitor. In the phase I/II trial, selpercatinib demonstrated clinically meaningful antitumor activity with manageable toxicity in heavily pre-treated and treatment-naive patients with RET-mutant medullary thyroid cancer (MTC). LIBRETTO-531 (NCT04211337) is a multicenter, open-label, randomized, controlled, phase III trial comparing selpercatinib to cabozantinib or vandetanib in patients with advanced/metastatic RET-mutant MTC. The primary objective is to compare progression-free survival (per RECIST 1.1) by blinded independent central review of patients with progressive, advanced, multikinase inhibitor-naive, RET-mutant MTC treated with selpercatinib versus cabozantinib or vandetanib. Key secondary objectives are to compare other efficacy outcomes (per RECIST 1.1) and tolerability of selpercatinib versus cabozantinib or vandetanib.
Keywords: medullary thyroid cancer, phase III trial, RET alteration, RET kinase inhibitor, RET mutation, selpercatinib, targeted therapy
Plain language summary
Selpercatinib (also known by the brand name Retevmo®/Retsevmo®) is a new treatment available in multiple countries for people with advanced or metastatic RET-mutant medullary thyroid cancer (MTC). Thyroid cancer starts in your thyroid gland and may spread or metastasize to other parts of the body, including lungs, bones, and occasionally the brain, which means the cancer is likely to be advanced. Advanced thyroid cancer can be driven by a gene in your body, one of which is RET. This is a summary of the LIBRETTO-531 study which compares selpercatinib, which is a strong and selective inhibitor of RET, with two approved drugs, cabozantinib and vandetanib. Patients with advanced or metastatic RET-mutant MTC who have not already received treatment with kinase inhibitors are being enrolled. This trial will evaluate how long people during and after treatment live with the disease without it getting worse. Selpercatinib may affect both healthy cells and tumor cells, which can result in side effects, which will also be evaluated in this study. This study is active and currently recruiting new patients.
Clinical Trial Registration: NCT04211337 (ClinicalTrials.gov)
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor originating from parafollicular C cells [1]. MTC occurs in hereditary (25%) and sporadic forms (75%) [2,3]. Germline mutations in the rearranged during transfection (RET) proto-oncogene have been identified in hereditary conditions. For example, multiple endocrine neoplasia (MEN) 2A and MEN2B [2,3]. Germline-activating RET mutations are found in 95% to 98% of hereditary MTC, and somatic RET mutations are present in more than half the cases of sporadic MTC [4,5]. In patients with advanced and progressive MTC, the prevalence of somatic RET mutations was found to be up to 86% [6].
The pathogenesis of MTC is closely related to the activation of the RET proto-oncogene, both in hereditary [7–10] and in sporadic cases [5,11,12]. The RET proto-oncogene encodes a transmembrane receptor tyrosine kinase that is constitutively activated through two primary mechanisms: point mutations that directly or indirectly activate the kinase, and chromosomal rearrangements leading to the fusion of RET to a 5′ upstream partner [4,13,14]. Codon 634 mutation (p.C634R) in exon 11 is described as the most common substitution RET mutation in MEN2A syndrome patients [15]. Kinase domain mutation p.M918T is described as the most common mutation in MEN2B syndrome patients and in sporadic MTC patients [15]. RET-mutated MTC is associated with more aggressive disease and poorer prognosis, and consequently tumors in a majority of patients with metastatic MTC harbor RET mutations [3,5]. National Comprehensive Cancer Network guidelines recommend germline RET testing for all patients diagnosed with MTC and somatic RET testing in patients who are RET germline unknown or negative [16].
The clinical course of MTC is highly heterogeneous, varying from indolent tumors that remain unchanged for many years to aggressive cancers associated with high mortality [17,18]. Initial treatment of MTC depends on its clinical presentation. Although surgery can be curative for patients who present with localized disease, approximately 50% of all patients diagnosed with MTC develop recurrent disease [19]. Metastatic MTC remains incurable.
