Abstract
Background
With recent developments in diabetes technology, attaining adequate glucose control is more achievable than ever. Despite these improvements, a significant proportion of individuals with type 1 diabetes do not reach recommended glycaemic goals. Sodium-glucose co-transporter-2 (SGLT2) inhibitors are glucose-lowering agents that inhibit the reabsorption of filtered glucose in the kidneys, thus promoting glucosuria. Because the glucose-lowering effect of SGLT2 inhibitors is achieved independently of insulin secretion, it has been speculated whether they could bridge the gap towards achieving glycaemic targets in individuals with type 1 diabetes.
Summary
Our main goal was to systematically map the current knowledge on the efficacy and safety of SGLT2 inhibitor use in adults with type 1 diabetes and present recent studies regarding the use of SGLT2 inhibitors in youth with type 1 diabetes. Using a scoping review approach, we searched MEDLINE to identify relevant clinical trials of SGLT2 inhibitors as adjunctive therapy to insulin in type 1 diabetes published from January 31, 2012, to January 31, 2022. We included the most relevant, large-scale, and long placebo-controlled clinical trials of SGLT2 inhibitors as an add-on therapy to insulin in adults with type 1 diabetes. Additionally, we included all relevant pilot studies evaluating the use of SGLT2 inhibitors as add-on therapy to insulin in youth with type 1 diabetes. We identified eight placebo-controlled clinical trials in adults with type 1 diabetes meeting our inclusion criteria and two relevant pilot studies in youth with type 1 diabetes. The clinical trials in adults with type 1 diabetes confirmed the efficacy of SGLT2 inhibitors as add-on therapy to insulin. However, this was associated with an increased incidence of diabetic ketoacidosis (DKA) versus placebo in all identified clinical trials. The two relevant pilot studies in youth with type 1 diabetes showed promising results of SGLT2 inhibitor use as an add-on therapy to insulin, especially when combined with a fully closed-loop system.
Key Messages
SGLT2 inhibitors, as an add-on therapy to insulin, improve glycaemic outcomes in adults with type 1 diabetes with a potential cost of increasing DKA risk. The use of add-on SGLT2 inhibitors to insulin shows promising results in youth with type 1 diabetes. Moreover, SGLT2 inhibitors as add-on therapy in combination with closed-loop insulin therapy could provide additional benefits in improving glycaemic control. The current role of SGLT2 inhibitors as an adjunct therapy to insulin in individuals with type 1 diabetes is yet to be determined.
Keywords: Type 1 diabetes, SGLT2 inhibitors, Diabetic ketoacidosis, Closed-loop system
Introduction
Type 1 diabetes is characterized by the autoimmune destruction of insulin-secreting pancreatic β cells leading to disturbed glucose regulation and manifest hyperglycaemia. Consequently, individuals with type 1 diabetes have a lifelong need for insulin replacement therapy [1]. The primary goal in the management of type 1 diabetes is to maintain blood glucose levels as close to normal as possible with the aim of preventing and/or delaying micro- and macrovascular complications [2, 3]. In addition to microvascular complications, cardiovascular disease is the main driver of morbidity and mortality in people with type 1 diabetes, and greater effort on cardioprotection is warranted, especially in individuals with early-onset type 1 diabetes due to increased risk for cardiovascular events and myocardial infarction [4]. A goal of glycated haemoglobin (HbA1c) below 7% (53 mmol/mol) is recommended for individuals with type 1 diabetes where comprehensive care is available, and a stricter goal of HbA1c below 6.5% (48 mmol/mol) might be targeted when possible as more stringent goals were associated with more favourable glycaemic outcomes without an increased rate of acute complications [5, 6, 7, 8].
The persistent development of innovations, including modern technologies and novel adjunctive drug therapies, has transformed diabetes care and offered the potential to optimize glycaemic control, improve quality of life, and reduce the burden of type 1 diabetes [9, 10, 11]. With recent advances in diabetes technology, including continuous glucose monitoring (CGM), advanced insulin delivery devices, and especially when used concomitantly to achieve a synergistic effect of glucose-responsive insulin therapy (closed-loop), attaining adequate glucose control is becoming increasingly more achievable [10, 12, 13, 14, 15]. However, precise lifelong diabetes care is challenging for all individuals living with type 1 diabetes, and achievement of HbA1c targets remains elusive for many living with type 1 diabetes [16].
Plasma glucose is freely filtered in the kidneys; under physiological conditions, however, none appears in the urine due to renal reabsorption of filtered glucose. High plasma glucose levels in individuals with diabetes lead to an increased glucose filtration load, which in turn overburdens the capacity to reabsorb glucose, resulting in glucose being leaked into the urine [17]. The main glucose transport protein assigned with the reabsorption of filtered glucose is sodium-glucose co-transporter-2 (SGLT2), which is found in the proximal tubule of the nephron. SGLT2 inhibitors are a class of drugs that take advantage of this mechanism and exert their function by preventing renal glucose reabsorption, thus facilitating glucosuria [18].
