Abstract
The marine bacterium Vibrio vulnificus is a human pathogen that can spontaneously switch between virulent opaque and avirulent translucent phenotypes. Here, we document an additional form, the rugose variant, which produces copious biofilms and which may contribute both to pathogenicity of V. vulnificus and to its survival under adverse environmental conditions.
Vibrio vulnificus is a marine bacterium that can cause human disease and has been implicated as the cause of over 95% of the deaths related to seafood consumption in the United States (13, 25). Infection usually occurs through the ingestion of raw oysters or by contact of an open wound with contaminated seawater. Susceptible individuals include those with liver disease, immune dysfunction, or elevated serum iron levels. When a susceptible individual consumes raw oysters contaminated with V. vulnificus, the result can be a rapidly fulminating septicemia, with mortality occurring in over 50% of cases (13, 25).
Some V. vulnificus isolates produce a polysaccharide capsule (3) which gives the colonies a smooth, mucoid, opaque phenotype. It has been reported that the capsule is a major virulence factor, presumably protecting the bacterium from the host immune system (23, 32). In culture, many opaque (O) strains spontaneously produce translucent (T) variants (28, 35). Opaque strains have been shown to be virulent in a mouse model, while translucent strains were avirulent in this model (23, 32, 35). Several studies have shown that capsular polysaccharide (CPS) expression varies between opaque and translucent strains as well as among different translucent strains (i.e., suggesting there are different degrees of translucence) (28, 29, 32). Transposon insertions have been shown to produce completely acapsular translucent strains (28, 29, 35).
Opaque strains of V. vulnificus have been reported to switch spontaneously to the translucent phenotype at frequencies of 10−5 to 10−4 when the cells were grown in rich medium (12, 29, 32). If grown in a peptone-based yet more-defined medium, the switch occurs at a higher frequency, with over 60% of the colonies exhibiting the translucent phenotype (23). The reverse switch, from translucent to opaque, has been reported to occur at frequencies of 9.2 × 10−3 (29), less than 10−4 (32), or not at all (12, 23). We have previously observed that opaque strains will spontaneously yield translucent variants much more often than the reverse. However, the translucent-to-opaque switch did occur on rare occasions when strains were routinely subcultured in heart infusion broth at 37°C and then left to stand at room temperature for several days before plating and incubation of plates at 37°C (unpublished results).
Vibrio cholerae, the etiological agent of cholera, is a close relative of V. vulnificus. Although the O1 strains of V. cholerae produce no CPS, they have been responsible for the first six pandemics of cholera. The current, seventh pandemic has been caused not only by O1 strains but also by O139 strains, which do produce CPS (19). In addition to the encapsulated and nonencapsulated smooth phenotypes, V. cholerae exhibits a third colony phenotype, rugose. First identified in the late 1930s (27), rugose (R) variants arise in both O1 and non-O1 V. cholerae strains and are wrinkled and dry compared to the smooth, mucoid phenotypes of the nonrugose strains. Typically, rugose variants are obtained following growth in minimal medium or under prolonged starvation conditions. Rugose variants have been shown to form well-developed biofilms and to be more resistant than the smooth forms to chlorine, hydrogen peroxide, UV light, complement activity in human serum, and osmotic stress (1, 18, 20, 22, 26, 31). It was suggested that the rugose phenotype may allow V. cholerae to survive under adverse environmental conditions (1, 26, 31).
Previously, we observed during subculturing of opaque and translucent strains of V. vulnificus that each could yield the other phenotype, although frequencies of these switching events were not determined. Here, we undertook a more comprehensive analysis to determine the frequency of conversion between opaque and translucent phase variants. Under carefully controlled conditions, we found high frequencies of conversion from the opaque to the translucent phenotype and also of translucent isolates switching to opacity. More significantly, our analysis revealed a new phenotypic form of V. vulnificus, the rugose variant. Further characterization of this variant revealed it to be relatively nonmotile, more resistant to serum killing than its parental opaque or translucent version, and capable of producing copious amounts of biofilm.
Appearance of a rugose phenotype for V. vulnificus.
