Abstract
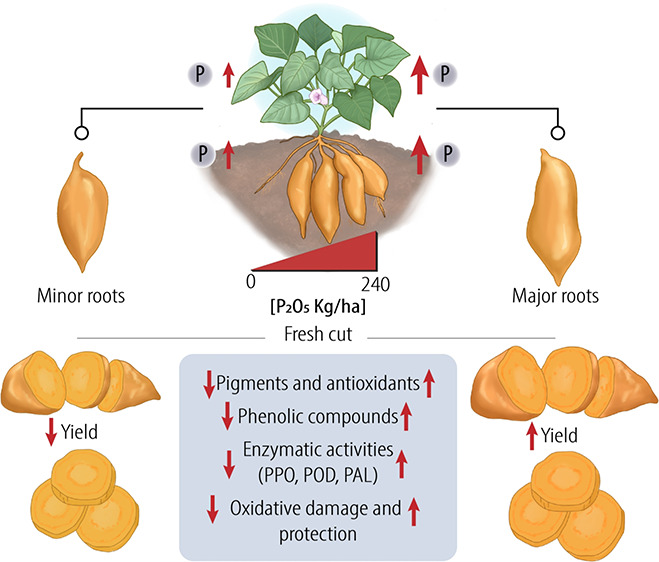
The present work aimed to study oxidative damage and protection, phenylpropanoid metabolism, and the quality of minimally processed colored sweet potatoes cultivated with increments in P2O5 fertilization. Sweet potato was cultivated with 0, 60, 120, 180, and 240 kg ha–1 of P2O5. The roots were harvested, and the P content in the roots and leaves was quantified. The roots were minimally processed and kept for 20 days at 5 °C. In general, the roots that were fertilized with P2O5 showed a higher content of the analyzed variables. The highest P dosage in the soil increased the P content in roots and leaves and the agro-industrial yield. Roots cultivated with P2O5 showed a higher content of hydrogen peroxide, phenolic compounds, vitamin C, yellow flavonoids, anthocyanins, and carotenoids, antioxidant capacity by the DPPH method, and higher activity of the enzymes polyphenol oxidase, peroxidase, and phenylalanine ammonia lyase. These results demonstrated the role of phosphorus in protecting against oxidative damage due to the accumulation of bioactive compounds, thus improving the physicochemical quality of minimally processed orange sweet potato.
Introduction
The sweet potato (Ipomoea batatas (L.) Lam.; I. batatas) ranks seventh in the world’s crop statistics outranked only by wheat, rice, maize, potato, cassava, and barley, making it an important food crop that serves as a source of energy and nutrition in many countries.1,2 It is considered a food safety crop due to its low input requirements, ease of production, and high-nutritional components.3,4 In Brazil, sweet potato has a strong social role as it is cultivated by small farmers with generally family-based labor. Perhaps for this reason, it is still an unexplored culture in the country mainly with regard to new technologies, information on management, fertilization, and the development of new cultivars adapted to different Brazilian regions.5
The nutritional composition and productive potential place the sweet potato among the interests of the industry regarding its use either as a minimally processed source of raw material for obtaining bioactive compounds or for obtaining industrialized products with nutritional quality or even for adding greater commercial value to other products.6 As pigmented pulp cultivars have high levels of carotenoids6,7 with antioxidant potential, sweet potato is a possible alternative as a food supplement to supply vitamin A deficiency and an abundant and low-cost source of β-carotene when compared to white and cream-fleshed cultivars.8 Sweet potato roots are also sources of phenolic compounds, which are antioxidants with pharmacological activity, and thus, the crop can potentially be used as a functional food. Consequently, the phenolic content in sweet potato roots can serve as a useful indicator of their antioxidant activity.9
The proper application of phosphorus can enhance sweet potato yields as the crop is very efficient in absorbing phosphorus. Due to the common deficiency in Brazilian soils of this nutrient, it must be applied in a readily available form and at the right time.10,11 Recently, another role was discovered for phosphorus in inducing the metabolism of phenylpropanoids and oxidation of phenolic compounds that cause browning in minimally processed cassava.12 This discovery complemented previous findings that provided more biochemical details on the participation of adenosine triphosphate (ATP) in the intra- and intercellular signaling and transduction process in the tissues of cut carrots that leads to wound-induced biosynthesis and accumulation of phenolic compounds.13
The findings by Eugênio et al.12 raised the question of whether the browning evidenced in minimally processed cassava was intensified because this root lacks or has small amounts of these antioxidant pigments, such as carotenoids and flavonoids among others. These phytochemicals can also mask oxidative reactions due to the coloring of these pigments, reducing the appearance of browning.14 Thus, this suggests that a root containing these pigments, such as sweet potato, may have enzymatic browning masked by them. This research aimed to study the use of increasing doses of P2O5 as an agronomic modulator for the induction of bioactive compounds and protection from oxidative damage in sweet potatoes after minimal processing.
Material and Methods
Characterization, Management of the Experimental Area, Fertilization, and Culture Management
Sweet potato plants were cultivated in the experimental unit of Fazenda Rafael Fernandes belonging to the Federal Rural University of the Semiarid region (UFERSA). The study area is located in the Alagoinha district in Mossoró in the state of Rio Grande do Norte, Brazil (5°03′31.00″ S, 37°23′47.57′′ W, and 80 m altitude). According to the Köppen classification, the characteristic climate of the region is semi-arid of the BSh type (dry and very hot)15 with the rainy season occurring between February and May. The average annual precipitation in the region is 670 mm, the relative humidity is 68.9%, and the dry period is from June to January.15 Meteorological data were collected throughout the experiment through the meteorological station installed on the experimental farm (Figure S1).
The soil in the experimental area is classified as typical dystrophic Red Latosol.16 Soil samples at depths of 0–0.20 m and 0.20–0.40 m were collected for chemical characterization17 (Table 1).
