Abstract
Butyrolactone I [α-oxo-β-(p-hydroxyphenyl)-γ-(p-hydroxy-m-3,3-dimethylallyl-benzyl)-γ-methoxycarbonyl-γ-butyrolactone] is produced as a secondary metabolite by Aspergillus terreus. Because small butyrolactone-containing molecules act as self-regulating factors in some bacteria, the effects of butyrolactone I on the producing organism were studied; specifically, changes in morphology, sporulation, and secondary metabolism were studied. Threefold or greater increases in hyphal branching (with concomitant decreases in the average hyphal growth unit), submerged sporulation, and secondary metabolism were observed when butyrolactone I was added to cultures of A. terreus. Among the secondary metabolites whose production was increased by this treatment was the therapeutically important compound lovastatin. These findings indicate that butyrolactone I induces morphological and sporulation changes in A. terreus and enhances secondary metabolite production in a manner similar to that previously reported for filamentous bacteria.
Like many filamentous fungi, Aspergillus terreus undergoes morphological and physiological changes when the abundance and complexity of nutrient sources are altered. When readily utilizable nutrients are abundant, the organism undergoes exponential growth, a phase known as primary metabolism. Primary metabolism includes the metabolic processes that are required for growth, maintenance, and survival of the organism and are basically similar for all living cells. Under conditions of nutrient limitation, such as deprivation of easily assimilated carbon, nitrogen, or phosphorus sources, the organism enters a period of slower growth, morphological alterations, and changes in metabolism known as secondary metabolism (10). Secondary metabolism is dispensable to normal metabolism, is widely variable in occurrence, and may or may not have a readily apparent biological function. A variety of clinically beneficial secondary metabolites are produced by fungi, such as the beta-lactam antibiotics penicillin and cephalosporin, the antifungal antibiotic griseofulvin, and the pharmacologically active compounds known as the ergot alkaloids.
A. terreus is an especially prolific producer of secondary metabolites. A few of the compounds that are produced by A. terreus are aspulvinone (30), asterric acid (8), asterriquinone (17), butyrolactone I (26), citrinin (27), emodin (7), geodin (20), itaconate (5), lovastatin (2, 12), questrin (8), sulochrin (33), and terrecyclic acid (24). Lovastatin, also known as mevinolin or monacolin K, is clinically useful for reducing serum cholesterol (2) and slowing the progression of atherosclerosis (34). Lovastatin and related compounds inhibit cholesterol synthesis by inhibiting the rate-limiting step in cellular cholesterol biosynthesis, namely, the conversion of hydroxymethylglutaryl coenzyme A to mevalonic acid by 3-hydroxy-3-methylglutaryl-coenzyme A reductase. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, such as lovastatin, reversibly inhibit cell proliferation by inducing a block in the G1 phase of the cell cycle in a wide variety of normal and tumorigenic mammalian cell lines (1, 19).
Butyrolactone I, another A. terreus secondary metabolite, inhibits eukaryotic cyclin-dependent kinases. The cyclin-dependent kinases are protein kinases that control cell cycle progression in all eukaryotes and are regulated by phosphorylation and dephosphorylation of critical serine, threonine, or tyrosine residues. Full kinase activity is dependent on the interaction of each cyclin-dependent kinase with a specific cyclin. Butyrolactone I is a selective inhibitor of cell cycle kinases (18, 25) but has little effect on other protein kinases, such as mitogen-activated protein kinase, protein kinase C, cyclic AMP-dependent kinase, or casein kinases. Butyrolactone I inhibits cyclin-dependent kinases cdk1 and cdk2 of mammalian cells with 50% inhibitory concentrations of 2.6 and 0.8 μM, respectively, while the 50% inhibitory concentrations for non-cyclin-dependent kinases are more than 100 μM (21).
Small γ-butyrolactone-containing molecules act as diffusible self-regulating factors in a number of bacteria and control many diverse functions, such as antibiotic production, biofilm formation, bioluminescence, virulence factor production, and plasmid conjugal transfer (4, 9). These compounds may provide a common means of bacterial cell-to-cell communication or signaling (16, 22). One well-studied compound, A-factor (2-isocapryloyl-3R-hydroxmethyl-γ-butyrolactone), regulates cellular differentiation and secondary metabolism in Streptomyces griseus (3, 14). Structurally related γ-butyrolactones are present in a variety of other Streptomyces species and are involved in morphological differentiation (aerial mycelium and spore formation) or secondary metabolism (4, 13). A number of γ-butyrolactone-containing compounds are also produced by different fungi; these compounds include aspulvinone in A. terreus (30), γ-decalactone in Sporobolomyces odorus (32), lachnumlactone A in Lachnum papyraceum (28), and multicolanic acid in Penicillium multicolor (32). However, the function of these compounds is not yet known. We undertook this study to determine if butyrolactone I, a γ-butyrolactone-containing secondary metabolite of the filamentous fungus A. terreus, functions in a manner analogous to the small γ-butyrolactone-containing compounds of the filamentous bacteria belonging to the genus Streptomyces. We show that as in prokaryotes, in A. terreus butyrolactone I has the ability to induce morphological changes, increases in spore formation, and increases in the production of A. terreus secondary metabolites.