The first SGLT2 inhibitors (dapagliflozin, canagliflozin) were approved for the treatment of type 2 diabetes approximately a decade ago, both in Europe and the USA [19]. Considered novel glucose-lowering drugs at the time, they have since become well established in treating persons with type 2 diabetes together with other glucose-lowering medications, including metformin, glucagon-like peptide 1 receptor agonists, dipeptidyl peptidase-4 inhibitors, sulfonylureas, and insulins. The Standards of Medical Care in Diabetes by the American Diabetes Association recommend using SGLT2 inhibitors with or without metformin as initial therapy for individuals with type 2 diabetes at higher risk for heart failure, chronic kidney disease, and/or atherosclerotic cardiovascular disease [20]. Furthermore, SGLT2 inhibitors have also been shown to exert other beneficial pleiotropic effects in individuals with type 2 diabetes, including a reduced risk of significant cardiovascular events and slower progression of kidney disease [21, 22, 23].
Despite being considered glucose-lowering agents for use in diabetes, SGLT2 inhibitors have recently been in the spotlight because studies have shown their potential in other conditions regardless of the presence of hyperglycaemia. Noteworthy, a recent study has demonstrated the potential of empagliflozin in heart failure with a preserved ejection fraction [24], and dapagliflozin was shown to significantly lower the risk of death from renal or cardiovascular causes in patients with chronic kidney disease [25]. It has also been reported that patients with heart failure with reduced ejection fraction have a significantly reduced risk of worsening heart failure or cardiovascular death when dapagliflozin is added to recommended therapy, regardless of diabetes status [26]. In addition, a recent extensive meta-analysis of 10 randomized clinical trials (71,553 participants), which included patients with and without diabetes, concluded that SGLT2 inhibitor use was associated with a lower occurrence of cardiovascular death or hospitalization for heart failure by 33% in high-risk patients [27]. In summary, SGLT2 inhibitors are emerging as potent agents with significant outcomes on cardiovascular morbidity and mortality, which seem to benefit both patients with and without diabetes.
A number of pharmacologic agents have been developed, and are approved, for the management of type 2 diabetes. However, most of the noninsulin adjunctive therapies approved for type 2 diabetes have not yet been proven to be effective in type 1 diabetes. One contemporary approach studied in the past years to improve outcomes in individuals with type 1 diabetes is the addition of SGLT2 inhibitors as an adjunct to insulin therapy. It has been speculated whether SGLT2 inhibitors could bridge the gap towards achieving glycaemic targets in individuals with type 1 diabetes on insulin therapy. Over the past years, there has been debate concerning the use of SGLT2 inhibitors in adults with type 1 diabetes [28]. Yet, little has been summarized about the recent studies focusing on SGLT2 inhibitor use in youth with type 1 diabetes. In order to systematically map the current knowledge on the efficacy and safety of SGLT2 inhibitor use in adults with type 1 diabetes, as well as to present recent studies regarding the use of SGLT2 inhibitors in youth patients with type 1 diabetes, a scoping review was conducted.
Methods
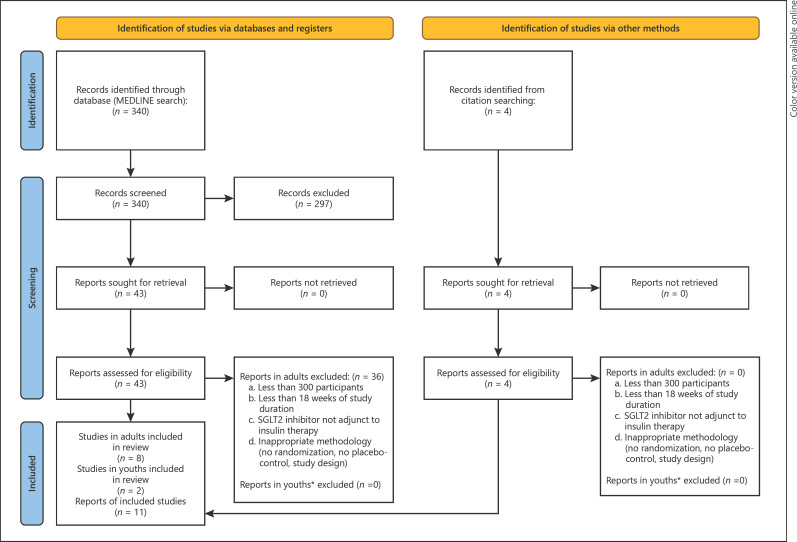
Our primary goal was to identify the most substantial, randomized, placebo-controlled clinical trials of each individual SGLT2 inhibitor as an add-on therapy to insulin in adults with type 1 diabetes. To achieve this, we searched MEDLINE using the terms “SGLT2 inhibitor” and “type 1 diabetes” to identify relevant clinical trials of SGLT2 inhibitors as adjunctive therapy to insulin in adults with type 1 diabetes published from January 31, 2012, to January 31, 2022 (PRISMA diagram in Fig. 1). The search was last executed on February 7, 2022. We excluded all studies in adults that had less than 300 participants, were less than 18 weeks in duration, did not investigate the effect of SGLT2 inhibitors in adjunct with insulin, were not written in English, and had inappropriate methodology [29]. In addition, given the presumed scarcity of studies concerning SGLT2 inhibitor use in youth with type 1 diabetes, we utilized our primary search also to identify studies in this population while refraining from strict inclusion criteria. Finally, we identified additional records through a citation search. Data charting was done independently from relevant study text or appropriate supplemental material, and no formal review protocol was registered.