Table 1. Chemical Analysis of the Soil in the Experimental Area of the Rafael Fernandes Experimental Farm in Mossoró, RN, Brazil.
| depth (cm) | pH (water) | CE (dS m–1) | P* (mg dm–3) | K+ (mg dm–3) | Na+ (mg dm–3) | Ca2+ (cmolc dm–3) | Mg2+ (cmolc dm–3) | Al3+ (cmolc dm–3) | SB (cmolc dm–3) | t (cmolc dm–3) | CTC (cmolc dm–3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 | 6.34 | 70.9 | 4.69 | 61.83 | 12.68 | 1.43 | 0.35 | 0 | 1.99 | 1.99 | 1.99 |
| 20–40 | 6.03 | 71 | 4.57 | 51.64 | 12.68 | 1.36 | 0.89 | 0 | 2.44 | 2.44 | 2.44 |
Vegetative propagation of sweet potato (I. batatas) cv. Paraná was used in cultivation. These were obtained from a multiplication field located at the Rafael Fernandes. Experimental phosphate fertilization was carried out via fertigation with monoammonium phosphate (MAP) (61% P2O5 and 12% N) at the following doses: 0, 60, 120, 180, and 240 kg ha–1. The field experiment was conducted from May to September 2021. The phosphate fertilizer was fully applied at planting. Fertilization with nitrogen (N) and potassium (K) was carried out according to the manual of the Instituto Agronômico de Pernambuco IPA (2008). The N and K fertilizations were split twice and applied via fertigation, having, as sources, urea (46% of N) and potassium chloride (KCl) (60% of K2O), respectively. Most nitrogen (80%) was applied 15 days after planting; the remaining 20% was not used to avoid shoot growth. Half of the potassium was applied at 20 days after planting, and the other 50% was applied 45 days after planting. The amounts of N and K were 1.677 and 1.608 kg per plot, respectively. The amount of N applied was averaged from the MAP that makes phosphorus and nitrogen available jointly. An overview of the shoot at 100 days is shown in Figure S2.
The irrigation system was a drip type with emitters spaced at 0.30 m applied to an average depth of 11 mm. Tensiometers were implanted to monitor the soil moisture. Daily irrigation was carried out up to 30 days after planting. From 30 to 75 days after planting, irrigation was performed when the tensiometers showed −20 k Pa. From 75 to 90 days after planting, irrigation was suspended then resumed and carried out once a week until harvest, which was carried out 154 days after planting considering the soil moisture.
Harvest, Minimal Processing, and Storage
The sweet potato roots were harvested and transported to the Center for Graduate Studies in Vegetal Production (PGPV) of the Academic Unit of Serra Talhada of the Federal Rural University of Pernambuco (UAST/UFRPE) located in the municipality of Serra Talhada in the state of Pernambuco, Brazil. The roots were harvested after 150 days and minimally processed according to Silva et al.14 In minimal processing, the roots were selected and washed, and the periderm was removed. Subsequently, they were sliced approximately 20 mm thick. The roots were then immersed in water at 5 °C for 10 s, sanitized for 10 min in chlorinated water (200 mg L–1) at 5 °C, and rinsed for 10 in chlorinated water (5 mg L–1) at 5 °C. After the water was drained for 10 min outdoors, using perforated plastic trays, the slices were placed in polypropylene packages (150 × 150 × 0.0005 mm thick) and stored at 5 °C for 20 days. Each experimental unit consisted of approximately 150 g of slices with three repetitions used per treatment (0, 60, 120, 180, and 240 kg ha–1 of P2O5).
Analyses were performed on days 0, 4, 8, 12, 16, and 20. On each sampling time, cortex samples ±2 mm thick were collected, frozen in liquid nitrogen, and stored in an ultrafreezer (−80 °C).
Agro-industrial Yield and Phosphorus Content
The agro-industrial yield was determined in percentage terms as described by Freire et al.18 using the following formula
| 1 |
where AY is the agro-industrial yield (%), FW is the final weight (g), and IW is the initial weight (g).
To measure the P contents (g kg–1) in the vegetative parts (leaf and root), 0.4 g samples were digested with sulfuric acid.19 Phosphorus was determined by colorimetry using the phosphomolybdic complex method in a reducing medium.19
Visual Assessment (General Appearance), Fresh Weight Loss, and Total Soluble Solids
The visual quality was assessed by a panel of raters to determine the presence or absence of dark spots, streaks, and discoloration on the surface of a slice of the potato flesh in addition to the presence of odor, proliferation of Pseudomonas spp., and whitening in the samples. Scores from 5 to 1 were subjectively assigned, as described by Simões et al.20
The relative loss of fresh mass, expressed in percentage terms, was calculated by the difference from the initial fresh weight according to the following formula
| 2 |
where IFW is the initial fresh weight after processing (g) and FFW is the final fresh weight obtained after two-day intervals. The packages were weighed on a semi-analytical scale (ARD 110, OHAUS Adventurer, Parsippany, USA).
For the determination of soluble solids, approximately 30 g of sweet potato slices was macerated with the aid of a mortar and porcelain pestle. The juice obtained was added to the prism of a refractometer, and the result was expressed as a percentage.
Total Carotenoids, Lycopene, and β-Carotene
The carotenoid content of the samples was determined according to the analytical methodology of separation and extraction of the compounds with organic solvents. Sweet potato samples (0.25 g) were weighed and added to 1.25 mL of acetone, 1.25 mL of methanol, and 2.5 mL of hexane. The extract was then kept at rest for 24 h away from light. After this period, the samples were centrifuged at 9000 rpm for 5 min at 4 °C. For lycopene determinations, the absorbance read at 470 nm was used, and for β-carotene, the absorbance read at 450 nm. Thus, carotenoids were determined according to eq 3.21,22
| 3 |
where A is the absorbance of the solution at 470 nm for lycopene and 450 nm for β-carotene, V is the final volume of the solution, ε is the molar extinction coefficient of each pigment in the specific solvent (3450 for lycopene and 2592 for β-carotene), and M is the mass of samples for analysis.
Yellow Flavonoids and Anthocyanins
The yellow flavonoids and anthocyanins were quantified according to the methodology proposed by Francis (1982)23 with slight modifications. A 1 g portion of sweet potato was weighed and homogenized with 30 mL of ethanol–HCl solution (1.5 N) for 2 min. The sample was filtered, and the volume was adjusted to 50 mL. Then, the extract was transferred to tubes wrapped in aluminum foil and kept at rest for 12 h at 4 °C. The blank was composed only of the ethanol–HCL solution (1.5N). Readings were taken in a spectrophotometer (Libra S8 model, Biochrom) at 535 nm for anthocyanins and 374 nm for flavonoids.
The calculations were made using the following formulas:
| 4 |
| 5 |
Extracts for evaluating the antioxidant activity in vitro were obtained by macerating 0.4 g of fresh samples with 2 mL of methanol and were left to rest for 24 h. After this period, the extracts were centrifuged at 9000g for 21 min at 5 °C, and the supernatant was used for subsequent antioxidant activity evaluations.
