Abstract
Background
Whether cesarean section (CS) is a risk factor for asthma in offspring is controversial. The purpose of this study was to investigate the association between CS and asthma in children/adolescents.
Methods
Pubmed, Embase, Web of Science, and Cochrane Library electronic databases were searched for cohort studies on the relationship between mode of delivery and asthma in children/adolescents up to February 2023. Birth via CS was considered an exposure factor. Asthma incidence was taken as a result.
Results
Thirty-five cohort studies (thirteen prospective and twenty-two retrospective cohort studies) were included. The results showed that the incidence of asthma was higher in CS offspring (odds ratio (OR) = 1.18, P < 0.001) than in the vaginal delivery (VD) group. Partial subgroup analyses showed a higher incidence of asthma in female offspring born via CS (OR = 1.26, P < 0.001) compared with the VD group, while there was no difference in males (OR = 1.07, P = 0.325). Asthma incidence was higher in CS offspring than in the VD group in Europe (OR = 1.20, P < 0.001), North America (OR = 1.15, P < 0.001), and Oceania (OR = 1.06, P = 0.008). This trend was not found in the Asian population (OR = 1.17, P = 0.102). The incidence of atopic asthma was higher in offspring born via CS (OR = 1.14, P < 0.001) compared to the VD group. The CS group had a higher incidence of persistent asthma, but the difference did not reach statistical significance (OR = 1.15, P = 0.063).
Conclusion
In this meta-analysis, CS may be a risk factor for asthma in offspring children/adolescents compared with VD. The relationship between CS and asthma was influenced by sex and region.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-023-04396-1.
Keywords: Cesarean section, Asthma, Child, Adolescent, Offspring, Meta-analysis
Introduction
Asthma is one of the leading causes of chronic respiratory disease-related death globally [1] and is the most common noncommunicable disease. Globally, asthma affects about 300 million people [2], and the prevalence of asthma in children and adolescents is approximately 10% [3]. Patients often suffer recurrent episodes of wheezing, coughing, chest tightness and other symptoms [4, 5]. Chronic airway inflammation is a common feature of asthma. It not only causes adverse physical and psychological feelings, but also lowers the quality of life and shortens life expectancy. Asthma is caused by the interaction of genetic and environmental factors [6]. Lung function defects, respiratory infections, and other factors are associated with the development of asthma [7]. Fetal exposure during pregnancy (such as cesarean section (CS) [8]) has been suggested as one of the determinants of immune system development [9].
In recent decades, the incidence of CS worldwide has continued to rise. The global CS rate is expected to increase to nearly 30% in 2030 [10]. Although rational use of CS in critical settings can reduce maternal and neonatal mortality and morbidity [11], excessive use of CS is not beneficial to the mother or the infant and will cause some waste of resources [12–14]. Even if the application of CS is mature, the short or long-term health damage of CS to infants is worth exploring, such as obesity [12, 15], type 1 diabetes [16, 17], and leukemia [18]. In addition, CS has been considered to increase the risk of asthma in offspring [19, 20]. Asthma is related to genetic factors [6] and varies from country to country, necessitating a comprehensive analysis of asthma risk in different regions. So far, the relationship between CS and asthma has been controversial. Some meta-analyses have shown that CS increases the risk of asthma in children. However, there are limitations, such as the small number or the small regional scope of included studies [12, 21]. Another meta-analysis from the European region found that due to the heterogeneity of results, CS cannot be explicitly considered a risk factor for asthma [22]. Therefore, this is an updated systematic review and meta-analysis that intends to include cohort studies with higher ability to test causality. The aim was to explore the relationship between CS and asthma in children/adolescents by reviewing previous relevant studies.
Method
Literature search strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [23] and Meta-analysis of Observational Studies in Epidemiology guideline [24]. Two researchers systematically searched data from studies on the relationship between CS and asthma in Pubmed, Embase, Web of Science, and Cochrane Library electronic databases until February 2023. Retrieve based on the following keywords: "Cesarean Section" [Mesh] and "Asthma" [Mesh]. Please refer to Supplementary Table 1 for detailed search strategies. In addition to this, the reference list of relevant literature was manually reviewed to avoid missing studies. The systematic review and meta-analysis Prospero registration number is CRD42023420333.
Inclusion and exclusion criteria
The following inclusion criteria were used in this systematic review and meta-analysis: (1) the article assessed the relationship between mode of delivery (CS vs. vaginal delivery (VD)) and asthma in offspring. (2) The exposed group was offspring born via CS and the control group was offspring born via VD. (3) The study was a cohort study.
Exclusion criteria were as follows: (1) the study was not published in English. (2) Relevant data could not be extracted. (3) The study was not conducted with children/adolescents (age > 18y). (4) Only the study protocol or ongoing study was available, or the full text was not available. When multiple updates of the same cohort study were published, the most comprehensive or recent article was included.
Quality assessment and data extraction
The Newcastle–Ottawa Quality Assessment Scale (NOS) checklist was used to assess the included cohort studies. Two researchers used a pre-designed form to extract the following information: author, year, country, study design, number of study subjects, age at diagnosis of asthma, asthma registry, data source, and adjustment factors. Third-party researchers resolved disputes.
Objectives and outcomes
The aim of this study was to assess the association between CS offspring and asthma incidence. The outcome was that participants were diagnosed with asthma during children/adolescences (age ≤ 18y). The pre-designed subgroups were CS type, offspring sex, and asthma type (atopic asthma, seasonal asthma, drug-induced asthma, pulmonary asthma, etc.).
Statistical analysis
Data were analyzed in Stata software version 12.0 to integrate estimates extracted from the included studies. The relationship between CS and asthma incidence was assessed by odds ratio (OR) and 95% confidence interval (CI). The Cochran Q chi-square test and the I2 statistical test were used to quantitatively assess heterogeneity between studies. A two-sided P < 0.1 of the Q test or I2 > 50% was considered statistically significant heterogeneity. Considering factors such as different CS types and ethnic populations, a random-effects model was used to improve the confidence in the results. Begg’s test was used to assess publication bias. Sensitivity analyses were used to determine the impact of individual studies on the overall risk assessment. A two-sided P < 0.05 was considered statistically significant.
