Abstract
The modification of metabolic pathways to allow for a dormant lifestyle appears to be an important feature for the survival of pathogenic bacteria within their host. One regulatory mechanism for persistent Mycobacterium tuberculosis infections is the stringent response. In this study, we analyze the stringent response of a nonpathogenic, saprophytic mycobacterial species, Mycobacterium smegmatis. The use of M. smegmatis as a tool for studying the mycobacterial stringent response was demonstrated by measuring the expression of two M. tuberculosis genes, hspX and eis, in M. smegmatis in the presence and absence of relMsm. The stringent response plays a role in M. smegmatis cellular and colony formation that is suggestive of changes in the bacterial cell wall structure.
The ability of Mycobacterium tuberculosis to persist in the human host is a major challenge for both vaccine- and drug-based strategies for controlling the spread of tuberculosis (TB) (17). Persistent M. tuberculosis cells are capable of initiating active growth in the host, a condition known as reactivation TB. Although it is somewhat controversial, it is generally believed that the site of viable M. tuberculosis in the host is inside caseous, necrotic, granulomatous lesions in the lungs. It has been reported that M. tuberculosis cells persisting inside granulomas lack the acid-fast staining characteristic of bacteria recovered from sputa and lesions of patents with active disease, indicating that changes occur in the bacterial cell wall during metabolic adaptation to a state of dormancy in the host (18, 22). Various models have been developed to create in vitro growth conditions that mimic the presumed state of M. tuberculosis inside granulomas, including oxygen limitation (32, 33) and nutrient starvation (1, 20). For example, when M. tuberculosis cultures are suspended in distilled water, they appear to lose their acid-fast staining ability, but the cells can remain viable for over 2 years in this extreme nutrient-deprived environment (22). This implies that in vitro observations of mycobacterial physiology may provide important insights into understanding how pathogenic mycobacteria survive in hosts. In addition, several mutants of M. tuberculosis that are impaired in de novo biosynthesis of various amino acids and vitamins (14) are attenuated in mouse models of tuberculosis infection, suggesting that the organism resides in a nutrient-poor environment. This supports the significance of in vitro nutrient starvation models for understanding in vivo persistence.
Recently, the stringent response of M. tuberculosis has been shown to play an important role in the in vitro and in vivo survival of this bacterium (9, 29). Escherichia coli has two homologous genes that are responsible for initiating the stringent response, namely, relA and spoT. Collectively, RelA and SpoT can sense nutrient deprivation and respond by synthesizing guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp), which can alter the promoter specificity of RNA polymerase. In mycobacteria, however, there is only one homologous gene, which by convention is referred to as relA. Deleting relMtb renders the tubercle bacillus less capable of surviving nutrient deprivation, and the mutant is also unable to persist in a mammalian host, in contrast to the isogenic wild-type strain (9, 29).
This report characterizes the stringent response of Mycobacterium smegmatis, a nonpathogenic, fast-growing species which is widely recognized as an excellent model system for studying various aspects of M. tuberculosis biology, such as gene expression (16), cell structure (7), and persistence in the face of nutrient starvation (31). The M. smegmatis stringent response was previously analyzed by overexpression of the relA gene of E. coli or relMtb in M. smegmatis (23). These strains produced elevated intracellular levels of ppGpp that led to altered cell morphologies and a modest decrease in the cell doubling time. In this study, we examine the effects of deleting the native relA homolog, relMsm, from M. smegmatis.
MATERIALS AND METHODS
Strains, media, and growth conditions for bacteria.
M. smegmatis (strain mc2155) was grown in 7H9 (Difco) medium supplemented with 0.2% glycerol and 0.05% Tween 80. M. tuberculosis cells (strains H37Rv and H37Rv ΔrelMtb) (29) were grown in 7H9 with 10% Middlebrook ADC (VWR), 0.2% glycerol, and 0.05% Tween 80. M. smegmatis ΔrelMsm was generated by allelic exchange. Briefly, M. smegmatis genomic DNA was digested with EcoRI, and fragments in the size range of 4 to 4.5 kb were cloned into pGEM3Zf(+). Colony blotting identified a clone (pGmsrelA) with a 4,229-bp EcoRI fragment containing the M. smegmatis relMsm gene. An internal BamHI-BglII fragment was replaced with the Tn903 aph cassette, removing 387 bp from the central region of the relMsm gene and leaving 991 and 1,021 bp of the open reading frame on the 5′ and 3′ ends of the insertion, respectively. A PAg85-lacZ Phsp60sacB cassette (25) was cloned into the XbaI site of this plasmid to create pGmsrelAKO. M. smegmatis was electroporated, and mutants were selected as previously described (3). DNA sequence analysis of the M. smegmatis genome indicated that the gene immediately downstream of relMsm is oriented in the opposite direction with respect to relMsm transcription, suggesting that it is highly unlikely that the insertion in relMsm inhibits transcription of this downstream gene via a polar effect. Therefore, the chances of a polar effect resulting from this deletion of relMsm are minimal.
