Abstract
Methylobacterium extorquens AM1 was used to explore the genetics of dephosphotetrahydromethanopterin (dH4MPT) biosynthesis. Strains with mutations in eight “archaeal-type” genes linked on the chromosome of M. extorquens AM1 were analyzed for the ability to synthesize dH4MPT, and six were found to be dH4MPT negative. Putative functions of these genes in dH4MPT biosynthesis are discussed.
Tetrahydromethanopterin (H4MPT) is a specific carrier of C1 units in the two widespread and environmentally important bioconversions, methanogenesis and methylotrophy (4, 5). Besides having a well-defined role in C1 transfer in methanogenic archaea and methylotrophic bacteria, H4MPT or its derivatives are implied to exist in organisms not known to be capable of these bioconversions, pointing to the possibility of H4MPT's involvement in other, yet-uncharacterized biochemical processes (4, 14). The presence and the distribution of H4MPT-linked reactions in the microbial world also provide important clues toward understanding the evolution of biochemical pathways.
While the biochemistry of H4MPT biosynthesis is well established (6, 23, 24), its genetics remain poorly understood. A number of genes were implied to be involved in H4MPT biosynthesis in archaea, based on gene overexpression and detection of the desired activity (7, 8, 10, 20, 25). However, mutant evidence confirming the functions in question is missing for archaea. The facultative methylotrophic bacterium Methylobacterium extorquens AM1 has been shown to contain a modified form of H4MPT, dephosphoH4MPT (dH4MPT) (5). Two genes have been shown to be involved so far in dH4MPT biosynthesis via gene overexpression, activity detection, and mutant analysis (1, 15, 18), demonstrating that M. extorquens AM1 presents an excellent model for identifying other genes in the dH4MPT biosynthetic pathway. This α-proteobacterium contains a cluster of genes whose translated polypeptides have homologs in archaea (3-5). While the functions of some genes in the cluster are well established in dH4MPT-linked C1 transfer (Fig. 1) (5, 9, 16, 17, 22), the functions of others remain unknown. We hypothesized that these genes of unknown function homologous to genes of unknown function in archaea might encode the yet-unidentified enzymes in the dH4MPT biosynthesis pathway. In this work, we mutate eight of the genes with unknown functions, show that mutations in all of them have a characteristic phenotype, and demonstrate the lack of dH4MPT production in six of these mutants.
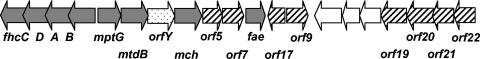
FIG. 1.
Cluster of archaeal-type genes in M. extorquens AM1. Functions of genes shown as grey arrows are known. fhcA, -B, -C, and -D encode the four subunits of the formyltransferase-hydrolase complex (16), mptG encodes ribofuranosylaminobenzene phosphate synthase (18), mtdB encodes methylene-H4MPT dehydrogenase (9), mch encodes methenyl-H4MPT cyclohydrolase (17), and fae encodes formaldehyde-activating enzyme (22). The function of ORFY remains unknown. Functions of genes shown in hatched arrows are assessed in this study. Genes shown as open arrows, as well as mtdB, have no homologs among Archaea.
New “archaeal-type” genes in M. extorquens AM1.
A cluster of genes in M. extorquens AM1 having homologs in archaea has been described previously (5). Some of these archaeal-type genes have been shown to catalyze reactions of dH4MPT-linked C1 transfers, similar to reactions of methanogenesis (9, 16, 17, 22). One was shown to catalyze the first reaction in dH4MPT biosynthesis (18), while the functions of others remained unknown. We sequenced a DNA region adjacent to this cluster and discovered four more genes whose translated polypeptides revealed high similarity to archaeal polypeptides of unknown function. These genes were designated open reading frame 19 (ORF19), ORF20, ORF21, and ORF22 (Fig. 1). A draft sequence (approximately 6.5-fold coverage) of the complete genome of M. extorquens AM1 is available, and its analysis has shown that only one archaeal-type gene island is present on the chromosome (3), that depicted in Fig. 1. The sequence of the chromosomal region involved in this study is available under GenBank accession numbers AF032114 (ORF5, ORF7, ORF17, and ORF9) and AY117134 (ORF19 to ORF22).
Function prediction based on protein family affiliation.