The opaque parental strain 1003(O), three spontaneous translucent derivatives [AZ(T), BG(T1), and BG(T2)], as well as two previously described (24, 35) transposon-induced translucent strains [ABZ1(T) and GMB4(T)] (Fig. 1 and Table 1) were subjected to culture conditions that allowed switching to the alternate phenotype. In an adaptation of a previously described procedure (15), V. vulnificus strains were tested for phenotypic switching by using heart infusion broth and heart infusion agar plates, both supplemented to 2% NaCl (HI). Briefly, 10-ml HI broth cultures were started from isolated colonies and, following overnight incubation at 34 to 35°C with shaking at 200 rpm, the first passage was started by diluting the original culture 1:100 into fresh broth followed by overnight incubation as before. Daily passages were continued for 5 to 6 days (one cycle), the final passage was serially diluted and plated on HI agar, and the plates were incubated at 30°C overnight, whereupon colonies and their phenotypes were enumerated. On the same day as the plating, another passage was made in case there was no phenotypic switching. In that case, daily passages were continued for another 5- to 6-day cycle, followed by dilutions and platings as before. A third cycle of passaging and plating was performed as necessary, and results were recorded as percentages [100 × (number of colonies showing a particular phenotype divided by the total number of colonies in all cycles)].
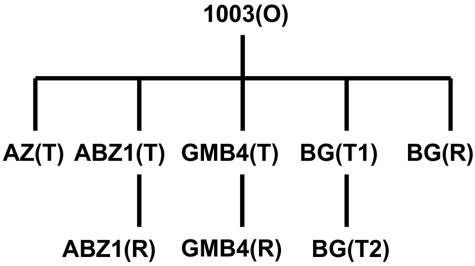
FIG. 1.
Relationship of phase variants used in this study. All first-level variants were derived directly from the Louisiana clinical isolate, V. vulnificus 1003(O). O, opaque; T, translucent; R, rugose.
TABLE 1.
Bacterial strains used in this study
| Strain | Isolation and characterizationa | Reference(s) or source |
|---|---|---|
| V. vulnificus 1003 | ||
| 1003(O) | Wound isolate, opaque, encapsulated, virulent; designated as wild type, smooth; CPS serotype 9, LPS serotype unk.; Colr | 16 |
| BG(R) | Spontaneous rugose derivative of 1003(O), dry, wrinkled | This study |
| BG(T1) | Spontaneous translucent derivative of 1003(O), smooth | This study |
| BG(T2) | Spontaneous highly translucent derivative of BG(T1), smooth | This study |
| AZ(T) | Spontaneous highly translucent derivative of 1003(O), avirulent, smooth, previously 1003(T) | 35 |
| ABZ1(T) | Translucent, avirulent; defective in capsule expression due to mini-Tn10 insertion in the wcvA gene of 1003(O), smooth; Kanr | 24, 35 |
| ABZ1(R) | Spontaneous rugose derivative of ABZ1(T), dry, wrinkled; Kanr | This study |
| GMB4(T) | Translucent, avirulent; mini-Tn10 insertion in ORF4 of 1003(O) super-integron region, smooth; previously ABZ44(T); Kanr | 24, 34 |
| GMB4(R) | Spontaneous rugose derivative of GMB4(T), dry, wrinkled; Kanr | This study |
| GMB4(O) | Spontaneous opaque derivative of GMB4(T), smooth; Kanr | This study |
| V. vulnificus | ||
| 1005(O) | Blood isolate, opaque, smooth; CPS serotype 1, LPS serotype 3 | 16 |
| 1005(R) | Spontaneous rugose derivative of 1005(O), dry, wrinkled | This study |
| 1007(O) | Blood isolate, opaque, smooth; CPS serotype 4, LPS serotype O1 | 16 |
| 1007(R) | Spontaneous rugose derivative of 1007(O), dry, wrinkled | This study |
| 1014(O) | Blood isolate, opaque, smooth; CPS serotype 3, LPS serotype 2 | 16 |
| 1014(R) | Spontaneous rugose derivative of 1014(O), dry, wrinkled | This study |
| 96-7-155(O) | Eel isolate, opaque, smooth; CPS serotype 9, LPS serotype unk. | Laboratory collection |
| 96-7-155(R) | Spontaneous rugose derivative of 96-7-155(O), dry, wrinkled | This study |
Colr, colistin resistance; Kanr, kanamycin resistance; LPS, lipopolysaccharide; unk., unknown.