Results
Study selection
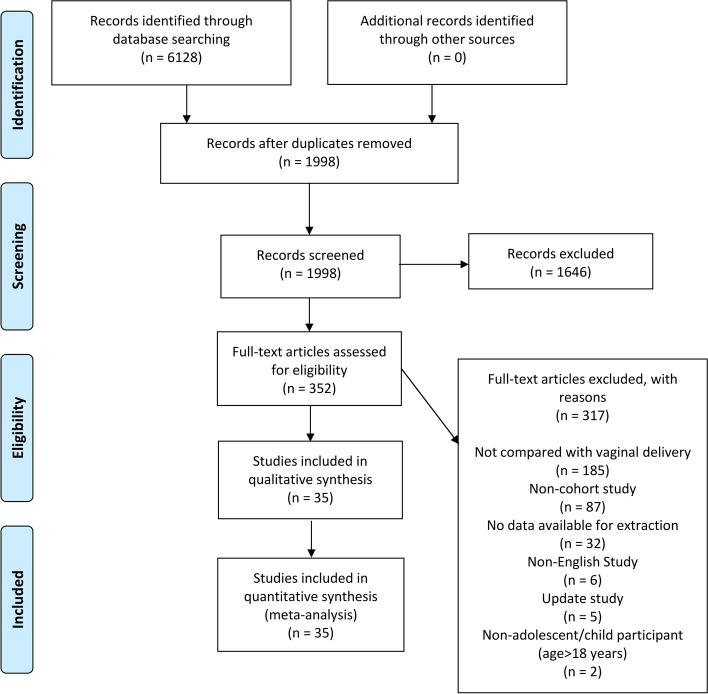
In this study, 6,128 relevant articles were identified in four electronic databases, with 1,998 articles remaining after removing duplicates. There were 352 articles that met the inclusion criteria for titles and abstracts. We carefully and thoroughly reviewed the articles, of which 317 were excluded for various reasons, and 35 articles [19, 20, 25–57] were finally included in the systematic review and meta-analysis. A detailed PRISMA flowchart is shown in Fig. 1.
Fig. 1.
PRISMA retrieval procedures flow chart
Study characteristics and quality assessment
Table 1 shows the basic characteristics of all included articles. Thirteen prospective cohort studies and twenty-two retrospective cohort studies were included in the systematic review and meta-analysis of the association between CS and asthma. These selected studies were distributed across different regions, with four studies conducted in Asia, twenty-three studies in Europe, five studies in North America, and three studies in Oceania. Participants were diagnosed with asthma between the ages of 0–18. Most articles adjusted for confounding factors such as sex, gestational age, birth weight, maternal age, and parity. Supplementary Table 2 provides details about the source of the participants, birth period, follow-up time, and asthma registry. The quality of the included studies was assessed by the NOS checklist with a score of ≥ 6. Detailed results of the quality assessment are provided in Supplementary Table 3.
Table 1.
Characteristics of all the studies included in the systematic review and meta-analysis
| Author | Year | Country | Continent | No. of Paticipants | Diagnostic age (year) | Ajusted factors | ||
|---|---|---|---|---|---|---|---|---|
| Child factors | Maternal factors | |||||||
| CS | VD | |||||||
| Nafstad [25] | 2000 | Norway | Europe | 279 | 2,193 | 4.3 | NA | |
| Xu [26] | 2000 | Finland | Europe | 1,147 | 6,386 | 7 | Sex, GA, BW, birth seasons | Age, parity, pregnancy (smoking, BMI before, weight gain), allergic disorders history, status, social class |
| Annesi-Maesano [27] | 2001 | UK | Europe | NA | NA | 1–18 | Sex, age, BW < 2.5 kg, prematurity, birth order, sibship size | Age, parity, pregnancy smoking, asthma |
| McKeever [29] | 2002 | UK | Europe | 4,073 | 18,573 | 0–11 | NA | |
| Kero [28] | 2002 | Finland | Europe | 8,826 | 51,039 | 7 | Sex, BW | Age, previous deliveries |
| Maitra [30] | 2004 | UK | Europe | 1,387 | 10,980 | 5–8 | Sex, BW, preterm delivery, breastfeeding duration | Age, asthma/eczema and hayfever, pregnancy smoking, environmental tobacco smoke exposure at 6 months, education, number of 0–15 years old in household, crowding in home, damp housing, household cats, ethnicity |
| Bernsen [31] | 2005 | Netherlands | Europe | 85 | 1,627 | 6 | Sex, sibship size, birth order, birth season, birth year, age at the check-up time, diphtheria tetanus pertussis poliomyelitis vaccination status | Age, atopic disease (parents), occupation of the breadwinner level, urbanization level, origin country |
| Renz-Polster [33] | 2005 | USA | North America | 1,286 | 6,586 | 3–10 | Sex, BW, birth order, diagnosis age | Age, pregnancy smoking, multiple gestation, asthma/hay fever medications use, exposure to antibiotics in the postpartum period, marital status, education, ethnicity |
| Juhn [32] | 2005 | USA | North America | 714 | 6,392 | 7 | Sex, BW | Age, education |
| Salam [34] | 2006 | USA | North America | 717 | 2,747 | 8–17 | Sex, GA, BW, birth order, birth calendar period, requirement for special care after birth | Age at childbirth, asthma and allergy history (parents), pregnancy smoking, environmental tobacco smoke exposure, disease history (pneumonia, bronchitis, bronchiolitis, croup), parents or guardians education, health insurance coverage, residence community, race |
| Werner [35] | 2007 | Denmark | Europe | 841 | 6,278 | ≤ 18 | Sex, breastfeeding | Age, previous deliveries, pregnancy smoking, educational level |
| Pistiner [36] | 2008 | USA | North America | 102 | 330 | 9 | NA | |
| Tollånes [37] | 2008 | Norway | Europe | 136,735 | 1,520,088 | 0–18 | Sex, birth order, birth year | Age, asthma, education |
| Roduit [38] | 2009 | Netherlands | Europe | 247 | 2,670 | 8 | Sex, BW, breastfeeding | BMI, education, allergy status (parents) |
| Park [40] | 2010 | Korea | Asia | 100 | 179 | 4.6 ± 3.8 | Sex, GA, BW, breastfeeding | Age, allergy (parents) |
| Davidson [39] | 2010 | UK | Europe | 18,462 | 230,150 | 2–11 | Sex, GA, BW, breastfed or not, Apgar 1, birth year | Age, parity, smoking, asthma, forceps delivery, marital status, social class |
| Magnus [41] | 2011 | Norway | Europe | 5,020 | 32,151 | 3 | Sex, GA, BW, exclusive breastfeeding duration, childcare attendance | Age, parity, pregnancy (smoking, BMI before, chronic conditions before, complications), indication of personal preference for CS, previous delivery by CS, atopy, membrane rupture indication, marital status, educational level |