Since the time archaeal-type genes were first discovered in M. extorquens AM1, protein databases have expanded dramatically, and function prediction based on protein family group affiliation has become a powerful tool. We used these tools to analyze the newly discovered archaeal-type genes and also to revisit function prediction for the genes described before (3). The general predicted function for each protein in accordance with COG (clusters of orthologous groups)/Pfam affiliations is shown in Table 1. More details are given below. ORF5 is predicted to encode a protein belonging to a family of ligases represented by RimK, CarB, and PurK, the enzymes of cofactor biosynthesis (10). The function of the ortholog of the ORF5 product in Methanosarcina and Methanococcus species has recently been predicted: modification of H4MPT into tetrahydrosarcinapterin (H4SPT) by the addition of a terminal glutamyl residue (10). The protein encoded by ORF7 is predicted to belong to a protein family including CitG, which is a kinase that participates in the biosynthesis of a prosthetic group of citrate lyase (19). ORF9 is predicted to encode a protein belonging to a family that includes hydantoinase and oxoprolinase, both reactions involving the hydrolysis of five-membered rings via hydrolysis of their internal imide bonds. The ORF17 product is predicted to be similar to members of the HisA family. HisA catalyzes phosphoribosylformimino-5-aminoimidazole carboxamide ribonucleotide isomerization. The ORF20 product is predicted to belong to the dihydropteroate synthase family, which includes an enzyme in the tetrahydrofolate biosynthesis pathway. The ORF21 product is predicted to be a kinase. No functional predictions could be made for the proteins encoded by ORF19 and ORF22.
TABLE 1.
Characteristics of genes and mutants involved in this study
| Gene | COG/Pfam-predicted general function | Methanol MIC (mM)a | Complementation forb:
|
MDH test result | dH4MPT test resultc | |
|---|---|---|---|---|---|---|
| Growth | C1 sensitivity | |||||
| ORF5 | Cofactor biosynthesis | 0.09 | − | + | + | − |
| ORF7 | Cofactor biosynthesis | 0.13 | + | + | + | + |
| ORF9 | C5 ring cleavage | 0.10 | − | − | + | − |
| ORF17 | Ribonucleotide isomerase | 0.20 | + | + | + | + |
| ORF19 | Unknown | 0.10 | + | + | + | − |
| ORF20 | Cofactor biosynthesis | 0.10 | + | + | + | − |
| ORF21 | Kinase | 0.09 | − | + | − | − |
| ORF22 | Unknown | 0.09 | − | + | Low | − |
For comparison, the wild-type strain is not inhibited by 100 mM methanol.
+, positive; −, negative. Results of expressing a gene in trans, from a low-activity or a high-activity promoter, are shown.
+, dH4MPT detected at or above the wild-type level of 64 μM dH4MPT; −, dH4MPT undetectable (detection limit, 3 μM dH4MPT).
Mutant generation and mutant phenotypes.
A number of mutants with mutations in the genes of the dH4MPT-linked formaldehyde oxidation pathway have been characterized, demonstrating that the pathway not only plays a central role in the C1 metabolism of M. extorquens AM1, but is also essential during growth on multicarbon substrates and is likely to function in formaldehyde detoxification (5, 9, 11, 15, 17, 22). Thus, mutating the dH4MPT pathway genes either causes a lethal effect on the organism (fhc genes and mch) or results in a dramatic phenotype, i.e., extreme sensitivity to C1 compounds (mptG, mtdB, fae, and dmrA). Therefore, while generating mutations in the newly discovered archaeal-type open reading frames, special care was taken to avoid any contact with vapors of methanol. The insertion mutagenesis technique described previously (2) (mutations in ORF7, ORF17, ORF20, and ORF21) or the deletion and insertion mutagenesis technique described previously (13) (mutations in ORF5, ORF9, ORF19, and ORF22) were employed. In our earlier work, mutants with mutations in ORF 5, ORF7, ORF9, and ORF17 were generated, but without special precautions being taken against the possible presence of methanol vapors. While ORF7 mutants were double-crossovers defective for growth on methanol, ORF9 and ORF17 mutants were obtained as single-crossover recombinants with a methanol-negative phenotype, while ORF5 mutants had the wild-type phenotype (5). We revisited the mutagenesis of these genes under methanol-free conditions and obtained double-crossover recombinants for all four genes. Double-crossover mutants were also obtained for the four new genes (ORF19 to ORF22). All new mutants were tested for growth on methanol by plating cells on methanol-containing plates and also for methanol sensitivity by streaking cells on succinate-containing plates and exposing them to methanol vapors added at final concentrations between 0.05 and 50 mM. Methanol (100% or appropriately diluted in water) was added to the lids of the inverted plates (1.2 to 50 μl; the concentration was calculated per the total volume of a plate equal to 75 ml), plates were immediately sealed with Parafilm and incubated for 2 days at 30°C before growth assessment. All new mutants were shown to be negative for growth on methanol, and all revealed high methanol sensitivity (Table 1).