Until recently, only two multikinase inhibitors (MKI), cabozantinib and vandetanib, had received regulatory approval for advanced MTC (irrespective of the presence or absence of a RET mutation), with tumor response rates of 28 and 45% and progression-free survival (PFS) improvements (over placebo) of 7.2 and 11.2 months, respectively [20,21]. However, the efficacy of these MKIs is ultimately limited by incomplete inhibition of RET, significant toxicity from more potent inhibition of other targets (e.g., KDR/VEGFR2, EGFR, MET) and poor pharmacokinetics (i.e., significant drug accumulation and long half-life contributing to toxicity but not efficacy). As a result, most patients treated with these agents experience significant toxicities requiring dose interruptions, reductions (35% with vandetanib, 79% with cabozantinib), and/or treatment cessation (12% with vandetanib, 16% with cabozantinib) [20,21]. Additionally, some RET disease-causing variants are non-responsive to MKI therapy, in other words, nonspecific RET inhibitor therapy. Thus, highly selective RET inhibitors have been developed to improve the efficacy and safety profile compared with MKIs. Selpercatinib [22–24] and pralsetinib [25–27] are two currently approved small molecule RET inhibitors.
Selpercatinib
Selpercatinib is a first-in-class highly selective and potent RET kinase inhibitor with nanomolar potency against wild type RET and RET alterations including M918T, MKI resistance-associated V804M, and others [28]. Selpercatinib showed robust and durable efficacy with a favorable safety profile in patients with advanced or metastatic RET-driven treatment-naive and previously treated cancers, irrespective of prior therapy [22,29–31]. Based on these findings, selpercatinib is approved in multiple countries for the treatment of RET-altered thyroid and lung cancers [24,32].
The phase I/II LIBRETTO-001 clinical trial demonstrated the safety and efficacy of selpercatinib in patients with RET-mutant MTC [22,23,33]. Treatment with selpercatinib resulted in a 69% objective response rate (ORR) (95% CI: 61–77%) by blinded independent central review (BICR) in patients previously treated with cabozantinib and/or vandetanib (n = 143) [33]. At a median follow-up of 17.5 months, 76% of responses were ongoing. In cabozantinib/vandetanib-naive patients with RET-mutant MTC, treatment with selpercatinib resulted in a 71% ORR (95% CI: 62–80%) by BICR (n = 112). At a median follow-up of 9.3 months, 94% of responses were ongoing.
At the updated data-cut of March 2020, the majority of adverse reactions were grade 1 or 2. Grades 3–4 events occurring in ≥2% of patients included hypertension (20%), increased ALT level (7%), increased AST level (6%), diarrhea (4%), prolonged QT corrected interval (4%), headache (2%) and abdominal pain (2%). Discontinuation rates were low with 2% of patients discontinuing due to treatment-related adverse events. The safety profile was similar to the overall safety profile for all patients who received selpercatinib, regardless of tumor type.
The LIBRETTO-531 clinical trial
LIBRETTO-531 (also referred to as J2G-MC-JZJB [JZJB]) is a multicenter, open-label, randomized, controlled, phase III trial comparing selpercatinib to cabozantinib or vandetanib in patients with advanced/metastatic RET-mutant MTC (NCT04211337), naive to any prior kinase inhibitor therapy. This study is active and recruiting participants, with initial planned enrollment of 250 patients at approximately 160 sites in 21 countries. Sample size re-estimation based on comparative data will be conducted at the interim efficacy analysis. The total number of patients could be increased from 250 up to a maximum of approximately 400, depending on the results of the interim efficacy analysis.
Although the LIBRETTO-001 phase I/II trial demonstrated that selpercatinib can produce high rates of objective responses that are durable in both MKI pretreated and MKI-naive MTC patients, it remains important to confirm these results and better understand the benefit of selpercatinib in the context of other available first-line treatments for advanced/metastatic MTC. This will be a key consideration for approval and reimbursement in many countries around the world. Furthermore, the study evaluates key secondary end points, including treatment failure-free survival (TFFS) and comparative tolerability, which could further characterize the potential benefits of selpercatinib relative to the current standard of care MKI inhibitors [34].