Determination of Antioxidant Activity through in Vitro Tests (DPPH and FRAP)
The antioxidant capacity by DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay was determined as proposed by Brand-Williams et al.24 with adaptations. For the assay, 840 μL of DPPH solution (0.1 mM) and 60 μL of the supernatant were combined. The control assay was prepared by adding 840 μL of the DPPH solution and 60 μL of the methanolic extract. After 30 min of reaction, readings were taken with a spectrophotometer (Biochrom, Libra S8, Cambridge, U.K.) at 517 nm and 25 °C. The decline in the absorbance of the samples generated the percentage of free radical scavenging (%FRS), which was calculated by the following equation:
| 6 |
The antioxidant activity evaluated by the iron reduction power was measured according to the methodology proposed by Benzie and Strain25 with adaptations. For the assay, a 900 μL aliquot of FRAP reagent (mixture containing 0.3 M acetate buffer, pH 3.6, 0.8 M TPTZ, and 1.66 mM ferric chloride) was mixed with 90 μL of distilled water and 30 μL of the supernatant. The solution was then homogenized in a tube shaker and kept in the dark for 30 min at 37 °C. After incubation, readings were performed with a spectrophotometer (Biochrom, Libra S8, Cam bridge, U.K.) at 594 nm and 25 °C. For the blank, 900 μL of FRAP reagent, 90 μL of distilled water, and 30 μL of an extractor (methanol) were used. The antioxidant potential of the extracts was determined from a calibration curve, traced with ferrous sulfate (FeSO4·7H2O) in concentrations from 0 to 1500 μM. The results were expressed in millimole Fe2+ per kilogram.
Total Soluble Phenols and Vitamin C
The extraction and quantification of total soluble phenols were performed according to the method proposed by Fernando Reyes et al.26 with adaptations. Vitamin C was quantified for the same extract as proposed by Sánchez-Rangel et al.27 A portion (0.25 g) of sweet potato was weighed in a mortar and pestle containing 1.5 mL of pure methanol. The extract remained at rest for 20 h in the dark at 4 °C and centrifuged at 10,000g at 2 °C for 21 min. A volume of 150 μL of the supernatant, 2,400 μL of distilled water, and 150 μL of Folin–Ciocalteu (0.25 N) were mixed. The mixture was homogenized for 3 min, and then the vitamin C reading was performed at 765 nm. The concentration of vitamin C was calculated from the standard curve of ascorbic acid from 0.1 to 3.0 mM, and the result was expressed in milligrams of ascorbic acid per gram of WF. After reading, 300 μL of sodium carbonate (1N) was added to the mixture containing the sample and Folin–Ciocalteu then was kept in the dark at room temperature for 2 h. Readings were performed in a spectrophotometer (Biochrom, Libra S8, Cambridge, U.K.) at 765 nm, and the result was expressed in milligrams of gallic acid per 100 grams of MF, compared to a standard curve of gallic acid for soluble phenols.
Electrolyte Leakage, Detection, and Quantification of Hydrogen Peroxide and Lipid Peroxidation
To determine the electrolyte leakage, cubes of approximately 2 g of sweet potato were added to test tubes containing 15 mL of ultrapure water and left to rest for 30 min. Then, the initial extravasation value was measured (V1). After this, the tubes were kept in a water bath at 95 °C for 30 min, and then the final extravasation reading (V2) was taken. Both readings were taken with an electronic conductivity meter. Thus, the electrolyte leakage was determined according to the following equation:
| 7 |
Hydrogen peroxide (H2O2) was histochemically detected by microscopy and by the staining technique described by Olson and Varner28 and Repka.29 Fractions of the superficial region of cassava tissue (2 × 2 × 5 mm) were vacuum-infiltrated with 3,3′-diaminobenzidine tetrachloride (DAB) in the dark at −25 Ba for 5 h. Then, the cellulose was washed with sodium hypochlorite for 1 min. Control samples were infiltrated with ascorbic acid (1 mM). All sample images were recorded with a semiprofessional digital camera (Nikon D3100) (14.2 megapixels) and recorded with a stereomicroscope (Labomed Luxeo 4D Zoom stereo digital inocular microscope with a stereo zoom under 10× magnification).
Quantification of hydrogen peroxide (H2O2) was carried out according to Kasnak and Palamutoglu30 with modifications. Samples of 0.1 g of tissue were macerated with 2.0 mL of 1% trichloroacetic acid (TCA). The extract was centrifuged at 1200 rpm for 15 min at 4 °C. For the reaction, 0.6 mL of the supernatant with 0.7 mL of potassium phosphate buffer and 0.7 mL of potassium iodide solution were combined and kept at rest for 30 min, and then readings were taken at 390 nm. The hydrogen peroxide content was estimated in milligrams per 100 grams of H2O2.
Lipid peroxidation was estimated by the content of reactive substances to thiobarbituric acid (TBARS), according to Heath and Packer.31 Then, 0.1 g tissue samples were macerated in a mortar with 1.0 mL of 6% trichloroacetic acid (TCA). The extract was centrifuged at 7960 rpm for 15 min at 4 °C. Then, 0.5 mL of the supernatant was added to 1.5 mL of the reaction medium containing 20% (w/v) TCA and 0.5% (w/v) TBA in closed tubes. The tubes were kept at 95 °C for 1 h followed by an ice bath at 5 °C. Then, readings were taken at 532 and 660 nm. The TBARS content was estimated using the molar extinction coefficient of 155 mM–1 cm–1 and expressed in nanomole per gram of MF.
Enzyme Extractions and Assays
Phenylalanine Ammonia Lyase (PAL)
PAL activity was determined following the methodology described by Ke and Saltveit32 with adaptations. Samples of 0.5 g of plant tissue were collected and homogenized in a mortar with 3 mL of sodium borate buffer (0.1 M, pH 8.8) composed of β-mercaptoethanol (5 mM), EDTA (2 mM), and 1% insoluble PVPP (polyvinylpyrrolidone) (w/v). Subsequently, the enzymatic extract was filtered and centrifuged at 25,000g for 20 min at 4 °C.
For the assay, 1.5 mL of l-phenylalanine (60 mM) was added to a borate buffer (0.1 M, pH 8.8), and the buffer was kept at 40 °C for 15 min. Then, 0.5 mL of the enzymatic extract was added. After 20 min of incubation at 40 °C, the absorbance was measured at 290 nm with a spectrophotometer (Biochrom, Libra S8, Cambridge, England). PAL enzyme activity was expressed as ΔAbs per hour per gram of FW.
Polyphenol Oxidase (PPO) and Peroxidase (POD)
The PPO and POD enzymes were extracted, and their activity was tested according to de Albuquerque et al.33 with adaptations. Samples (0.25 g) from the superficial region of the tissue were macerated and homogenized in 1.5 mL of 0.2 M potassium phosphate buffer (pH 6.0). The extract was centrifuged at 10,000g for 21 min at 4 °C.