MATERIALS AND METHODS
Materials.
Glucose, lactose, buffers, and other reagents were obtained from Sigma Chemical Co. (St. Louis, Mo.). Yeast extract and malt extract were obtained from Difco Laboratories (Detroit, Mich.); peptonized milk was obtained from Quest International (Norwich, N.Y.); and corn steep liquor was obtained from Grain Processing Corp. (Muscatine, Iowa). Solvents were obtained from Fisher Scientific Co. (Pittsburgh, Pa.). Purified butyrolactone I and authentic samples of lovastatin and sulochrin were provided by William Saum (Chemistry Section, Technical Operations, Merck and Co., Inc., Elkton, Va.), and the butyrolactone I standard was provided by Henry Joshua (Merck Research Laboratories, Rahway, N.J.).
Cultures and growth conditions.
A. terreus ATCC 20542 was obtained from the Merck Culture Collection (Rahway, N.J.). A reisolate, M8, was used throughout this study as it exhibited less variability in growth and secondary metabolism than the parent strain. The culture was maintained on yeast extract-malt extract (YME) agar slants containing 0.4% yeast extract, 1% malt extract, 0.4% glucose, and 2% agar (pH 7.0). Freshly inoculated slants were incubated at 28°C for 5 days, after which they were stored at 4°C. Frozen spore suspensions were prepared by washing the agar slants with 5 ml of a sterile 5% (wt/vol) glycerol–10% (wt/vol) lactose solution, combining the washes, and freezing (−70°C) 2-ml aliquots. Spores were propagated from 1 ml of a frozen spore suspension by using the inoculum medium (medium A) described by Alberts et al. (2). Medium A contained (per liter) 5 g of corn steep liquor, 40 g of tomato paste, 10 g of oat flour, 10 g of dextrose, and 10 ml of a trace element solution; the pH was 6.8. The trace element solution contained (per liter) 1 g of FeSO4 · 7H2O, 1 g of MnSO4 · 4H2O, 25 mg of CuCl2 · 2H2O, 100 mg of CaCl2 · 2H2O, 56 mg of H3BO3, 19 mg of (NH4)6Mo7O24 · 4H2O, and 200 mg of ZnSO4 · 7H2O. The secondary metabolite production medium contained glucose, peptonized milk, and yeast extract, as well as additional lactose (6). This medium, which was designated GPY-L, contained (per liter) 25 g of glucose, 24 g of peptonized milk, 2.5 g of yeast extract, 50 g of lactose, and 2.5 ml of P2000 defoamer; the pH was 7.4. The inoculum used for secondary metabolite production was prepared by inoculating 250-ml Erlenmeyer flasks containing 40 ml of medium A with 1-ml portions of frozen spore suspension (approximately 2 × 106 spores). The flasks were incubated at 27°C on a rotatory shaker at 220 rpm for 25 h, and then 2 ml of each inoculum was used to inoculate 20 ml of production medium. The flasks were then incubated at 27°C on a rotatory shaker at 220 rpm for up to 10 days.
Evaluation of morphological changes: branching.
Portions (1 ml) of the spore suspensions (approximately 2 × 106 spores) were used to inoculate flasks containing 40 ml of inoculum sporulation medium (medium A), which were incubated at 27°C on a rotatory shaker at 220 rpm for 18 h. Butyrolactone I was added to individual 18-h flasks in 500 μl of sterile ethanol so that final butyrolactone I concentrations of 0, 63, 125, 250, 500, and 1,000 μM were obtained. Each concentration was tested in triplicate. After 2 h of incubation at 27°C with agitation, the numbers of branches and hyphae were determined with a light microscope (Standard 16; Carl Zeiss, Inc., Thomwood, N.Y.). Three aliquots from three different samples at each concentration were taken, and a total of at least 30 hyphae from three different fields for each aliquot were counted. Controls were treated with sterile ethanol alone.
Evaluation of morphological changes: HGU.