| Almqvist [42] | 2012 | Sweden | Europe | 16,460 | 145,341 | > 10 | Sex, GA, BW, birth order, Apgar score, hypoxia/asphyxia | Age, pregnancy smoking, birth country, BMI, living with child father |
| Bråbäck [43] | 2013 | Sweden | Europe | 29,925 | 143,347 | 2–5; 6–9 | Sex, GA (small or large), birth year, meconium aspiration, neonatal respiratory distress, transient tachypnoea | Age, smoking, education, BMI, chorioamnionitis, history of diabetes and hypertension, premature rupture of the membranes, preeclampsia, pregnancy diabetes, HDP, fever during labour, asthma medication (parents), social welfare, urban/rural living, county |
| Pyrhönen [44] | 2013 | Finland | Europe | 551 | 2,630 | 1–4 | Sex, BW, birth order, breastfeeding duration | Pregnancy (smoking, duration), allergy (parents) |
| Black [45] | 2015 | UK | Europe | 68,370 | 252,917 | 5 | GA, BW, birth year, male infant, breastfeeding at 6 weeks | Age, smoking status, salbutamol prescription, Carstairs decile |
| Brüske [46] | 2015 | Germany | Europe | 389 | 1,461 | 15 | Sex, GA | Education level (parents), study center |
| Kristensen [48] | 2016 | Denmark | Europe | 124,130 | 666,439 | 0–14 | Sex, GA, BW | Age, pregnancy smoking, pregnancy complications (preeclampsia, eclampsia, hemorrhage, and hyperemesis) |
| Sevelsted [49] | 2016 | Denmark | Europe | 163,462 | 696,864 | 0–15 | Sex, GA, BW, calendar year | Age, multiple births, parity, pregnancy (smoking, antibiotics), employment status, asthma |
| Black [47] | 2016 | UK | Europe | 26,766 | 13,379 | > 6 | GA, male infant, BW, birth year, breastfeeding at 6 weeks | Age, smoking status, salbutamol prescription, Carstairs decile |
| Rusconi [51] | 2017 | mutiple countries | Europe | NA | NA | 5–9 | Sex, GA, weight for GA, birth year, birth country | Age, parity, education, pregnancy (smoking, BMI before, and diabetes), HDP, asthma |
| Lavin [20] | 2017 | India | Asia | 289 | 1,717 | 8 | Sex, term low BW | Age, liveborn parity, household smoking, cooking fuel, geographic location, livestock ownership, housing quality, household size, wealth index |
| Vietnam | 178 | 1,760 | ||||||
| Chen [50] | 2017 | China | Asia | 6,556 | 13,143 | 5.5 | Sex, GA, birth order | Age, education level, family monthly income |
| Peters [52] | 2018 | Australia | Oceania | 107,560 | 185,883 | 5 | Sex, GA (small or large), BW, birth trauma | Age, parity, pharmacological pain medication or anesthesia at birth, birth country, socioeconomic status |
| Liao [53] | 2020 | Australia | Oceania | 2,138 | 4,651 | 6–7 | GA, BW | Age, pregnancy smoking, birth country, Socio-economic Indexes for Areas |
| Soullane [55] | 2021 | Canada | North America | 216,547 | 645,427 | ≤ 13 | Sex | Age, parity, atopy, HDP, diabetes (pregnancy or preexisting), illicit use (drug, alcohol, and tobacco), socioeconomic disadvantage, time period |
| Brew [54] | 2021 | Australia | Oceania | 5,755 | 20,018 | 1–4 | NA | Asthma, medical conditions, remoteness |
| Salem [56] | 2022 | Switzerland | Europe | 65 | 306 | 6 | Sex, GA, BW, exclusive breastfeeding duration, older siblings, childcare attendance | Age, pregnancy smoking, atopy status, current smoking (parents) |
| Wang [19] | 2023 | China | Asia | 305,890 | 583,303 | 9 | GA, BW, birth length | Age, parity, allergic diseases, pregnancy diabetes, preeclampsia, urbanization levels |
| O’Connor [57] | 2023 | UK | Europe | 3,873 | 14,340 | 7, 11, 14 | GA (small) | Age, BMI, asthma, pregnancy smoking, HDP, diabetes, education, ethnicity, income quintile |
CS cesarean section, VD vaginal delivery, GA gestational age, BW birth weight, BMI body mass index, HDP hypertensive disorders of pregnancy, NA not available
Association of cesarean section and asthma
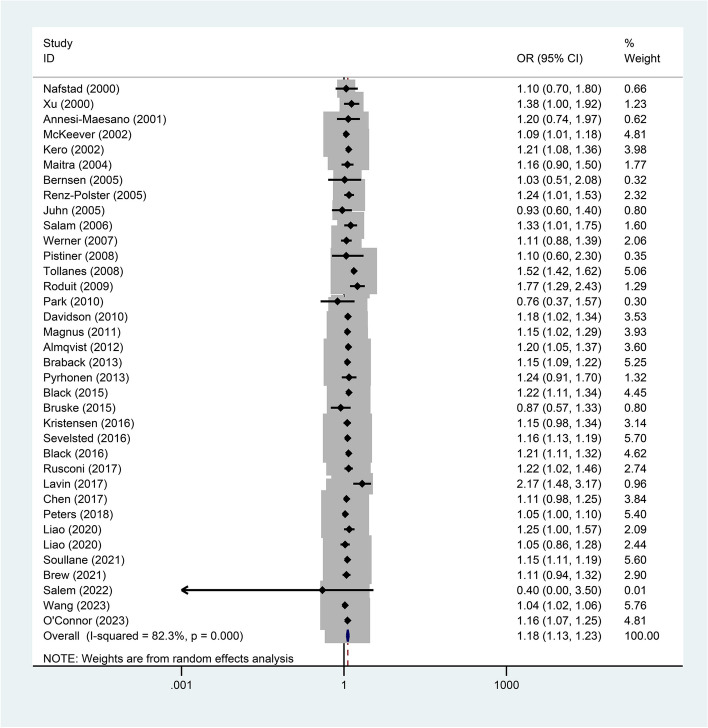
Thirty-five articles reported on the association between mode of delivery and asthma. The results showed that the incidence of asthma was higher in offspring born via CS than those born via VD (OR = 1.18, 95%CI = 1.13–1.23, P < 0.001, I2 = 82.3%) (Fig. 2). To explore the sources of heterogeneity, the following subgroup analyses were designed (Table 2). First, subgroup analysis based on CS type showed that offspring born via elective CS (OR = 1.18, 95%CI = 1.11–1.25) and emergency CS (OR = 1.18, 95%CI = 1.10–1.27) had a higher incidence of asthma than the VD group, which was consistent with the overall results. According to sex-grouped data, female offspring born via CS (OR = 1.26, 95%CI = 1.13–1.42) had a higher incidence of asthma compared to the VD group, but there was no difference in males (OR = 1.07, 95%CI = 0.94–1.22). According to the continental divisions in the different study regions, there was no difference in asthma incidence between Asian populations born via CS (OR = 1.17, 95%CI = 0.97–1.42) and offspring born via VD. Those born via CS in Europe, North America and Oceania all had a higher incidence of asthma than the VD group (P < 0.05).