The results of three independent switching assays are given as the average percentage of each resulting phenotype (Table 2). Not only did the opaque strain 1003(O) often switch to the translucent phenotype but, as with most of the other strains, it also produced a small, wrinkled, and dry colony type (Fig. 2) that resembled the rugose phenotype reported for V. cholerae (18, 20, 26, 31) and other bacterial species (4, 8). Translucent strains meanwhile varied considerably in their conversion to the alternate phenotypes. Of the spontaneously isolated strains, BG(T1) produced opaque colonies at a frequency 10-fold greater than that of its more highly translucent derivative, BG(T2). Strain AZ(T), the most translucent of the spontaneous translucent strains used here, produced 35-fold fewer opaque colonies than BG(T1) and never produced a rugose colony during any of the three switching assays. AZ(T) has been shown to have a chromosomal rearrangement involving an insertion sequence (24) when compared to 1003(O). While it is possible that this rearrangement may have rendered the strain unable to yield a rugose phenotype, it is also possible that, as this strain produces opaque variants only rarely, it may also produce rugose variants, but at a low level undetected in our switching assay.
TABLE 2.
Phenotypic switching of V. vulnificus 1003 phase variants
| Parent straina | Resulting phenotypes (avg % of total colonies)b
|
||
|---|---|---|---|
| Opaque (SE) | Translucent (SE) | Rugose (SE) | |
| 1003(O) | 55.2 (23.0) | 30.6 (16.2) | 13.0 (3.0) |
| BG(T1) | 17.3 (12.4) | 74.7 (15.9) | 5.8 (2.9) |
| BG(T2) | 1.7 (0.9) | 94.3 (2.1) | 3.8 (2.2) |
| AZ(T) | 0.5 (0.4) | 98.1 (1.7) | 0 (0.0) |
| ABZ1(T) | 0 (0.0) | 78.2 (16.0) | 18.2 (12.8) |
| GMB4(T) | 7.9 (7.7) | 79.8 (10.5) | 8.3 (5.5) |
| BG(R), 30°C | 15.8 (3.2) | 0.1 (0.04) | 53.8 (2.8) |
| BG(R), 37°C | 26.3 (15.5) | 0 (0.0) | 53.1 (21.8) |
| ABZ1(R), 30°C | 0 (0.0) | 42.9 (21.0) | 42.0 (13.0) |
| ABZ1(R), 37°C | 0 (0.0) | 3.7 (3.7) | 94.2 (5.0) |
O, opaque; T, translucent; R, rugose. Switching assays were conducted for one to three cycles of culture passaging and plating as described in the text, with O and T broth cultures incubated at 34 to 35°C and plates incubated at 30°C; R broth cultures and plates were incubated at either 30 or 37°C as indicated.
The total percentage of colonies for a given strain does not equal 100 because some colonies were sectored while others were of an intermediate phenotype.
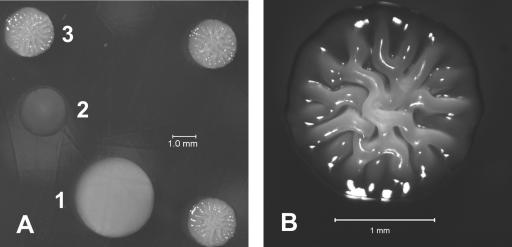
FIG. 2.
V. vulnificus 1003 phase variants. All colonies were grown on HI agar plates for 24 to 48 h at 30°C and viewed with a dissecting microscope. (A) Opaque (1), translucent (2), and rugose (3) colonies. (B) A rugose colony at higher magnification.
Two previously described translucent variants of parental strain 1003(O) that were isolated following transposon mutagenesis (24, 35) were also examined for their switching frequencies. The first of these, ABZ1(T), contains an insertion in a gene (wcvA) that is essential for capsule production and, as expected, it showed no conversion to opacity (Table 2). It did, however, yield rugose colonies, which demonstrates that the rugose phenotype in V. vulnificus can occur independently from capsule production. In contrast, strain GMB4(T), which contains an insertion in ORF4 of the super-integron region of the V. vulnificus genome (6), produced not only a rugose form but, surprisingly, also converted to the opaque phenotype. An isolated opaque derivative of GMB4(T) [i.e., GMB4(O)] retained both the kanamycin resistance and the mutant ORF4 PCR product that are characteristic of the translucent parent (data not shown). Also, introduction of an uninterrupted version of ORF4 on a plasmid into GMB4(T) did not restore the opaque phenotype of the original parental strain (data not shown). Based on these data, it appears that GMB4(T) arose spontaneously from 1003(O) and coincidentally acquired the transposon during the original transposon mutagenesis experiments (34).