The PPO assay was performed by adding 100 μL of supernatant to a reaction medium containing 1.5 mL of potassium phosphate buffer (0.2 M, pH 6.0) and 1.3 mL of catechol (0.2 M), which was previously maintained at 25 °C in a dry bath. Readings were taken at intervals of 30 s over 2 min with a spectrophotometer (Biochrom, Libra S8, Cambridge, England) at 425 nm at a temperature of 25 °C. PPO activity was calculated based on the molar extinction coefficient of 3400 M cm–1 for catechol and was expressed in micromole catechol per minute per gram of MF.
The POD assay was performed by adding 100 μL of the supernatant to a reaction medium containing 1 mL of potassium phosphate buffer (0.2 M, pH 6.0), 100 μL of guaiacol (40 mM), and 100 μL of sodium peroxide hydrogen (23 mM) that was previously maintained at a temperature of 25 °C in a dry bath. Absorbances were read at intervals of 30 s over 2 min using a spectrophotometer (Biochrom, Libra S8, Cambridge, England) at 470 nm. POD activity was calculated based on a guaiacol molar extinction coefficient of 26.6 mM–1 cm–1 and is expressed in micromole guaiacol per minute per gram of WF.
Experimental Design and Statistical Analysis
The experiment was implemented in a completely randomized design with a 5 × 6 factorial arrangement represented by five treatments (phosphate fertilization: 0, 60, 120, 180, and 240 kg ha–1 P2O5) and six evaluation days (0, 4, 8, 12, 16, and 20 days) with three replications. Each experimental unit was represented by a package containing approximately 150 g of minimally processed sliced sweet potato roots. The H2O2 detection analysis was performed only on days 0 and 20 of conservation. Data were subjected to normality (Shapiro-Wilk) and homoscedasticity (Levene) tests, analysis of variance, and Tukey’s test at 5% probability using SAS software, and graphs were created using Sigma Plot software, version 11.0.
Results
Appearance, Phosphorus Content, and Agro-industrial yield
Fertilization starting at 120 kg ha–1 of P2O5 resulted in visible changes in the roots, making them larger (Figure 1). This was accompanied by a higher P2O5 content in the leaves and roots (Table 2). For the agro-industrial yield in potatoes after minimal processing, a significant increase occurred with the dosage of 120 kg ha–1 of P2O5 (Table 2).
Figure 1.

(A) Roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5). (B) Visual appearance illustrations and (C) appearance of minimally processed sweet potato after 8 days of storage, immediately removed from the package (I) and later hydrated (II), grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Images are provided by the author.
Table 2. Agro-industrial Yield (%) and Phosphorus Content (g kg–1) in Different Parts of the Sweet Potato Plant cv. Paraná Submitted to Different Levels of Phosphate Fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5); Letters Represent Significant Differences by Tukey’s Test at 5% Probability, and Capital Letters Compare the Different Phosphate Fertilization Levels.
| phosphorus
content (g kg–1) |
|||
|---|---|---|---|
| fertilizer dose (kg ha–1 P2O5) | agro-industrial yield (%) | leaf | root |
| 0 | 35.61 ± 3.72BC | 1.75 ± 0.16B | 1.99 ± 0.25A |
| 60 | 30.49 ± 2.35C | 1.73 ± 0.22B | 2.10 ± 0.20A |
| 120 | 38.14 ± 6.9B | 2.36 ± 0.14A | 2.27 ± 0.67A |
| 180 | 34.10 ± 5.07BC | 2.69 ± 0.29A | 2.33 ± 0.15B |
| 240 | 50.97 ± 4.89A | 2.5 ± 0.04B | 3.10 ± 0.27A |
Fresh Weight Loss, Soluble Solids, and Visual Evaluation
Fresh weight loss increased during storage in all minimally processed roots (Figure 2A). Interestingly, the greatest losses were for the pieces that had their roots fertilized with 120 and 180 kg ha–1 of P2O5; the differences were significant at 8, 12, and 16 days (Figure 2 B). In the case of soluble solids, fertilizations with P2O5 at doses of 60, 120, and 240 kg ha–1 of P2O5 tended to increase in their average value compared to the control; this was observed at the beginning (day zero) and at 4, 12, and 20 days (Figure 2B). The scores corresponding to the visual evaluation dropped during conservation regardless of P2O5 fertilization (Figure 2C). The minimally processed potatoes took a maximum period of 8 days to reach commercial grade with the potatoes that were fertilized with 180 and 240 kg ha–1 of P2O5 achieving a slightly higher grade (Figure 2C). A sharp drop after a short time was due to whitening symptoms (Figure 1). However, during storage, at 20 days, there were still small, blackened spots, mainly in the potatoes that had received higher levels of phosphorus fertilization; however, this was not considered to be the main problem of quality loss (Figures 1B and 2).
Figure 2.
(A) Fresh weight loss (%), (B) visual score (5–1), and (C) soluble solids (°Brix) in roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Bars represent the standard deviation of the mean. Letters represent significant differences by Tukey’s test at 5% probability. Capital letters compare the different phosphate fertilization levels, and lowercase letters compare storage days.
Total Carotenoids, β-Carotene, and Lycopene
It was evident that the sweet potato pieces from roots not fertilized with P2O5 at the during storage had significant lower levels of total carotenoids, β-carotene, and lycopene (Figure 3A–C). At 20 days, potatoes fertilized with 120 and 180 kg ha–1 P2O5 showed the highest levels of these pigments (Figure 3A–C).
Figure 3.
(A) Total carotenoids (mg 100 g–1 FW), (B) β-carotene (mg 100 g–1 FW), and (C) lycopene (mg 100 g–1 FW) in roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Bars represent the standard deviation of the mean. Letters represent significant differences by Tukey’s test at 5% probability. Capital letters compare the different phosphate fertilization levels, and lowercase letters compare storage days.
Yellow Flavonoids and Anthocyanins
The levels of yellow flavonoids and anthocyanins were significantly higher from the beginning to the end of the experiment for the roots fertilized with P2O5 compared to the control (Figure 4A,B). A higher content of yellow flavonoids was observed in the pieces of roots treated with the highest level (240 kg ha–1) of phosphate after 8, 12, and 16 days of processing.
Figure 4.
(A) Yellow flavonoids (mg 100 g–1 FW), (B) anthocyanins (mg 100 g–1 FW) in roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Bars represent the standard deviation of the mean. Letters represent significant differences by Tukey’s test at 5% probability. Capital letters compare the different phosphate fertilization levels, and lowercase letters compare storage days.
Antioxidant Capacity (DPPH and FRAP)
The level of antioxidant capacity measured by the DPPH method was significantly higher from the beginning to the end of the experiment for the roots fertilized with P2O5 compared to the control (Figures 5 B). This pattern was not observed for antioxidant capacity by the FRAP method (Figure 5 B), although the same trend continued at the end of 20 days (Figure 5 B).
Figure 5.