Study design
This phase III study is a head-to-head comparison of selpercatinib versus physician's choice of cabozantinib or vandetanib in patients with advanced or metastatic MTC. The study includes a screening phase, an on-study treatment phase with an optional crossover treatment phase (only arm B patients), and a post-treatment follow-up phase (Figure 1). During the screening phase, baseline patient characteristics and eligibility criteria will be assessed using medical history, physical exam, clinical labs and tumor evaluations. Prior to enrollment, the presence of the RET gene alteration must be confirmed in a tumor, germline DNA or blood sample. An unstained, archived tumor tissue sample in a quantity sufficient to allow for retrospective central analysis confirmation of RET mutation status is required. Patients will be stratified by RET mutation (M918T vs other) and intended treatment if randomized to arm B (cabozantinib vs vandetanib). The selection of cabozantinib or vandetanib for each eligible patient is required by the investigator prior to randomization. Treatment will continue until disease progression, development of unacceptable toxicity, the start of a new anticancer therapy, withdrawal of patient consent, death, or completion of the study. Patients who were randomized to arm B who have radiographic disease progression confirmed by BICR may be eligible for crossover to selpercatinib if they meet the eligibility criteria.
Figure 1. . LIBRETTO-531 study design.
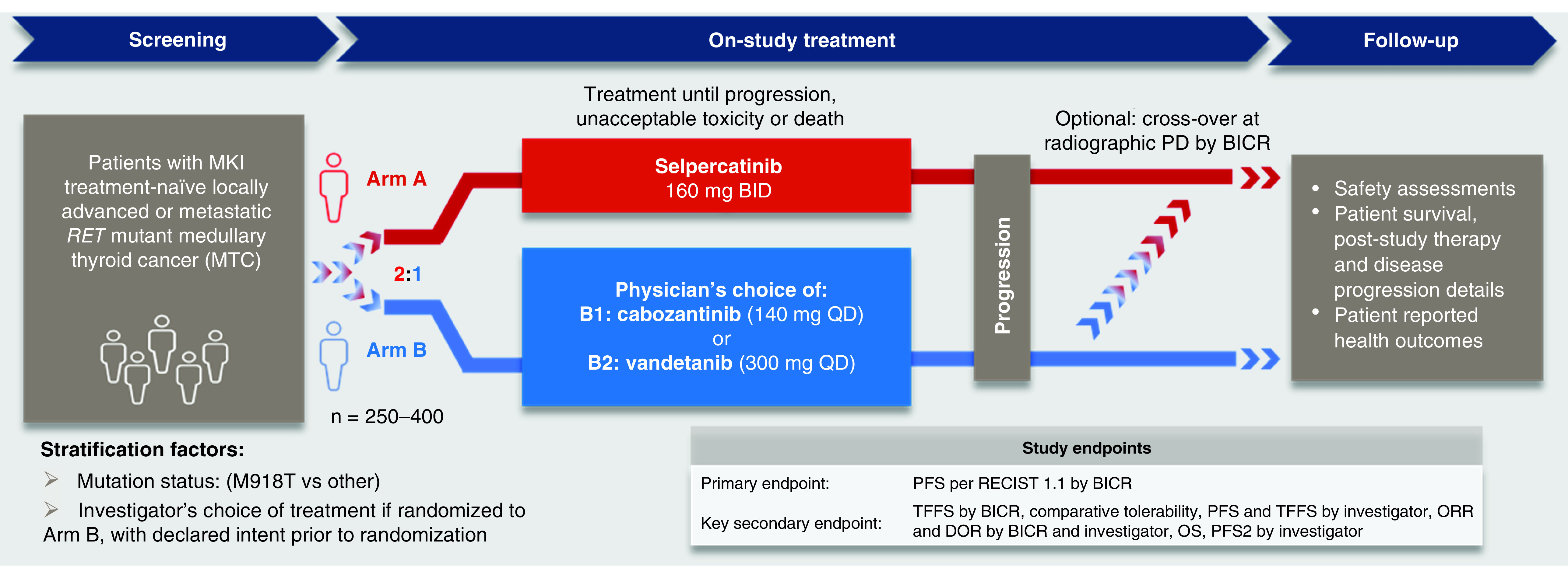
BICR: Blinded independent committee review; BID: Twice daily; DOR: Duration of response; MKI: Mulitkinase inhibitor; ORR: Objective response rate; OS: Overall survival; PD: Progressive disease; PFS: Progression-free survival; PFS2: Progression after the next line of therapy; QD: Once daily; RECIST 1.1: Response Evaluation Criteria in Solid Tumors version 1.1; TFFS: Treatment failure free survival, which incorporates radiographic PD, unacceptable toxicity (predefined by protocol) or death.