Eighteen-hour-old inoculum flasks were prepared as described above. Butyrolactone I or lovastatin was added to each flask to a final concentration of 0, 50, 100, 200, or 500 μM. Duplicate flasks containing each concentration were incubated for 4 h at 27°C with agitation. Two aliquots were taken from each flask, and 8 to 12 fields were examined with a light microscope and captured digitally by using Picture Publisher software (Micrografx, Richardson, Tex.). The number of branches and the length of approximately 10 hyphae in each field were determined. The results were plotted as the average hyphal length divided by the number of branches in each 10-hypha group in order to derive the hyphal growth unit (HGU) (the average length of the hypha associated with each branch). The mean and standard error of the mean for 8 to 13 groups at each concentration were determined.
Submerged sporulation studies.
For each concentration tested, 1 ml of spore suspension (approximately 2 × 106 spores) was used to inoculate 40 ml of medium A. Butyrolactone I or lovastatin was added at different times to individual flasks to provide the final concentration needed in each experiment. The inoculum was incubated at 27°C with agitation at 220 rpm until day 8, and then the flasks were assayed to determine spore production. The number of visible spores was determined by taking two aliquots from each flask, diluting each aliquot 10-fold, and counting the spores with a light microscope by using a hemacytometer. Two samples from each aliquot were counted to provide four replicates for each concentration. The number of viable spores was determined by plating diluted samples from each flask onto YME agar plates; four replicates were examined at each concentration. The plates were incubated at 27°C for 5 days.
Secondary metabolite production studies.
Flasks containing 20 ml of GPY-L were inoculated with 2 ml of 25-h-old inoculum (see above) and incubated at 27°C with agitation at 220 rpm. Butyrolactone I was added after 24 h to one-half of the flasks to a final concentration of 500 μM. Two or more of the butyrolactone-containing flasks were harvested along with two or more of the non-butyrolactone-containing controls at various times to evaluate lovastatin production. Lovastatin was quantified from methanol extracts of culture broth by using reverse-phase chromatography on an octyldecyl silane Hypersil column (length, 100 mm; particle size, 5 μm; Hewlett-Packard, Palo Alto, Calif.). The mobile phase, 0.1% aqueous phosphoric acid–acetonitrile (45:55, vol/vol), was added isocratically at a flow rate of 1 ml/min, and detection was at 238 nm. Authentic lovastatin was used to confirm the retention time and the quantities of lovastatin in the culture extracts.
A full factorial experiment was performed by using Design Expert software (Stat-Ease, Minneapolis, Minn.) to examine the effect of addition of butyrolactone I or lovastatin on secondary metabolism. Flasks containing 20 ml of GPY-L were inoculated with 2 ml of inoculum (as described above) and incubated at 27°C as described above. Butyrolactone I or lovastatin was added to replicate flasks after 120, 145, and 170 h to a final concentration of 0, 10, 50, or 100 μM. Each flask was harvested after 12 days. Each concentration at each time point was tested by using at least four replicate flasks; 16 replicates were used for the designated midpoint of the experiment, 50 μM at 145 h, in order to determine experimental variability. The concentrations of lovastatin and sulochrin in methanol extracts of culture broth were quantified by high-performance liquid chromatography as described above.
Dry cell weight determinations.
The dry cell weight was determined by filtering 20 ml of culture broth through filter paper (Whatman 934-AH; Fisher Scientific), washing the preparation three times with distilled water, and drying the remaining mycelial mat under a vacuum overnight at 60°C.
RESULTS AND DISCUSSION
Morphological changes.