(A) FRAP (mmol Fe2+ kg FW) and (B) DPPH scavenging activity (%) in roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Bars represent the standard deviation of the mean. Letters represent significant differences by Tukey’s test at 5% probability. Capital letters compare the different phosphate fertilization levels, and lowercase letters compare storage days.
Vitamin C and Total Phenolic Compounds
Concerning the content of vitamin C and total phenolic compounds, during storage, the phosphate-fertilized potatoes showed significantly higher values of these phytochemicals (Figure 6 A). Furthermore, in the case of total phenolic compounds, the harvested sweet potatoes grown at the highest P2O5 dosages resulted in the highest phenolic content (Figure 6 B).
Figure 6.
(A) Vitamin C (mg of ascorbic acid g–1 FW), (B) total soluble phenols (mg of gallic acid 100 g–1 MF) in roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Bars represent the standard deviation of the mean. Letters represent significant differences by Tukey’s test at 5% probability. Capital letters compare the different phosphate fertilization levels, and lowercase letters compare storage days.
TBARS, Electrolyte Leakage, and Hydrogen Peroxide Content
TBARS and hydrogen peroxide levels and electrolyte leakage were similar at the beginning of the experiment regardless of whether the roots were fertilized with P2O5 (Figure 7A–C). During storage of the sweet potato slices, these measures increased. No significant differences were observed in the case of electrolyte leakage and TBARS levels (Figure 7A,B). However, for H2O2 levels, the roots fertilized with P2O5 showed the highest levels of H2O2 from eight days onward (Figure 7 C).
Figure 7.
(A) TBARS content (μmol g–1 MF), (B) electrolyte leakage (%), (C) hydrogen peroxide content (g 100 g–1 FW) in roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Bars represent the standard deviation of the mean. Letters represent significant differences by Tukey’s test at 5% probability. Capital letters compare the different phosphate fertilization levels, and lowercase letters compare storage days.
H2O2 Detection
For the detection of H2O2, on day 0, the pieces from nonfertilized roots showed little difference in dark tones whether treated with DAB alone or together with ASA (Figure 8). This was in contrast to the roots that were cultivated with increased phosphorus fertilization, where the pieces treated with DAB alone always had a darker hue compared to those treated with DAB + ASA (Figure 8). A more intense darkening in the pieces with DAB was observed in samples collected at 20 days (Figure 8).
Figure 8.

Detection of hydrogen peroxide (H2O2) at days 0 and 20 of storage by vacuum infiltration with diaminobenzidine tetrahydrochloride (DAB) + ascorbate (control) and only DAB in segments (0–5 mm) of roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Images are provided by the author.
Peroxidase (POD), Polyphenol Oxidase (PPO), and Phenylalanine Ammonia (PAL) Activity
At the beginning of conservation (day 0), POD and PPO activities of potatoes derived from phosphate fertilization were significantly higher than those of the control (Figure 9A,B). In the same period, PAL activity was significantly increased only in the samples of potatoes derived from fertilization of 240 kg ha–1 with P2O5 (Figure 9 C). During storage, the PPO activity was significantly lower in the control sweet potato tissues (Figure 9 B). For POD and PAL, these differences were accentuated at 12 and 16 days for the control roots (Figure 9A,C).
Figure 9.
(A) POD activity (μmol of guaiacol min–1 kg–1 of FW), (B) PPO activity (μmol of catechol min–1 kg–1 of FW) and phenylalanine ammonia lyase (μmol min–1 g–1 FW) in roots of sweet potato cv. Paraná grown at different levels of phosphate fertilization (0, 60, 120, 180, and 240 kg ha–1 P2O5), minimally processed, and stored for 20 days at 5 ± 2 °C and 90 ± 5% RH. Bars represent the standard deviation of the mean. Letters represent significant differences by Tukey’s test at 5% probability. Capital letters compare the different phosphate fertilization levels, and lowercase letters compare storage days.
Discussion
The present work investigated the effect of increasing doses of P2O5-based fertilization on oxidative damage and protection and the accumulation of important antioxidants for the quality of minimally processed orange sweet potato roots. It is believed that phosphate fertilization can accelerate phenylpropanoid metabolism, resulting in the accumulation of phenolic compounds.12 Furthermore, it is assumed that, depending on the sensitivity of the tissue to browning, these increases in antioxidant metabolites, instead of being positive, become a problem as they stimulate the evolution of enzymatic browning as seen in minimally processed manioc.12 In the present research, the plant model to investigate this phenomenon was the root of an orange sweet potato called Paraná, a commercial variety grown in Brazil, accepted as a table sweet potato being sweet and14 fast-cooking32 and with an orange hue attractive to the consumer.14
The main problem that led to the loss of quality of the minimally processed sweet potatoes in this study was the disorder caused by whitening where the pieces became aged regardless of the P2O5 dose to which the potatoes had been subjected (Figure 1C). Changes in the visual quality were quantified by assigning a visual score between 5 and 1 in which grade 3 was considered the acceptable limit.20 A trained panel gave this score for samples stored within a limit of eight days regardless of whether the roots were derived from phosphate fertilization (Figure 2 C). Results indicated no relationship between the phosphorus doses and the whitening symptoms. It is worth noting that, although these potatoes presented whitening symptoms that could compromise marketing, when hydrated, they return to the initial orange color similar to that observed in the first days of storage (Figure 1 C). Sweet potatoes are usually steamed or boiled in water. For this reason, storage was extended for up to 20 days even knowing that bleaching could compromise the visual quality of the uncooked sweet potato.
The sweet potato samples from soil treated with phosphate fertilizers after minimal processing showed increased levels of β-carotene, lycopene, and total carotenoids in their tissues in addition to yellow flavonoids and anthocyanins compared to the control sweet potatoes (Figures 3A–C and 4A,B). However, even with these pigment differences, there were no differences in the perception of whiteness even though another study by Ahmed et al.34 related whiteness to pigment degradation. It is believed that the main cause of whitening is a physical phenomenon related to dehydration,35 as also studied by Cisneros-Zevalllos et al.36 Furthermore, it is worth noting that the method of Francis et al.23 was used for anthocyanins in which the values were obtained as total anthocyanins. Unlike that reported by Lee et al.,37 using more robust methods, Tang et al.38 expressed their results as monomeric anthocyanin cyanidin-3-glucoside equivalent. However, when we compare total anthocyanins, our data are in the range of 40–80 mg 100 g–1, the data from Tang et al.38 are in values close to 20 mg CyE/g. We believe that this difference may be due to the plant material and also the technique mentioned. In the present study, the percentage loss of fresh mass was estimated in the packaged pieces from the different P2O5 fertilizations, where an increase in the mass loss was noted. At the end of the conservation period, however, the doses of 120 and 180 kg of P2O5 ha–1 were the ones that showed the highest moisture loss (Figure 2A). However, this is difficult to explain in practice as there was no difference in whitening between treatments.
