Abstract
The pivotal role of the tumor microenvironment (TME) in the initiation and advancement of hepatocellular carcinoma (HCC) is widely acknowledged, as it fosters the proliferation and metastasis of HCC cells. Within the intricate TME of HCC, tumor-associated macrophages (TAMs) represent a significant constituent of non-malignant cells. TAMs engage in direct communication with cancer cells in HCC, while also exerting influence on other immune cells to adopt a tumor-supportive phenotype that facilitates tumor progression. Among the multifaceted mechanisms at play, the metabolic reprogramming of both tumor cells and macrophages leads to phenotypic alterations and functional modifications in macrophages. This comprehensive review elucidates the intricate interplay between cellular metabolism and macrophage phenotype/polarization, while also providing an overview of the associated signaling molecules and potential therapeutic strategies for HCC.
Keywords: Macrophage, Metabolism, Phenotype, Function, Tumor microenvironment, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a prevalent malignancy with significant global mortality rates. The etiology and progression of HCC are influenced by various factors, such as hepatitis B virus (HBV) and HCV infection, aflatoxin exposure, alcohol consumption, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and cirrhosis. While surgical resection, ablation, and liver transplantation are currently the most efficacious treatments for early-stage HCC, the diagnosis of HCC often occurs at an advanced stage, resulting in poor prognosis [1–3]. The advent of systemic therapeutics for advanced HCC, including immune checkpoint inhibitors (ICIs) [4, 5], tyrosine kinase inhibitors (TKIs) [6–8], and anti-angiogenic antibodies [9, 10], has improved patient outcomes by demonstrating some anti-tumor effects [1, 2, 11]. However, the efficacy of these therapeutics is hindered by primary and secondary resistance, posing a significant challenge [12].
The tumor microenvironment (TME) plays a critical role in modulating the response to anti-tumor therapy in HCC. By orchestrating alterations in the surrounding milieu of HCC cells, including endothelial cells, stromal cells, immune cells, and associated cytokines, a pro-tumor TME is established, thereby facilitating HCC cell proliferation and impeding the efficacy of anti-tumor interventions [13–15]. Additionally, extracellular metabolites within the TME serve as both energy sources and mediators of intercellular communication. Notably, tumor cells exploit these metabolites to subvert immune cells, redirecting their function from anti-tumor to pro-tumor activities [16]. Concurrently, non-malignant cells contribute to tumor support by releasing metabolites into the TME [17].
Macrophages constitute a significant proportion of immune cells and exert crucial functions within the TME of HCC. Notably, the intrinsic characteristics of tumor cells and extrinsic environmental factors induce alterations in the metabolism, phenotype, and biological functions of macrophages [18–20], thereby enabling their reprogramming to support tumor growth. Of particular importance, changes in cellular metabolism can profoundly impact the functional properties of macrophages [21–23]. Consequently, it is imperative to investigate the intricate interplay between macrophage metabolism, phenotype, and function. In this comprehensive review, we present the latest advancements in understanding macrophage phenotypes, functions, and metabolism, while also exploring potential therapeutic strategies for HCC that target key molecules within these immune cells.
Phenotype and function of macrophages
Macrophages, as a crucial component of the innate immune system, are ubiquitously distributed in the bloodstream and various tissues throughout the body. Their significance extends to autoimmune disorders and malignancies, where they play a pivotal role in processes such as angiogenesis and tumor progression. Originating from monocytes in the peripheral blood, macrophages undergo differentiation into distinct functional subpopulations upon local recruitment and stimulation by specific chemokines [24]. Additionally, a subset of macrophages arises from tissue-resident macrophages. These versatile immune cells exhibit a diverse range of functions, including cellular debris clearance, promotion of angiogenesis for tissue repair, cytokine release, phagocytosis-mediated inflammatory responses, and tumor cell elimination [25–28]. Meanwhile, macrophages possess a high degree of plasticity and can adapt to environmental changes by modulating their cellular metabolism and functional phenotype [29].
Macrophages have been classified into two primary categories based on their activation status and biological functions: classically activated macrophages (M1-like phenotype) and alternatively activated macrophages (M2-like phenotype) [30]. The differentiation of these macrophage subtypes occurs under distinct conditions. While recent advancements in single-cell RNA sequencing techniques have revealed the existence of additional macrophage phenotypes, the M1 and M2 categories remain the predominant phenotypes used to distinguish macrophage populations.
M1-like phenotype macrophages
Stimulation with cytokines such as lipopolysaccharide (LPS), Toll-like receptor (TLR) ligands, and interferon-γ (IFN-γ) elicits the induction of a pro-inflammatory M1-like phenotype in macrophages. These M1-like macrophages exhibit heightened expression of T-lymphocyte activating antigens (CD80 and CD86) and possess a multitude of capabilities, including the secretion of pro-inflammatory cytokines, generation of reactive oxygen species (ROS), expression of inducible nitric oxide synthase (iNOS) and subsequent production of nitric oxide (NO), as well as the synthesis of interleukin 12 (IL-12) to activate other immune cells. This activation cascade ultimately enhances the antigen-presenting capacity of macrophages, facilitating the activation of cytotoxic T lymphocytes and promoting the eradication of tumor cells [25, 30–32] (Fig. 1).
Fig. 1.
The differentiation of macrophages into distinct phenotypes, namely M1 and M2-like, is influenced by various stimulators. For instance, the exposure to LPS and IFN-γ induces macrophages to adopt an M1-like phenotype. M1-like macrophages express specific markers such as CD80, CD86, CD16, CD32, CD64, among others. These M1-like macrophages are capable of secreting pro-inflammatory factors including TNF-α, NO, IL-12, among others. Furthermore, they can activate T cells and NK cells, thereby mediating anti-tumor immunity against HCC cells. Conversely, the presence of IL-4, IL-10, and IL-13 can induce macrophages to undergo M2-like polarization. M2-like macrophages express specific markers such as CD163, CD206, CD209, TGF-β, IL-10, among others. These M2-like macrophages play a role in promoting angiogenesis, inhibiting T cell activation, and inducing T cell apoptosis, thereby assisting HCC cells in evading immune surveillance
Bufalin has demonstrated its ability to modulate anti-tumor immunity by inhibiting the expression of nuclear factor-κB (NF-κB) p50, leading to an increase in p65–p50 heterodimerization. This subsequently activates NF-κB signaling, resulting in the production of immune stimulatory factors. These factors induce the reprogramming of M2-like macrophages to M1-like phenotypes, thereby facilitating T cell-associated anti-tumor immunity [33]. Additionally, the matricellular protein SPON2 acts as a suppressor of HCC by activating RhoA and Rac1 through the integrin SPON2-α5β1. This activation promotes F-actin recombination, which in turn facilitates the infiltration of M1-like macrophages [34]. Furthermore, forkhead box protein O1 (FOXO1), derived from HCC, not only targets tumor cells but also synchronizes with re-educated macrophages. This synchronization is partially dependent on FOXO1 transcriptionally modulating the interferon regulatory factor 1 (IRF1)/nitric oxide (NO) axis in macrophages, resulting in the suppression of HCC cells [35].
M2-like phenotype macrophages
Conversely, the cytokines IL-4, IL-10, and IL-13 have been shown to drive the polarization of macrophages towards an M2-like phenotype with anti-inflammatory characteristics. M2-like macrophages are characterized by high expression of scavenger receptors (CD163) and mannose receptors (CD206) (Fig. 1). These macrophages secrete anti-inflammatory cytokines that play a crucial role in suppressing immune responses, regulating hypoxia, promoting angiogenesis, facilitating tumor invasion, metastasis, and progression, as well as aiding tumor cells in evading anti-tumor immune responses [21, 36–40].
The PKC/ZFP64/CSF1 axis within the HCC-regulated TME is characterized by its ability to induce polarization of M2-like macrophages and mediate immunosuppression, resulting in resistance to anti-PD1 therapy. Mechanistically, PKC phosphorylates ZFP64, leading to its nuclear translocation and subsequent activation of CSF1 at the transcriptional level. This activation of CSF1 transforms macrophages into M2-like phenotypes. Consequently, the protein kinase inhibitor Gö6976 and the multiple kinase inhibitor lenvatinib are able to disrupt this axis, thereby reversing the immunosuppressive TME and reprogramming the macrophage phenotype to increase susceptibility to anti-PD1 therapy [41]. Additionally, the IRF2/β-catenin pathway plays a regulatory role in lenvatinib resistance in HCC cells. Targeting this pathway may hold promise in improving the therapeutic efficacy of lenvatinib for HCC treatment [42].
Meanwhile, the polarization of macrophages towards an M2-like phenotype was observed in the presence of TREM1 expression. Notably, the down-regulation of TREM1 resulted in the transformation of M2-like macrophages into an M1-like phenotype, thereby exerting a preventive effect on the invasion and metastasis of HCC cells. This transformation was achieved through the suppression of the PI3K/AKT/mTOR signaling pathway [43].
Communication of TAMs with other immune cells in HCC
Macrophages present within the TME are commonly referred to as TAMs, constituting the predominant immune cell population infiltrating various tumor types. TAMs typically exhibit M2-like phenotypes, characterized by their secretion of anti-inflammatory cytokines and angiogenetic factors, thereby creating an inflammatory milieu that supports cancer cell survival. Concurrently, TAMs facilitate angiogenesis and tumor cell proliferation, while also bolstering tumor cell resistance to chemotherapy agents, including cytotoxic agents and checkpoint inhibitors. Additionally, TAMs play a role in suppressing anti-tumor immune responses. These multifaceted functions are often mediated through the cross-talk between TAMs and other immune cells [44–46].
Communication of M2-like TAMs with other immune cells in HCC
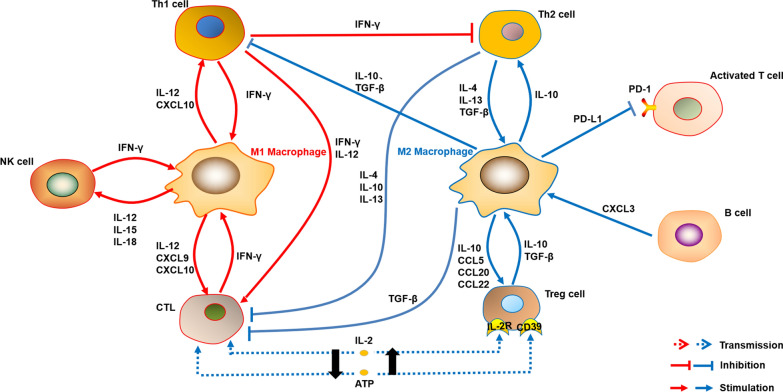
TAMs exhibit close interactions with other immune cells within the TME. Notably, TAMs have the ability to dampen the anti-tumor immune responses mediated by M1-like macrophages [47]. Additionally, TAMs secrete IL-10, which serves to suppress the function of Th1 cells while promoting the growth and development of Th2 cells [48]. Th2 cells, in turn, produce IL-4, thereby facilitating the development of TAMs. Functionally, Th2 cells exhibit characteristics that are opposite to Th1 cells, as they actively suppress the activation of cytotoxic T lymphocytes (CTLs). Simultaneously, the IL-10 produced by TAMs activates regulatory T (Treg) cells, leading to the inhibition of macrophage response to LPS and consequently suppressing the immune response, thereby promoting tumor progression. Furthermore, TAMs produce transforming growth factor-beta (TGF-β), which hinders the development of Th1 cells and CTLs, subsequently diminishing their anti-tumor activities [49] (Fig. 2).