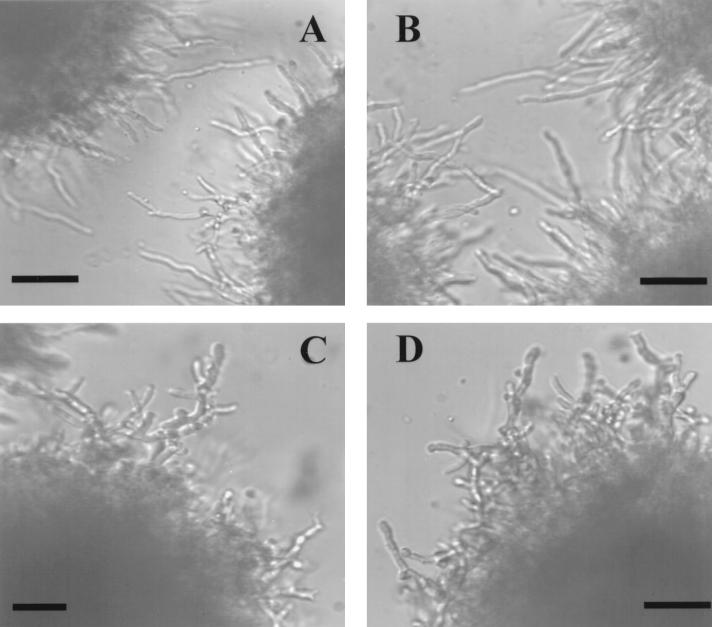
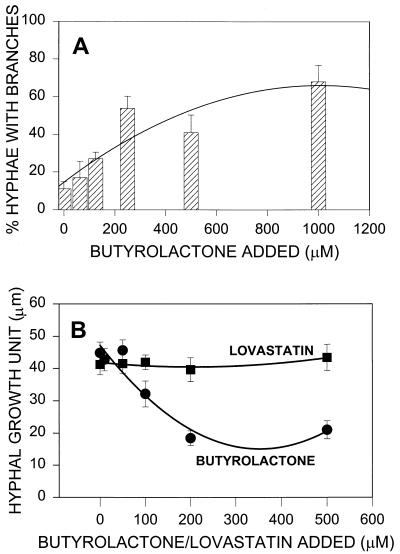
Several A-factor-related compounds have an effect on aerial mycelium morphogenesis in a number of filamentous Streptomyces species. A. terreus, a filamentous fungus, was therefore examined for possible morphological changes in response to γ-butyrolactone treatment. In strain M8, no overt morphological changes in aerial hyphae and conidiophores were observed by microscopy when butyrolactone I was added exogenously. However, Fig. 1 shows that an increase in the overall hyphal branching was discerned when butyrolactone I was added to 18-h-old submerged cultures and the cultures were incubated for an additional 2 h. At that time, the percentage of hyphae with branches was determined and compared to the percentage in the control cultures. The results indicated that there was a dose-dependent increase in the number of hyphae with branches, which peaked in response to approximately 500 μM butyrolactone I, as shown by second-order regression analysis (Fig. 2A). Monitoring changes in branching alone did not take into account any changes in hyphal extension that may have been induced by butyrolactone I. For this reason, the effect of butyrolactone I on the HGU, which was the average length of the hypha associated with each branch (31), was determined. Lovastatin was used as a control compound, as it has been found to be active at concentrations ranging from 20 to 100 μM in inhibiting cell cycle progression in a wide variety of mammalian cells (19) and is produced as a secondary metabolite concurrently with butyrolactone I in A. terreus M8. Figure 2B shows that addition of 200 to 500 μM butyrolactone I resulted in a statistically significant decrease in the HGU, while addition of lovastatin at the same concentrations resulted in no change in the HGU. These changes in fungal morphology are consistent with previous reports on antheridiol, a butyrolactone-containing diffusible hormone of the saphrophytic water mold Achlya ambisexualis, which causes cessation of growth and profuse hyphal branching (11).
FIG. 1.
A. terreus hyphal filaments without (A and B) and with (C and D) butyrolactone I added. Butyrolactone I in sterile ethanol was added to a final concentration of 200 μM to 18-h cultures. Aliquots of the cultures were taken after 5 h, and photomicrographs were made. Bars = 50 μm.
FIG. 2.
Effect of butyrolactone I on morphology. (A) Percentage of hyphae with branches after butyrolactone I was added to aliquots of a 20-h culture. Each bar represents the mean obtained with at least 10 hyphae in three samples at each concentration. (B) Changes in HGU (the average length of hypha associated with each branch) of fungal pellets treated with butyrolactone I or lovastatin. The data for both 200 μM butyrolactone and 500 μM butyrolactone I are significant at the P < 0.001 level.
Effect of butyrolactone I on submerged sporulation.