The application of P2O5 at any dose resulted in higher levels of yellow flavonoids and anthocyanins as well as greater antioxidant capacity by the DPPH method, vitamin C, and total phenolic compounds, compared to the control (Figures 4A,B, 5D, and 6A,B). In addition, the highest doses of P2O5 resulted in minimally processed tissues richer in phenolic compounds (Figure 6B), which can be explained in part by the higher PAL activity (Figure 9 C), a key enzyme in the phenylpropanoid metabolism, where phenolic compounds are biosynthesized.39 This fact shows the participation of phosphorus in the accumulation of phenolic compounds in sweet potato cv. Paraná as also observed in minimally processed cassava.12 Interestingly, in the case of the sweet potato under study, the tissue did not darken intensely as in the cited work by Eugênio et al.,12 even observing greater PPO and POD activities in the tissues that were higher in soil fertilized with phosphorus compared to the control (Figure 9A,B). It is believed that the presence of pigments or antioxidants in the tissue of the sweet potato cv. Paraná, as seen in Figures 2–5, made the plant more tolerant to enzymatic browning, as evidenced in the study performed by Silva et al.14
Therefore, we highlight an important role of phosphate fertilization in reducing enzymatic browning in minimally processed sweet potato roots. More investigations must be carried out to elucidate the functions of phosphorus to complement the knowledge evidenced by Jacobo-Vellazquez and Cisneros-Zevallos40 and by Eugênio et al.12 However, it has been reported that phosphorus applied via fertilization is a nutrient that increases bioactive compounds in strawberries41 and Vitex negundo Linn.42
Regarding oxidative damage, some parameters indicative of cell damage, such as TBARS and hydrogen peroxide content, and the in situ detection of H2O2 were measured (Figures 8 and 9). In general, phosphate fertilization did not increase membrane damage or TBARS content (Figure 7A,B). However, H2O2 levels were significantly higher in the tissues of minimally processed roots derived from P2O5-based fertilization (Figure 7C). Moreover, H2O2 was detected in the tissues using the DAB reagent and the addition of ascorbate as a control. Interestingly, a slightly darker tissue was detected in the roots resulting from phosphate fertilization at the beginning and at 20 days (Figure 8). Hydrogen peroxide is required for polyphenolic formation during wound healing and suberization in tubers. Higher amounts of H2O2 may be related to stress-induced phenylpropanoid metabolism in phosphate-treated samples. The level of stress might not be sufficient to cause cell damage especially because of the counteracting effect of increased levels of hydrosoluble and liposoluble antioxidants. These results may be associated with the higher PAL and PPO activities detected in the roots resulting from fertilization, suggesting that, although there exists an oxidation of phenolics, contributing to the darker color, the enzymatic activity of PAL is also high, allowing the accumulation of soluble phenolics. This response could be mediated by extracellular ATP release from the cytoplasm of the wounding tissue, which binds to ATP receptors and triggers the production of secondary signaling molecules, such as H2O2, in response to phosphorus applied to the soil.40 This ATP-dependent wound response could be increased due to the higher availability of phosphate in sweet potatoes grown under P fertilization, as previously suggested for cassava.12,13
Furthermore, these findings contribute to the discussion that phosphate fertilization can accelerate the loss of homeostasis when associated with abiotic stress caused by minimal processing, as reported by Cisneros-Zevallos and Jacobo-Velazquez.43 This supports the idea that the orange sweet potato with its antioxidant tissues can be an adequate model system to study the effect of P fertilization on the accumulation of bioactive compounds during postharvest storage of fresh-cut tissue. More investigations in this field of study must be carried out to understand better the relationship between P and the loss of cellular homeostasis that destabilizes oxidative reactions.
The findings of the present work showed that agronomic biofortification through fertilization with P2O5 could be a feasible tool to increase the levels of bioactive compounds in sweet potatoes with colored flesh, which could be increased due to wounding stress during storage. The recommended P2O5 fertilization for soils of the Brazilian semi-arid region for sweet potatoes is 60 kg ha–1 P P2O5.41 Given that the studied soil had, on average, a layer of up to 40 cm 4.5 mg dm–3, it is possible that this favored a greater absorption and the use of P by the roots. Studying other horticultural products (roots or not) with strong antioxidant potential is necessary. Sweet potato is still not exploited for the production of carotenoids. This is a vast field for exploration given the possibility of using carotene as a natural dye additive in the food industry.44,45 In the pharmaceutical and nutraceutical industry, carotene can prevent photosensitivity disorders, cardiovascular diseases, diabetes, vision disorders, cancer, neurological disorders, and immune diseases.46 In addition to the enhanced content of phytochemicals due to P fertilization, it is also important to point out the increase in the root size (Figure 1 A) and agro-industrial yield (Table 1) as crucial parameters for agronomic and industrial sustainability. The present study demonstrated the role of phosphorus in protecting plants from oxidative damage by inducing the synthesis of antioxidant compounds and enhancing the quality of minimally processed colored sweet potatoes. Fertilization with P2O5 is associated with greater detection and content of hydrogen peroxide, which was accompanied by an increase in phenolic compounds, vitamin C, yellow flavonoids, anthocyanins, carotenoids, antioxidant capacity (DPPH), and activities of the enzymes polyphenol oxidase, peroxidase, and phenylalanine ammonia lyase. This finding on the use of P fertilization to improve the quality of sweet potatoes has applications in the fresh-cut industry to generate products with a higher content of bioactive molecules and higher acceptability by consumers. However, additional applications of technologies such as edible coatings are still needed to minimize the whitening of minimally processed roots.
Acknowledgments
This research was supported by Coordenaca̧ o de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Proc. no. 88881-159183/2017-01), Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE, no. PQ-0795-5.01/16), Universidade Federal Rural de Pernambuco (UFRPE, no. PRPPG 015/2018), Universidade Federal Rural do Semi-Árido and Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq, no. 423100/ ´2018-1).
Glossary
Abbreviations
- SOD
superoxide dismutase
- CAT
catalase
- APX
ascorbate peroxidase
- PAL
phenylalanine ammonia lyase
- ROS
reactive oxygen species
- NADPH oxidase
reduced nicotinamide adenine dinucleotide phosphate
- ATP
adenosine triphosphate
- PPO
polyphenoloxidase
- POD
peroxidase
- DPPH
2,2- diphenyl-1-picrylhydrazyl
- FRAP
ferric reducing antioxidant Power
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c04196.