Fig. 2.
Communication of macrophages with other immune cells. M1-like macrophages and M2-like macrophages possess different effects on other immune cells in the TME, respectively. Moreover, the other immune cells regulate macrophage polarization through their specific ways
Furthermore, TAMs exhibit high expression of the CD48 protein, which interacts with the 2B4 receptor (CD244) present on natural killer (NK) cells. This interaction ultimately leads to the rapid activation, exhaustion, and subsequent demise of NK cells [50]. Simultaneously, TAMs contribute to tumor progression by releasing anti-inflammatory cytokines, such as prostaglandin E2 (PGE2), and expressing programmed death ligand 1 (PD-L1) on their surface. PD-L1 engages with the PD-1 receptor on activated T cells, inducing apoptosis and thereby impeding immune responses [51, 52] (Fig. 2).
Communication of M1-like TAMs with other immune cells in HCC
In addition to the presence of M2-like TAMs, the TAM population also includes M1-like macrophages, which exert distinct effects on immune cells. Unlike M2-like macrophages, M1-like TAMs play a crucial role in promoting anti-tumor responses by activating NK cells, Th1 cells, and CTL. Additionally, they secrete IL-15 and IL-18 to stimulate NK cells [53]. The activated NK cells, in turn, produce IFN-γ, which induces macrophages to polarize towards the M1 phenotype [54]. Furthermore, various immune cells, including CD8 T cells, specific subsets of CD4 T cells, and γδ T cells, are capable of producing IFN-γ, thereby promoting M1-like polarization [55]. Thus, this reciprocal feedback loop contributes to the establishment of an anti-tumor immune microenvironment (Fig. 2). In light of these findings, inhibiting M2-like TAM polarization may represent a promising strategy for HCC immunotherapy.
TAMs and associated clinical application in HCC
The characteristic of TAMs
As previously mentioned, TAMs do not solely exhibit the M2-like phenotype. Extensive investigations into macrophages in human cancers have revealed that TAMs express markers associated with both M1-like and M2-like phenotypes, indicating a mixed population [23, 56–58]. Since M1-like and M2-like macrophages exert pro-inflammatory and anti-inflammatory effects, respectively, the phenotypes and functions of TAMs can undergo transformation under various intrinsic and extrinsic conditions [52, 59, 60].
During tumorigenesis, M2-like macrophages can undergo conversion to the M1-like phenotype, enabling them to combat tumor cells. Conversely, tumors have the ability to induce the conversion of the M1-like phenotype to the M2-like phenotype, thereby shielding themselves from immune cell-mediated damage [61–63]. Hence, the different subpopulations of macrophages can undergo interconversion rather than maturation and differentiation into distinct subpopulations. Moreover, a high density of TAMs in the tumor microenvironment has been correlated with a poor prognosis in patients, further confirming the tumor-promoting role of TAMs [51, 64, 65]. Consequently, targeting TAMs may represent a viable approach for tumor immunotherapy [66, 67].
TAMs-associated therapy in HCC
Targeting TAMs for tumor immunotherapy has been explored in clinical trials for the treatment of HCC. In one such trial, the combination of a phosphatidylserine (PS) targeting agent (2aG4) with sorafenib has demonstrated increased apoptosis of tumor cells and elevated levels of M1-like macrophages, while simultaneously reducing tumor microvasculature genesis and density of M2-like macrophages. This ongoing Phase I clinical study focuses on HCC patients and holds great promise as a therapeutic target [68].
Furthermore, the combination of tyrosine kinase inhibitors (TKIs) with immune checkpoint inhibitors (ICIs) has shown potential in enhancing anti-tumor immunity and inhibiting M2-like polarization. For instance, regorafenib has been found to enhance M1-like TAM polarization and increase the M1/M2 ratio. When combined with an ICI, regorafenib exhibits enhanced anti-HCC effects [69, 70]. Additionally, the compound kushen injection (CKI) has been shown to activate the TNFR1/NF-κB/p38/MAPK pathway, thereby reducing the immunosuppressive effects of TAMs and promoting T cell-associated cytotoxicity, leading to apoptosis of HCC cells. CKI also improves the efficacy of sorafenib, and the combination treatment demonstrates stronger anti-HCC responses [71].
Additionally, the activation of the Wnt2b/β-linked c-Myc signaling pathway has been found to promote the conversion of M2-like TAMs, thereby enhancing the progression of HCC. Conversely, the down-regulation of Wnt2b/β-linked protein expression through the use of Toll-like receptor 9 (TLR9) agonist CpG ODN has been shown to inhibit M2-like TAM polarization and exert anti-tumor effects [72]. Moreover, the long-stranded noncoding RNA (lncRNA) known as miR4458HG, derived from tumor cells and encapsulated in exosomes, has been identified as an oncogenic factor that promotes the conversion of TAMs into an M2-like phenotype. This conversion is achieved through the upregulation of arginase 1 (ARG1) expression, creating a microenvironment conducive to HCC progression [73]. Also, the Nogo-B-Yap/Taz axis has been implicated in the polarization of M2-like TAMs, leading to a tumor-promoting effect and worse prognosis in HCC patients. Knockdown of Nogo-B or the use of Verteporfin, an inhibitor of Yap, has been shown to significantly inhibit the activation of the Nogo-B-Yap/Taz axis-mediated M2-like macrophages, thereby suppressing HCC cell proliferation [74]. Collectively, these pathways present promising therapeutic options for the treatment of HCC.
Cell metabolism and function
Tumors exhibit a strong correlation between cellular metabolism and various cell biological functions. The maintenance of essential cellular functions relies on the availability of energy and nutrients derived from metabolites, such as adenosine 5′-triphosphate (ATP) and various biomolecules like lipids, amino acids, and nucleotides. Numerous studies have extensively explored the impact of metabolism on the biological functions of immune cells, along with the underlying molecular mechanisms involved. Importantly, the metabolic phenotype of macrophages undergoes reprogramming, which significantly influences their biological behavior in HCC (Fig. 3). The physiological pathways associated with metabolism play a crucial role in modulating macrophage function and polarization, and will be comprehensively discussed in the subsequent sections.
Fig. 3.
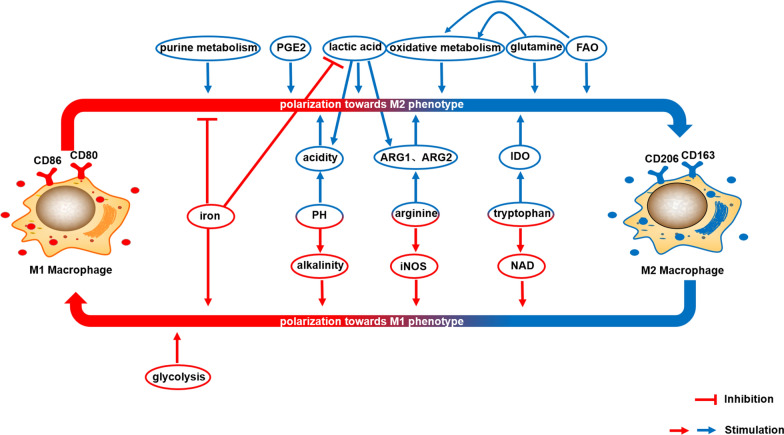
Metabolic processes play a crucial role in regulating the polarization of macrophages. The phenotype of macrophages is intricately linked to their metabolic state. Various physiological pathways involved in metabolism have the ability to influence macrophage function and polarization. Notably, glycolysis, iNOS-associated arginine metabolism, NAD-associated tryptophan metabolism, alkaline environment, and iron uptake have been identified as inducers of M1-like polarization. Conversely, FAO, PGE2 production, oxidative metabolism, lactate production, glutamine-associated metabolism, ARG-associated arginine metabolism, IDO-associated tryptophan metabolism, purine metabolism, and acidic environment have been implicated in the promotion of M2-like polarization. These metabolic pathways and environmental factors contribute to the determination of macrophage polarization and subsequent functional outcomes
Glucose metabolism
ATP serves as the primary source of cellular energy, without which cells are unable to execute their biological functions. The production of ATP primarily occurs when cells uptake extracellular glucose during the process of catabolism. Two distinct pathways, namely glycolysis and oxidative phosphorylation (OXPHOS), are responsible for the degradation of glucose and subsequent ATP generation. Glycolysis represents a common stage in which organisms undergo glucose catabolism, while OXPHOS serves as the principal mechanism for glucose oxidation, thereby providing energy for organisms [75]. The energy supply for most normal cells heavily relies on ATP derived from OXPHOS.
Upon stimulation by M1-like phenotypic activators such as LPS and IFN-γ, macrophages undergo a transformation in their cellular function accompanied by metabolic alterations. LPS induces polarization towards the M1-like phenotype by activating downstream TLR-induced pathways, leading to the stabilization of hypoxia-inducible factor 1a (HIF1α) expression in macrophages. This, in turn, enhances the activity of mechanistic target of rapamycin (mTOR), promoting glycolytic activity, augmenting the pentose phosphate pathway (PPP), and facilitating de novo synthesis of fatty acids. The significance of glycolysis in M1-like macrophages has been further substantiated by studies demonstrating that inhibitors of glycolysis, such as mTOR inhibitors, can suppress M1-like polarization. Conversely, inhibition of mitochondrial OXPHOS activity and tricarboxylic acid (TCA) cycle by suppressing succinate catabolism in macrophages does not decrease the M1-like phenotype. This suggests that M1-like macrophages do not rely on OXPHOS, but rather their metabolic mode is dependent on glycolysis [76]. Thus, the metabolic characteristics of M1-like macrophages primarily encompass enhanced glycolysis, increased synthesis of fatty acids, and inhibition of the TCA cycle [77, 78].
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a crucial enzyme in glycolysis, exhibits significantly decreased activity in M2-like macrophages compared to M1-like macrophages, indicating a reduced reliance on glycolytic metabolism in M2 macrophages [79]. Within the TME, liver cancer cells and macrophages compete for limited glucose resources. To avoid this competition, M2-like macrophages downregulate their own glucose metabolism, leading to an elevated glucose level in the TME. Consequently, cancer cells are able to utilize more glucose for energy supply, thereby promoting their growth. The diminished intracellular glucose metabolism in macrophages facilitates M2-like polarization, which subsequently contributes to tumor progression. Overall, alterations in glucose metabolism play a transformative role in shaping macrophage phenotype and function.