Observations on recombination frequencies and also growth patterns of ORF5, ORF9, ORF21, and ORF22 recombinants suggested that, in order to allow for the double-crossover recombination event, a second compensatory mutation might have been required. Double-crossover recombinants for these genes appeared at very low frequencies (10−11 to 10−10) and grew extremely slowly at first but eventually formed colonies that grew more competitively. To test the hypothesis about the presence of secondary compensatory mutations in these mutants, we introduced copies of the genes in each of the mutants in trans, transcribed from either the low-activity or the high-activity promoter (12). While ORF7, ORF17, ORF19, and ORF20 mutants were complemented for both growth and methanol sensitivity by respective genes when the genes were transcribed at both low and high levels, ORF5, ORF21, and ORF22 mutants were complemented only for methanol sensitivity and not growth, supporting the idea of the presence of secondary, spontaneous mutations. ORF9 mutants were not complemented for either growth or methanol sensitivity (Table 1). One kind of a compensatory mutation that allows the growth of a methanol-sensitive mutant is a mutation in the methanol dehydrogenase (MDH) gene, as we demonstrated before (9, 11). We tested MDH activity in the mutants in question by visualizing MDH in gels, after isoelectric focusing (pIs 3 to 9) in PhastGels (Amersham). The reaction mixture for MDH staining contained 100 mM Tris-HCl buffer (pH 9.0), 1 mM phenasinemetasulfate, 1 mM nitroblue tetrazolium, 1 mM (NH4)2SO4, and 5 mM methanol (all reagents were purchased from Sigma). We found that the ORF21 mutant contained no MDH activity and that the ORF22 mutant contained lowered MDH activity, while ORF5 and ORF9 mutants contained wild-type MDH activity (Table 1). There are many other sites for such compensatory mutations, which we have not tested in this work; for example, mutations in cytochrome c or in the gene for heme biosynthesis would lead to similar compensatory effects.
To determine whether the mutants were capable of synthesizing dH4MPT, we used an enzymatic assay developed previously to detect H4MPT analogs in archaea and M. extorquens AM1 (18). Of the eight mutants tested, only ORF7 and ORF17 mutants produced dH4MPT, while ORF5, ORF9, ORF19, ORF20, ORF21, and ORF22 mutants did not produce detectable levels of dH4MPT (Table 1). These results are consistent with a role for the last six genes in the biosynthesis of dH4MPT. We also performed dH4MPT tests with a subset of mutants complemented with the respective genes in trans and found them positive for dH4MPT (data not shown).
Dephospho-H4MPT biosynthesis.
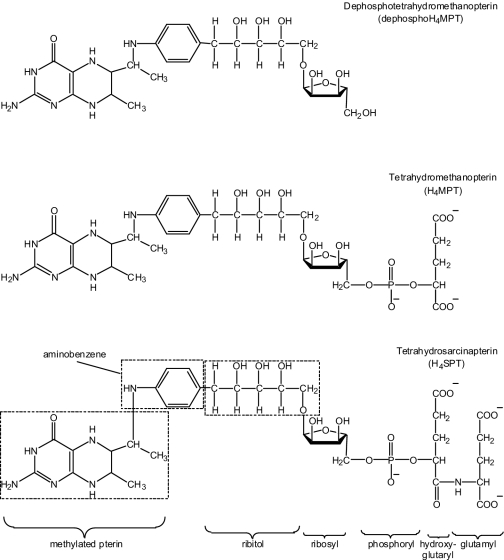
In methanogenic archaea, 18 enzymes have been proposed to be involved in H4MPT or H4SPT biosynthesis, and five genes have been suggested so far to be involved in these reactions (7, 8, 10, 20, 23-25). As shown in Fig. 2, dH4MPT from M. extorquens AM1 lacks the phosphate, hydroxyglutaryl, and terminal glutamate residues present in the archaeal coenzyme H4SPT (5). Therefore, it is anticipated that the biosynthetic pathway of dH4MPT shares some steps in common with the archaeal pathway but that it contains fewer enzymatic steps. The enzymes involved in the first and last proposed steps of dH4MPT biosynthesis in M. extorquens AM1 have already been identified. Ribofuranosylaminobenzene 5′-phosphate (RFAP) synthase catalyzing the first step of H4MPT biosynthesis, the reaction of p-aminobenzoic acid with phosphoribosylpyrophosphate, is encoded by mptG (formerly ORF4), an archaeal-type gene found in the dH4MPT-linked C1 transfer gene cluster of M. extorquens AM1 (Fig. 1) (18). The last reaction of the pathway is the reduction of dephospho-dihydromethanopterin to the active tetrahydro form (dH4MPT) catalyzed by dihydromethanopterin reductase, which is encoded by dmrA (1, 15). By analogy to the second reaction of H4SPT biosynthesis in archaea (23), the second proposed reaction of dH4MPT biosynthesis in bacteria is the condensation of RFAP with 6-hydroxymethyldihydropterin