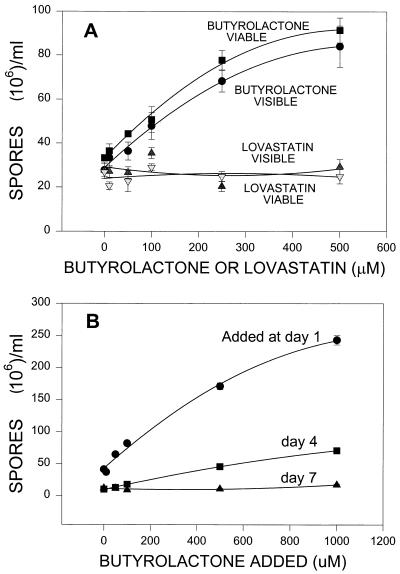
The effect of butyrolactone I on submerged spore production in A. terreus was tested. Sporulation in submerged liquid cultures occurs when conidia are produced directly from hyphae without prior differentiation into conidiophores and can be induced by carbon or nitrogen starvation (23, 29). Different concentrations of butyrolactone I were added to submerged spore-producing inoculum cultures in medium A. These cultures were examined after 8 days of incubation both for visible spores as determined by microscopic examination and for viable spore production as determined by CFU on YME agar plates. Figure 3A shows that addition of butyrolactone I resulted in increases in the numbers of both visible and viable spores, while addition of lovastatin resulted in no changes in spore number at the concentrations tested. Addition of butyrolactone I to a final concentration of 500 μM yielded approximately threefold more spores (visible or viable) than the number of spores in the control samples. Analysis of visible spores provided a straightforward and quick assay whose results strongly correlated with the number of viable spores detected on agar plates after 5 days of incubation (R = 0.992). Therefore, the visible spore assay was used to determine the effect of addition of butyrolactone I on 8 days of spore production when it was added on days 1, 4, and 7. Figure 3B shows that there was a greater increase in the day 8 spore number when butyrolactone I was added on day 1 than when it was added on day 4 or 7. By day 7, addition of butyrolactone I had little or no effect on spore production. Even at the highest concentration used, 1,000 μM, addition on day 7 produced only 40% more spores, while addition of the same concentration on day 1 resulted in more than sixfold more spores than the number in the control.
FIG. 3.
Effect of butyrolactone I on submerged spore production. (A) Number of visible spores as determined with the hemacytometer and number of viable spores as determined by CFU counts on agar plates after butyrolactone I or lovastatin was added at different final concentrations on day 2. Spores were counted on day 8. The data for both 250 μM butyrolactone and 500 μM butyrolactone I are significant at the P < 0.001 level. (B) Number of visible spores produced when butyrolactone I was added on different days and spores were counted on day 8.
Effect of butyrolactone I on secondary metabolism.
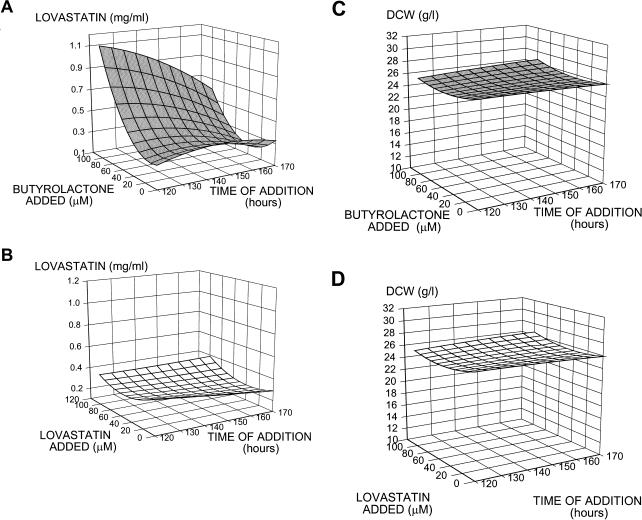
Since A. terreus produces many secondary metabolites, including the clinically useful compound lovastatin, we wanted to determine if addition of butyrolactone I had an effect on secondary metabolism in the producing organism. A full factorial experiment was designed to examine the combination of time of addition and concentration in order to evaluate the effect of both metabolites on secondary metabolite production. Butyrolactone I or lovastatin was added to a final concentration of 0, 10, 50, or 100 μM during secondary metabolism (at 120, 145, or 170 h postinoculation), and each culture was incubated until day 12; then each culture was assayed for metabolite production. As shown in the response surface map generated from the results of the factorial experiment (Fig. 4A), addition of butyrolactone I increased lovastatin production. The mean titer of lovastatin for six replicates to which butyrolactone I (diluted in sterile ethanol) was added to a final concentration of 100 μM at 120 h was 0.94 g/liter (standard error of the mean, 0.08 g/liter), while the mean for the controls to which only sterile ethanol was added was 0.32 g of lovastatin per liter (standard error of the mean, 0.06 g/liter). The same factorial experiment was performed with lovastatin (rather than butyrolactone I) added to a final concentration of 0, 50, or 100 μM at 120, 145, or 170 h postinoculation. These levels of lovastatin represent amounts that have been reported to influence cell cycle activity in mammalian cells but are less than the amount of lovastatin produced by A. terreus M8. Figure 4B shows that no increase in the final lovastatin titer was detected at the time of harvest when lovastatin was added between 120 and 170 h after inoculation. Figure 4C shows that only a slight increase in cell mass occurred when butyrolactone I was added, indicating that the increase in lovastatin production upon addition of butyrolactone I was probably not due to the small increase in cell mass. The dry cell weights of the treated cultures were 93.6 to 108.5% of the untreated control dry cell weights. The large change in secondary metabolism (an increase of more than twofold for lovastatin) was probably not due to the small change in cell mass (less than 9%). The final cell mass was approximately the same whether butyrolactone I (Fig. 4C) or lovastatin (Fig. 4D) was added. Butyrolactone I not only affected lovastatin production but also increased the production of sulochrin, another secondary metabolite of A. terreus produced concurrently with lovastatin. Table 1 shows that addition of 100 μM butyrolactone I at 120 h postinoculation increased sulochrin production by 86% compared with the controls, while no increase was observed in the lovastatin-treated cultures.