Lipid metabolism
Fatty acid oxidation
In addition to glucose metabolism, lipid catabolism, specifically fatty acid oxidation (FAO), plays a pivotal role in cellular metabolism. Fatty acids (FA) serve as indispensable constituents of cell membrane structure and serve as crucial sources of energy supply. They contribute to various cellular processes including cell membrane composition, homeostasis, and motility. FAO is prominently observed in metabolically active tissues such as cardiac muscle, skeletal muscle, and liver. Under oxygen-rich conditions, FAO generates a greater amount of ATP through the TCA cycle compared to glucose oxidation, thereby serving as a vital energy source for cellular development and function [12, 75, 80]. The accumulation of lipids promotes the proliferation and growth of HCC cells, exacerbating the progression of HCC. Enzymes involved in FAO exhibit tumor-inhibitory effects in HCC. Enhancing β-oxidation can diminish the availability of FA in HCC cells, thereby offering a potential therapeutic approach for HCC treatment [81].
M2-like macrophages exhibit distinct metabolic profiles in comparison to M1-like macrophages. Activation of macrophages with M2-like phenotypic activators, such as IL-4, IL-10, and IL-13, leads to a significant increase in FAO and OXPHOS rates, while glycolysis rates are reduced. These metabolic changes are mediated through the PGC1β signaling pathway, indicating a reliance on lipid metabolism by M2-like macrophages [82–84]. Conversely, FAO is diminished in the M1-like phenotype [85]. Nuclear receptors, including peroxisome proliferator-activated receptor (PPAR) and liver x receptor (LXR), play a regulatory role in lipid metabolism and consequently influence macrophage polarization [76, 86, 87]. Collectively, the metabolic characteristics of M2-like macrophages encompass decreased glycolysis, enhanced FAO, and OXPHOS [77, 78].
Furthermore, it has been demonstrated that Sirtuin 4 (SIRT4) plays a role in lipid metabolism, and the knockdown of SIRT4 leads to an upregulation of FAO genes in TAMs, including PPARδ. Knockdown of SIRT4 results in an elevation of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) protein levels in TAMs, which is essential for the polarization of M2 phenotype. The PPARδ and PPARδ-STAT3 axis actively promotes the polarization of M2-like macrophages. Conversely, inhibition of PPARδ reverses the M2-like polarization induced by SIRT4 knockdown. Additionally, SIRT4 knockdown enhances apoptosis of M1 TAMs, leading to an increase in the M2/M1 ratio. Consequently, the inhibition of SIRT4 in macrophages induces M2-like polarization, augments the M2/M1 ratio through the FAO/PPAR/STAT3 pathway, and ultimately facilitates HCC progression [88]. These findings highlight the potential of targeting fatty acid metabolism in macrophages within the HCC tumor microenvironment as a promising therapeutic strategy.
Arachidonic acid metabolism
Arachidonic acid (AA), a polyunsaturated fatty acid, serves as a structural lipid in cellular membranes and acts as a precursor for various bioactive molecules involved in the regulation of biological processes. Among these molecules, prostaglandin E2 (PGE2) plays a crucial role. PGE2 exhibits both pro-tumorigenic and immunosuppressive effects and serves as a key factor in the polarization of macrophages towards an M2-like phenotype [89].
Furthermore, PGE2 induces the upregulation of PD-L1 in macrophages, which subsequently dampens the anti-tumor response of T cells and facilitates tumor growth [90]. Within the TME of HCC, tumor cells produce PGE2, which triggers the polarization of macrophages towards an M2-like phenotype via the cyclic adenosine monophosphate (cAMP) pathway, leading to immune suppression. Conversely, inhibition of PGE2 synthesis can reverse this process and enhance anti-tumor effects [89, 91]. Therefore, targeting agents that modulate PGE2 production holds promise for activating anti-cancer immunity.
Redox metabolism
The maintenance of redox homeostasis is closely linked to the development of HCC. Notably, studies have demonstrated that the deletion of NADPH oxidase NOX4 triggers the activation of the MYC pathway, resulting in a perturbation of redox homeostasis, perturbed oxidative metabolism, and ultimately facilitating the progression of HCC [92].
As previously mentioned, the polarization of macrophages towards an M2-like phenotype is influenced by intracellular OXPHOS responses, indicating the involvement of redox processes. Suppression of OXPHOS and FAO levels leads to a decrease in M2-like macrophage markers. This can be attributed to the fact that oxidative metabolism fulfills the long-term bioenergetic demands of macrophages during M2 polarization. In contrast, M1 macrophages primarily rely on glycolytic metabolism for rapid energy production. Hence, the regulation of OXPHOS and FAO levels plays a crucial role in macrophage energy utilization, thereby governing M2 polarization [93].
Furthermore, IL-4 significantly enhances the expression of enzymes involved in mitochondrial FAO in macrophages, thereby modulating mitochondrial function and exerting anti-inflammatory effects. The impact of IL-4 on mitochondrial function was confirmed by the reduction in this effect upon the use of mitochondrial inhibitors, further validating the regulatory role of IL-4. STAT6 acts as a transcriptional regulator of the Th2 response in macrophages and coordinates macrophage metabolic programs in response to IL-4. IL-4 triggers STAT6 activation and co-regulates M2 polarization of macrophages [93]. Thus, modulation of oxidative metabolism has the potential to influence the polarization of macrophages. Diminishing the oxidative response of macrophages would result in a decline in their immunosuppressive function, while concurrently enhancing their anti-tumor immune response.
Lactate metabolism
Cancer cells exhibit a propensity for glycolytic metabolism, wherein they avidly uptake extracellular glucose to generate ATP. Notably, cancer cells exhibit a preference for aerobic glycolysis, commonly known as the Warburg Effect, even in the presence of ample oxygen. This metabolic adaptation, although inefficient in terms of ATP production [94, 95], enables cancer cells to rapidly consume glucose, leading to the accumulation of lactate. Consequently, this metabolic shift impacts immune cell functionality, resulting in the conversion of immune cells into an immunosuppressive phenotype [96].
Excessive accumulation of lactate has been identified as a suppressor of anti-tumor immunity, impeding the proliferation and secretion of cytotoxic cytokines by CTLs. Intracellular acidification resulting from heightened lactic acid consumption by NK cells and T cells has been observed to inhibit IFN-γ production [97]. Meanwhile, lactic acid has the ability to induce the expression of G protein-coupled receptor 132 (Gpr132) in macrophages [16], subsequently promoting the conversion of M1-like macrophages to M2-like macrophages. The ensuing release of trophic factors, metabolic regulators, and immunosuppressive molecules by M2-like macrophages, including vascular endothelial growth factor A (VEGFA), PGE2, IL-6, IL-10, among others, ultimately accelerates tumor progression [12, 95, 98]. Moreover, lactic acid stimulation leads to increased expression of arginase1 (ARG1) and arginase2 (ARG2) in TAMs, thereby facilitating the release of pro-tumorigenic substances and impairing the activity of other immune cells. Consequently, inhibiting Gpr132 can modulate the impact of lactate on macrophage polarization, thereby restraining the metastasis of cancer cells [99].
Furthermore, the pro-tumorigenic impact of macrophages is induced by lactic acid. Targeting the M2-like polarization of macrophages presents a potential strategy to counteract the immunosuppressive effects. Notably, in HCC, macrophages demonstrate elevated expression levels of carbonic anhydrase XII (CA12), which is associated with the survival of M2-like macrophages in an acidic milieu, thereby facilitating HCC progression [100]. Moreover, the production of lactic acid by HCC cells intensifies the acidic microenvironment and exerts significant effects on macrophages.
Amino acid metabolism
Amino acids play an important role as metabolites closely intertwined with the phenotypic and biological functions of macrophages. A comprehensive summary of amino acid metabolism and its consequential impact on macrophages will be presented.
Glutamine metabolism
Glutamine serves as a substrate for various nitrogenous compounds involved in biosynthesis, thereby generating nitrogen for the synthesis of non-essential amino acids and nucleotides. The metabolism of glutamine assumes critical importance in HCC proliferation, as the growth of HCC cells escalates the demand for glutamine and augments its catabolism. This heightened glutamine metabolism represents a significant characteristic of HCC cells [2]. Furthermore, M2 macrophages exhibit elevated levels of glutamine metabolism, glutamine transporter protein, and associated metabolic enzyme expressions [101].
In order to facilitate M2 polarization, TAMs engage in the conversion of glutamate to glutamine. Inhibition of this conversion leads to an upregulation of glycolysis in TAMs, resulting in the reprogramming of macrophages towards a pro-inflammatory M1-like phenotype. Additionally, glutamine enters the TCA cycle through OXPHOS in macrophages, thereby promoting the acquisition of an M2-like polarization phenotype. Notably, restricting glutamine availability has been shown to diminish M2-like polarization [29]. These findings indicate that targeting glutamine metabolism could serve as a potential approach to modulate macrophage phenotype and function.
Furthermore, a previous investigation has demonstrated that fructose stimulation triggers glutamine catabolism, thereby inducing LPS-mediated inflammation and the production of pro-inflammatory cytokines in macrophages [102]. This observation highlights the ability of fructose to induce pro-inflammatory M1-like polarization, thereby enhancing anti-tumor immunity through its impact on glutamine metabolism.
Arginine metabolism
Arginine, a metabolite with the ability to undergo catabolism to generate ornithine, serves as a precursor for various biologically significant molecules. For instance, ornithine plays a crucial role in the synthesis of proline and polyamines, which exert an influence on cell division processes [89]. The process of macrophage polarization can be modulated by this metabolic pathway.
Investigations have demonstrated that polyamines contribute to M2-like polarization and inhibit the expression of genes associated with M1-like polarization induced by LPS. Furthermore, the absence of a rate-limiting enzyme involved in polyamine metabolism has been linked to M1-like polarization. As discussed, lactate has been shown to enhance the activity of ARG1 and ARG2 enzymes in TAMs, leading to the catabolism of arginine and the production of compounds that promote cancer cell proliferation. However, it is important to note that both M1 and M2 macrophages utilize arginine, albeit through distinct mechanisms [103].
Moreover, M1-like macrophages employ iNOS to convert arginine into NO. iNOS also upregulates glycolysis to induce M1-like polarization, while concurrently suppressing TCA cycle and OXPHOS processes associated with an M2-like phenotype [29]. Conversely, ARG1 and ARG2 serve as key enzymes utilized by M2-like macrophages to metabolize arginine, thereby attenuating the anti-tumor effects through reduced NO production.