Southern blot analysis.
Southern blot analysis of the M. smegmatis relMsm gene was performed as previously described (30). Genomic DNAs were isolated from wild-type M. smegmatis and from the candidate ΔrelMsm strain as previously described (2). Genomic DNAs were first digested with ApaI for 24 h before the buffer was adjusted and they were further digested with EcoRV for 24 h. The 1-kb region downstream of the M. smegmatis relMsm gene was amplified by a PCR with 5′-CATCTTAAGCGCACTGTTCGTCGTGTGGGC-3′ and 5′-TGCTCTAGAAGCGCGGCCTGATCGAGCG-3′ as forward and reverse primers, respectively. This PCR product was gel purified and used as a template in a PCR involving random oligonucleotide primers and [32P]CTP.
RNA extraction and analysis.
Fifty-milliliter quantities of M. smegmatis strains were grown to stationary phase (4 days in 7H9 medium with 0.05% Tween 80) before centrifugation. Cell pellets were resuspended in 1 ml of Trizol and lysed by the use of 0.1-mm-diameter glass beads (Biospec Products, Inc.) and a FastPrep FP120 bead-beating device (Bio 101). The cells were disrupted three times at a speed setting of 6.5 for 45 s, with intermittent incubation on ice for 5 min. Lysed samples were centrifuged at 12,000 × g for 10 min to remove cellular debris, and the supernatant was transferred to RNase-free 1.5-ml tubes containing 300 μl of chloroform-isoamyl alcohol (24:1) and phase-lock gel (Sigma). The solution was mixed by inversion for 2 min at room temperature before being centrifuged at 12,000 × g for 10 min. The upper aqueous layer was removed and added to 600 μl of isopropanol in a fresh tube, which was inverted several times and incubated at 4°C overnight. Nucleic acids were pelleted by centrifugation at 12,000 × g for 10 min and were washed with 70% ethanol. After the pellets were air dried for 10 min, they were redissolved in 70 μl of RNase-free water. RNA samples were mixed with an ethidium bromide-containing loading dye and separated in 1.5% formaldehyde-agarose.
Measurement of (p)ppGpp.
Three strains were examined for the induction of radiolabeled (p)ppGpp synthesis, namely, E. coli JM105, wild-type M. smegmatis, and M. smegmatis ΔrelMsm. Cells were inoculated into 3 ml of minimal medium (MOPS [morpholinepropanesulfonic acid] buffer, 7.4 μM vitamin B12, 2% glucose; pH 7.2) and incubated overnight. These cultures were used to seed 25-ml cultures in minimal medium that were allowed to grow to an optical density at 600 nm (OD600) of 0.3 before each culture was split in half; one part was used to continue monitoring changes in the OD600, and one part received radiolabeled phosphoric acid at a final concentration of 100 μCi/ml. The cultures were allowed to go through two doublings before the stringent response was induced by the addition of either 1 mg of d,l-serine hydroxamate/ml to E. coli (4) or 1 mg of d,l-norvaline/ml to the M. smegmatis strains (11). Both E. coli and M. smegmatis cells were induced for 6 h. Cells were harvested by centrifugation and washed twice in phosphate-buffered saline to remove the unincorporated label. Cell pellets were resuspended in 1 ml of 2 M formic acid, disrupted three times with glass beads in a bead beater, and centrifuged at 12,000 × g for 10 min to remove cellular debris. Supernatants were spotted onto 20- by 20-cm, 100-μm-thick cellulose polyethyleneimine thin-layer chromatography plates (Selecto Scientific). Thin-layer chromatography plates were equilibrated by soaking in distilled water and then drying at room temperature. Plates were spotted with samples 1 cm from the bottom of the plates. Commercially available radiolabeled ATP was spotted at a 1:1,000 dilution to identify the relative positions of radiolabeled spots. The samples were resolved with a 1.5 M potassium phosphate running buffer until the solvent front migrated 15 cm (about 2.5 h). The plates were air dried and exposed to phosphorimager screens for 7 days. These experiments were performed independently two times.
Electron microscopy.
Cells were grown to stationary phase (4 days growth) and pelleted before being fixed with 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer with 6 M sucrose. Samples were prepared and analyzed for scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as previously described (8).
SDS sensitivity assay.
Wild-type and ΔrelMsm cells were compared for their ability to grow in the presence of 0.005% sodium dodecyl sulfate (SDS), as previously described (19). Cells were grown in 7H9 medium with Tween 80 to an OD600 of 0.5 and then serially diluted 10-fold onto Luria-Bertani agar plates, with or without 0.005% SDS. The plates were scored after 4 days of growth at 37°C.
Competition starvation assays.