FIG. 4.
Effect of butyrolactone I on secondary metabolism. (A) Response surface map showing the effects of different concentrations of butyrolactone I added at different times. For the sum of squares (a measure of variability between groups), P < 0.001. (B) Response surface map showing the effect of lovastatin addition on lovastatin production. (C and D) Response surface maps showing the effect of butyrolactone I and lovastatin additions on cell mass, respectively. DCW, dry cell weight.
TABLE 1.
Sulochrin production after addition of butyrolactone I or lovastatin
| Treatment | n | Sulochrin concn (μg/ml) | % of control value |
|---|---|---|---|
| Control | 6 | 121 ± 18a | 100 |
| Butyrolactone I | 6 | 226 ± 16 | 187b |
| Control | 7 | 140 ± 11 | 100 |
| Lovastatin | 3 | 123 ± 14 | 88c |
Mean ± standard error of the mean.
The increase is statistically significant at P < 0.002.
The decrease is not statistically significant compared to the lovastatin control values.
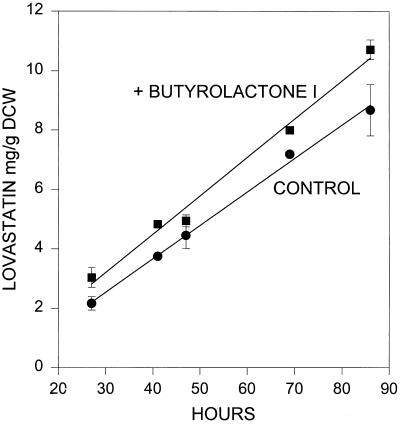
When added during the primary metabolism phase, butyrolactone I did not have the dramatic effect on lovastatin production seen when it was added during secondary metabolism. Figure 5 shows that adding butyrolactone I to cultures during exponential growth, even to a concentration fivefold greater than the concentration shown to be effective during secondary metabolism (500 μM), shifted lovastatin production ahead by approximately 5 to 10 h, while the rate of lovastatin production remained approximately the same. Addition of butyrolactone I during the growth phase advanced the timing of secondary metabolism by approximately 5 to 10 h. This is comparable to the advance in secondary metabolite production when the γ-butyrolactone compound virginiae butanolide-C was added to Streptomyces virginiae (35) or A-factor was added to S. griseus during growth (14). These results suggest that butyrolactone I may play a role in regulating the amount of lovastatin produced by A. terreus.
FIG. 5.
Effect of butyrolactone I addition on lovastatin production when butyrolactone I was added to 24-h cultures of A. terreus during primary metabolism. Each point represents at least two replicates, and the error bars represent the standard errors of the means for three replicates. The correlation coefficient for both lines is >0.993. DCW, dry cell weight.
Although further work is needed to determine if butyrolactone I belongs to the class of compounds that constitute the microbial hormones, treatment with butyrolactone I induced changes in the host organism that are similar to those induced by the more completely characterized microbial hormones. A practical application of these findings is the possibility that butyrolactone I could be used to increase or promote the production of desired secondary metabolites in A. terreus. It has been proposed that the A-factor-related microbial signals might be useful in controlling secondary metabolism in streptomycetes (4), and this has been demonstrated by using the autoregulator virginiae butanolide-C to control the induction time of virginiamycin production and the amount of virginiamycin produced. A twofold increase in the amount of virginiamycin was observed when virginiae butanolide-C was added during secondary metabolism just after growth had slowed (35). Similarly, in this study, a threefold increase in the amount of lovastatin and an approximately twofold increase in sulochrin production were observed after butyrolactone I was added to postexponentially growing cultures.
Footnotes
This article is dedicated to the memory of William Saum, whose untimely passing was a great loss for family, colleagues, and friends. He will be remembered for his great dedication to helping others.