Precipitation (mm), air temperature (°C), relative humidity (%), and wind speed (m s–1) between the months of April to August 2021 in the municipality of Mossoró, Rio Gande do Norte, Brazil, and sweet potato cultivars plants fertilized with different doses of phosphorus (PDF)
Author Present Address
∥ Tecnologico de Monterrey, Escuela de Ingenieria y Ciencias, Av. General Ramón Corona 2514, Nuevo Mexico, Zapopan C.P. 45138, Jalisco, Mexico
Author Present Address
⊥ Tecnologico de Monterrey, Institute for Obesity Research, Av. General Ramón Corona 2514, Nuevo Mexico, Zapopan C.P. 45138, Jalisco, Mexico.
Author Contributions
V.N.S.S. handled the conceptualization, methodology, software, formal analysis, investigation, data curation, and writing - original draft. P.H.A.O. and W.A.R.L. contributed in conceptualization, methodology, formal analysis, writing - review and editing, supervision, and project administration. A.L.S., N.L.F., and A.S.A.N.M. participated in the methodology, investigation, and resources. S.A.S. conducted the methodology and validation. F.A.L.B. and D.A.J.V. participated in the methodology and validation. A.P.B.J. and L.M.S. contributed in writing - review and editing, visualization, supervision, project administration, and funding acquisition. A.N.S. contributed in conceptualization, methodology, writing - review and editing, visualization, supervision, project administration, and funding acquisition.
The authors declare no competing financial interest.
Supplementary Material
References
- Lin K. H.; Lai Y. C.; Chang K. Y.; Chen Y. F.; Hwang S. Y.; Lo H. F. Improving breeding efficiency for quality and yield of sweet potato. Bot. Stud. 2007, 48, 283–292. [Google Scholar]
- Jung J. K.; Lee S. U.; Kozukue N.; Levin C. E.; Friedman M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24 (29), 29–37. 10.1016/j.jfca.2010.03.025. [DOI] [Google Scholar]
- Chakraborty C.; Roychowdhury R.; Chakraborty S.; Chakravorty P.; Ghosh D. A. Review on Post-Harvest Profile of Sweet Potato. Int. J. Curr. Microbiol. Appl. 2017, 6 (5), 1894–1903. 10.20546/ijcmas.2017.605.210. [DOI] [Google Scholar]
- Roesler P. V. S. O.; Gomes S. D.; Moro E.; Kummer A. C. B.; Cereda M. P. Produção e qualidade de raiz tuberosa de cultivares de batata-doce no Oeste do Paraná. Acta Sci. Agron. 2008, 30 (1), 117–122. 10.4025/actasciagron.v30i1.1159. [DOI] [Google Scholar]
- Block G. Nutrient sources of provitamin A carotenoides in the American diet. Am. J. Epidemiol. 1994, 139, 290–293. 10.1093/oxfordjournals.aje.a116996. [DOI] [PubMed] [Google Scholar]
- Mitra S. Nutritional status of orange-fleshed sweet potatoes in alleviating vitamin A malnutrition through a food-based approach. J. Nutr. Food Sci. 2012, 2 (8), 160. 10.4172/2155-9600.1000160. [DOI] [Google Scholar]
- Akhtar S.; Ahmed A.; Randhawa M. A.; Atukorala S.; Arlappa N.; Ismail T.; Ali Z. Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies. J. Health, Popul., Nutr. 2014, 31 (4), 413. 10.3329/jhpn.v31i4.19975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S. N.; Nusrat T.; Begum P.; Ahsan M. Carotenoids and β-carotene in orange fleshed sweet potato: A possible solution to vitamin A deficiency. Food Chem. 2016, 199, 628–631. 10.1016/j.foodchem.2015.12.057. [DOI] [PubMed] [Google Scholar]
- Teow C. C.; Truong V. D.; McFeeters R. F.; Thompson R. L.; Pecota K. V.; Yencho G. C. Antioxidant activities, phenolic and b-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. 10.1016/j.foodchem.2006.09.033. [DOI] [Google Scholar]
- Filgueira F. A. R.Novo Manual de Olericultura: Agrotecnologia moderna na produção e comercialização de hortaliças, Viçosa, MG: UFV 200. 402 p. [Google Scholar]
- Empresa Brasileira De Pesquisa Agropecuária. Centro Nacional de Pesquisa de Hortaliças . A Cultura da batata-doce. Brasília: EMBRAPA-SPI. 95p. (Coleção Plantar; 30). Centro Nacional de Pesquisa de Hortaliças. Cultivo da batata-doce (Ipomoea batatas (L.) Lam). produções técnicas do CNP Hortaliças. 3ed. Brasília: EMBRAPA. 1995. [Google Scholar]
- Eugênio D. S.; Fonseca K. S.; Marcelino A. S. A. N.; Silva V. N. S.; Ferreira-Silva S. L.; Barros-Júnior A. P.; Silveira F. P. M.; Lopes W. A. R.; Santos H. R. B.; Simões A. N. Phosphate fertilization as a modulator of enzymatic browning in minimally processed cassava. J. Agric. Food Chem. 2021, 69, 10058–10068. 10.1021/acs.jafc.1c02590. [DOI] [PubMed] [Google Scholar]
- Jacobo-Velázquez D. A.; Martínez-Hernández G. B.; del C. Rodríguez S.; Cao C. M.; Cisneros-Zevallos L. Plants as biofactories: physiological role ofreactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59 (12), 6583–6593. 10.1021/jf2006529. [DOI] [PubMed] [Google Scholar]
- Silva V. N. S.; Fonseca K. S.; De Sá S. A.; Souza J. F. N.; Barros-Júnior A. P.; Simões A. N. Harvest time as a modulator of phytochemicals in sweet potato cultivars for the industry. Revista Caatinga 2022, 35, 956–963. 10.1590/1983-21252022v35n423rc. [DOI] [Google Scholar]
- Alvares C. A.; Stape J. L.; Sentelhas P. C.; de Moraes Gonçalves J. L.; Sparovek G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. 10.1127/0941-2948/2013/0507. [DOI] [Google Scholar]
- Silva J. B. C.; Lopes C. A.; Magalhães J. S.. Cultivo da batata-doce. EMBRAPA-CNPH: Brasília, Sistemas de Produção 6, ISSN 1678–880X. 2009. [Google Scholar]
- Donagemma G. K.; Campos D. V. B.; Calderano S. B.; Teixeira W. G.; Viana J. H. M.. Manual de métodos de análise de solo. 2nd ed. 2011http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/990374.