The post-treatment phase includes a short-term follow-up period that begins once the patient and investigator decide the patient will end study therapy. Long-term follow-up begins when the patient completes the (30 ± 7 days) short-term follow-up period and ends with the patient's death, upon study withdrawal, upon loss to follow-up, or upon study completion. To evaluate patient reported health outcomes (quality of life, patient functioning, health status, disease symptoms and toxicities) patient-reported surveys will be administered.
Objectives & end points
The study objectives are detailed in Table 1. The primary objective is to compare PFS (per RECIST 1.1) by BICR. PFS by BICR will act as a gatekeeper for the key secondary end point of TFFS by BICR, in other words, this key secondary end point will be tested conditionally on achieving a statistical significance for the primary end point. TFFS incorporates radiographic progressive disease, unacceptable toxicity (predefined by protocol) or death, of patients with progressive, advanced, kinase inhibitor naive, RET-mutant MTC treated with selpercatinib versus cabozantinib or vandetanib. TFFS was selected as this end point takes into account potential improvement in both efficacy and toxicity profile relative to comparator agents. Additional secondary end points are comparative tolerability, investigator-assessed PFS, investigator-assessed TFFS, investigator- and BICR-assessed ORR/duration of response, overall survival, investigator-assessed progression after the next line of therapy, safety/tolerability and pharmacokinetics of selpercatinib.
Table 1. . Study objectives.
| Objectives | End points |
|---|---|
| Primary | |
| To compare PFS of patients with progressive, advanced, kinase inhibitor-naive, RET-mutant MTC treated with selpercatinib vs cabozantinib or vandetanib | • PFS by BICR |
| Secondary | |
| To compare other efficacy outcomes, based on RECIST 1.1 criteria, observed in patients with progressive, advanced, kinase inhibitor-naive, RET-mutant MTC treated with selpercatinib vs cabozantinib or vandetanib |
• TFFS by BICR • TFFS by investigator • PFS by investigator • ORR by investigator and BICR • DOR by investigator and BICR • OS • PFS2 by investigator |
| To evaluate the safety and tolerability of selpercatinib compared with cabozantinib or vandetanib | • Safety per CTCAE v5.0 (including but not limited to): incidence and severity of TEAEs, SAEs, deaths, and clinical laboratory abnormalities |
| To compare the tolerability of selpercatinib vs cabozantinib or vandetanib | • Proportion of time with high side-effect bother based on FACT-GP5 |
| To assess/evaluate performance of local RET laboratory tests compared with a single, central test | • RET mutation status |
| To assess the pharmacokinetics of selpercatinib in patients receiving selpercatinib | • Predose plasma concentrations at Day 8 of Cycle 1, and at Day 1 of Cycles 2 through 6 |
BICR: Blinded independent committee review; DOR: Duration of response; FACT-GP5: Functional Assessment of Cancer Therapy general scale to assess overall side effect burden; MTC: Medullary thyroid cancer; ORR: Objective response rate; OS: Overall survival; PFS: Progression-free survival; PFS2: Progression after the next line of therapy; SAE: Serious adverse event; TEAE: Treatment-emergent adverse event; TFFS: Treatment failure free survival, which incorporates radiographic disease progression, unacceptable toxicity (predefined by protocol) or death.
Key eligibility criteria
Key eligibility criteria are summarized in Table 2 and below. To be eligible for the trial, participants must have histologically or cytologically confirmed unresectable, locally advanced/metastatic MTC, a RET gene alteration, documented RECIST 1.1 progression within 14 months by BICR, measurable disease per RECIST 1.1, an Eastern Cooperative Oncology Group performance status of 0 to 2, a life expectancy of at least 3 months, adequate organ function, and the ability to swallow capsules. Exclusion criteria include: presence of additional validated oncogenic drivers in MTC, having received prior systemic treatment with kinase inhibitor(s), taking a concomitant medication that is known to cause QTc prolongation, or having symptomatic central nervous system involvement, or active cardiovascular disease.