Comparisons of wild-type M. smegmatis transformed with pMV306 (hygromycin-resistant integrating plasmid) and M. smegmatis ΔrelMsm (kanamycin resistant) for survival under nutrient and oxygen deprivation were performed as described previously (29). Briefly, each strain was grown to mid-log phase before being subjected to the following conditions. For oxygen starvation conditions, the mid-log strains were kept in their original medium, mixed in a 1:1 ratio, and divided into aliquots in a series of 1.5-ml Sarstedt screw-cap tubes containing gas-impermeable seals, with a 300-μl headspace for the cultures. A control culture containing 1.5 μg of methylene blue/ml indicated that oxygen was depleted within 36 h, as measured by the complete decolorization of the dye.
Western blot analysis of Eis.
Detection of the Eis protein (Rv2416c) was performed as previously described, with a slight modification (10). M. tuberculosis strains H37Rv and H37Rv ΔrelMtb were grown from early log phase to stationary phase, with aliquots removed at various time points to determine culture densities (OD600) and prepare protein lysates. For analyses of Eis levels in M. smegmatis, strains transformed with either pOLYG (12) or p69 (35) were grown in 7H9 medium with 0.05% Tween 80 for 4 days before the cells were lysed and analyzed for Eis protein as previously described (10). In addition, M. smegmatis lysates separated by SDS-12% polyacrylamide gel electrophoresis were stained with Coomassie brilliant blue as previously described (35). A comparative quantitation of band intensities in Western blots was made by densitometry with an AlphaImager 2000 (Alpha Innotech Corp.).
Western blot analysis of HspX.
An analysis of HspX (α-crystallin) (Rv2031c) production was performed as previously described (10). H37Rv and H37Rv ΔrelMtb cells were grown to an OD600 of 1.6 before being harvested and used to prepare protein lysates as described above. M. smegmatis strains were grown for 4 days (OD600, 1.8) before the cells were harvested and lysed. For the expression of hspX in M. smegmatis, the gene was amplified by PCR from H37Rv chromosomal DNA with the primers 5′-CCCAAGCTTGACGGTGGCCCTCGGTGAC-3′ and 5′-GCTCTAGAGCCGCTGCGGTCATCAGCAC-3′. The 976-bp PCR product containing hspX and its promoter was digested with HindIII and XbaI and ligated into HindIII- and XbaI-digested pOLYG to generate the plasmid phspX. Both pOLYG and phspX were electroporated into wild-type and ΔrelMsm strains of M. smegmatis.
RESULTS
Deletion of relMsm from M. smegmatis.
The relMsm gene was inactivated in M. smegmatis by allelic replacement of the wild-type copy with one containing an internal, in-frame deletion and an internal kanamycin resistance marker (aph). Southern blot analysis and PCR analysis (results not shown) confirmed that the M. smegmatis ΔrelMsm strain lacked a 387-bp internal fragment and contained a 1.27-kbp insertion (aph) in relMsm.
The disruption of the relMsm gene caused a loss of RelA function, as shown by the loss of a radiolabeled spot for a formic acid extract of M. smegmatis ΔrelMsm that corresponded with the d,l-serine hydroxamate-induced ppGpp spot seen for E. coli (Fig. 1A, arrowhead). d,l-Serine hydroxamate was not used to induce the M. smegmatis stringent response since this amino acid analog has previously been shown to be ineffective at inducing ppGpp synthesis in M. tuberculosis (29), possibly due to impermeability of the mycobacterial cell wall to this analog. Therefore, d,l-norvaline was chosen to induce the stringent response since it was previously shown to initiate a stringent response in Bacillus subtilis (11) and M. tuberculosis (our unpublished data). d,l-Norvaline induced the synthesis of a molecule (Fig. 1A, lane 5) that was present in the wild-type strain of M. smegmatis but was significantly reduced in the ΔrelMsm strain (lane 6). The position of this spot matched that for radiolabeled ppGpp produced in E. coli. The identity of an additional 32P-labeled spot migrating below ppGpp in M. smegmatis formic acid extracts is unknown. This spot is not present in E. coli extracts, but it has been previously seen in extracts of M. smegmatis radiolabeled with 32P (23).
FIG. 1.
Inactivation of relMsm in M. smegmatis. (A) Thin-layer chromatography of radiolabeled (p)ppGpp. E. coli strain JM105 (lane 1) was induced for the stringent response by d,l-serine hydroxamate (lane 2). The appearance of labeled (p)ppGpp is indicated with an arrowhead. Wild-type M. smegmatis (lane 3) and M. smegmatis ΔrelMsm (lane 4) were induced for the stringent response by d,l-norvaline (lanes 5 and 6, respectively). Radiolabeled (p)ppGpp is present in lane 5 (wild-type strain) but absent from lane 6 (ΔrelMsm strain). (B) Agarose gel electrophoresis of RNAs obtained from either wild-type M. smegmatis (lane 1) or M. smegmatis ΔrelMsm (lane 2) cells that had been grown to stationary phase (3-day-old cultures). A 1-kb DNA ladder is present in lane L. rRNA bands are indicated (arrowheads).