Fig. 2.
Forest plot of the association between cesarean section and asthma (P < 0.001)
Table 2.
Subgroup analyses of the association between cesarean section and asthma
| No. of studies | OR | 95%CI | p | Heterogeneity (I2) (%) | |
|---|---|---|---|---|---|
| Type of CS | |||||
| Elective CS | 13 | 1.18 | 1.11–1.25 | < 0.001 | 71.4 |
| Emergency CS | 13 | 1.18 | 1.10–1.27 | < 0.001 | 83.3 |
| Sex | |||||
| Female | 3 | 1.26 | 1.13–1.42 | < 0.001 | 0 |
| Male | 3 | 1.07 | 0.94–1.22 | 0.325 | 0 |
| Continent | |||||
| Asia | 4 | 1.17 | 0.97–1.42 | 0.102 | 81.3 |
| Europe | 23 | 1.20 | 1.15–1.26 | < 0.001 | 70.4 |
| North America | 5 | 1.15 | 1.11–1.19 | < 0.001 | 0 |
| Oceania | 3 | 1.06 | 1.02–1.11 | 0.008 | 0 |
| Atopic asthma | 6 | 1.14 | 1.11–1.18 | < 0.001 | 0 |
| Persistent asthma | 3 | 1.15 | 0.99–1.33 | 0.063 | 65.3 |
| Cohort design | |||||
| Prospective cohort | 13 | 1.19 | 1.11–1.28 | < 0.001 | 40.4 |
| Retrospective cohort | 22 | 1.17 | 1.11–1.23 | < 0.001 | 86.9 |
OR odds ratio, CI confidence interval, CS cesarean section
Offspring born via CS had a higher incidence of atopic asthma (OR = 1.14, 95%CI = 1.11–1.18) compared to the VD group. The CS group had a higher incidence of persistent asthma (OR = 1.15, P = 0.063) than the VD group, but this difference was not statistically significant. Finally, in prospective cohort studies (OR = 1.19, 95%CI = 1.11–1.28) and retrospective cohort studies (OR = 1.17, 95%CI = 1.11–1.23), the incidence of asthma in offspring born via CS was higher than that in controls.
Publication bias and sensitivity analyses
The statistical results of the Begg’s funnel plot showed no potential publication bias in the forest plot of the relationship between mode of delivery and incidence of asthma (P = 0.066) (SFig. 1). After excluding articles one by one, sensitivity analysis showed stable results (SFig. 2).
Discussion
The purpose of this study was to investigate the statistical association between CS and asthma in children/adolescents, which was used to infer whether there is an effect of CS on asthma. The results of the systematic review and meta-analysis showed that children/adolescents born via CS were at increased incidence of developing asthma compared to VD. Further subgroup analyses showed that the relationship between CS and asthma was not affected by CS type, asthma type, or cohort design. But the increased incidence of asthma in children/adolescents born via CS may be influenced by sex and region.
The etiology of asthma has not been determined, and some studies believe that both genetic factors and environmental factors affect the occurrence of asthma [6]. Most immune system dysplasia is caused by environmental factors [58], which is an important cause of the epidemic of noncommunicable diseases [59]. Herein, the possible mechanisms by which CS increases asthma incidence are speculated from the following aspects.
First, childbirth is one of the early exposures for newborns. As is known to all, newborns born via CS are exposed to a different external environment for the first time compared to VD. Neonates born via VD are primarily exposed to bacteria in and around the maternal birth canal, whereas neonates born via CS are predominantly exposed to external bacteria [60]. Animal studies have shown that CS affects the diversity and density of the intestinal flora [61]. Infants born with CS have reduced numbers of Bacteroides and microbial sphingolipids in their faeces, so infants are more susceptible to asthma [62].
Second, compared to VD, CS was considered to postpone the onset of breastfeeding and to shorten the duration of exclusive breastfeeding [63], which may result in infants having insufficient exposure to breast milk. Breast milk contains high amounts of immunoglobulin (Ig) A, glycans [64], bioactive enzymes, and hormones that benefit the development of the immature immune defense system [65]. Breast milk has been found to transfer airborne antigens to newborn mice. Due to the presence of transforming growth factor-β mediated by CD4 + T lymphocytes in breast milk, its signalling is dependent on T cells. This induces antigen tolerance in newborns and provides specific protection against some allergic airway diseases, such as asthma [66]. Moreover, adequate breastfeeding is thought to facilitate the growth of infants’ lungs [67]. Therefore, insufficient breastfeeding may increase the risk of asthma.
Third, CS increases the binding of the progeny dopamine D1-like-receptor [68]. The conduction signal of D1-like-receptor facilitate the activation of the B-cell activating transcription factor, thereby increasing the transcription of the retinoic acid receptor-related orphan receptor-γ-t, and promoting the differentiation of T helper cell (Th) 17. Correspondingly, more Th17 were found in the spleen cells of mice in the asthma group than in the control group [69]. Th17 participate in antigen-induced aggregation of neutrophils and eosinophils in the airways, which play an important role in asthma [70, 71]. Antagonizing D1-like-receptor will inhibit the Th17-mediated inflammatory response in the lungs [72], but this evidence has not yet been validated in humans.