Table 2. . Key eligibility criteria for LIBRETTO-531.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Locally advanced or metastatic MTC ○ Histologically confirmed, unresectable locally advanced or metastatic disease and no prior history of treatment with kinase inhibitors for advanced/metastatic disease Patient characteristics ○ Age ≥12, where allowed ○ Documented RECIST 1.1 progression within 14 months by BICR ○ Measurable disease by RECIST 1.1 ○ ECOG performance status of 0–2 ○ Adequate organ function ○ Able to swallow capsules ○ Not pregnant/agreeable to highly effective birth control/no breastfeeding RET alteration ○ RET mutation identified via local NGS or PCR testing on germline DNA, tumor or blood ○ Sufficient tissue for central analysis of RET mutation |
Medical conditions ○ An additional validated oncogenic driver in MTC ○ Symptomatic CNS metastases, leptomeningeal carcinomatosis, or untreated spinal cord compression ○ Clinically significant active cardiovascular disease or history of myocardial infarction within 6 months prior to planned start of study treatment or prolongation of the QT interval corrected for heart rate using Fridericia's formula (QTcF) >470 msec on more than one ECG during screening ○ Active uncontrolled systemic bacterial, viral or fungal infection or serious ongoing intercurrent illness, such as hypertension or diabetes, despite optimal treatment (screening for chronic conditions is not required) ○ Clinically significant active malabsorption syndrome or other condition likely to affect gastrointestinal absorption of the study drug. ○ Active hemorrhage or at significant risk for hemorrhage Prior/Concomitant Therapy ○ Prior systemic treatment with kinase inhibitor(s) ○ Are taking a concomitant medication that is known to cause QTc prolongation Prior/Concurrent Clinical Study Experience ○ Have participated, within the last 30 days (4 months for studies conducted in Japan; 3 months for studies conducted in the UK), in a clinical study involving an investigational product |
BICR: Blinded independent committee review; CNS: Central nervous system; ECOG: Eastern Cooperative Oncology Group; MTC: Medullary thyroid cancer; NGS: Next-generation sequencing; PCR: Polymerase chain reaction; RECIST 1.1: Response Evaluation Criteria in Solid Tumors version 1.1.
Dose & schedule of therapy
Patients in Arm A will receive selpercatinib at a starting dose of 160 mg twice daily. Patients in arm B will receive cabozantinib at a starting dose of 140 mg once daily (QD) or vandetanib at a starting dose of 300 mg QD. All treatments are administered orally, and a cycle is defined as 28 days in length, regardless of treatment arm assignment. Treatment will continue until radiographic disease progression, unacceptable toxicity, withdrawal of consent or death. Dosing and treatment durations for all intervention groups are outlined in Table 3.
Table 3. . Doses and dosing schedule.
| Intervention | Arm A (selpercatinib) |
Arm B (investigator's discretion of B1: cabozantinib or B2: vandetanib) | |
|---|---|---|---|
| Selpercatinib | Cabozantinib | Vandetanib† | |
| Dose | 160 mg | 140 mg | 300 mg |
| Schedule | BID continuously in 28-day cycles | QD continuously in 28-day cycles | QD continuously in 28-day cycles |
| Route | Oral | Oral | Oral |
Starting dose of vandetanib for patients with creatinine clearance <50 ml/min is 200 mg QD.
BID: Twice daily; QD: Once daily.
Patients in Arm A will receive selpercatinib at a starting dose of 160 mg twice daily. Patients in arm B will receive cabozantinib at a starting dose of 140 mg once daily (QD) or vandetanib at a starting dose of 300 mg QD. All treatments are administered orally, and a cycle is defined as 28 days in length, regardless of treatment arm assignment. Treatment will continue until radiographic disease progression, unacceptable toxicity, withdrawal of consent or death. Dosing and treatment durations for all intervention groups are outlined in Table 3.