REFERENCES
- 1.Addeo R, Altucci L, Battista T, Bonapace M, Cancemi M, Cicatiello L, Germano D, Pacilio C, Salzano S, Bresciani F, Weisz A. Stimulation of human breast cancer MCF-7 cells with estrogen prevents cell cycle arrest by HMG-CoA reductase inhibitors. Biochem Biophys Res Commun. 1996;220:864–870. doi: 10.1006/bbrc.1996.0494. [DOI] [PubMed] [Google Scholar]
- 2.Alberts A W, Kuron J, Hunt V, Huff J, Hoffman J, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beppu T. Secondary metabolites as chemical signals for cellular differentiation. Gene. 1992;115:159–165. doi: 10.1016/0378-1119(92)90554-3. [DOI] [PubMed] [Google Scholar]
- 4.Beppu T. Signal transduction and secondary metabolism: prospect for controlling productivity. Trends Biotechnol. 1995;13:264–269. doi: 10.1016/S0167-7799(00)88961-1. [DOI] [PubMed] [Google Scholar]
- 5.Bonnarme P, Gillet B, Sepulchre A M, Role C, Beloeil J C, Ducrocq C. Itaconate biosynthesis in Aspergillus terreus. J Bacteriol. 1995;177:3573–3578. doi: 10.1128/jb.177.12.3573-3578.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckland B, Gbewonyo K, Hallada T, Kaplan L, Masurekar P. Production of lovastatin, an inhibitor of cholesterol accumulation in humans. In: Demain A L, Somkuti G A, Hunter-Cevera J C, Rossmore H W, editors. Novel microbial products for medicine and agriculture. New York, N.Y: Elsevier Science Publishing; 1989. pp. 161–169. [Google Scholar]
- 7.Chen Z G, Fujii I, Ebizuka Y, Sankawa U. Emodin O-methyl-transferase from Aspergillus terreus. Arch Microbiol. 1992;158:29–34. doi: 10.1007/BF00249062. [DOI] [PubMed] [Google Scholar]
- 8.Curtis R F, Hassall C H, Jones D W, Williams T W. The biosynthesis of phenols. II. Asterric acid, a metabolic product of Aspergillus terreus. Thom J Chem Soc. 1960;1960:4836–4842. [Google Scholar]
- 9.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 10.Drew S, Demain A. Effects of primary metabolites on secondary metabolism. Annu Rev Microbiol. 1977;31:343–356. doi: 10.1146/annurev.mi.31.100177.002015. [DOI] [PubMed] [Google Scholar]
- 11.Gooday G W, Adams D J. Sex hormones and fungi. Adv Microb Physiol. 1993;43:65–145. doi: 10.1016/s0065-2911(08)60028-4. [DOI] [PubMed] [Google Scholar]
- 12.Greenspan M D, Yudkovitz J B. Mevinolinic acid biosynthesis by Aspergillus terreus and its relationship to fatty acid biosynthesis. J Bacteriol. 1985;162:704–707. doi: 10.1128/jb.162.2.704-707.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horinouchi S, Beppu T. Autoregulatory factors and communication in actinomycetes. Annu Rev Microbiol. 1992;46:377–398. doi: 10.1146/annurev.mi.46.100192.002113. [DOI] [PubMed] [Google Scholar]
- 14.Horinouchi S, Beppu T. A-factor as a microbial hormone that controls cellular differentiation and secondary metabolism in Streptomyces griseus. Mol Microbiol. 1994;12:859–864. doi: 10.1111/j.1365-2958.1994.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 15.Hosoi T, Uchiyama M, Okumura E, Saito T, Ishiguro K, Uchida T, Okuyama A, Kishimoto T, Hisanaga S. Evidence for cdk5 as a major phosphorylating tau protein in porcine brain extract. J Biochem. 1995;117:741–749. doi: 10.1093/oxfordjournals.jbchem.a124771. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser D. Bacteria also vote. Science. 1996;272:1598–1599. doi: 10.1126/science.272.5268.1598. [DOI] [PubMed] [Google Scholar]
- 17.Kaji A, Iwata T, Kiriyama N, Wakusawa S, Miyamoto K. Four new metabolites of Aspergillus terreus. Chem Pharm Bull (Tokyo) 1994;42:1682–1684. doi: 10.1248/cpb.42.1682. [DOI] [PubMed] [Google Scholar]
- 18.Kanemitsu M Y, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ. 1998;9:13–21. [PubMed] [Google Scholar]