Further evidence for the inactivation of relMsm was seen by the accumulation of RNAs in stationary-phase M. smegmatis ΔrelMsm (Fig. 1B), which was analogous to that reported for E. coli (6). Stable RNAs (23S and 16S rRNAs) were clearly visible with the M. smegmatis ΔrelMsm strain induced for the stringent response (Fig. 1B, arrowheads).
The stringent response alters M. smegmatis morphology.
Several structural differences were observed between the wild-type M. smegmatis and M. smegmatis ΔrelMsm strains. Cultured ΔrelMsm cells clumped significantly more than wild-type cells in liquid cultures (data not shown). This same phenotypic difference was also noticed between H37Rv ΔrelMtb and H37Rv (data not shown). It has previously been demonstrated that relMtb controls several genes that are associated with biogenesis or the structure of the cell wall (9), which may correlate with the apparent changes in cell wall characteristics. These genes include those known to play roles in cell invasion, peptidoglycan synthesis, mycolic acid biosynthesis, and transport as well as those encoding a number of putative lipoproteins of unknown function and PE/PGRS family members. The Chatterji laboratory recently reported that M. smegmatis can alter its profile of surface-associated glycopeptidolipids (GPLs) during carbon starvation (24). To analyze if GPLs were responsible for the clumping of the M. smegmatis ΔrelMsm strain, we isolated and compared GPLs from the mutant and parental strains as previously described (24). There was no detectable difference between GPLs of the wild-type and ΔrelMsm strains (data not shown), which indicates that the GPLs are not likely responsible for the clumping phenotype.
Individual colony morphologies also varied, as shown in Fig. 2A. The wild-type strain formed colonies characteristic of M. smegmatis, with protruding edges on the perimeter and a relatively uniform orange pigmentation (Fig. 2A, panel 1). When viewed from the angle of the agar surface, these wild-type colonies were relatively flat. Colonies of the M. smegmatis ΔrelMsm strain, however, had smoother perimeter edges and less pigmentation in the perimeter of the colonies and formed central, elevated peaks when viewed from the side (Fig. 2A, panel 2).
FIG. 2.
Effects of stringent response upon the appearance of M. smegmatis. Wild-type M. smegmatis (panels 1) and M. smegmatis ΔrelMsm (panels 2) are shown. (A) Colonies grown on 7H9 agar for 2 weeks. Bars = 5 mm. (B) SEM analysis of cells grown for 3 days in 7H9 liquid medium. Bars = 3.8 μm. (C) TEM analysis of cells grown for 3 days in 7H9 liquid medium. “N” indicates a nucleoid region, and arrowheads indicate two septa in a single cell. The inset shows a magnification of the two septa. Bars = 200 nm. (D) TEM analysis showing an enlarged cell (EC) compared to the smaller (in diameter) wild-type cells. Ghost cells (G) and pear-shaped cells (PS) were seen routinely for M. smegmatis ΔrelMsm but were absent from the wild-type cells. Bars = 500 nm.
Individual cell morphologies also varied between the two M. smegmatis strains. Wild-type cells viewed by SEM appeared as short rods (Fig. 2B, panel 1) (average length, 1.2 μm), while ΔrelMsm cells appeared significantly longer (Fig. 2B, panel 2) (average length, 3.5 μm).
TEM analysis of the two strains confirmed the short lengths of wild-type cells compared to ΔrelMsm cells (Fig. 2C). Numerous elongated M. smegmatis ΔrelMsm cells contained multiple division septa per cell (Fig. 2C, panel 2). These results are similar to those reported for ppGpp-free E. coli cells (36). Notably, the M. smegmatis ΔrelMsm strain also produced round enlarged cells (EC) as well as cells with a pear-shaped (PS) appearance that diverged from the typical bacillus shape (Fig. 2D). TEM analysis of the M. smegmatis ΔrelMsm strain also revealed a large number of cell wall “ghosts” (G) that appeared to be remnants of cells lacking cytoplasm (Fig. 2D). These ghosts were not seen in wild-type cells grown for an equivalent length of time. Despite the differences in cell clumping (data not shown) and cell shape (Fig. 2B, C, and D), there was no detectable difference in cell wall structure between the two strains, as observed by TEM at a magnification of ×200,000 (data not shown).
The stringent response does not affect the M. smegmatis growth rate.
It was previously shown that eliminating the stringent response in M. tuberculosis results in decreased growth at 37°C and an inability to grow at 42°C (29). M. smegmatis ΔrelMsm grown at 37 or 42°C showed no significant difference from the wild-type parental strain (data not shown). Both of these observations are in contrast to those for M. tuberculosis.