Finally, infants born via CS exhibit higher DNA methylation of cord blood leukocytes [73]. DNA methylation has been shown to play an important role in fetal development and may be an important cause of susceptibility to certain diseases [74]. DNA methylation may alter the composition of immune cells by regulating gene expression, putting CS offspring at higher risk of asthma. Undesirable methylation may disrupt the balance of Th1 and Th2, thereby increasing the risk of immune disease, which may be a regulatory mechanism for allergic asthma [75]. Allergic asthma is also known as atopic asthma, and the subgroup results in this study show that CS may be a risk factor for atopic asthma.
Subgroup results showed that the incidence of asthma appears to vary by sex. Compared to VD, CS is a risk factor for asthma in female rather than in male. There may be three possible reasons for this. (1) Asthma is a heterogeneous condition that may be sex-specific. Biological differences in development in the womb may explain the sex differences in asthma incidence. Sex influences the physiology and development of the infant’s lungs [76, 77]. In addition, females will appear more asthma attacks and asthma symptoms compared to males, and bronchial hyperresponsiveness is more common in females [78]. Asthma attacks in female appear to be closely related to menstrual periods. Asthma symptoms worsen during ovulation and menstruation [79]. Asthma patients have high markers of inflammation during the menstrual cycle, so asthma may be associated with female physiological hormones [80]. (2) Female infants have better viability than male infants when faced with adverse birth circumstances such as prematurity [81], which may result in more female babies surviving than male infants. Thus, more female than male infants are registered as having asthma. This may be one of the reasons why no association was found between CS male offspring and asthma. (3) The number of relevant studies that could be included was small and the results were subject to some chance.
The prevalence of childhood asthma varies considerably between countries [82]. It is well known that the developed countries are mainly distributed in Europe. Of the studies included in this systematic review and meta-analysis, the largest number of studies, up to twenty-three, were conducted in Europe. Notably, these studies were carried out in developed countries. In addition, research in North America and Oceania was also carried out in developed countries. Our results show that infants born via CS have a higher incidence of asthma compared to VD births in Europe, North America, and Oceania. Interestingly, no such association was found in infants born in Asia. The following three reasons are considered: (1) race may have influenced the onset of asthma. The relative prevalence of asthma varies by ethnic group [83]. (2) Developed countries have a high level of medical care and pay more attention to the health damage caused by diseases. People actively seek medical treatment, which is conducive to the diagnosis of diseases. This may be one of the reasons for the largest increase in asthma incidence in developed countries [84]. Developing country studies make up the majority of Asian regional studies. Disease diagnosis rates in developing countries may be lower due to a variety of factors. (3) And it should also be considered that the criteria for performing CS may vary between developing and developed countries.
Reviewing past systematic review and meta-analyses, Keag et al.concluded that CS was associated with asthma in children under 12 years of age. However, the number of studies included in this meta-analysis is relatively small [12]. A subsequent study focused on the relationship between CS and asthma. In addition to cohort studies, this meta-analysis included case–control studies and cross-sectional studies [85], which may have reduced the level of evidence for the results. Another meta-analysis on a European population took into account the heterogeneity of results and concluded that CS could not be clearly recognized as a risk factor for asthma in children [22].
The strengths of this study are as follows: (1) the number of included studies was comprehensive. It was an update and supplement to previous meta-analyses with detailed subgroup analyses. (2) This article was a systematic review and meta-analysis based on cohort studies. The high certainty of the evidence from the cohort studies contributed to the credibility of this study. However, there are some limitations: (1) there was selection bias and follow-up bias in the original studies. (2) The number of relevant studies in some subgroups was small.
Conclusions: CS seems to be associated with asthma in children/adolescent offspring compared to VD. However, the result has a relatively high degree of heterogeneity and require further validation. Subgroup analyses showed that sex may influence the relationship between CS and asthma, with the risk of asthma in CS offspring only present in females. The risk of CS for asthma appears to differ across regions. CS may be related to childhood/adolescent asthma in populations in Europe, North America, and Oceania.
Supplementary Information
Additional file 1: Supplementary Table 1. Search Strategy.
Additional file 2: Supplementary Table 2.Characteristics of all the studies included in the systematic review and meta-analysis.
Additional file 3: Supplementary Table 3. Quality assessment of cohort studies included.
Additional file 4: SFigure 1. Publication bias of the association between cesarean section and asthma (P=0.066).
Additional file 5: SFigure 2. Sensitivity analysis of the association between cesarean section and asthma.
Acknowledgements
Not applicable.
Abbreviations
- CS
Cesarean section
- VD
Vaginal delivery
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NOS
Newcastle-Ottawa Quality Assessment Scale
- OR
Odds ratio
- CI
Confidence interval
- Ig
Immunoglobulin
- Th
T helper cell
- GA
Gestational age
- BW
Birth weight
- BMI
Body mass index
- HDP
Hypertensive disorders of pregnancy
- LSAC
The Longitudinal Study of Australian Children
- P
Prospective cohort
- R
Retrospective cohort
- ICD
The International Classification of Diseases
- CM
Clinical Modification
- AM
Australian Modification
- ISAAC
The International Study of Asthma and Allergies in Childhood
- ICS
Inhaled corticosteroid
- NA
Not available
Authors’ contributions
Wei Ren designed the framework and supervised this study; Ziwei Zhong, Meiling Chen, and Senjie Dai conducted the statistical analysis, and drafted the manuscript. Yu Wang, Jie Yao, Haojie Shentu, Jianing Huang, Chiyuan Yu, Hongrui Zhang, and Tianyue Wang participated data process and analysis. All the authors have read and approved the final manuscript.
Funding
The study received no fund support.
Availability of data and materials
The datasets supporting this article’s conclusions are included within the article and its additional files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ziwei Zhong, Meiling Chen and Senjie Dai contributed equally to this work and share the first authorship.