The 16S rRNA genes from rugose variants isolated from both opaque and translucent parental types were amplified by PCR using previously described primers (21), the amplified products were subjected to automated sequencing, and the resulting sequences were used in BLASTN (2) searches of relevant databases. The submitted sequences (GenBank accession numbers AY676130, AY676132, and AY676133) showed greatest identity (97 to 99%) to 16S rRNA genes from known V. vulnificus strains (data not shown).
Characterization of V. vulnificus rugose variants.
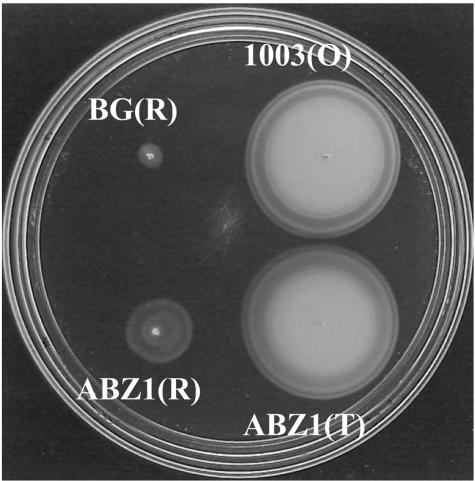
Because rugose isolates of V. cholerae show decreased motility relative to their smooth counterparts (1), rugose strains of V. vulnificus were tested for their motility characteristics relative to their opaque or translucent parents. Following inoculation from colonies onto HI plates containing 0.3% agar and incubation for 6 h at 30°C, both BG(R) and ABZ1(R) showed much lower motility than their respective opaque [1003(O)] and translucent [ABZ1(T)] parental strains, which produced wide motility zones of similar sizes (Fig. 3). Interestingly, ABZ1(R) consistently demonstrated a greater-than-twofold motility increase over the opaque-derived BG(R) (Fig. 3), and similar results were observed for rugose isolates derived from other translucent parents (data not shown). Despite their decreased motility in 0.3% agar, both BG(R) and ABZ1(R) cells were observed by transmission electron microscopy to possess a polar flagellum and under phase-contrast microscopy to actively swim in phosphate-buffered saline (data not shown). The presence of a polar flagellum for rugose isolates is similar to the findings for rugose V. cholerae as described by Ali et al. (1).
FIG. 3.
Swimming motility of V. vulnificus 1003 strains. Motility agar plates were inoculated from isolated colonies of each phase variant and incubated at 30°C for 6 h. Thirty-five colonies of each variant were tested.
Rugose V. vulnificus isolates BG(R) and ABZ1(R) were analyzed for their resistance to complement-mediated serum killing by exposing cells grown in broth culture to normal human serum (NHS; Sigma) at 37°C as described previously (5). Each culture was started from a representative colony of a given phenotype, and the experiments were conducted twice with the averages reported. As expected, the parental opaque strain 1003(O) showed much greater resistance to serum than the translucent parent ABZ1(T). Specifically, 1003(O) demonstrated serum resistance of approximately 57% (standard error [SE], 11.5%) (i.e., percent survival of cells exposed to NHS relative to the survival of cells exposed to heat-inactivated NHS), while ABZ1(T) was over 300-fold less resistant at 0.18% (SE, 0.08%) survival. While serum resistance relative to that of the specific parents appeared slightly elevated for rugose isolates BG(R) (87%; SE, 35.3%) and ABZ1(R) (1.0%; SE, 0.6%), the latter translucent-derived rugose isolate was still more sensitive by over 50-fold compared to the opaque strain 1003(O) and by over 80-fold compared to the opaque-derived rugose derivative BG(R). Taken together, the motility and serum resistance data demonstrate that although rugose isolates derived from opaque and translucent parents appear identical in colony morphology, they show significant differences with respect to other characteristics.