- Freire C. S.; Simões A. N.; Vieira M. R. S.; Barros-Júnior A. P.; Costa F. B.; et al. Quality of minimally processed sweet cassava in minitolete and rubiene shapes. Revista Caatinga 2014, 27 (4), 95. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuaria Centro Nacional ´ de Pesquisa de Solos Manual de Metodos de Análise de Solos ´, 2nd ed.; Empresa Brasileira de Pesquisa Agropecuaria (EMBRAPA), Centro ´ Nacional de Pesquisa de Solos (CNPS): Rio de Janeiro, Brazil, 1997; p 212. [Google Scholar]
- Simões A. d. N.; Almeida S. L.; Borges C. V.; Fonseca K. S.; Barros Júnior A. P.; Albuquerque J. R. T.; Corrêa C. R.; Minatel I. O.; Morais M. A. d. S.; Diamante M. S.; Lima G. P. P. Delaying the harvest induces bioactive compounds and maintains the quality of sweet potatoes. J. Food Biochem. 2020, 44, 1–13. 10.1111/jfbc.13322. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Amaya D. B.A guide to carotenoid analysis in foods; Internacional Life Sciences Institute Press: WA, 200171. 64 p. [Google Scholar]
- Rodriguez-Amaya D. B.; Kimura M.. Handbook for carotenoid analysis. HarvestPlus: WA, 2004. 58 p. (HarvestPlus Technical Monograph, Series 2). [Google Scholar]
- Francis F. J. Analysis of anthocyanins. In Markakis P. (Ed.), Anthocyanins as Food Colors pp 181. Academic Press: London, UK. 1982 10.1016/b978-0-12-472550-8.50011-1. [DOI] [Google Scholar]
- Brand-Williams W.; Cuvelier M. E.; Berset C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28 (1), 25–30. 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- Benzie I. F. F.; Strain J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239 (1), 70–76. 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Fernando Reyes L.; Emilio Villarreal J.; Cisneros-Zevallos L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2006, 101 (3), 1254–1262. 10.1016/j.foodchem.2006.03.032. [DOI] [Google Scholar]
- Sánchez-Rangel J. C.; Benavides J.; Heredia J. B.; Cisneros-Zevallos L.; Jacobo-Velázquez D. A. The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5 (21), 5990. 10.1039/c3ay41125g. [DOI] [Google Scholar]
- Olson P. D.; Varner J. E. Hydrogen peroxide and lignification. Plant J. 1993, 4 (5), 887–892. 10.1046/j.1365-313X.1993.04050887.x. [DOI] [Google Scholar]
- Repka V. Improved histochemical test for in situ detection of hydrogen peroxide in cells undergoing oxidative burst or lignification. Biol. Plant. 1999, 42 (4), 599–607. 10.1023/A:1002687603731. [DOI] [Google Scholar]
- Kasnak C.; Palamutoglu R. Effect of Yogurt Serum on Enzymatic and Oxidative Activity inFresh-Cut Potatoes. Food Science & Technology. 2021, 1, 1842–1848. 10.1021/acsfoodscitech.1c00222. [DOI] [Google Scholar]
- Heath R. L.; Packer L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Ke D.; Saltveit M. E. Effects of calcium and auxin on russet spotting and phenylalanine ammonia-lyase activity in iceberg lettuce. HortScience 1986, 21 (5), 1169–1171. 10.21273/HORTSCI.21.5.1169. [DOI] [Google Scholar]
- de Albuquerque J. R. T.; Ribeiro R. M. P.; de Sousa L. V.; Oliveira G. B. S.; Lins H.Á.; Barros-Júnior A. P.; dos Santos E. C.; Morais P. L. D.; Simões A. N. Quality of sweet potato cultivars planted in two seasons and harvested at different times. Aust. J. Crop Sci. 2018, 12, 898. 10.21475/ajcs.18.12.06.PNE884. [DOI] [Google Scholar]
- Ahmed J.; Shivhare U. S.; Sandhu K. S. Thermal degradation kinetics of 395 carotenoids and visual color of papaya puree. J. Food Sci. 2002, 67, 2692–2695. 10.1111/j.1365-2621.2002.tb08800.x. [DOI] [Google Scholar]
- Simões A. d. N.; Puiatti M.; Salomão L. C.; Mosquim P. R.; Puschmann R. Effect in the quality of intact and minimally processed leaves of collard greens stored at different temperatures. Hortic. Bras. 2010, 28 (1), 81–86. 10.1590/S0102-05362010000100015. [DOI] [Google Scholar]
- Cisneros-Zevallos L.; Saltveit M. E.; Krochta J. M. Mechanism of surface white discoloration of peeled (minimally processed) carrots during storage. J. Food Sci. 1995, 60, 320–323. 10.1111/j.1365-2621.1995.tb05664.x. [DOI] [Google Scholar]
- Lee J.; Durst R. W.; Wrolstad R. E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88 (5), 1269. 10.1093/jaoac/88.5.1269. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Cai W.; Xu B. Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin China. Food Sci. Hum. Wellness 2015, 4, 123–132. 10.1016/j.fshw.2015.07.003. [DOI] [Google Scholar]
- Dixon R. A.; Paiva N. L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7 (7), 1085–1097. 10.2307/3870059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Velázquez D. A.; Cisneros-Zevallos L. An alternative use of horticultural crops: stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2 (3), 259–271. 10.3390/agriculture2030259. [DOI] [Google Scholar]
- Valentinuzzi F.; Mason M.; Scampicchio M.; Andreotti C.; Cesco S.; Mimmo T. Enhancement of the bioactive compound content in strawberry fruits grown under iron and phosphorus deficiency. J. Sci. Food Agric. 2015, 95, 2088–2094. 10.1002/jsfa.6924. [DOI] [PubMed] [Google Scholar]
- Jin L.; Liu L.; Guo Q. Phosphorus and iron in soil play dominating roles in regulating bioactive compounds of Glechoma longituba (Nakai) Kupr. Sci. Hortic. 2019, 256, 108534. 10.1016/j.scienta.2019.05.061. [DOI] [Google Scholar]
- Cisneros-Zevallos L.; Jacobo-Velázquez D. A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme stresses and the impact on nutraceuticals and quality during pre-and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68 (43), 11877–11879. 10.1021/acs.jafc.0c06029. [DOI] [PubMed] [Google Scholar]
- Soares K. T.; Melo A. S. D.; Matias E. C.. A Culturada batata-doce (Ipomoea batatas (L.) Lam). EMEPA-PB: João Pessoa, 2002, 26 p. [Google Scholar]
- Kalra R.; Gaur S.; Goel M. Microalgae bioremediation: A perspective towards wastewater treatment along with industrial carotenoids production. J. Water Process Eng. 2021, 40, 101794. 10.1016/j.jwpe.2020.101794. [DOI] [Google Scholar]
- Przybylska S. Lycopene – a bioactive carotenoid offering multiple health benefits: a review. J. Food Technol. 2020, 55, 11–32. 10.1111/ijfs.14260. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