SDS sensitivity.
Sensitivity to SDS has previously been shown for M. smegmatis strains with altered cell surfaces (19). The presence of 0.005% SDS in Luria-Bertani agar reduced the growth of both the wild-type and ΔrelMsm strains, but the reduction was especially apparent with the ΔrelMsm cells (Fig. 3). The ΔrelMsm colonies were much smaller and had edges that were raised. These colonies seemed to grow in a way that minimized contact with the agar surface, while the wild-type colonies were seen to be spreading out.
FIG. 3.
SDS sensitivity test. The photomicrographs show that both wild-type and ΔrelMsm strains (A and B, respectively) grow equally well in the absence of SDS. However, when SDS was present, the wild-type cells (C) showed a marked increase in growth on the agar surface compared to the ΔrelMsm cells (D). Bars = 0.01 mm.
The stringent response is required for long-term survival under conditions of nutrient deprivation.
Based on previous studies of the stringent response in M. tuberculosis (29), we predicted that the relMsm function would contribute to M. smegmatis persistence in vitro under conditions of prolonged nutrient or oxygen starvation. Wild-type M. smegmatis containing an integrated copy of pMV306 (Hygr) and M. smegmatis ΔrelMsm (Kanr) were grown separately to mid-log phase, mixed at a 1:1 ratio, and subjected to various starvation conditions. After 30 days of starvation, there were dramatic decreases in the viability of the M. smegmatis ΔrelMsm strain compared to the wild-type strain. These included a 4.5-log decrease in viability when cells were grown to stationary phase in rich medium (Fig. 4A), a >2-log decrease when cells were subjected to oxygen limitation only (Fig. 4B), and a 2.5-log decrease when cells were suddenly and completely starved for nutrients in Tris-buffered saline with Tween 80 (TBST) (Fig. 4C). The cessation of growth of M. smegmatis after 36 h in gas-impermeable tubes was due to the consumption of all available oxygen, as demonstrated by the complete decolorization of methylene blue in control cultures. These differences in viability between the wild-type and M. smegmatis ΔrelMsm strains at 30 days exceed those reported between the H37Rv and H37Rv ΔrelMtb strains after starvation for 60 days (29).
FIG. 4.
Competition survival assays comparing wild-type M. smegmatis (open squares) and M. smegmatis ΔrelMsm (closed circles). (A) Cells were grown to stationary phase in 7H9 liquid medium with 0.05% Tween. Alternatively, cells were grown to mid-log phase (OD600, 0.15) before being subjected to anaerobic conditions (B) or resuspended in TBST (C).
M. tuberculosis eis and hspX genes are regulated by the stringent response.
A microarray analysis of M. tuberculosis revealed several genes which were up or down regulated in response to RelMtb activation (9). Induction of the stringent response in wild-type and ΔrelMtb strains of M. tuberculosis by incubation of the cells in TBST for 6 h resulted in a 6.4-fold higher expression level for the hspX gene in the wild-type strain and an 18-fold higher expression level for the eis genes in relMtb strains. For verification that these transcriptional changes were reflective of differences in protein levels, Western blot analyses were performed to determine the amounts of hspX and eis gene products in M. tuberculosis. In contrast to the case for transcriptional profiling studies (9), M. tuberculosis strains were gradually starved for amino acids by growing to stationary phase. This allowed for a gradual depletion of amino acids that would induce the stringent response but not impair the bacterium's ability to reconstruct its proteome. It was anticipated that a gradual depletion of available nutrients would more closely mimic the development of a restrictive environment in developing granulomas in vivo. Figure 5A shows that during gradual starvation in 7H9 medium with ADC and Tween 80, the Eis protein accumulated 6.3-fold in M. tuberculosis ΔrelMtb, while Eis levels remained constant throughout the growth curve of the wild-type strain (compare lanes 9 for H37Rv and H37Rv ΔrelMtb). Western blot analysis was used to examine hspX expression in M. tuberculosis strains grown to stationary phase. In the presence of relMtb, HspX levels were fivefold higher than those in the ΔrelMtb mutant strain (Fig. 6, lanes 2 and 3).
FIG. 5.
Regulation of eis (Rv2416c) by mycobacterial stringent response. (A) M. tuberculosis strains H37Rv and H37Rv ΔrelMtb were grown from early log (lanes 1 and 2) to mid-log (lanes 3 to 6) and stationary (lanes 7 to 9) phase. Protein lysates were equilibrated (22 μg/lane) and examined for eis expression by Western blot analysis. (B) Coomassie-stained proteins (25 μg/lane) are shown for M. smegmatis/pOLYG (lane 1), M. smegmatis ΔrelMsm/pOLYG (lane 2), M. smegmatis/p69 (lane 3), and M. smegmatis ΔrelMsm/p69 (lane 4). (C) Western blot analysis of Eis with the same protein lysates as those used for panel B.