To demonstrate that the rugose phenotype is not unique to V. vulnificus 1003 and its derivatives, 26 other V. vulnificus strains, from environmental and clinical sources, were tested qualitatively for the ability to form rugose colonies. Upon growth overnight in HI broth at 34 to 35°C followed by streaking onto HI agar and incubation of plates at 30°C overnight, the rugose phenotype was shown by three Louisiana clinical isolates (1005, 1007, and 1014) and one eel isolate (96-7-155) from Denmark (data not shown). The parent phenotype of these four strains is opaque. The other 22 strains produced only opaque or translucent colonies in this shortened assay.
Rugose V. vulnificus produces copious biofilms.
When inoculated into broth and incubated at 30°C with shaking, all V. vulnificus 1003 phase variants grew suspended in the broth, although rugose isolates formed an aggregate ring at the air-broth-glass interface. When the broth was incubated statically at 30°C (Fig. 4), opaque and translucent strains grew suspended in the broth while rugose variants formed a pellicle at the air-broth interface as well as the aggregate ring and did not grow in suspension; rather, aggregates formed at the pellicle, sank to the bottom of the tube, and accumulated there while the broth remained clear. The aggregate ring and pellicle did not form when rugose isolates were incubated at 37°C (data not shown).
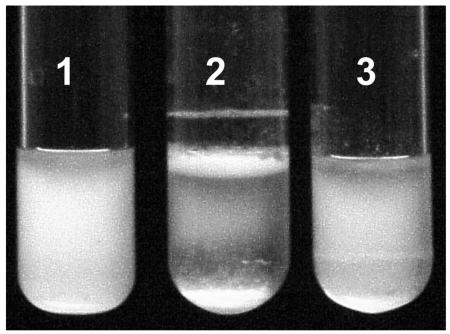
FIG. 4.
Distribution of V. vulnificus 1003 phase variants in broth culture. The rugose variant BG(R) (tube 2) forms a pellicle and aggregate ring after static growth in HI broth for 24 h at 30°C. As cells accumulate in the pellicle, aggregates break off and sink to the bottom of the tube. Both the 1003(O) (tube 1) and AZ(T) (tube 3) strains grow dispersed in the broth and do not form a pellicle or aggregates.
The aggregate ring produced by rugose strains of V. vulnificus at the air-broth-glass interface in culture tubes resembled biofilms produced by V. cholerae rugose variants (31). Biofilms produced by V. vulnificus phase variants that were grown in glass culture tubes at 30°C statically for 48 h were quantified by staining with crystal violet, resuspending the stained biofilms in dimethyl sulfoxide, and measuring the absorbance of the extracted stain at 570 nm (1, 33). Rugose variants produced significantly more biofilm than did opaque or translucent strains (Table 3). Strains BG(R), ABZ1(R), and GMB4(R) produced 16 (P < 0.005), 20 (P < 0.05), and 17 (P < 0.0005) times, respectively, the amount of biofilm produced by the opaque strain 1003(O). Meanwhile, 1003(O) produced relatively minimal biofilms, which appeared to be consistently greater than those produced by its translucent derivatives; in most cases, the difference in biofilm formation between opaque and translucent strains was not statistically significant (Table 3).
TABLE 3.
Biofilm production by V. vulnificus 1003 phase variants
| Strain | Absorbance at 570 nm (SE)a | Relative biofilm productionb |
|---|---|---|
| 1003(O) | 0.279 (0.041) | 1.00 |
| AZ(T) | 0.140 (0.019) | 0.51 |
| ABZ1(T) | 0.163 (0.045) | 0.58 |
| GMB4(T) | 0.191 (0.033) | 0.68 |
| BG(R) | 4.429 (0.528) | 15.87 |
| ABZ1(R) | 5.602 (1.322) | 20.19 |
| GMB4(R) | 4.857 (0.421) | 17.41 |
Absorbance is the average of triplicate measurements obtained in biofilm tube assays as described in the text. Absorbance values of incubated H1 broth also were measured and subtracted from the experimental sample values to correct for background.
Relative biofilm production is calculated relative to that produced by 1003(O).