Efficacy evaluations
All patients are required to undergo imaging at baseline and subsequent serial imaging at disease assessment time points. Scans should be obtained within 28 days of baseline and every 8 weeks after treatment initiation through week 24, and every 12 weeks thereafter until progression, the start of a new anticancer therapy, death, or study completion. The BICR will conduct assessment of tumor response by RECIST 1.1.
Safety evaluations
For each patient, safety assessments including physical examination, vital signs, electrocardiogram and clinical laboratory tests will be evaluated at predefined intervals. Adverse events will be continuously evaluated throughout the duration of the study.
Conclusion
The LIBRETTO-531 phase III trial outlined here will evaluate selpercatinib versus cabozantinib or vandetanib in patients with advanced/metastatic RET-mutant MTC. The findings of this study will help further define the benefit of selpercatinib used as first-line therapy in patients with RET-mutant MTC, naive to prior kinase inhibitor therapy.
Executive summary.
Background
Medullary thyroid carcinoma (MTC) is a rare malignancy of the thyroid that accounts for 5–10% of all thyroid malignancies.
Germline-activating RET mutations are found in 95–98% of hereditary MTC, and somatic RET mutations are present in >50% of the cases of sporadic MTC.
Multikinase inhibitors (MKI) with anti-RET activity have limited efficacy due to incomplete inhibition and significant toxicity.
Selpercatinib
Selpercatinib, a first-in-class highly selective and potent RET kinase inhibitor, is approved in multiple countries for the treatment of RET-altered lung or thyroid cancers.
Selpercatinib has nanomolar potency against wild type RET and RET alterations including M918T, MKI resistance-associated V804M, and others.
In the LIBRETTO-001 phase I/II trial, selpercatinib treatment demonstrated clinically meaningful responses and sustained antitumor activity with a manageable toxicity profile in both heavily pre-treated and treatment-naive patients with RET-mutant MTC.
LIBRETTO-531 study
The global, multicenter, open-label, randomized, controlled, phase III LIBRETTO-531 trial, will evaluate selpercatinib versus cabozantinib or vandetanib in patients with MKI-naive locally advanced or metastatic RET-mutant MTC (NCT04211337).
First ever randomized Phase III clinical trial in patients with MKI treatment naive RET-mutant MTC.
Conclusion
The results of this key trial will help further define the role of selpercatinib as a front-line treatment for people living with advanced or metastatic RET-mutant MTC.
Supplementary Material
Acknowledgments
The authors thank the patients and their caregivers for their participation in this study, the study investigators and their staff, the independent data monitoring committee, and the entire LIBRETTO-531 clinical trial team.
Footnotes
Supplementary data
An infographic accompanies this paper. To view or download this infographic in your browser please click here: https://www.futuremedicine.com/doi/suppl/10.2217/fon-2022-0657
Author contributions
All authors were involved in the conception, design or planning of the study, and critically reviewed and revised the manuscript for intellectual content as well as read and approved the final version to be published.
Financial & competing interests disclosure
Funding for this research was provided by LOXO Oncology Inc., a wholly owned subsidiary of Eli Lilly and Company. LJ Wirth reports receiving consulting fees from Bayer, Eli Lilly and Company, Exelixis, and Merck. MS Brose reports receiving grant funding to institution from Eli Lilly and Company, Bayer/LOXO Oncology, Exelixis, and Blueprint Medicines; receiving consulting fees from Bayer and Eli Lilly and company; and participating on a data safety monitoring or advisory board for Eli Lilly and Company, Bayer, Exelixis, and Aadi. R Elisei reports receiving consulting fees from Eisai, Ipsen, Eli Lilly and Company, LOXO Oncology, Bayer, and Roche; and participating on a data safety monitoring or advisory board for Eisai, Ipsen, Eli Lilly and Company, LOXO Oncology, Bayer, and Roche. J Capdevila reports receiving payment or honoraria from Bayer, Exelixis, Eli Lilly and Company, Eisai, Sanofi, Merk Serono, Amgen, Roche, Adacap, Esteve, Advanz, Ipsen, Pfizer, Novartis, and Huchmed; receiving support for attending meetings and/or travel from Ipsen and Eli Lilly and Company; and serving as a chair of the Spanish Taskforce for Neuroendocrine and Endocrine Tumors (GETNE); executive member of the European Society of Neuroendocrine Tumors (ENETS); treasurer of the Spanish Multidisciplinary Taskforce for Digestive Cancers (GEMCAD). AO Hoff reports receiving research support to