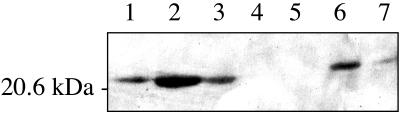
FIG. 6.
Regulation of hspX (Rv2031c) by mycobacterial stringent response. Western blot analysis was performed with an anti-HspX antibody. Lane 1, 3 μg of purified HspX; lane 2, M. tuberculosis H37Rv; lane 3, H37Rv ΔrelMtb; lane 4, M. smegmatis/pOLYG; lane 5, M. smegmatis ΔrelMsm/pOLYG; lane 6, M. smegmatis/phspX; and lane 7, M. smegmatis ΔrelMsm/phspX. Lanes 2 to 7 contained 22 μg of protein/lane.
For verification that the expression of the eis and hspX genes is stringently controlled, these genes were expressed from multicopy plasmids in M. smegmatis. Each gene was under the control of its native promoter. M. smegmatis does not have a gene homologous to eis (35). Therefore, eis expression in M. smegmatis was made possible by transforming wild-type and ΔrelMsm strains of M. smegmatis with the plasmid p69 (a pOLYG derivative containing eis from M. tuberculosis under the control of its native promoter) (35). The expression of the Eis protein by cells carrying p69 was sixfold higher for the ΔrelMsm strain than for the wild-type strain (Fig. 5B and C, compare lanes 4 with lanes 3). Therefore, the eis promoter appears to be negatively regulated by the stringent response in both M. tuberculosis and the heterologous host M. smegmatis.
The transformation of M. smegmatis with a plasmid containing the hspX gene with its native promoter (phspX) resulted in the expression of the gene in a relMsm-dependent manner (Fig. 6, lanes 6 and 7). The levels of HspX were 4.5-fold higher in wild-type M. smegmatis than in the ΔrelMsm strain, which is comparable to the difference seen between the M. tuberculosis strains. As expected, no Eis or HspX was expressed in cells transformed with the vector (pOLYG) control, as determined by Coomassie blue staining and Western blot analysis (Fig. 5B, lanes 1 and 2; Fig. 5C, lanes 1 and 2; and Fig. 6, lanes 4 and 5).
DISCUSSION
Undetectable levels of ppGpp in our M. smegmatis ΔrelMsm mutant correlated with alterations in the M. smegmatis cellular morphology (Fig. 2B, C, and D). Elongated cells and coccoidal cells were previously reported for wild-type M. smegmatis that expressed E. coli relA from a heat shock promoter (23). A concern with the previous study is that the ppGpp levels were elevated by transferring M. smegmatis cultures from 30 to 44°C, and it is possible that the 14°C temperature shift contributed to the changes in cell shape. However, our results show that these changes can be attributed to intracellular ppGpp levels. We present the first high-resolution electron microscopy images of mycobacterial changes in cell morphology due to different ppGpp levels. These alterations in cell shape corroborate changes in E. coli cell morphologies due to a lack of ppGpp (36).
Mutations that affect mycobacterial cell wall surfaces have a correlating increase in SDS sensitivity (5, 19, 21). The M. smegmatis ΔrelMsm strain had an increased sensitivity to the anionic detergent SDS (Fig. 3). This suggests that the mutant has altered cell wall surface properties compared to the wild-type strain. In fact, the small raised M. smegmatis colonies that grew in the presence of SDS were remarkably similar to those seen for an M. smegmatis strain that was deficient in cell surface Erp proteins (19).
Our results indicate that, similar to the cases of E. coli (6) and M. tuberculosis (29), the stringent response allows M. smegmatis to adapt to nutrient deprivation. It is possible that the stringent response is involved during the adaptation of mycobacteria to anaerobiosis, since a gradual nutrient starvation of cells was associated with increased resistance to hypoxia (34). Our results show that under conditions in which oxygen is the only limiting factor (Fig. 4B), the presence of the relMsm gene is associated with anaerobic survival, as seen by the >100-fold decrease in survival of a ΔrelMsm mutant in a competition experiment with its parental counterpart.
A noticeable difference exists between the stringent responses of E. coli and mycobacteria. Recently, DksA was shown to act in synergy with ppGpp during the regulation of RNA polymerase activity (28). DksA is essential for the stringent control of rRNA in E. coli (27), but no DksA homologs exist in the known genomes of mycobacteria. Therefore, the tight regulation of rRNA by M. smegmatis (Fig. 1B) and M. tuberculosis (9) suggests that there is some DksA-independent mechanism.