References
- 1.Labaki WW, Han MK. Chronic respiratory diseases: a global view. Lancet Respir Med. 2020;8(6):531–533. doi: 10.1016/S2213-2600(20)30157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42(1):5–15. doi: 10.1007/s00281-020-00785-1. [DOI] [PubMed] [Google Scholar]
- 3.García-Marcos L, Asher MI, Pearce N, Ellwood E, Bissell K, Chiang CY, El Sony A, Ellwood P, Marks GB, Mortimer K, et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur Respir J. 2022;60(3):2102866. doi: 10.1183/13993003.02866-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007;42(8):723–728. doi: 10.1002/ppul.20644. [DOI] [PubMed] [Google Scholar]
- 5.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140(4):895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, Bai C, Kang J, Ran P, Shen H, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet (London, England) 2019;394(10196):407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5(3):224–234. doi: 10.1016/S2213-2600(16)30187-4. [DOI] [PubMed] [Google Scholar]
- 8.Mitselou N, Hallberg J, Stephansson O, Almqvist C, Melén E, Ludvigsson JF. Adverse pregnancy outcomes and risk of later allergic rhinitis-Nationwide Swedish cohort study. Pediatr Allergy Immunol. 2020;31(5):471–479. doi: 10.1111/pai.13230. [DOI] [PubMed] [Google Scholar]
- 9.Parazzini F, Cipriani S, Zinetti C, Chatenoud L, Frigerio L, Amuso G, Ciammella M, Di Landro A, Naldi L. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25(1):43–50. doi: 10.1111/pai.12165. [DOI] [PubMed] [Google Scholar]
- 10.Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. 2021;6(6):e005671. doi: 10.1136/bmjgh-2021-005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, Moller AB, Say L, Hosseinpoor AR, Yi M, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet (London, England) 2018;392(10155):1341–1348. doi: 10.1016/S0140-6736(18)31928-7. [DOI] [PubMed] [Google Scholar]
- 12.Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 2018;15(1):e1002494. doi: 10.1371/journal.pmed.1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, Gibbons D, Kelly NM, Kennedy HP, Kidanto H, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet (London, England) 2018;392(10155):1349–1357. doi: 10.1016/S0140-6736(18)31930-5. [DOI] [PubMed] [Google Scholar]
- 14.Sobhy S, Arroyo-Manzano D, Murugesu N, Karthikeyan G, Kumar V, Kaur I, Fernandez E, Gundabattula SR, Betran AP, Khan K, et al. Maternal and perinatal mortality and complications associated with caesarean section in low-income and middle-income countries: a systematic review and meta-analysis. Lancet (London, England) 2019;393(10184):1973–1982. doi: 10.1016/S0140-6736(18)32386-9. [DOI] [PubMed] [Google Scholar]
- 15.Kuhle S, Tong OS, Woolcott CG. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev. 2015;16(4):295–303. doi: 10.1111/obr.12267. [DOI] [PubMed] [Google Scholar]
- 16.Cardwell CR, Stene LC, Joner G, Cinek O, Svensson J, Goldacre MJ, Parslow RC, Pozzilli P, Brigis G, Stoyanov D, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–735. doi: 10.1007/s00125-008-0941-z. [DOI] [PubMed] [Google Scholar]
- 17.Lee HY, Lu CL, Chen HF, Su HF, Li CY. Perinatal and childhood risk factors for early-onset type 1 diabetes: a population-based case-control study in Taiwan. Eur J Pub Health. 2015;25(6):1024–1029. doi: 10.1093/eurpub/ckv059. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Yu C, Fu R, Xia S, Ni H, He Y, Zhu K, Sun Q. Association of cesarean section with risk of childhood leukemia: a meta-analysis from an observational study. Hematol Oncol. 2023;41(1):182–191. doi: 10.1002/hon.3070. [DOI] [PubMed] [Google Scholar]
- 19.Wang CM, Yang ST, Yang CC, Chiu HY, Lin HY, Tsai ML, Lin HC, Chang YC. Maternal and neonatal risk factors of asthma in children: Nationwide population based study. J Microbiol Immunol Infect. 2023;56(1):182–191. doi: 10.1016/j.jmii.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Lavin T, Franklin P, Preen DB. Association between caesarean delivery and childhood asthma in India and Vietnam. Paediatr Perinat Epidemiol. 2017;31(1):47–54. doi: 10.1111/ppe.12324. [DOI] [PubMed] [Google Scholar]
- 21.Sio YY, Chew FT. Risk factors of asthma in the Asian population: a systematic review and meta-analysis. J Physiol Anthropol. 2021;40(1):22. doi: 10.1186/s40101-021-00273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wypych-Ślusarska A, Niewiadomska E, Oleksiuk K, Krupa-Kotara K, Głogowska-Ligus J, Słowiński J. Caesarean delivery and risk of childhood asthma development: meta-analysis. Postepy dermatologii i alergologii. 2021;38(5):819–826. doi: 10.5114/ada.2020.96703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.Nafstad P, Magnus P, Jaakkola JJK. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. J Allergy Clin Immunol. 2000;106(5):867–873. doi: 10.1067/mai.2000.110558. [DOI] [PubMed] [Google Scholar]
- 26.Xu B, Pekkanen J, Järvelin MR. Obstetric complications and asthma in childhood. J Asthma. 2000;37(7):589–594. doi: 10.3109/02770900009090814. [DOI] [PubMed] [Google Scholar]
- 27.Annesi-Maesano I, Moreau D, Strachan D. In utero and perinatal complications preceding asthma. Allergy. 2001;56(6):491–497. doi: 10.1034/j.1398-9995.2001.056006491.x. [DOI] [PubMed] [Google Scholar]
- 28.Kero J, Gissler M, Grönlund MM, Kero P, Koskinen P, Hemminki E, Isolauri E. Mode of delivery and asthma – is there a connection? Pediatr Res. 2002;52(1):6–11. doi: 10.1203/00006450-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 29.McKeever TM, Lewis SA, Smith C, Hubbard R. Mode of delivery and risk of developing allergic disease. J Allergy Clin Immunol. 2002;109(5):800–802. doi: 10.1067/mai.2002.124046. [DOI] [PubMed] [Google Scholar]
- 30.Maitra A, Sherriff A, Strachan D, Henderson J. Mode of delivery is not associated with asthma or atopy in childhood. Clin Exp Allergy. 2004;34(9):1349–1355. doi: 10.1111/j.1365-2222.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 31.Bernsen RM, de Jongste JC, Koes BW, Aardoom HA, van der Wouden JC. Perinatal characteristics and obstetric complications as risk factors for asthma, allergy and eczema at the age of 6 years. Clin Exp Allergy. 2005;35(9):1135–1140. doi: 10.1111/j.1365-2222.2005.2155.x. [DOI] [PubMed] [Google Scholar]