institution from Exelixis, Eli Lilly and Company, Eisai, and Roche; receiving consulting fees from Eli Lilly and Company, Exelixis and Bayer; receiving honoraria from Bayer, United and Genzyme; and participating on a data safety monitoring or advisory board for Eli Lilly and Company and Bayer. M Hu reports receiving research funding to institution from Eli Lilly and Company; receiving consulting fees from Veracyte; receiving honoraria from Eli Lilly and Company and Blueprint Medicines; receiving travel support from Eli Lilly and Company; participating on a data safety monitoring or advisory board for LOXO Oncology, Blueprint Medicines, Eli Lilly and Company; and serving as leadership for ThyCa Medical Advisory Group Member. M Tahara reports receiving consulting fees from Ono Pharmaceutical, MSD, BMS, and Merck Biopharma; and receiving payment or honoraria from Eisai, Ono Pharmaceutical, Merck Biopharma, Rakuten Medical, BMS, Eli Lilly and Company, and MSD. B Robinson reports consulting for Eisai and LOXO Oncology; stock ownership for Cochlear and Mayne Pharma; Speaker's Bureau for Eisai; receiving travel fees from Eisai; consulting or advising for Blueprint Medicines, Cota Healthcare, Eisai, Goldilocks, Eli Lilly and Company, Regeneron, and UpToDate; receiving research funding from Plexxikon and Regeneron; and serving as leadership for Cochlear and Mayne Pharma. M Xia and P Maeda are employees of Eli Lilly and Company and own stock in the company. E Sherman reports consulting or advising for Blueprint Medicines, Cota Healthcare, Eisai, Goldilocks, Eli Lilly and Company, Regeneron, UpToDate; and receiving research funding from Plexxikon and Regeneron. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by HM Messersmith of Eli Lilly and Company. Eli Lilly and Company contracted with Syneos Health for editorial support provided by Antonia Baldo.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Wolfe HJ, Melvin KE, Cervi-Skinner SJ et al. C-cell hyperplasia preceding medullary thyroid carcinoma. N. Engl. J. Med. 289(9), 437–441 (1973). [DOI] [PubMed] [Google Scholar]
- 2.Kouvaraki MA, Shapiro SE, Perrier ND et al. RET proto-oncogene: a review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid 15(6), 531–544 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Accardo G, Conzo G, Esposito D et al. Genetics of medullary thyroid cancer: an overview. Int. J. Surg. 41(Suppl. 1), S2–S6 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Salvatore D, Santoro M, Schlumberger M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat. Rev. Endocrinol. 17(5), 296–306 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Ciampi R, Romei C, Ramone T et al. Genetic landscape of somatic mutations in a large cohort of sporadic medullary thyroid carcinomas studied by next-generation targeted sequencing. iScience 20, 324–336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romei C, Casella F, Tacito A et al. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J. Med. Genet. 53(11), 729–734 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Elisei R, Tacito A, Ramone T et al. Twenty-five years experience on RET genetic screening on hereditary MTC: an update on the prevalence of germline RET mutations. Genes (Basel) 10(9), 698 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donis-Keller H, Dou S, Chi D et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 2(7), 851–856 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Mulligan LM, Kwok JB, Healey CS et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363(6428), 458–460 (1993). [DOI] [PubMed] [Google Scholar]
- 10.Mulligan LM, Marsh DJ, Robinson BG et al. Genotype-phenotype correlation in multiple endocrine neoplasia type 2: report of the International RET Mutation Consortium. J. Intern. Med. 238(4), 343–346 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 12(4), 192–202 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Elisei R, Cosci B, Romei C et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J. Clin. Endocrinol. Metab. 93(3), 682–687 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Yue CH, Oner M, Chiu CY et al. RET regulates human medullary thyroid cancer cell proliferation through CDK5 and STAT3 activation. Biomolecules 11(6), (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plaza-Menacho I, Mologni L, Mcdonald NQ. Mechanisms of RET signaling in cancer: current and future implications for targeted therapy. Cell. Signal. 26(8), 1743–1752 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Belli C, Penault-Llorca F, Ladanyi M et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann. Oncol. 32(3), 337–350 (2021). [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Thyroid Cancer Version (2020).