A separate investigation revealed that the knockdown of lncRNA cox-2 resulted in a reduction of iNOS and TNF-α levels in M1-like macrophages. This reduction in iNOS and TNF-α levels led to the suppression of the macrophages’ ability to inhibit the proliferation of HCC cells and promote apoptosis. Conversely, the knockdown of lncRNA cox-2 increased the expression of ARG1 and IL-10 in M2-like macrophages. This increase in ARG1 and IL-10 expression promoted the proliferation of HCC cells and inhibited apoptosis in M2-like macrophages, respectively [104]. These findings provide evidence that the knockdown of lncRNA cox-2 can weaken the anti-tumor immune response by modulating macrophage polarization in HCC. The underlying molecular mechanism involves the differential impact of lncRNA cox-2 on iNOS and ARG1. Consequently, altering the utilization of arginine by macrophages to specifically induce iNOS or inhibit ARG1 and ARG2 may have implications for macrophage phenotypes.
Tryptophan metabolism
The involvement of tryptophan metabolism in HCC cells is of significant importance. Specifically, the presence of tryptophan 2, 3-dioxygenase (TDO2) in HCC cells has been linked to malignant characteristics in patients with HCC. TDO2 facilitates the metabolic conversion of tryptophan, resulting in the production of kynurenine along the kynurenine (Kyn) pathway. The activation of the aryl hydrocarbon receptor (AhR) by kynurenine plays a regulatory role in the growth and invasion of HCC [105].
Moreover, indoleamine 2, 3-dioxygenase (IDO) can oxidize tryptophan in TAMs, leading to a reduction in tryptophan availability for T cells and the generation of metabolites that suppress T cell functions [89]. The restoration of T cell function through the use of IDO inhibitors further supports the close association between tryptophan and M2-like polarization [29]. These findings provide additional evidence for the contribution of tryptophan metabolism to macrophage polarization and function in HCC.
Furthermore, it has been observed that the metabolite of tryptophan, nicotinamide adenine nucleotide (NAD), has the ability to influence the levels of the inflammatory cytokine TNF-α in macrophages [106]. Obviously, an elevated NAD level is associated with an up-regulation of TNF-α, which is indicative of M1-like polarization. These findings collectively suggest that the utilization of tryptophan by macrophages holds promise for exerting anti-tumor effects. Consequently, an increase in NAD levels or a decrease in IDO activity would likely support an anti-tumor response.
Purine metabolism
Purine, a crucial metabolite involved in energy production, metabolic regulation, and various other physiological processes, undergoes conversion into uric acid for elimination. Notably, investigations have revealed a substantial activation of purine metabolism and heightened activity within the purine biosynthesis pathway in patients with HCC [107]. Furthermore, the upregulation of genes associated with the purine metabolism pathway has been observed, and this dysregulation has been linked to an unfavorable prognosis in HCC patients [108].
Purine metabolism exhibits a close association with the biological functionality of macrophages, particularly in the context of TAMs. Studies have demonstrated a direct correlation between heightened purine metabolism in TAMs and compromised efficacy of immunotherapeutic interventions, as well as the promotion of pro-tumorigenic activities. The augmented purine metabolism in TAMs is characterized by a diminished capacity for antigen presentation, resulting in the suppression of M1-like macrophages. This suppression is attributed to the downregulation of antigen presentation-related genes in macrophages with elevated purine metabolism. Remarkably, the expression levels of purine metabolism genes exhibit an inverse relationship with those of antigen-presenting genes [109].
Furthermore, the immunosuppressive molecule TREM2, expressed in macrophages, displays a positive correlation with the purine metabolism score. Macrophages expressing TREM2 exhibit reduced levels of antigen presentation-related proteins, such as MHC-II (I-A/I-E) and MHC-I (H-2D), indicative of impaired antigen presentation capabilities. These findings further underscore the negative regulatory role of purine metabolism in macrophage antigen presentation [109]. Meanwhile, heightened purine metabolism confers enhanced immunosuppressive and angiogenic capacities, thereby bolstering the functions associated with M2-like macrophages. Thus, the intricate interplay between purine metabolism and M2-like polarization emerges as a promising avenue for harnessing the anti-tumor potential.
Acid–base metabolism
The maintenance of acid–base homeostasis is of paramount importance for cellular survival, as cells necessitate an environment with optimal pH levels. As previously discussed, the presence of lactic acid contributes to the establishment of an acidic milieu that fosters tumor progression. Perturbations in pH levels exert a profound influence on the biological functionality of macrophages.
Emerging evidence highlights a close correlation between the pH value and the phenotype and function of macrophages. For instance, the administration of chloroquine (CQ) has been shown to elevate the pH level within macrophage lysosomes, thereby eliciting M1-like polarization through calcium-mediated mechanisms. Furthermore, CQ induces a metabolic shift in macrophages from OXPHOS to glycolysis [110, 111]. Moreover, the manipulation of hydrogen binding and removal within the microenvironment, facilitated by specific agents like calcium carbonate (CaCO3), promotes an alkaline milieu that favors M1-like polarization [112].
In addition, TAMs have the ability to adapt and survive within the acidic TME of HCC through the production of vacuolar-type ATPase (V-ATPase). Notably, the inhibition of V-ATPase has been demonstrated to induce a shift in macrophage polarization from M2-like to M1-like, accompanied by an upregulation of pro-inflammatory cytokine expression, such as TNF-α [113]. These findings collectively underscore the impact of acid–base balance on macrophage polarization. M1-like macrophages exhibit a preference for an alkaline milieu, while M2-like phenotypes display an affinity for acidic conditions. Consequently, an elevation in pH levels can prompt macrophages to transition towards an M1-like phenotype, thereby fostering an environment conducive to anti-tumor immune responses.
Iron metabolism
Inorganic elements play a crucial role in maintaining cellular functions, with iron (Fe) being intricately linked to macrophage polarization. The distinct iron utilization patterns of M1 and M2 macrophages contribute to their respective functions. M2 macrophages are involved in tissue healing and employ scavenger receptors like CD163 to uptake and process heme, thereby generating iron and producing anti-inflammatory factors. Conversely, iron release supports tumor progression by promoting tissue repair, cellular proliferation, and immune modulation. In contrast, M1-type macrophages primarily uptake and store iron, thereby attenuating the immunosuppressive effects associated with iron release [103, 114].
As mentioned, HCC cells generate lactate, which induces M2-like polarization. However, the introduction of ferric citrate has been shown to suppress lactate-mediated M2-like polarization [115]. Therefore, increased iron uptake by macrophages facilitates M1-like polarization, thereby enhancing anti-tumor immunity.
Treatment prospects in targeting macrophage metabolism
Numerous strategies have been explored to effectively target TAMs in HCC. One promising immunotherapeutic approach involves the augmentation of M1-like polarization, which holds potential for the treatment of HCC (Fig. 1). Additionally, the modulation of metabolic molecules and associated enzymes represents another avenue to regulate the pro-inflammatory and anti-inflammatory functions of macrophages, thereby facilitating an anti-tumor response. Subsequently, we will delve into therapeutic interventions that target macrophage metabolism pathways, providing a comprehensive analysis of their potential implications in HCC treatment.
ATO combined with CTS therapy for HCC
Arsenic trioxide (ATO) has emerged as a primary therapeutic option for acute promyelocytic leukemia (APL). Meanwhile, Cryptotanshinone (CTS), an extract derived from the traditional Chinese medicine Salvia miltiorrhiza, has gained attention for its potential in HCC treatment. Notably, the combination of ATO and CTS (ACCS) has been employed in HCC therapy. In a recent study, it was demonstrated that ACCS administration led to an elevation in AMPK phosphorylation, thereby activating the AMPK pathway. This activation, in turn, induced glycolysis in macrophages and promoted M1-like macrophage polarization. Furthermore, ACCS exhibited the ability to suppress glycolysis in HCC cells by inhibiting the NF-κB/HIF-1α pathway [116] (Fig. 4A). Consequently, ACCS represents a promising therapeutic approach for HCC, as it effectively targets both macrophages and cancer cells.
Fig. 4.
The mechanisms of therapeutics for HCC through regulating macrophage metabolism. A ACCS modulates glycolysis metabolism, leading to the induction of M1-like polarization in macrophages. This metabolic shift not only suppresses HCC cell survival but also exerts potent anti-tumor effects. B The combination of decitabine and etomoxir acts on macrophages by regulating FAO and amino acid metabolism, thereby promoting M1-like polarization. This synergistic effect contributes to the therapeutic potential against HCC. C Celecoxib, through the inhibition of COX-2-related PGE2 production, facilitates the promotion of M1-like polarization in macrophages
Decitabine combined with etomoxir therapy for HCC
Receptor-interacting protein kinase 3 (RIPK3) is a serine/threonine kinase that plays a crucial role in activating immunological responses and facilitating necrosis [117]. RIPK3 expression was found to be down-regulated in TAMs within HCC. This down-regulation of RIPK3 was observed to stimulate the PPAR pathway, thereby inducing fatty acid metabolism through the suppression of the ROS-Caspase-1 pathway. The latter pathway is known to be associated with the immunological function of TAMs and inhibits HCC progression [118, 119].
In Phase II clinical trials (NCT02264873), decitabine, a therapeutic agent, has been demonstrated to effectively inhibit myelodysplastic syndrome, liver metastasis, and tumor cell proliferation. Decitabine treatment was found to enhance RIPK3 expression in TAMs while suppressing PPAR expression. This suppression of PPAR prevented FAO by reducing the levels of carnitine palmitoyltransferase 1 A (CPT1A) and CPT1B, which are rate-limiting enzymes in fatty acid metabolism. Furthermore, decitabine treatment resulted in decreased expression of ARG1, a marker associated with M2-like TAMs, and increased expression of iNOS, a marker associated with M1-like TAMs. These findings confirmed that decitabine treatment prevented M2 polarization of TAMs and promoted M1 polarization through the modulation of RIPK3. Consequently, decitabine effectively reversed the pro-tumoral effects induced by RIPK3 deficiency in HCC [118, 119].
Furthermore, it has been demonstrated that the inhibition of CPT1 by etomoxir resulted in a decrease in ARG1 expression. This decrease in ARG1 expression subsequently interfered with the expression of PPARA and PPARG, thereby impacting FAO metabolism. In parallel, etomoxir treatment upregulated the expression of iNOS in TAMs, ultimately facilitating an anti-tumor effect. Consequently, the combination therapy of decitabine with etomoxir holds promise in controlling fatty acid metabolism, leading to the inflammatory polarization of TAMs, as depicted in Fig. 4B [118, 119].
Celecoxib regulates macrophage metabolism and function in tumors
The abundant expression of cyclooxygenase-2 (COX-2) in various cancers, including HCC, is closely associated with inflammation, which plays a crucial role in the initiation and progression of HCC and is indicative of a poor prognosis in patients. COX-2 also facilitates the production of PGE2, thereby exerting pro-tumoral effects. Consequently, the potential anti-cancer function of COX-2 knockdown has been identified [120, 121].