- 19.Keyomarsi K, Sandoval L, Band V, Pardee A. Synchronization of tumor and normal cells for G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 20.Kiriyama N, Nitta K, Sakaguchi Y, Tagushi Y, Yamamoto Y. Studies on the metabolic products of Aspergillus terreus. III. Metabolites of the strain IFO 8835. Chem Pharm Bull (Tokyo) 1977;25:2593–2601. [Google Scholar]
- 21.Kitagawa M, Okabe T, Ogino H, Matsumoto H, Suzuki-Takahashi I, Kokubo T, Higashi H, Saitoh S, Taya Y, Yasuda H, Ohba Y, Nishimura S, Tanaka N, Okuyama A. Butyrolactone I, a selective inhibitor of cdk2 and cdc2 kinase. Oncogene. 1993;8:2425–2432. [PubMed] [Google Scholar]
- 22.Kolter R, Losick R. One for all and all for one. Science. 1998;280:226–227. doi: 10.1126/science.280.5361.226. [DOI] [PubMed] [Google Scholar]
- 23.Martinelli S D. Conidiation of Aspergillus nidulans in submerged culture. Trans Br Mycol Soc. 1976;67:121–128. [Google Scholar]
- 24.Nakagawa M, Hirota A, Sakai H, Isogai A. Terrecyclic acid A, a new antibiotic from Aspergillus terreus. I. Taxonomy, production, and chemical and biological properties. J Antibiot. 1982;35:778–782. doi: 10.7164/antibiotics.35.778. [DOI] [PubMed] [Google Scholar]
- 25.Nishio K, Ishida T, Arioka H, Kurokawa H, Fukuoka K, Nomoto T, Fukumoto H, Yokote H, Saijo N. Antitumor effects of butyrolactone I, a selective cdc2 kinase inhibitor, on human lung cancer cell lines. Anticancer Res. 1996;16:3387–3395. [PubMed] [Google Scholar]
- 26.Nitta K, Fujita N, Yoshimura T, Arai K, Yamamoto U. Metabolic products of Aspergillus terreus. IX. Biosynthesis of butyrolactone derivatives isolated from strain IFO 8835 and 4100. Chem Pharm Bull (Tokyo) 1983;31:1528–1533. [Google Scholar]
- 27.Sankawa U, Ebizuka Y, Noguchi H, Isikawa Y, Kitaghawa S, Yamamoto Y, Kobayashi T, Iitak Y. Biosynthesis of citrinin in Aspergillus terreus. Tetrahedron. 1983;39:3583–3591. [Google Scholar]
- 28.Shan R, Stadler M, Sterner O, Anke H. New metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. VIII. Isolation, structure determination and biological activities of minor metabolites structurally related to mycorrhizin A. J Antibiot. 1996;49:447–452. doi: 10.7164/antibiotics.49.447. [DOI] [PubMed] [Google Scholar]
- 29.Skromne I, Sanchez O, Aguirre J. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology. 1995;141:21–28. doi: 10.1099/00221287-141-1-21. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi I, Ojima N, Ogura K, Seto S. Purification and characterization of dimethylallyl pyrophosphate:aspulvinone dimethylallyltransferase from Aspergillus terreus. Biochemistry. 1978;17:2696–2702. doi: 10.1021/bi00606a037. [DOI] [PubMed] [Google Scholar]
- 31.Trinci A P J. A study of the kinetics of hyphal extention and branch initiation of fungal mycelia. J Gen Microbiol. 1974;81:225–236. doi: 10.1099/00221287-81-1-225. [DOI] [PubMed] [Google Scholar]
- 32.Turner W B, Aldridge D C. Fungal metabolites II. New York, N.Y: Academic Press; 1983. Polyketides; pp. 55–223. [Google Scholar]
- 33.Vinci V A, Hoerner T D, Coffman A D, Schimmel T G, Dabora R L, Kirpekar A C, Ruby C L, Stieber R W. Mutants of a lovastatin-hyperproducing Aspergillus terreus deficient in the production of sulochrin. J Ind Microbiol. 1991;8:113–120. [Google Scholar]
- 34.Waters D, Higginson L, Gladstone P, Kimball B, LeMay M, Boccuzzi S J, Lesperance J the CCAIT Study Group. Effects of monotherapy with an HMG-CoA reductase inhibitor on the progression of coronary atherosclerosis as assessed by serial quantiative arteriography: the Canadian coronary atherosclerosis intervention trial. Circulation. 1994;89:959–968. doi: 10.1161/01.cir.89.3.959. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y K, Morikawa M, Shimizu H, Shioya S, Suga K, Nihira T, Yamada Y. Maximum virginiamycin production by optimization of cultivation conditions in batch culture with autoregulator addition. Biotechnol Bioeng. 1996;49:437–444. doi: 10.1002/(SICI)1097-0290(19960220)49:4<437::AID-BIT11>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]