- 32.Juhn YJ, Weaver A, Katusic S, Yunginger J. Mode of delivery at birth and development of asthma: a population-based cohort study. J Allergy Clin Immunol. 2005;116(3):510–516. doi: 10.1016/j.jaci.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 33.Renz-Polster H, David MR, Buist AS, Vollmer WM, O’Connor EA, Frazier EA, Wall MA. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35(11):1466–1472. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 34.Salam M, Margolis H, McConnell R, McGregor J, Avol E, Gilliland F. Mode of delivery is associated with asthma and allergy occurrences in children. Ann Epidemiol. 2006;16(5):341–346. doi: 10.1016/j.annepidem.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 35.Werner A, Ramlau-Hansen CH, Jeppesen SK, Thulstrup AM, Olsen J. Caesarean delivery and risk of developing asthma in the offspring. Acta Paediatr. 2007;96(4):595–595. doi: 10.1111/j.1651-2227.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 36.Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122(2):274–279. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tollånes MC, Moster D, Daltveit AK, Irgens LM. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J Pediatr. 2008;153(1):112–116.e111. doi: 10.1016/j.jpeds.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Roduit C, Scholtens S, de Jongste JC, Wijga AH, Gerritsen J, Postma DS, Brunekreef B, Hoekstra MO, Aalberse R, Smit HA. Asthma at 8 years of age in children born by caesarean section. Thorax. 2009;64(2):107–113. doi: 10.1136/thx.2008.100875. [DOI] [PubMed] [Google Scholar]
- 39.Davidson R, Roberts SE, Wotton CJ, Goldacre MJ. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med. 2010;10:14. doi: 10.1186/1471-2466-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park YH, Kim KW, Choi BS, Jee HM, Sohn MH, Kim K-E. Relationship between mode of delivery in childbirth and prevalence of allergic diseases in Korean children. Allergy Asthma Immunol Res. 2010;2(1):28–33. doi: 10.4168/aair.2010.2.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magnus MC, Haberg SE, Stigum H, Nafstad P, London SJ, Vangen S, Nystad W. Delivery by cesarean section and early childhood respiratory symptoms and disorders: the Norwegian mother and child cohort study. Am J Epidemiol. 2011;174(11):1275–1285. doi: 10.1093/aje/kwr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almqvist C, Cnattingius S, Lichtenstein P, Lundholm C. The impact of birth mode of delivery on childhood asthma and allergic diseases–a sibling study. Clin Exp Allergy. 2012;42(9):1369–1376. doi: 10.1111/j.1365-2222.2012.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bråbäck L, Ekéus C, Lowe AJ, Hjern A. Confounding with familial determinants affects the association between mode of delivery and childhood asthma medication - a national cohort study. Allergy Asthma Clin Immunol. 2013;9(1):14. doi: 10.1186/1710-1492-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pyrhönen K, Näyhä S, Hiltunen L, Läärä E. Caesarean section and allergic manifestations: insufficient evidence of association found in population-based study of children aged 1 to 4 years. Acta Paediatr. 2013;102(10):982–989. doi: 10.1111/apa.12342. [DOI] [PubMed] [Google Scholar]
- 45.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314(21):2271–9. doi: 10.1001/jama.2015.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brüske I, Pei Z, Thiering E, Flexeder C, Berdel D, von Berg A, Koletzko S, Bauer C-P, Hoffmann B, Heinrich J, et al. Caesarean Section has no impact on lung function at the age of 15 years. Pediatr Pulmonol. 2015;50(12):1262–1269. doi: 10.1002/ppul.23196. [DOI] [PubMed] [Google Scholar]
- 47.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned repeat cesarean section at term and adverse childhood health outcomes: a record-linkage study. PLoS Med. 2016;13(3):e1001973. doi: 10.1371/journal.pmed.1001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol. 2016;137(2):587–590. doi: 10.1016/j.jaci.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 49.Sevelsted A, Stokholm J, Bisgaard H. Risk of asthma from cesarean delivery depends on membrane rupture. J Pediatr. 2016;171:38–42.e34. doi: 10.1016/j.jpeds.2015.12.066. [DOI] [PubMed] [Google Scholar]
- 50.Chen G, Chiang W-L, Shu B-C, Guo YL, Chiou S-T. Chiang T-l: Associations of caesarean delivery and the occurrence of neurodevelopmental disorders, asthma or obesity in childhood based on Taiwan birth cohort study. BMJ Open. 2017;7(9):e017086. doi: 10.1136/bmjopen-2017-017086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rusconi F, Zugna D, Annesi-Maesano I, Baïz N, Barros H, Correia S, Duijts L, Forastiere F, Inskip H, Kelleher CC, et al. Mode of delivery and asthma at school age in 9 European birth cohorts. Am J Epidemiol. 2017;185(6):465–473. doi: 10.1093/aje/kwx021. [DOI] [PubMed] [Google Scholar]
- 52.Peters LL, Thornton C, de Jonge A, Khashan A, Tracy M, Downe S, Feijen-de Jong EI, Dahlen HG. The effect of medical and operative birth interventions on child health outcomes in the first 28 days and up to 5 years of age: a linked data population-based cohort study. Birth. 2018;45(4):347–357. doi: 10.1111/birt.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao Z, Lamb KE, Burgner D, Ranganathan S, Miller JE, Koplin JJ, Dharmage SC, Lowe AJ, Ponsonby A-L, Tang MLK, et al. No obvious impact of caesarean delivery on childhood allergic outcomes: findings from Australian cohorts. Arch Dis Child. 2020;105(7):664–670. doi: 10.1136/archdischild-2019-317485. [DOI] [PubMed] [Google Scholar]
- 54.Brew B, Gibberd A, Marks GB, Strobel N, Allen CW, Jorm L, Chambers G, Eades S, McNamara B. Identifying preventable risk factors for hospitalised asthma in young Aboriginal children: a whole-population cohort study. Thorax. 2021;76(6):539–546. doi: 10.1136/thoraxjnl-2020-216189. [DOI] [PubMed] [Google Scholar]
- 55.Soullane S, Bégin P, Lewin A, Lee GE, Auger N. Increased risk of allergy hospitalization after cesarean delivery: a longitudinal study of 950,000 children. Ann Allergy Asthma Immunol. 2021;127(1):142–144. doi: 10.1016/j.anai.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Salem Y, Oestreich M-A, Fuchs O, Usemann J, Frey U, Surbek D, Amylidi-Mohr S, Latzin P, Ramsey K, Yammine S. Are children born by cesarean delivery at higher risk for respiratory sequelae? Am J Obstet Gynecol. 2022;226(2):257.e251–257.e211. doi: 10.1016/j.ajog.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 57.O’Connor A, McCarthy FP, Kelly L, Khashan AS, Maher GM. Mode of delivery and asthma in childhood and adolescence: findings from the millennium cohort study. Clin Exp Allergy. 2023;53(4):459–464. doi: 10.1111/cea.14282. [DOI] [PubMed] [Google Scholar]