Furthermore, the elevated expression of COX-2 in HCC cells has been experimentally confirmed to induce M2-like polarization, leading to the suppression of T cell cytotoxicity. The administration of celecoxib, a COX-2 inhibitor, effectively inhibits PGE2 formation [122], consequently reducing the presence of M2-like TAMs as depicted in Fig. 4C. Simultaneously, celecoxib enhances the anti-tumor function by augmenting T cell cytotoxicity [123]. Therefore, the targeting of COX-2/PGE2-based metabolic molecules holds promise in improving immunotherapy outcomes for HCC.
Other therapies for regulating macrophage metabolism and function in tumor
The additional studies that have explored the use of macrophage metabolism-targeting drugs for tumor therapy are summarized in Table 1.
Table 1.
Drugs that target metabolism in macrophages
| Drug | Mechanism | References |
|---|---|---|
| Glucose metabolism | ||
| Insulin | Promoting glycolysis in monocyte-phagocytes to enhance phagocytosis on tumor cells | [124] |
| β-Glucan | Promoting glycolysis in macrophages and thereby inducing M1-like polarization | [125] |
| Fucoidan | Inhibiting the oxidation reaction of macrophages, promoting glycolysis, inducing M1 polarization, and playing an anti-tumor effect | [126] |
| Ibrutinib | Inhibiting glycolysis in monocyte-phagocyte and weakening its phagocytosis on tumor cells | [124] |
| Lipid metabolism | ||
| Simvastatin | Consuming lipids and transforming M2-like macrophages to M1-like | [127] |
| Rapamycin + Hydroxychloroquine | Disrupting FAO in macrophages to inhibit M2-like polarization | [128] |
| Perhexiline | Inhibiting oxidative phosphorylation and fatty acid metabolism to promote M1-like polarization | [129] |
| Metformin | Inhibiting FAO to induce macrophage polarization to M1 phenotype, suppressing anti-inflammatory macrophage infiltration through decreasing COX2 and PGE2 | [29, 130] |
| Indomethacin | Inhibiting COX and PGE2, inducing anti-tumor effect by macrophages | [131] |
| Isoliquiritigenin | Inhibition of PGE2 production and reduction of M2-like polarization | [132] |
| Salvia miltiorrhiza Bunge aqueous extract | Inhibiting COX-2, reducing PGE2 production, decreasing tumor-promoting macrophage infiltration, and mediating anti-tumor immune responses | [133] |
| Fe-5,5′-azosalicylic acid nanoscale coordination polymer nanomedicines | Producing 5-aminosalicylic acid to reduce COX-2 and PGE2 expression, conversely, generating Fe3 + to induce M1-like polarization | [134] |
| 5-Aminolevulinic Acid | Inhibiting COX-2 and PGE2 expressions, suppressing tumor by macrophages | [135] |
| Lactic acid metabolism | ||
| 3-Bromopyruvate | Inhibiting tumor-promoting macrophages by decreasing lactate production | [136] |
| Albiziabioside A + Dichloroacetate acid | Inhibiting lactate accumulation to reduce M2 macrophages and reprogram anti-tumor microenvironment | [137] |
| Dual PI3Kδ/γ Inhibitor RP6530 | Reducing lactate, inducing M1-like polarization and inhibiting tumor progression | [138] |
| Amino acid metabolism | ||
| 6-Diazo-5-oxo-l-norleucine | Inhibiting glutamine metabolism, suppressing IDO expression, and inducing pro-inflammatory macrophages | [139] |
| 6-Gingerol | Inhibiting ARG expression, promoting iNOS and NO expression, enhancing M1-like polarization, and exerting anti-tumor effect | [140] |
| Triptolide | Reducing ARG1 expression and decreasing M2-like polarization | [141] |
| 1,3-Diaryl-pyrazin-6-one-5-carboxamides | Inhibiting IDO level and reducing immunosuppressive macrophage infiltration | [142] |
| Sulfasalazine | Inhibiting cystine-glutamate exchange (xCT) and thereby inducing M2-like polarization | [143] |
| Acid–base metabolism | ||
| Anti‐V‐ATPase‐V0a2 antibody | Inhibiting proton pump activity to induce M1-like macrophage | [144] |
| Pantoprazole | Inhibiting proton pump to induce M1-like polarization and activating anti-tumor immunity | [145] |
| Iron metabolism | ||
| Iron oxide nanoparticles | Iron absorbed by macrophages and thereby replenished to promote M1-like polarization | [146] |
| Iron chelated melanin-like nanoparticles | Iron supplemented by macrophages to induce M1-like polarization | [147] |
| Intracellular iron chelator (TC3-S)2 | Transforming macrophage to iron-absorbing M1-like phenotype to play anti-tumor effects | [148] |
Conclusion and perspective
It is well-established that cellular metabolism plays a crucial role in determining cell function and the TME in HCC. The TME, in turn, can influence the metabolism and polarization of macrophages, thereby impacting their phenotype and function. Notably, these altered macrophages play a pivotal role in promoting the carcinogenesis and progression of HCC, as outlined in Table 2. Consequently, targeting M2-like TAMs and their associated metabolism holds significant potential in enhancing the efficacy of anti-tumor therapy, with several studies confirming its clinical value. Therefore, it is imperative to continue investigating the intricate relationship between metabolism and the resulting alterations in the phenotype and function of TAMs. Such investigations may pave the way for the development of novel strategies aimed at promoting anti-tumor immunity in HCC.
Table 2.
The relationship between macrophage and HCC involved pathway and clinical outcome
| Cell | Pathway | Clinical outcome | References |
|---|---|---|---|
| The effect of HCC cells on macrophages | |||
| HCC cells | FOXO1/ IRF1/ NO axis | Reprogramming macrophages and inhibiting HCC progression | [35] |
| HCC cells | PKC/ZFP64/CSF1 axis | Inducing M2-like polarization to mediate immune suppression and resistance to anti-PD1 therapy | [42] |
| HCC cells | MiR4458HG/ARG1 axis | Promoting M2-like polarization and creating an environment conducive to HCC cells | [73] |
| HCC cells | PGE2/PD-L1; PGE2/cAMP pathway | Reducing anti-tumor response of T cells, promoting M2-like polarization and inducing HCC growth | [89–91] |
| HCC cells | Lactic acid/Gpr132; Lactic acid/ARG1, ARG2 axis | Transforming M1-like macrophages into M2-like; Promoting the release of tumor substances, damaging the activity of other immune cells, and enhancing the metastasis of cancer cells | [12, 95, 98, 99] |
| The effect of macrophages on HCC | |||
| Macrophages | TREM1/PI3K/AKT/mTOR pathway down-regulation | Transforming M2-like macrophages into M1-like, preventing HCC cell invasion and metastasis | [43] |
| Macrophages | TNFR1/NF- κ B/p38/MAPK pathway | Reducing immunosuppressive effect of TAMs, promoting T cell related cytotoxicity, and inducing HCC cell apoptosis | [71] |
| Macrophage | Wnt2b/β/C-Myc pathway | Promoting the conversion of M2-like TAMs and enhancing HCC progression | [72] |
| Macrophage | Nogo-B-Yap/Taz axis | Promoting M2-like TAMs polarization and HCC cells proliferation | [74] |
| Macrophage | LPS/HIF1 α/mTOR axis | Promoting glycolytic activity, leading to M1-like phenotype polarization, and inhibiting tumor progression | [76] |
| Macrophage | SIRT4/FAO/PPAR/STAT3 pathway | Promoting M2-like polarization and HCC progression | [88] |
| Macrophage | Glutamic acid/glutamine/OXPHOS pathway | Promoting M2-like polarization and tumor progression | [29] |
| Macrophage | Arginine/iNOS/NO, glycolysis pathway | Promoting M1-like polarization and inhibiting tumor progression | [29] |
| Macrophage | LncRNA cox-2/iNOS, TNF- α axis | Inhibiting HCC cell proliferation and promoting cell apoptosis | [104] |
| Macrophage | Tryptophan/IDO pathway | Reducing tryptophan used by T cells, producing metabolites to inhibit T cell function, and inhibiting anti-tumor immunity | [89] |
Acknowledgements
Not applicable.
Abbreviations
- ACCS
ATO Joint CTS
- AhR
Aryl hydrocarbon receptor
- APL
Acute promyelocytic leukemia
- ARG1
Arginase 1
- ARG2
Arginase 2
- ATO
Arsenic trioxide
- ATP
Adenosine 5′-triphosphate
- CA12
Carbonic anhydrase XII
- cAMP
Cyclic adenosine monophosphate
- CD163
Scavenger receptor cysteine-rich type 1 protein M130
- CD206
Mannose receptor C-type 1
- CD244
Natural killer cell receptor 2B4
- CD48
Signaling lymphocytic activation molecule 2
- CD80
T-lymphocyte activation antigen CD80
- CD86
T-lymphocyte activation antigen CD86
- CKI
Cmpound kushen injection
- c-Myc
MYC proto-oncogene
- BHLH
Transcription Factor
- COX-2
Cyclooxygenase-2
- CPT1A
Carnitine palmitoyltransferase 1 A
- CQ
Chloroquine
- CSF1
Colony-stimulating factor 1
- CTLs
Cytotoxic T lymphocytes
- CTS
Cryptotanshinone
- FA
Fatty acids
- FAO
Fatty acid oxidation
- FOXO1
Forkhead box protein O1
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- ICIs
Immune checkpoint inhibitors
- IDO
Indoleamine 2,3 dioxygenase
- IFN-γ
Interferon-γ
- IL-10
Interleukin 10
- IL-12
Interleukin 12
- IL-13
Interleukin 13
- IL-4
Interleukin 4
- iNOS
Inducible nitric oxide synthase
- IRF1
Interferon regulatory factor 1
- Kyn
Kynurenine
- LPS
Lipopolysaccharide
- LXR
Liver x receptor
- MAPK
Mitogen-activated protein kinase
- mTOR
Mechanistic target of rapamycin
- NAD
Nicotinamide adenine nucleotide
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NF-κB
Nuclear factor-κB
- NK cell
Natural killer cell
- NO
Nitric oxide
- Nogo-B
Reticulon 4B
- OXPHOS
Oxidative phosphorylation
- p38
Tumor necrosis factor receptor superfamily member 1
- PD-1
Programmed cell death 1
- PD-L1
Programmed death ligand 1
- PGE2
Prostaglandin E2
- PKCα
Protein kinase C alpha
- PPAR
Proliferator-activated receptor
- PPP
Pentose phosphate pathway
- PS
Phosphatidylserine
- p-STAT3
Phosphorylated signal transducer and activator of transcription 3
- RIPK3
Receptor-interacting protein kinase 3
- ROS
Reactive oxygen species
- SIRT4
Sirtuin 4
- SPON2
Spondin 2
- TAMs
Tumor-associated macrophages
- Taz
Tafazzin
- TCA cycle
Tricarboxylic acid cycle
- TDO2
Tryptophan 2,3-dioxygenase
- TGF-β
Transforming growth factor beta
- TKIs
Tyrosine kinase inhibitors
- TLR
Toll-like receptor
- TLR9
Toll-like receptor 9
- TME
Tumor microenvironment
- TNFR1
Tumor necrosis factor receptor superfamily member 1
- Treg
Regulatory T cells
- TREM1
Triggering receptor expressed on myeloid cells 1
- VEGFA
Vascular endothelial growth factor A
- Wnt2b
Wnt family member 2B
- Yap
Yes-associated protein
- ZFP64
Zinc finger protein 64
Author contributions
JH, QW and YY discussed the viewpoints and wrote this manuscript. DAG revised the manuscript. All authors have read and agreed to approve the final manuscript.