Our study corroborates previous findings that the expression of the hspX and eis genes is under opposite control by the stringent response, as evidenced by the increased expression of HspX and Eis proteins in the wild-type and ΔrelMtb mutants of M. tuberculosis, respectively (Fig. 5 and 6). Eis was previously shown to be immunogenic in TB patients (10). This is similar to the case for several other immunodominant M. tuberculosis antigens which have been shown to be relMtb dependent, including the 19-kDa antigen Esat6 and members of the antigen 85 complex (9). Although the function of the Eis protein is unknown, reverse position-specific BLAST, three-dimensional position-specific scoring matrix, and multiple EM for motif elicitation programs have revealed that Eis is a member of the GCN5 superfamily of N-acetyltransferases (Richard Friedman, personal communication). This family of proteins regulates several different cellular functions, including transcriptional activity and antibiotic resistance.
In contrast to the unknown function of Eis, the HspX protein has been shown to act as a heat shock protein (Hsp) and can prevent the thermal denaturation of alcohol dehydrogenase (37). HspX is also involved in Mycobacterium bovis BCG and M. tuberculosis cell wall thickening under prolonged anaerobic growth (7). The hspX gene is a member of the DosR-regulated dormancy regulon (26), and its expression has been shown to increase during stationary-phase growth (15), during hypoxia (38), or when cultures are exposed to nitric oxide (13). We provided evidence that hspX additionally belongs to the RelA regulon in mycobacteria. We believe that this regulation is independent of sigF since the expression of this alternative sigma factor in M. tuberculosis is not regulated by the stringent response (unpublished data). The inability of the H37Rv ΔrelMtb strain to grow at 42°C (29) may be linked to the decreased expression of HspX. The absence of temperature sensitivity in the M. smegmatis ΔrelMsm strain may indicate that heat shock proteins with a similar role in protection against thermal denaturation are not under stringent control in this organism. RelMtb-mediated expression of HspX during nutrient restriction in the host environment may assist in persistent survival since this protein has been shown to play a role in vitro in structural adaptations to a persistent phenotype (7).
Our results have shown that the key elements of stringent control are conserved between M. tuberculosis and M. smegmatis and that M. smegmatis is a useful heterologous host for investigating critical elements of this bacterial response. In addition, we have shown that the stringent response plays a role in adaptation to both nutrient and oxygen starvation. Understanding the mechanisms that allow M. smegmatis to enter a state of apparent dormancy is essential for identifying targets that are necessary for the persistent survival of organisms in patients harboring latent disease. However, the genome size of M. smegmatis (about 7 Mb) is considerably larger than that of M. tuberculosis (4.4 Mb), and thus it is likely that studying the stringent response of this nonpathogenic species may produce interesting relA-dependent mechanisms that are not seen in the pathogen.
Acknowledgments
This research was supported by a grant from the American Lung Association (RG-022-N) and by a new investigator matching grant from the National Foundation for Infectious Diseases. Purified HspX protein was provided by Colorado State University, courtesy of NIH, NIAID contract no. AI-75320, entitled “Tuberculosis Research Materials and Vaccine Testing.”
We are appreciative of Christine Davitt and Valerie Lynch-Holm of Washington State University for their assistance with electron microscopy analysis. We thank Linoj Samuel and Richard Friedman for discussions about the function of Eis, William Louie and Nancy Tacconi for helpful comments, and Robert Kadner for proofreading the manuscript.
REFERENCES
- 1.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 2.Bose, M., A. Chander, and D. H. Das. 1993. A rapid and gentle method for the isolation of genomic DNA from mycobacteria. Nucleic Acids Res. 21:2529-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boshoff, H. I., and V. Mizrahi. 2000. Expression of Mycobacterium smegmatis pyrazinamidase in Mycobacterium tuberculosis confers hypersensitivity to pyrazinamide and related amides. J. Bacteriol. 182:5479-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, L., D. Gentry, T. Elliott, and M. Cashel. 2002. DksA affects ppGpp induction of RpoS at a translational level. J. Bacteriol. 184:4455-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camacho, L. R., P. Constant, C. Raynaud, M. A. Laneelle, J. A. Triccas, B. Gicquel, M. Daffe, and C. Guilhot. 2001. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. J. Biol. Chem. 276:19845-19854. [DOI] [PubMed] [Google Scholar]
- 6.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. ASM Press, Washington, D.C.