pyrophosphate to form dihydropterin-RFAP. This reaction is similar to the dihydropteroate synthase reaction of folate biosynthesis in which 6-hydroxymethyldihydropterin pyrophosphate and p-aminobenzoate react to produce dihydropteroate (21). In the archaeon Methanocaldococcus jannaschii, the second reaction of H4MPT biosynthesis has been proposed to be catalyzed by a protein designated MJ0301 (25). While no homolog for MJ0301 is identifiable in the M. extorquens AM1 genome, one of the putative dH4MPT biosynthesis genes, ORF20, codes for a protein in the dihydropteroate synthase family of enzymes, making it a candidate for the second enzyme of the dH4MPT synthesis pathway. The enzymes catalyzing the next three reactions of H4SPT biosynthesis in archaea are currently unknown. In these reactions, the ribose ring of dihydropterin-RFAP is opened and reduced to produce a linear ribitol phosphate moiety, a phosphate group is removed, and a second phosphoribosyl group is added. At this point, because the structure of dH4MPT is truncated relative to H4SPT (Fig. 2), the bacterial and archaeal pathways are likely to diverge. Only three modifications would be required to convert the product of the fifth step to dH4MPT: removal of a phosphate group, methylation of the dihydropterin at two positions, and reduction of the methylated dihydropterin to produce dH4MPT. Thus, the proposed pathway for dH4MPT biosynthesis in bacteria consists of eight steps, and the enzymes catalyzing six of the steps are unknown. Experiments are in progress to determine whether the putative dH4MPT biosynthetic genes identified in this study encode proteins that catalyze some of these enzymatic reactions.
FIG. 2.
Structures of H4MPT and H4MPT analogs discussed in this study.
Conclusions.
In summary, we have provided evidence that the genes designated ORF5, ORF9, ORF19, ORF20, ORF21, and ORF22 in M. extorquens AM1 function in the biosynthesis of the coenzyme dH4MPT. While the ORF20 product is likely to catalyze the second reaction in the biosynthetic pathway, exact functions of other enzymes remain unknown. ORF7 and ORF17 mutants were able to produce dH4MPT; thus, their function in dH4MPT-linked C1 transfer remains unknown. Biochemical characterization of the corresponding enzymes will contribute to elucidating the specific roles of the genes in bacterial dH4MPT biosynthesis and may serve as a model system for identifying unknown H4MPT biosynthesis enzymes in archaea.
Acknowledgments
This work was supported by a grant from the NIH (GM58933) to M.E.L. and by a grant from the NSF (MCB-9876212) to M.E.R.
REFERENCES
- 1.Caccamo, M. A., C. M. Malone, and M. E. Rasche. 2004. Biochemical characterization of a dihydromethanopterin reductase involved in tetrahydromethanopterin biosynthesis in Methylobacterium extorquens AM1. J. Bacteriol. 186:2068-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chistoserdov, A. Y., L. V. Chistoserdova, W. S. McIntire, and M. E. Lidstrom. 1994. The genetic organization of the mau gene cluster in Methylobacterium extorquens AM1: complete nucleotide sequence and generation and characteristics of mau mutants. J. Bacteriol. 176:4052-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chistoserdova, L., S.-W. Chen, A. Lapidus, and M. E. Lidstrom. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J. Bacteriol. 185:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chistoserdova, L., C. Jenkins, M. G. Kalyuzhnaya, C. J. Marx, A. Lapidus, J. A. Vorholt, J. T. Staley, and M. E. Lidstrom. 2004. The enigmatic planctomycetes may hold a key to the origins of methanogenesis and methylotrophy. Mol. Biol. Evol. 21:1234-1241. [DOI] [PubMed] [Google Scholar]
- 5.Chistoserdova, L., J. A. Vorholt, R. K. Thauer, and M. E. Lidstrom. 1998. C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic archaea. Science 281:99-102. [DOI] [PubMed] [Google Scholar]
- 6.Graham, D. E., and R. H. White. 2002. Elucidation of methanogenic coenzyme biosyntheses: from spectroscopy to genomics. Nat. Prod. Rep. 19:133-147. [DOI] [PubMed] [Google Scholar]