Funding
This work was supported by The National Natural Science Foundation of China (82360526, Y.Y.), The Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation (2023GXNSFDA026011, Y.Y.), NIH P30DK120531–01 (D.A.G.) and The Victor and Anna Mae Beghini Charitable Foundation (D.A.G).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Competing interests
The author declares no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David A. Geller, Email: gellerda@upmc.edu
Yihe Yan, Email: yanyihe12@sr.gxmu.edu.cn.
References
- 1.Xia H, Huang Z, Wang Z, Liu S, Zhao X, You J, Xu Y, Yam JWP, Cui Y. Glucometabolic reprogramming: From trigger to therapeutic target in hepatocellular carcinoma. Front Oncol. 2022;12:953668. doi: 10.3389/fonc.2022.953668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, Hao H, Xiong J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B. 2022;12:558–580. doi: 10.1016/j.apsb.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5.Dyhl-Polk A, Mikkelsen MK, Ladekarl M, Nielsen DL. Clinical trials of immune checkpoint inhibitors in hepatocellular carcinoma. J Clin Med. 2021;10:2662. doi: 10.3390/jcm10122662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, Baron A, Park J-W, Han G, Jassem J, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu AX, Park JO, Ryoo B-Y, Yen C-J, Poon R, Pastorelli D, Blanc J-F, Chung HC, Baron AD, Pfiffer TEF, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 10.Personeni N, Pressiani T, Bozzarelli S, Rimassa L. Targeted agents for second-line treatment of advanced hepatocellular carcinoma. World J Gastrointest Oncol. 2019;11:788–803. doi: 10.4251/wjgo.v11.i10.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel A, Bathon M, Saborowski A. Advances in systemic therapy for the first-line treatment of unresectable HCC. Expert Rev Anticancer Ther. 2021;21:621–628. doi: 10.1080/14737140.2021.1882855. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Li Y. Cancer metabolism and intervention therapy. Mol Biomed. 2021;2:5. doi: 10.1186/s43556-020-00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren M, Zheng X, Gao H, Jiang A, Yao Y, He W. Nanomedicines targeting metabolism in the tumor microenvironment. Front Bioeng Biotechnol. 2022;10:943906. doi: 10.3389/fbioe.2022.943906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, He Q, Li C, Alsharafi BLM, Deng L, Long Z, Gan Y. Focus on the tumor microenvironment: a seedbed for neuroendocrine prostate cancer. Front Cell Dev Biol. 2022;10:955669. doi: 10.3389/fcell.2022.955669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wozniakova M, Skarda J, Raska M. The role of tumor microenvironment and immune response in colorectal cancer development and prognosis. Pathol Oncol Res. 2022;28:1610502. doi: 10.3389/pore.2022.1610502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, Siegwart DJ, Wan Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci USA. 2017;114:580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitale I, Manic G, Galassi C, Galluzzi L. Stress responses in stromal cells and tumor homeostasis. Pharmacol Ther. 2019;200:55–68. doi: 10.1016/j.pharmthera.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Alvarez A, Hernando J, Carmona-Alonso A, Capdevila J. What is the status of immunotherapy in thyroid neoplasms? Front Endocrinol. 2022;13:929091. doi: 10.3389/fendo.2022.929091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Yu H, Dai X, Zhang X. CMTM6 and CMTM4 as two novel regulators of PD-L1 modulate the tumor microenvironment. Front Immunol. 2022;13:971428. doi: 10.3389/fimmu.2022.971428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo M, Nastasi C. Targeting the tumor microenvironment: a close up of tumor-associated macrophages and neutrophils. Front Oncol. 2022;12:871513. doi: 10.3389/fonc.2022.871513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Liu G, Li Y, Pan Y. Metabolic reprogramming induces macrophage polarization in the tumor microenvironment. Front Immunol. 2022;13:840029. doi: 10.3389/fimmu.2022.840029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Z, Zhang S. Tumor-associated macrophages and their functional transformation in the hypoxic tumor microenvironment. Front Immunol. 2021;12:741305. doi: 10.3389/fimmu.2021.741305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasan MN, Capuk O, Patel SM, Sun D. The role of metabolic plasticity of tumor-associated macrophages in shaping the tumor microenvironment immunity. Cancers. 2022;14:3331. doi: 10.3390/cancers14143331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marelli G, Morina N, Portale F, Pandini M, Iovino M, Di Conza G, Ho PC, Di Mitri D. Lipid-loaded macrophages as new therapeutic target in cancer. J Immunother Cancer. 2022;10:e004584. doi: 10.1136/jitc-2022-004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabold K, Netea MG, Adema GJ, Netea-Maier RT. Cellular metabolism of tumor-associated macrophages—functional impact and consequences. FEBS Lett. 2017;591:3022–3041. doi: 10.1002/1873-3468.12771. [DOI] [PubMed] [Google Scholar]
- 30.Geiss C, Salas E, Guevara-Coto J, Regnier-Vigouroux A, Mora-Rodriguez RA. Multistability in macrophage activation pathways and metabolic implications. Cells. 2022;11:404. doi: 10.3390/cells11030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Hou J, Liu J, Bhushan S, Wu G. The origins of resident macrophages in mammary gland influence the tumorigenesis of breast cancer. Int Immunopharmacol. 2022;110:109047. doi: 10.1016/j.intimp.2022.109047. [DOI] [PubMed] [Google Scholar]
- 32.Radmark O. Formation of eicosanoids and other oxylipins in human macrophages. Biochem Pharmacol. 2022;204:115210. doi: 10.1016/j.bcp.2022.115210. [DOI] [PubMed] [Google Scholar]
- 33.Yu Z, Li Y, Li Y, Zhang J, Li M, Ji L, Tang Y, Zheng Y, Sheng J, Han Q, et al. Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma. J Immunother Cancer. 2022;10:e004297. doi: 10.1136/jitc-2021-004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang YL, Li Q, Yang XM, Fang F, Li J, Wang YH, Yang Q, Zhu L, Nie HZ, Zhang XL, et al. SPON2 promotes m1-like macrophage recruitment and inhibits hepatocellular carcinoma metastasis by distinct integrin-rho GTPase-Hippo pathways. Cancer Res. 2018;78:2305–2317. doi: 10.1158/0008-5472.CAN-17-2867. [DOI] [PubMed] [Google Scholar]
- 35.Cui X, Zhao H, Wei S, Du Q, Dong K, Yan Y, Geller DA. Hepatocellular carcinoma-derived FOXO1 inhibits tumor progression by suppressing IL-6 secretion from macrophages. Neoplasia. 2023;40:100900. doi: 10.1016/j.neo.2023.100900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Li S, Zhang H, Zhu J, Che T, Yan B, Li J, Liu C. The effect of macrophage polarization on the expression of the oxytocin signalling system in enteric neurons. J Neuroinflammation. 2021;18:261. doi: 10.1186/s12974-021-02313-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuntzel T, Bagnard D. Manipulating macrophage/microglia polarization to treat glioblastoma or multiple sclerosis. Pharmaceutics. 2022;14:344. doi: 10.3390/pharmaceutics14020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao J, Liang Y, Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. 2022;13:888713. doi: 10.3389/fimmu.2022.888713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow SK, Wong CH, Cui C, Li MM, Wong RMY, Cheung WH. Modulating macrophage polarization for the enhancement of fracture healing, a systematic review. J Orthop Transl. 2022;36:83–90. doi: 10.1016/j.jot.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Sadoun H. Macrophage phenotypes in normal and diabetic wound healing and therapeutic interventions. Cells. 2022;11:2430. doi: 10.3390/cells11152430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei CY, Zhu MX, Zhang PF, Huang XY, Wan JK, Yao XZ, Hu ZT, Chai XQ, Peng R, Yang X, et al. PKCalpha/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J Hepatol. 2022;77:163–176. doi: 10.1016/j.jhep.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Guo Y, Xu J, Du Q, Yan Y, Geller DA. IRF2 regulates cellular survival and Lenvatinib-sensitivity of hepatocellular carcinoma (HCC) through regulating beta-catenin. Transl Oncol. 2021;14:101059. doi: 10.1016/j.tranon.2021.101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M, Lai R, Lin X, Chen W, Wu H, Zheng Q. Downregulation of triggering receptor expressed on myeloid cells 1 inhibits invasion and migration of liver cancer cells by mediating macrophage polarization. Oncol Rep. 2021;45:37. doi: 10.3892/or.2021.7988. [DOI] [PubMed] [Google Scholar]
- 44.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan Y, Zhao L, Yang YG, Liu W. The role of osteopontin in tumor progression through tumor-associated macrophages. Front Oncol. 2022;12:953283. doi: 10.3389/fonc.2022.953283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franzen AS, Raftery MJ, Pecher G. Implications for immunotherapy of breast cancer by understanding the microenvironment of a solid tumor. Cancers. 2022;14:3178. doi: 10.3390/cancers14133178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassetta L, Kitamura T. Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology. 2018;155:285–293. doi: 10.1111/imm.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh SA, Li MO. TGF-beta: guardian of T cell function. J Immunol. 2013;191:3973–3979. doi: 10.4049/jimmunol.1301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng L, He K, Pan Y, Wang H, Luo Y, Xia Q. The role of tumor-associated macrophages in primary hepatocellular carcinoma and its related targeting therapy. Int J Med Sci. 2021;18:2109–2116. doi: 10.7150/ijms.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schweer D, McAtee A, Neupane K, Richards C, Ueland F, Kolesar J. Tumor-associated macrophages and ovarian cancer: implications for therapy. Cancers. 2022;14:2220. doi: 10.3390/cancers14092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sajid M, Liu L, Sun C. The dynamic role of NK cells in liver cancers: role in HCC and HBV associated HCC and Its therapeutic implications. Front Immunol. 2022;13:887186. doi: 10.3389/fimmu.2022.887186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tosello-Trampont A, Surette FA, Ewald SE, Hahn YS. Immunoregulatory role of NK cells in tissue inflammation and regeneration. Front Immunol. 2017;8:301. doi: 10.3389/fimmu.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41:815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–1308.e1236. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Xu Y, Sun Q, Zhou X, Ma W, Wu J, Zhuang J, Sun C. New insights from the single-cell level: tumor associated macrophages heterogeneity and personalized therapy. Biomed Pharmacother. 2022;153:113343. doi: 10.1016/j.biopha.2022.113343. [DOI] [PubMed] [Google Scholar]