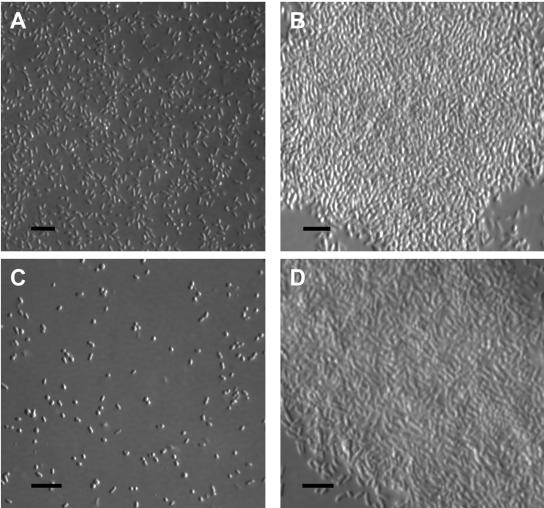
To confirm and extend the results from the tube assay, V. vulnificus phase variants were grown statically in HI broth in six-well culture plates at 30°C for up to 6 h, and the resulting biofilms that formed at the air-broth interface were harvested by their attachment upon contact to collodion-coated glass coverslips as described previously (10) and viewed by differential interference contrast microscopy. Figure 5 shows that while the rugose strains BG(R) and ABZ1(R) (Fig. 5B and D, respectively) produced a thick pellicle and adhered to the collodion-coated coverslip as a biofilm, their respective parental strains 1003(O) and ABZ1(T) (Fig. 5A and C, respectively) did not produce pellicles and adhered as individual cells. Compared to the quantitative results for the tube assay, the difference in biofilm formation between the opaque and translucent strains here appears to be greater; however, it is noteworthy that while the earlier assay involved staining biofilms that had adhered to glass tubes at the air-broth interface, the latter method involved capturing previously formed biofilms upon contact with collodion-coated coverslips.
FIG. 5.
Differential interference contrast micrographs of V. vulnificus 1003 biofilms collected with a collodion-coated coverslip. Fresh medium was inoculated 1:100 into six-well plates from the appropriate overnight cultures, and the resulting cultures were incubated statically for up to 6 h at 30°C. Biofilms that formed at the air-broth interface were harvested upon contact with collodion-coated coverslips. Each strain was grown in triplicate cultures, and the assay was conducted at least twice for each strain. (A) 1003(O); (B) BG(R); (C) ABZ1(T); (D) ABZ1(R). Bars, 10 μm.
Production of biofilms has been reported previously for V. vulnificus (11, 14, 17). In particular, a recent study reported that various translucent strains of V. vulnificus produced over three times more biofilm than their parent opaque strain (11). Although the basis for the apparent discrepancy between that study and ours remains undetermined, it is possible that strain differences and/or differences in the media and experimental protocols used for biofilm formation may be contributing factors. Previously, a significant difference in biofilm production was observed for different smooth strains of V. cholerae O1 El Tor (30).
Switching of rugose forms back to opaque and translucent colony forms.
V. vulnificus strains have been grown routinely at 37°C in our laboratory, and no rugose phenotype was previously observed. This included switching assays involving opaque and translucent isogenic pairs, which were performed similarly to ours, except that alkaline peptone water minimal medium and a consistent temperature of 37°C were used for all culture and plate incubations (15). In our study, the rugose variants appeared when HI broth cultures were incubated at temperatures below 37°C and plates were incubated at 30°C. To examine the persistence of rugose isolates at different temperatures, strains BG(R) and ABZ1(R) were subjected to switching assays where all inoculations, passages, and platings were done as described with incubations at either 30 or 37°C throughout the assays. The results (Table 2) showed that significant numbers of the opaque-derived rugose isolate BG(R) could persist at both 30 and 37°C. Switching of BG(R) back to opaque variants was also observed while, interestingly, conversion to a translucent form was barely detectable (at 30°C) or was not seen at all (at 37°C). Persistence of ABZ1(R), the rugose derivative of the acapsular transposon mutant ABZ1(T), was also detected at 30°C and even more so at 37°C. As expected, no switching back to the opaque phenotype was observed for ABZ1(R), while conversion to translucence was observed at both temperatures. Consistent with their new phenotypes, opaque and translucent derivatives of rugose strains obtained during these assays showed increased motility and lost the ability to form pellicles during static growth in broth (data not shown).