- 7.Graham, D. E., H. Xu, and R. H. White. 2002. A member of a new class of GTP cyclohydrolases produces formylaminopyrimidine nucleotide monophosphates. Biochemistry 41:15074-15084. [DOI] [PubMed] [Google Scholar]
- 8.Graupner, M., H. Xu, and R. H. White. 2000. Identification of an archaeal 2-hydroxy acid dehydrogenase catalyzing reactions involved in coenzyme biosynthesis in methanoarchaea. J. Bacteriol. 182:3688-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagemeier, C. H., L. Chistoserdova, M. E. Lidstrom, R. K. Thauer, and J. A. Vorholt. 2000. Characterization of a second methylene tetrahydromethanopterin dehydrogenase from Methylobacterium extorquens AM1. FEBS Lett. 267:3762-3769. [DOI] [PubMed] [Google Scholar]
- 10.Li, H., H. Xu, D. E. Graham, and R. H. White. 2003. Glutathione synthetase homologs encode alpha-l-glutamate ligases for methanogenic coenzyme F420 and tetrahydrosarcinapterin biosyntheses. Proc. Natl. Acad. Sci. USA 100:9785-9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx, C. J., L. Chistoserdova, and M. E. Lidstrom. 2003. Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J. Bacteriol. 185:7160-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marx, C. J., and M. E. Lidstrom. 2001. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065-2075. [DOI] [PubMed] [Google Scholar]
- 13.Marx, C. J., and M. E. Lidstrom. 2002. A broad-host-range cre-lox system for antibiotic marker recycling in Gram-negative bacteria. BioTechniques 33:1062-1067. [DOI] [PubMed] [Google Scholar]
- 14.Marx, C. J., J. A. Miller, L. Chistoserdova, and M. E. Lidstrom. 2004. Multiple formaldehyde oxidation/detoxification pathways in Burkholderia fungorum LB400. J. Bacteriol. 186:2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx, C. J., B. N. O'Brien, J. Breezee, and M. E. Lidstrom. 2003. Novel methylotrophy genes of Methylobacterium extorquens AM1 identified by using transposon mutagenesis including a putative dihydromethanopterin reductase. J. Bacteriol. 185:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pomper, B. K., O. Saurel, A. Milon, and J. A. Vorholt. 2002. Generation of formate by the formyltransferase/hydrolase complex (Fhc) from Methylobacterium extorquens AM1. FEBS Lett. 523:133-137. [DOI] [PubMed] [Google Scholar]
- 17.Pomper, B. K., J. A. Vorholt, L. Chistoserdova, M. E. Lidstrom, and R. K. Thauer. 1999. A methenyl tetrahydromethanopterin cyclohydrolase and a methenyl tetrahydrofolate cyclohydrolase in Methylobacterium extorquens AM1. Eur. J. Biochem. 261:475-480. [DOI] [PubMed] [Google Scholar]
- 18.Rasche, M. E., S. A. Havemann, and M. Rosenzvaig. 2004. Characterization of two methanopterin biosynthesis mutants of Methylobacterium extorquens AM1 by use of a tetrahydromethanopterin bioassay. J. Bacteriol. 186:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider, K., P. Dimroth, and M. Bott. 2000. Identification of triphosphoribosyl-dephospho-CoA as precursor of the citrate lyase prosthetic group. FEBS Lett. 483:165-168. [DOI] [PubMed] [Google Scholar]
- 20.Scott, J. W., and M. E. Rasche. 2002. Purification, overproduction, and partial characterization of β-RFA-P synthase, a key enzyme in the pathway of methanopterin biosynthesis. J. Bacteriol. 184:4442-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiota, T. 1984. Biosynthesis of folate from pterin precursors, p. 61-120. In R. L. Blakley and S. J. Benkovic (ed.), Folates and pterins, vol. 1: chemistry and biochemistry of folates. John Wiley & Sons, New York, N.Y.
- 22.Vorholt, J. A., C. J. Marx, M. E. Lidstrom, and R. K. Thauer. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J. Bacteriol. 182:6645-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White, R. H. 1996. Biosynthesis of methanopterin. Biochemistry 19:3447-3456. [DOI] [PubMed] [Google Scholar]
- 24.White, R. H. 2001. Biosynthesis of the methanogenic cofactors. Vitam. Horm. 61:299-337. [DOI] [PubMed] [Google Scholar]
- 25.Xu, H., R. Aurora, G. D. Rose, and R. H. White. 1999. Identifying two ancient enzymes in Archaea using predicted secondary structure alignment. Nat. Struct. Biol. 6:750-754. [DOI] [PubMed] [Google Scholar]