- 60.Tian L, Lei A, Tan T, Zhu M, Zhang L, Mou H, Zhang J. Macrophage-based combination therapies as a new strategy for cancer immunotherapy. Kidney Dis. 2022;8:26–43. doi: 10.1159/000518664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Habanjar O, Diab-Assaf M, Caldefie-Chezet F, Delort L. The impact of obesity, adipose tissue, and tumor microenvironment on macrophage polarization and metastasis. Biology. 2022;11:339. doi: 10.3390/biology11020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He Y, de Araujo Junior RF, Cruz LJ, Eich C. Functionalized nanoparticles targeting tumor-associated macrophages as cancer therapy. Pharmaceutics. 2021;13:1670. doi: 10.3390/pharmaceutics13101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995. doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kondoh N, Mizuno-Kamiya M, Umemura N, Takayama E, Kawaki H, Mitsudo K, Muramatsu Y, Sumitomo S. Immunomodulatory aspects in the progression and treatment of oral malignancy. Jpn Dent Sci Rev. 2019;55:113–120. doi: 10.1016/j.jdsr.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta (BBA) Rev Cancer. 2020;1874:188434. doi: 10.1016/j.bbcan.2020.188434. [DOI] [PubMed] [Google Scholar]
- 67.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221. doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng X, Li L, Thorpe PE, Yopp AC, Brekken RA, Huang X. Antibody-mediated blockade of phosphatidylserine enhances the antitumor effect of sorafenib in hepatocellular carcinomas xenografts. Ann Surg Oncol. 2016;23:583–591. doi: 10.1245/s10434-016-5107-5. [DOI] [PubMed] [Google Scholar]
- 69.Chen R, Li Q, Xu S, Ye C, Tian T, Jiang Q, Shan J, Ruan J. Modulation of the tumour microenvironment in hepatocellular carcinoma by tyrosine kinase inhibitors: from modulation to combination therapy targeting the microenvironment. Cancer Cell Int. 2022;22:73. doi: 10.1186/s12935-021-02435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ou DL, Chen CW, Hsu CL, Chung CH, Feng ZR, Lee BS, Cheng AL, Yang MH, Hsu C. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J Immunother Cancer. 2021;9:e001657. doi: 10.1136/jitc-2020-001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, Li J, Gao Z, Qiu L, You R, et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8:e000317. doi: 10.1136/jitc-2019-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang Y, Han Q, Zhao H, Zhang J. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/beta-catenin/c-Myc signaling and reprogramming glycolysis. J Exp Clin Cancer Res. 2021;40:13. doi: 10.1186/s13046-020-01808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye Y, Wang M, Wang G, Mai Z, Zhou B, Han Y, Zhuang J, Xia W. lncRNA miR4458HG modulates hepatocellular carcinoma progression by activating m6A-dependent glycolysis and promoting the polarization of tumor-associated macrophages. Cell Mol Life Sci. 2023;80:99. doi: 10.1007/s00018-023-04741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao X, Wang X, You Y, Wen D, Feng Z, Zhou Y, Que K, Gong J, Liu Z. Nogo-B fosters HCC progression by enhancing Yap/Taz-mediated tumor-associated macrophages M2 polarization. Exp Cell Res. 2020;391:111979. doi: 10.1016/j.yexcr.2020.111979. [DOI] [PubMed] [Google Scholar]
- 75.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho PC, Liu PS. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J Immunother Cancer. 2016;4:4. doi: 10.1186/s40425-016-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mills EL, O'Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol. 2016;46:13–21. doi: 10.1002/eji.201445427. [DOI] [PubMed] [Google Scholar]
- 78.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller A, Nagy C, Knapp B, Laengle J, Ponweiser E, Groeger M, Starkl P, Bergmann M, Wagner O, Haschemi A. Exploring metabolic configurations of single cells within complex tissue microenvironments. Cell Metab. 2017;26:788–800.e786. doi: 10.1016/j.cmet.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Liu X, Du H, Sun Y, Shao L. Role of abnormal energy metabolism in the progression of chronic kidney disease and drug intervention. Ren Fail. 2022;44:790–805. doi: 10.1080/0886022X.2022.2072743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma APY, Yeung CLS, Tey SK, Mao X, Wong SWK, Ng TH, Ko FCF, Kwong EML, Tang AHN, Ng IO, et al. Suppression of ACADM-mediated fatty acid oxidation promotes hepatocellular carcinoma via aberrant CAV1/SREBP1 signaling. Cancer Res. 2021;81:3679–3692. doi: 10.1158/0008-5472.CAN-20-3944. [DOI] [PubMed] [Google Scholar]
- 82.Tian F, Chen H, Zhang J, He W. Reprogramming metabolism of macrophages as a target for kidney dysfunction treatment in autoimmune diseases. Int J Mol Sci. 2022;23:8024. doi: 10.3390/ijms23148024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das Gupta K, Shakespear MR, Curson JEB, Murthy AMV, Iyer A, Hodson MP, Ramnath D, Tillu VA, von Pein JB, Reid RC, et al. Class IIa histone deacetylases drive toll-like receptor-inducible glycolysis and macrophage inflammatory responses via pyruvate kinase M2. Cell Rep. 2020;30:2712–2728.e2718. doi: 10.1016/j.celrep.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Eshghjoo S, Kim DM, Jayaraman A, Sun Y, Alaniz RC. Macrophage polarization in atherosclerosis. Genes. 2022;501:142–146. doi: 10.3390/genes13050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1alpha-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediators Inflamm. 2017;2017:9029327. doi: 10.1155/2017/9029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun JX, Xu XH, Jin L. Effects of metabolism on macrophage polarization under different disease backgrounds. Front Immunol. 2022;13:880286. doi: 10.3389/fimmu.2022.880286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanjay, Park M, Lee HJ. Roles of fatty acids in microglial polarization: evidence from in vitro and in vivo studies on neurodegenerative diseases. Int J Mol Sci. 2022;23:7300. doi: 10.3390/ijms23137300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z, Li H, Zhao ZB, Zhu W, Feng PP, Zhu XW, Gong JP. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARdelta signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res. 2019;38:469. doi: 10.1186/s13046-019-1456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mazzone M, Menga A, Castegna A. Metabolism and TAM functions-it takes two to tango. FEBS J. 2018;285:700–716. doi: 10.1111/febs.14295. [DOI] [PubMed] [Google Scholar]
- 90.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci USA. 2017;114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Penuelas-Haro I, Espinosa-Sotelo R, Crosas-Molist E, Herranz-Iturbide M, Caballero-Diaz D, Alay A, Sole X, Ramos E, Serrano T, Martinez-Chantar ML, et al. The NADPH oxidase NOX4 regulates redox and metabolic homeostasis preventing HCC progression. Hepatology. 2023;78:416–433. doi: 10.1002/hep.32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bose S, Le A. Glucose metabolism in cancer. Adv Exp Med Biol. 2018;1063:3–12. doi: 10.1007/978-3-319-77736-8_1. [DOI] [PubMed] [Google Scholar]
- 96.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 97.Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, Wang Y. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. 2020;21:8363. doi: 10.3390/ijms21218363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohashi T, Aoki M, Tomita H, Akazawa T, Sato K, Kuze B, Mizuta K, Hara A, Nagaoka H, Inoue N, Ito Y. M2-like macrophage polarization in high lactic acid-producing head and neck cancer. Cancer Sci. 2017;108:1128–1134. doi: 10.1111/cas.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ning WR, Jiang D, Liu XC, Huang YF, Peng ZP, Jiang ZZ, Kang T, Zhuang SM, Wu Y, Zheng L. Carbonic anhydrase XII mediates the survival and prometastatic functions of macrophages in human hepatocellular carcinoma. J Clin Invest. 2022;132:e153110. doi: 10.1172/JCI153110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi J, Stradmann-Bellinghausen B, Yakubov E, Savaskan NE, Regnier-Vigouroux A. Glioblastoma cells induce differential glutamatergic gene expressions in human tumor-associated microglia/macrophages and monocyte-derived macrophages. Cancer Biol Ther. 2015;16:1205–1213. doi: 10.1080/15384047.2015.1056406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones N, Blagih J, Zani F, Rees A, Hill DG, Jenkins BJ, Bull CJ, Moreira D, Bantan AIM, Cronin JG, et al. Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation. Nat Commun. 2021;12:1209. doi: 10.1038/s41467-021-21461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 104.Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, Luo X, Chen R, Chen T. Long non-coding RNA cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J Cell Biochem. 2018;119:2951–2963. doi: 10.1002/jcb.26509. [DOI] [PubMed] [Google Scholar]