Here, we have described a new phenotypic version of V. vulnificus, the rugose variant, which is produced at high frequencies from both opaque and translucent parental strains when they are cultured repeatedly at temperatures below 37°C and which, once formed, persists even at 37°C. Rugose cells are capable of forming prodigious biofilms, and rugose isolates derived from the opaque parent were highly resistant to the complement-mediated killing of human serum. Based on these characteristics, the rugose form of V. vulnificus may play an important role not only in pathogenesis within the human host but also in survival within the cooler temperatures of its natural marine environment. Biofilm formation in the latter case may allow the organism to persist under conditions that are often nutritionally deficient or are otherwise unfavorable (7, 9).
Previous studies of phase variation in V. vulnificus involved inoculating rich medium with a single colony, incubating it overnight, and plating dilutions the next day. None of these studies reported the existence of a rugose phenotype (12, 23, 29, 32). Most of the studies that resulted in isolation of the V. cholerae rugose variant involved incubating starvation media cultures for extended times (18, 26, 31); however, a high-frequency rugose-producing strain of V. cholerae was isolated after only 24 to 48 h of incubation (1). In our study, the aggregate ring appeared in the culture tubes as early as the third day of the incubation cycle. Our method of inoculating rich medium and subculturing each day for a total of 5 to 6 days before plating seems to allow not only for high-frequency opaque and translucent phase variation, but also for high-frequency rugose production by both opaque and translucent strains. Though rich medium was used and cells were not under the continuous nutritional stress of growing in starvation medium, cultures did undergo many hours in stationary phase each day prior to their next passage. Such nutritional deprivation, though temporary, may have contributed to the observed high frequency of switching events.
Clearly more study is needed to elucidate the mechanisms that underlie the ability of V. vulnificus to convert between encapsulated opaque and nonencapsulated translucent forms, as well as to switch from these variants to the newly documented rugose form. Alteration of its morphology among these distinct phenotypes has obvious and important consequences for the ability of V. vulnificus to survive and cause disease.
Acknowledgments
We thank Eric Achberger and Richard Cooper for their support and guidance. We are grateful to Mark Batzer, Randy Garber, and Gail Kilroy for assistance with nucleotide sequencing. We also thank Sheri Dixon Schully for assistance with StatView 5.
This research was funded in part by the Louisiana Agricultural Experimental Station Hatch Project LAB03329 (to R. J. Siebeling, then to G.S.P.). Additional funding was supplied by the Louisiana Sea Grant College Program, grant number NA16RG2249 (to G.S.P.). The Louisiana Sea Grant College Program is a part of the National Sea Grant College Program maintained by the National Oceanic and Atmospheric Administration of the U.S. Department of Commerce. Microscopy was conducted in the Socolofsky Microscopy Center, Department of Biological Sciences, Louisiana State University.
Footnotes
This work is dedicated to the memory of Ronald J. Siebeling and V. R. Srinivasan, both of whom were tireless in their enthusiastic teaching of microbiology.
Approved for publication by the director of the Louisiana Agricultural Experiment Station as manuscript 04-20-0639.