- 58.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1–2):37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dietert RR. Developmental immunotoxicity, perinatal programming, and noncommunicable diseases: focus on human studies. Adv Med. 2014;2014:867805. doi: 10.1155/2014/867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siggers RH, Thymann T, Jensen BB, Mølbak L, Heegaard PM, Schmidt M, Buddington RK, Sangild PT. Elective cesarean delivery affects gut maturation and delays microbial colonization but does not increase necrotizing enterocolitis in preterm pigs. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R929–938. doi: 10.1152/ajpregu.00705.2007. [DOI] [PubMed] [Google Scholar]
- 62.Lee-Sarwar KA, Chen YC, Chen YY, Kozyrskyj AL, Mandhane PJ, Turvey SE, Subbarao P, Bisgaard H, Stokholm J, Chawes B, et al. The maternal prenatal and offspring early-life gut microbiome of childhood asthma phenotypes. Allergy. 2023;78(2):418–428. doi: 10.1111/all.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Wan W, Zhu C. Breastfeeding after a cesarean section: a literature review. Midwifery. 2021;103:103117. doi: 10.1016/j.midw.2021.103117. [DOI] [PubMed] [Google Scholar]
- 64.Friedman NJ, Zeiger RS. The role of breast-feeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005;115(6):1238–1248. doi: 10.1016/j.jaci.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 65.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 66.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14(2):170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 67.Guilbert TW, Stern DA, Morgan WJ, Martinez FD, Wright AL. Effect of breastfeeding on lung function in childhood and modulation by maternal asthma and atopy. Am J Respir Crit Care Med. 2007;176(9):843–848. doi: 10.1164/rccm.200610-1507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boksa P, Zhang Y, Bestawros A. Dopamine D1 receptor changes due to caesarean section birth: effects of anesthesia, developmental time course, and functional consequences. Exp Neurol. 2002;175(2):388–397. doi: 10.1006/exnr.2002.7896. [DOI] [PubMed] [Google Scholar]
- 69.Gong S, Li J, Ma L, Li K, Zhang L, Wang G, Liu Y, Ji X, Liu X, Chen P, et al. Blockade of dopamine D1-like receptor signalling protects mice against OVA-induced acute asthma by inhibiting B-cell activating transcription factor signalling and Th17 function. FEBS J. 2013;280(23):6262–6273. doi: 10.1111/febs.12549. [DOI] [PubMed] [Google Scholar]
- 70.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 71.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178(10):1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 72.Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol. 2012;158(Suppl 1):96–102. doi: 10.1159/000337801. [DOI] [PubMed] [Google Scholar]
- 73.Schlinzig T, Johansson S, Gunnar A, Ekström TJ, Norman M. Epigenetic modulation at birth - altered DNA-methylation in white blood cells after Caesarean section. Acta Paediatr (Oslo, Norway: 1992) 2009;98(7):1096–1099. doi: 10.1111/j.1651-2227.2009.01371.x. [DOI] [PubMed] [Google Scholar]
- 74.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy. 2010;65(1):7–15. doi: 10.1111/j.1398-9995.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- 76.Postma DS. Gender differences in asthma development and progression. Gender Med. 2007;4(Suppl B):S133–146. doi: 10.1016/S1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- 77.Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, Zeldin DC. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18(8):308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manfreda J, Sears MR, Becklake MR, Chan-Yeung M, Dimich-Ward H, Siersted HC, Ernst P, Sweet L, Van Til L, Bowie DM, et al. Geographic and gender variability in the prevalence of bronchial responsiveness in Canada. Chest. 2004;125(5):1657–1664. doi: 10.1378/chest.125.5.1657. [DOI] [PubMed] [Google Scholar]
- 79.Brenner BE, Holmes TM, Mazal B, Camargo CA., Jr Relation between phase of the menstrual cycle and asthma presentations in the emergency department. Thorax. 2005;60(10):806–809. doi: 10.1136/thx.2004.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakasato H, Ohrui T, Sekizawa K, Matsui T, Yamaya M, Tamura G, Sasaki H. Prevention of severe premenstrual asthma attacks by leukotriene receptor antagonist. J Allergy Clin Immunol. 1999;104(3 Pt 1):585–588. doi: 10.1016/S0091-6749(99)70327-1. [DOI] [PubMed] [Google Scholar]
- 81.Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120(6):1337–1351. doi: 10.1213/ANE.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serebrisky D, Wiznia A. Pediatric asthma: a global epidemic. Ann Glob Health. 2019;85(1):6. doi: 10.5334/aogh.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moraes TJ, Sears MR, Subbarao P. Epidemiology of Asthma and Influence of Ethnicity. Semin Respir Crit Care Med. 2018;39(1):3–11. doi: 10.1055/s-0037-1618568. [DOI] [PubMed] [Google Scholar]
- 84.Chang C. Asthma in children and adolescents: a comprehensive approach to diagnosis and management. Clin Rev Allergy Immunol. 2012;43(1–2):98–137. doi: 10.1007/s12016-011-8261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darabi B, Rahmati S, HafeziAhmadi MR, Badfar G, Azami M. The association between caesarean section and childhood asthma: an updated systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2019;15:62. doi: 10.1186/s13223-019-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Search Strategy.
Additional file 2: Supplementary Table 2.Characteristics of all the studies included in the systematic review and meta-analysis.
Additional file 3: Supplementary Table 3. Quality assessment of cohort studies included.
Additional file 4: SFigure 1. Publication bias of the association between cesarean section and asthma (P=0.066).
Additional file 5: SFigure 2. Sensitivity analysis of the association between cesarean section and asthma.
Data Availability Statement
The datasets supporting this article’s conclusions are included within the article and its additional files.