- 105.Li L, Wang T, Li S, Chen Z, Wu J, Cao W, Wo Q, Qin X, Xu J. TDO2 promotes the EMT of hepatocellular carcinoma through Kyn-AhR pathway. Front Oncol. 2020;10:562823. doi: 10.3389/fonc.2020.562823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Gool F, Galli M, Gueydan C, Kruys V, Prevot PP, Bedalov A, Mostoslavsky R, Alt FW, De Smedt T, Leo O. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206–210. doi: 10.1038/nm.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang S, Zhang B, Tan W, Qi L, Ma X, Wang X. A novel purine and uric metabolism signature predicting the prognosis of hepatocellular carcinoma. Front Genet. 2022;13:942267. doi: 10.3389/fgene.2022.942267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Su WJ, Lu PZ, Wu Y, Kalpana K, Yang CK, Lu GD. Identification of key genes in purine metabolism as prognostic biomarker for hepatocellular carcinoma. Front Oncol. 2020;10:583053. doi: 10.3389/fonc.2020.583053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S, Yu J, Huber A, Kryczek I, Wang Z, Jiang L, Li X, Du W, Li G, Wei S, et al. Metabolism drives macrophage heterogeneity in the tumor microenvironment. Cell Rep. 2022;39:110609. doi: 10.1016/j.celrep.2022.110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 111.Chen D, Xie J, Fiskesund R, Dong W, Liang X, Lv J, Jin X, Liu J, Mo S, Zhang T, et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun. 2018;9:873. doi: 10.1038/s41467-018-03225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, Wang J, Wen D, Zhang Y, Lu Y, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2018;14:89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 113.Kuchuk O, Tuccitto A, Citterio D, Huber V, Camisaschi C, Milione M, Vergani B, Villa A, Alison MR, Carradori S, et al. pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. Oncoimmunology. 2018;7:e1445452. doi: 10.1080/2162402X.2018.1445452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32:241–247. doi: 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 115.Sun JL, Zhang NP, Xu RC, Zhang GC, Liu ZY, Abuduwaili W, Wang F, Yu XN, Shi X, Song GQ, et al. Tumor cell-imposed iron restriction drives immunosuppressive polarization of tumor-associated macrophages. J Transl Med. 2021;19:347. doi: 10.1186/s12967-021-03034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang T, Huang JB, Xu CY, Lv YL, Lu J, Zhao ZQ, Yang DQ, Lou ZH, Zhang GJ. Arsenic trioxide cooperate cryptotanshinone exerts antitumor effect by medicating macrophage polarization through glycolysis. J Immunol Res. 2022;2022:2619781. doi: 10.1155/2022/2619781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orozco S, Oberst A. RIPK3 in cell death and inflammation: the good, the bad, and the ugly. Immunol Rev. 2017;277:102–112. doi: 10.1111/imr.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng H, Peng X, Yang S, Li X, Huang M, Wei S, Zhang S, He G, Liu J, Fan Q, et al. Targeting tumor-associated macrophages in hepatocellular carcinoma: biology, strategy, and immunotherapy. Cell Death Discov. 2023;9:65. doi: 10.1038/s41420-023-01356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu L, Zhang X, Zheng L, Zhao H, Yan G, Zhang Q, Zhou Y, Lei J, Zhang J, Wang J, et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol Res. 2020;8:710–721. doi: 10.1158/2326-6066.CIR-19-0261. [DOI] [PubMed] [Google Scholar]
- 120.Lv X, Chen Z, Li S, Xie H. Knockdown of cyclooxygenase-2 leads to growth inhibition and cell cycle arrest in hepatocellular carcinoma cells. Onco Targets Ther. 2019;12:4341–4349. doi: 10.2147/OTT.S196822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review. J Cell Physiol. 2019;234:5683–5699. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- 122.Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149:1884–1895.e1884. doi: 10.1053/j.gastro.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen C, Guan J, Gu X, Chu Q, Zhu H. Prostaglandin E2 and receptors: insight into tumorigenesis, tumor progression, and treatment of hepatocellular carcinoma. Front Cell Dev Biol. 2022;10:834859. doi: 10.3389/fcell.2022.834859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qorraj M, Bruns H, Bottcher M, Weigand L, Saul D, Mackensen A, Jitschin R, Mougiakakos D. The PD-1/PD-L1 axis contributes to immune metabolic dysfunctions of monocytes in chronic lymphocytic leukemia. Leukemia. 2017;31:470–478. doi: 10.1038/leu.2016.214. [DOI] [PubMed] [Google Scholar]
- 125.Liu M, Luo F, Ding C, Albeituni S, Hu X, Ma Y, Cai Y, McNally L, Sanders MA, Jain D, et al. Dectin-1 activation by a natural product beta-glucan converts immunosuppressive macrophages into an M1-like phenotype. J Immunol. 2015;195:5055–5065. doi: 10.4049/jimmunol.1501158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deng Z, Wu N, Suo Q, Wang J, Yue Y, Geng L, Zhang Q. Fucoidan, as an immunostimulator promotes M1 macrophage differentiation and enhances the chemotherapeutic sensitivity of capecitabine in colon cancer. Int J Biol Macromol. 2022;222:562–572. doi: 10.1016/j.ijbiomac.2022.09.201. [DOI] [PubMed] [Google Scholar]
- 127.Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J, Huang Y. Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin beta3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics. 2019;9:265–278. doi: 10.7150/thno.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsu SPC, Chen YC, Chiang HC, Huang YC, Huang CC, Wang HE, Wang YS, Chi KH. Rapamycin and hydroxychloroquine combination alters macrophage polarization and sensitizes glioblastoma to immune checkpoint inhibitors. J Neurooncol. 2020;146:417–426. doi: 10.1007/s11060-019-03360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dhakal B, Li CMY, Ramezanpour M, Houtak G, Li R, Bouras G, Collela A, Chegeni N, Chataway TK, Drew P, et al. Proteomic characterisation of perhexiline treatment on THP-1 M1 macrophage differentiation. Front Immunol. 2023;14:1054588. doi: 10.3389/fimmu.2023.1054588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Q, Tong D, Liu G, Gao J, Wang LA, Xu J, Yang X, Xie Q, Huang Y, Pang J, et al. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin Cancer Res. 2018;24:5622–5634. doi: 10.1158/1078-0432.CCR-18-0420. [DOI] [PubMed] [Google Scholar]
- 131.Totary-Jain H, Sionov RV, Gallily R. Indomethacin sensitizes resistant transformed cells to macrophage cytotoxicity. Immunol Lett. 2016;176:1–7. doi: 10.1016/j.imlet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao H, Zhang X, Chen X, Li Y, Ke Z, Tang T, Chai H, Guo AM, Chen H, Yang J. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6. Toxicol Appl Pharmacol. 2014;279:311–321. doi: 10.1016/j.taap.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Song M, Qian C, Zhang T, Tang Y, Zhou Y, Wei Z, Wang A, Zhong C, Zhao Y, Lu Y. Salvia mitiorrhiza Bunge aqueous extract attenuates infiltration of tumor-associated macrophages and potentiates anti-PD-L1 immunotherapy in colorectal cancer through modulating Cox2/PGE2 cascade. J Ethnopharmacol. 2023;316:116735. doi: 10.1016/j.jep.2023.116735. [DOI] [PubMed] [Google Scholar]
- 134.Sun K, Yu J, Hu J, Chen J, Song J, Chen Z, Cai Z, Lu Z, Zhang L, Wang Z. Salicylic acid-based hypoxia-responsive chemodynamic nanomedicines boost antitumor immunotherapy by modulating immunosuppressive tumor microenvironment. Acta Biomater. 2022;148:230–243. doi: 10.1016/j.actbio.2022.06.026. [DOI] [PubMed] [Google Scholar]
- 135.Nakano Y, Kitagawa T, Osada Y, Tanaka T, Nishizawa S, Yamamoto J. 5-aminolevulinic acid suppresses prostaglandin E2 production by murine macrophages and enhances macrophage cytotoxicity against glioma. World Neurosurg. 2019;127:e669–e676. doi: 10.1016/j.wneu.2019.03.240. [DOI] [PubMed] [Google Scholar]
- 136.Sheng Y, Jiang Q, Dong X, Liu J, Liu L, Wang H, Wang L, Li H, Yang X, Dong J. 3-Bromopyruvate inhibits the malignant phenotype of malignantly transformed macrophages and dendritic cells induced by glioma stem cells in the glioma microenvironment via miR-449a/MCT1. Biomed Pharmacother. 2020;121:109610. doi: 10.1016/j.biopha.2019.109610. [DOI] [PubMed] [Google Scholar]
- 137.Wei G, Sun J, Luan W, Hou Z, Wang S, Cui S, Cheng M, Liu Y. Natural product albiziabioside A conjugated with pyruvate dehydrogenase kinase inhibitor dichloroacetate to induce apoptosis-ferroptosis-M2-TAMs polarization for combined cancer therapy. J Med Chem. 2019;62:8760–8772. doi: 10.1021/acs.jmedchem.9b00644. [DOI] [PubMed] [Google Scholar]
- 138.Locatelli SL, Careddu G, Serio S, Consonni FM, Maeda A, Viswanadha S, Vakkalanka S, Castagna L, Santoro A, Allavena P, et al. Targeting cancer cells and tumor microenvironment in preclinical and clinical models of hodgkin lymphoma using the dual PI3Kdelta/gamma inhibitor RP6530. Clin Cancer Res. 2019;25:1098–1112. doi: 10.1158/1078-0432.CCR-18-1133. [DOI] [PubMed] [Google Scholar]
- 139.Oh MH, Sun IH, Zhao L, Leone RD, Sun IM, Xu W, Collins SL, Tam AJ, Blosser RL, Patel CH, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest. 2020;130:3865–3884. doi: 10.1172/JCI131859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yao J, Du Z, Li Z, Zhang S, Lin Y, Li H, Zhou L, Wang Y, Yan G, Wu X, et al. 6-Gingerol as an arginase inhibitor prevents urethane-induced lung carcinogenesis by reprogramming tumor supporting M2 macrophages to M1 phenotype. Food Funct. 2018;9:4611–4620. doi: 10.1039/C8FO01147H. [DOI] [PubMed] [Google Scholar]
- 141.Jiang X, Cao G, Gao G, Wang W, Zhao J, Gao C. Triptolide decreases tumor-associated macrophages infiltration and M2 polarization to remodel colon cancer immune microenvironment via inhibiting tumor-derived CXCL12. J Cell Physiol. 2021;236:193–204. doi: 10.1002/jcp.29833. [DOI] [PubMed] [Google Scholar]
- 142.Sun L. Recent advances in the development of AHR antagonists in immuno-oncology. RSC Med Chem. 2021;12:902–914. doi: 10.1039/D1MD00015B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu N, Zhang J, Yin M, Liu H, Zhang X, Li J, Yan B, Guo Y, Zhou J, Tao J, et al. Inhibition of xCT suppresses the efficacy of anti-PD-1/L1 melanoma treatment through exosomal PD-L1-induced macrophage M2 polarization. Mol Ther. 2021;29:2321–2334. doi: 10.1016/j.ymthe.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kulshrestha A, Katara GK, Ibrahim SA, Riehl VE, Schneiderman S, Bilal M, Young AN, Levine S, Fleetwood S, Dolan J, et al. In vivo anti-V-ATPase antibody treatment delays ovarian tumor growth by increasing antitumor immune responses. Mol Oncol. 2020;14:2436–2454. doi: 10.1002/1878-0261.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vishvakarma NK, Singh SM. Immunopotentiating effect of proton pump inhibitor pantoprazole in a lymphoma-bearing murine host: Implication in antitumor activation of tumor-associated macrophages. Immunol Lett. 2010;134:83–92. doi: 10.1016/j.imlet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 146.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rong L, Zhang Y, Li WS, Su Z, Fadhil JI, Zhang C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials. 2019;225:119515. doi: 10.1016/j.biomaterials.2019.119515. [DOI] [PubMed] [Google Scholar]
- 148.Mertens C, Akam EA, Rehwald C, Brune B, Tomat E, Jung M. Intracellular iron chelation modulates the macrophage iron phenotype with consequences on tumor progression. PLoS ONE. 2016;11:e0166164. doi: 10.1371/journal.pone.0166164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.