- 7.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl, J. L. 2004. Electron microscopy analysis of Mycobacterium tuberculosis cell division. FEMS Microbiol. Lett. 240:15-20. [DOI] [PubMed] [Google Scholar]
- 9.Dahl, J. L., C. N. Kraus, H. I. Boshoff, B. Doan, K. Foley, D. Avarbock, G. Kaplan, V. Mizrahi, H. Rubin, and C. E. Barry III. 2003. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 100:10026-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl, J. L., J. Wei, J. W. Moulder, S. Laal, and R. L. Friedman. 2001. Subcellular localization of the intracellular survival-enhancing Eis protein of Mycobacterium tuberculosis. Infect. Immun. 69:4295-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eymann, C., G. Homuth, C. Scharf, and M. Hecker. 2002. Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J. Bacteriol. 184:2500-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garbe, T. R., J. Barathi, S. Barnini, Y. Zhang, C. Abou-Zeid, D. Tang, R. Mukherjee, and D. B. Young. 1994. Transformation of mycobacterial species using hygromycin resistance as selectable marker. Microbiology 140:133-138. [DOI] [PubMed] [Google Scholar]
- 13.Garbe, T. R., N. S. Hibler, and V. Deretic. 1999. Response to reactive nitrogen intermediates in Mycobacterium tuberculosis: induction of the 16-kilodalton alpha-crystallin homolog by exposure to nitric oxide donors. Infect. Immun. 67:460-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hingley-Wilson, S. M., V. K. Sambandamurthy, and W. R. Jacobs, Jr. 2003. Survival perspectives from the world's most successful pathogen, Mycobacterium tuberculosis. Nat. Immunol. 4:949-955. [DOI] [PubMed] [Google Scholar]
- 15.Hu, Y., and A. R. Coates. 1999. Transcription of the stationary-phase-associated hspX gene of Mycobacterium tuberculosis is inversely related to synthesis of the 16-kilodalton protein. J. Bacteriol. 181:1380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs, W. R., Jr., M. Tuckman, and B. R. Bloom. 1987. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature 327:532-535. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann, S. H. E., and I. D. A. van Embden. 1993. Tuberculosis: a neglected disease strikes back. Trends Microbiol. 1:2-5. [DOI] [PubMed] [Google Scholar]
- 18.Khomenko, A. G. 1987. The variability of Mycobacterium tuberculosis in patients with cavitary pulmonary tuberculosis in the course of chemotherapy. Tubercle 68:243-253. [DOI] [PubMed] [Google Scholar]
- 19.Kocincova, D., B. Sonden, L. de Mendoca-Lima, B. Gicquel, and J.-M. Reyrat. 2004. The Erp protein is anchored at the surface by a carboxy-terminal hydrophobic domain and is important for cell-wall structure in Mycobacterium smegmatis. FEMS Microbiol. Lett. 231:191-196. [DOI] [PubMed] [Google Scholar]
- 20.Lobel, R. O., E. Shorr, and H. B. Richardson. 1933. The influence of adverse conditions upon the respiratory metabolism and growth of human tubercle bacilli. J. Bacteriol. 26:167-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyka, W. 1974. Studies on the effect of starvation on mycobacteria. Infect. Immun. 9:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojha, A. K., T. K. Mukherjee, and D. Chatterji. 2000. High intracellular level of guanosine tetraphosphate in Mycobacterium smegmatis changes the morphology of the bacterium. Infect. Immun. 68:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojha, A. K., S. Varma, and D. Chatterji. 2002. Synthesis of an unusual polar glycopeptidolipid in glucose-limited culture of Mycobacterium smegmatis. Microbiology 148:3039-3048. [DOI] [PubMed] [Google Scholar]
- 25.Parish, T., and N. G. Stoker. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146:1969-1975. [DOI] [PubMed] [Google Scholar]
- 26.Park, H. D., K. M. Guinn, M. I. Harrell, R. Liao, M. I. Voskuil, M. Tompa, G. K. Schoolnik, and D. R. Sherman. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul, B. J., M. M. Barker, W. Ross, D. A. Schneider, C. Webb, J. W. Foster, and R. L. Gourse. 2004. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118:311-322. [DOI] [PubMed] [Google Scholar]
- 28.Perederina, A., V. Svetlov, M. N. Vassylyeva, T. H. Tahirov, S. Yokoyama, I. Artsimovitch, and D. G. Vassylyev. 2004. Regulation through the secondary channel—structural framework for ppGpp-DksA synergism during transcription. Cell 118:297-309. [DOI] [PubMed] [Google Scholar]
- 29.Primm, T. P., S. J. Andersen, V. Mizrahi, D. Avarbock, H. Rubin, and C. E. Barry III. 2000. The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182:4889-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Smeulders, M. J., J. Keer, R. A. Speight, and H. D. Williams. 1999. Adaptation of Mycobacterium smegmatis to stationary phase. J. Bacteriol. 181:270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wayne, L. G. 1994. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 13:908-914. [DOI] [PubMed] [Google Scholar]
- 33.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wayne, L. G., and K. Lin. 1982. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect. Immun. 37:1042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei, J., J. L. Dahl, J. W. Moulder, E. A. Roberts, P. O'Gaora, D. B. Young, and R. L. Friedman. 2000. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J. Bacteriol. 182:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]
- 37.Yuan, Y., D. D. Crane, and C. E. Barry III. 1996. Stationary-phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J. Bacteriol. 178:4484-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan, Y., D. D. Crane, R. M. Simpson, Y. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry III. 1998. The 16-kDa α-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 95:9578-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]