REFERENCES
- 1.Ali, A., M. H. Rashid, and D. K. R. Karaolis. 2002. High-frequency rugose exopolysaccharide production by Vibro cholerae. Appl. Environ. Microbiol. 68:5773-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amako, K., K. Okada, and S. Miake. 1984. Evidence for the presence of a capsule in Vibrio vulnificus. J. Gen. Microbiol. 130:2741-2743. [DOI] [PubMed] [Google Scholar]
- 4.Anriany, Y. A., R. M. Weiner, J. A. Johnson, C. E. De Rezende, and S. W. Joseph. 2001. Salmonella enterica serovar Typhimurium DT104 displays a rugose phenotype. Appl. Environ. Microbiol. 67:4048-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carruthers, M. M., and W. J. Kabat. 1981. Vibrio vulnificus (lactose-positive vibrio) and Vibrio parahaemolyticus differ in their susceptibilities to human serum. Infect. Immun. 32:964-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C. Y., K. M. Wu, Y. C. Chang, C. H. Chang, H. C. Tsai, T. L. Liao, Y. M. Liu, H. J. Chen, A. B. Shen, J. C. Li, T. L. Su, C. P. Shao, C. T. Lee, L. I. Hor, and S. F. Tsai. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 13:2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henk, M. C. 2004. Method for collecting air-water interface microbes suitable for subsequent microscopy and molecular analysis in both research and teaching laboratories. Appl. Environ. Microbiol. 70:2486-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph, L. A., and A. C. Wright. 2004. Expression of Vibrio vulnificus capsular polysaccharide inhibits biofilm formation. J. Bacteriol. 186:889-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, C. M., K. C. Jeong, J. H. Rhee, and S. H. Choi. 1997. Thermal-death times of opaque and translucent morphotypes of Vibrio vulnificus. Appl. Environ. Microbiol. 63:3308-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 14.Marco-Noales, E., M. Milán, B. Fouz, E. Sanjuán, and C. Amaro. 2001. Transmission to eels, portals of entry, and putative reservoirs of Vibrio vulnificus serovar E (biotype 2). Appl. Environ. Microbiol. 67:4717-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, S. J. 1989. Lipopolysaccharide serotyping of Vibrio vulnificus. Ph.D. dissertation. Louisiana State University, Baton Rouge.
- 16.Martin, S. J., and R. J. Siebeling. 1991. Identification of Vibrio vulnificus O serovars with antilipopolysaccharide monoclonal antibody. J. Clin. Microbiol. 29:1684-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDougald, D., S. A. Rice, and S. Kjelleberg. 2001. SmcR-dependent regulation of adaptive phenotypes in Vibrio vulnificus. J. Bacteriol. 183:758-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizunoe, Y., S. N. Wai, A. Takade, and S.-I. Yoshida. 1999. Isolation and characterization of rugose form of Vibrio cholerae O139 strain MO10. Infect. Immun. 67:958-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris, J. G., Jr. 2003. Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin. Infect. Dis. 37:272-280. [DOI] [PubMed] [Google Scholar]
- 20.Morris, J. G., Jr., M. B. Sztein, E. W. Rice, J. P. Nataro, G. A. Losonsky, P. Panigrahi, C. O. Tacket, and J. A. Johnson. 1996. Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J. Infect. Dis. 174:1364-1368. [DOI] [PubMed] [Google Scholar]
- 21.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 22.Rice, E. W., C. J. Johnson, R. M. Clark, K. R. Fox, D. J. Reasoner, M. E. Dunnigan, P. Panigrahi, J. A. Johnson, and J. G. Morris, Jr. 1992. Chlorine and survival of “rugose” Vibrio cholerae. Lancet 340:740. [DOI] [PubMed] [Google Scholar]
- 23.Simpson, L. M., V. K. White, S. F. Zane, and J. D. Oliver. 1987. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 55:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, A. B., and R. J. Siebeling. 2003. Identification of genetic loci required for capsular expression in Vibrio vulnificus. Infect. Immun. 71:1091-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strom, M. S., and R. N. Paranjype. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 26.Wai, S. N., Y. Mizunoe, A. Takade, S.-I. Kawabata, and S.-I. Yoshida. 1998. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64:3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White, P. B. 1938. The rugose variant of vibrios. J. Pathol. Bacteriol. 46:1-6. [Google Scholar]
- 28.Wright, A. C., J. L. Powell, M. K. Tanner, L. A. Ensor, A. B. Karpas, J. G. Morris, Jr., and M. B. Sztein. 1999. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect. Immun. 67:2250-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright, A. C., L. M. Simpson, J. D. Oliver, and J. G. Morris, Jr. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida, S.-I., M. Ogawa, and Y. Mizuguchi. 1985. Relation of capsular materials and colony opacity to virulence in Vibrio vulnificus. Infect. Immun. 47:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuppardo, A. B. 1997. The polysaccharide capsule of Vibrio vulnificus—virulence, transposon mutagenesis, and identification of an essential gene. Ph.D. dissertation. Louisiana State University, Baton Rouge.
- 35.Zuppardo, A. B., and R. J. Siebeling. 1998. An epimerase gene essential for capsule synthesis in Vibrio vulnificus. Infect. Immun. 66:2601-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]