- 17.Maxwell JE, Sherman SK, O'dorisio TM, Howe JR. Medical management of metastatic medullary thyroid cancer. Cancer 120(21), 3287–3301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorusso L, Cappagli V, Valerio L et al. Thyroid cancers: from surgery to current and future systemic therapies through their molecular identities. Int. J. Mol. Sci. 22(6), 3317 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Sippel RS, O'dorisio MS et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 39(6), 775–783 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells SA Jr, Robinson BG, Gagel RF et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30(2), 134–141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elisei R, Schlumberger MJ, Muller SP et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 31(29), 3639–3646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirth LJ, Sherman E, Robinson B et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N. Engl. J. Med. 383(9), 825–835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Findings from the multicenter, global, phase I/II LIBRETTO-001 trial reporting the safety and efficacy of selpercatinib in patients with RET-altered thyroid cancers.
- 23.Sherman EJ, Wirth LJ, Shah MH et al. Selpercatinib efficacy and safety in patients with RET-altered thyroid cancer: a clinical trial update. J. Clin. Oncol. 39(Suppl. 15), 6073–6073 (2021). [Google Scholar]; •• Updated findings from the multicenter, global, phase I/II LIBRETTO-001 trial reporting the safety and efficacy of selpercatinib in patients with RET-altered thyroid cancers.
- 24.US Food and Drug Administration. Retevmo (selpercatinib) (2022). www.accessdata.fda.gov/drugsatfda_docs/label/2020/213246s000lbl.pdf
- 25.Subbiah V, Gainor JF, Rahal R et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 8(7), 836–849 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Subbiah V, Hu MI, Wirth LJ et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 9(8), 491–501 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Us Food and Drug Administration. Gavreto (Pralsetinib). www.accessdata.fda.gov/drugsatfda_docs/label/2020/213721s000lbl.pdf (Accessed July 19).
- 28.Subbiah V, Velcheti V, Tuch BB et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 29(8), 1869–1876 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the preclinical antitumor activity of LOXO-292 and provides clinical proof-of-concept for selective RET inhibition with LOXO-292 for patients with RET-altered cancers.
- 29.Drilon A, Oxnard GR, Tan DSW et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N. Engl. J. Med. 383(9), 813–824 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Findings from the global phase 1/2 study (LIBRETTO-001) reporting the safety and efficacy of selpercatinib in patients with RET-postive non-small-cell lung cancer.
- 30.Matrone A, Prete A, Sartini MS, Elisei R. Significant response of medullary thyroid cancer choroidal metastases to highly selective RET inhibitor selpercatinib: a case report. Ann. Oncol. 32(11), 1447–1449 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Elisei R, Ciampi R, Matrone A et al. Somatic RET indels in sporadic medullary thyroid cancer: prevalence and response to selpercatinib. J. Clin. Endocrinol. Metab. 107(8), 2195–2202 (2022). [DOI] [PubMed] [Google Scholar]
- 32.European Medicines Agency, 2021. Retsevmo (selpercatinib) (2022). www.ema.europa.eu/en/medicines/human/EPAR/retsevmo#product-information-section
- 33.Wirth LJ, Sherman EJ, Weiler D et al. Efficacy of selpercatinib after prior systemic therapy in patients with RET mutant medullary thyroid cancer. J. Clin. Oncol. 39(Suppl. 15), 6074–6074 (2021). [Google Scholar]; •• Updated results from the phase I/II LIBRETTO-001 trial reporting the safety and efficacy of selpercatinib in patients with RET-mutant MTC who received a prior systemic therapy.
- 34.Gill J, Prasad V. When are randomized controlled trials needed to assess novel anticancer drugs? An illustration based on the development of selpercatinib, a RET inhibitor. Ann. Oncol. 31(3), 328–330 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