Fig. 1.
PRISMA diagram of the study selection process. *Given the scarcity of data on SGLT2 inhibitor use in youth with type 1 diabetes, no strict exclusion criteria were used. SGLT2, sodium-glucose co-transporter-2.
Aiming to compile data on glycaemic outcomes and safety concerns from relevant adult studies, we systematically gathered data on the following variables: duration of study, HbA1c, time in range (TIR), and diabetic ketoacidosis (DKA) incidence in both the study and placebo groups. Additionally, in the relevant youth studies, with regard to the specific nature of the pilot studies, we systematically gathered data on inulin dose, urinary glucose excretion, TIR, and β-hydroxybutyrate levels in both the study and placebo groups. All relevant studies were presented in text summaries, and relevant variable data were presented in two tables for each studied age group.
Results
SGLT2 Inhibitors in Adults with Type 1 Diabetes
SGLT2 inhibitors' glucose-lowering effect is achieved independently of insulin secretion, which is one of the foundations behind the idea of implementing these drugs as complementary to insulin therapy when managing type 1 diabetes. Multiple pilot studies suggest positive glycaemic outcomes in adults with type 1 diabetes receiving SGLT2 inhibitors in addition to insulin [30, 31, 32]. The process and results of our primary search for relevant studies on SGLT2 inhibitor use in adults with type 1 diabetes are presented in Figure 1 (PRISMA flow diagram). We identified eight randomized, placebo-controlled clinical trials of four different SGLT2 inhibitors used as add-on therapy to insulin in adults with type 1 diabetes [33, 34, 35, 36, 37, 38, 39]. The main glycaemic outcomes and DKA incidence rates of these relevant adult studies are summarized in Table 1.
Table 1.
Significant randomized, placebo-controlled studies of different SGLT2 inhibitors as an add-on therapy to insulin in adults with type 1 diabetes
| Study | SGLT2 Inhibitor (mg) | Duration (weeks) | HbA1ca (%) | Time in rangea 3.9–10 mmol/L (70–180 mg/dL) (%) | DKA (%) |
|
|---|---|---|---|---|---|---|
| SGLT2 inhibitor | Placebo | |||||
| Henry et al. [33] | Canagliflozin 100 | 18 | −0.29 | − | 4.3 | 0 |
| Canagliflozin 300 | −0.25 | 6.0 | ||||
|
| ||||||
| DEPICT-1 [34, 40] | Dapagliflozin 5 | 52 | −0.33 | +9.1 | 4.0 | 1.9 |
| Dapagliflozin 10 | −0.36 | +10.7 | 3.4 | |||
|
| ||||||
| DEPICT-2 [39, 41] | Dapagliflozin 5 | 52 | −0.20 | +9.0 | 4.1 | 0.4 |
| Dapagliflozin 10 | −0.25 | +10.7 | 3.7 | |||
|
| ||||||
| inTandem1 [35] | Sotagliflozin 200 | 52 | −0.25 | +3.0 | 3.4 | 0.4 |
| Sotagliflozin 400 | −0.31 | +10.4 | 4.2 | |||
|
| ||||||
| inTandem2 [36] | Sotagliflozin 200 | 52 | −0.21 | +8.4 | 2.3 | 0 |
| Sotagliflozin 400 | −0.32 | +13.4 | 3.4 | |||
|
| ||||||
| inTandem3 [37] | Sotagliflozin 400 | 24 | −0.46 | − | 3.0 | 0.6 |
|
| ||||||
| EASE-2 [38] | Empagliflozin 10 | 52 | −0.39 | +12.2 | 4.3b | 1.2 |
| Empagliflozin 25 | −0.45 | +12.5 | 3.3b | |||
|
|
||||||
| EASE-3 [38] | Empagliflozin 2.5 | 26 | −0.28 | +4.3 | 0.8 | |
| Empagliflozin 10 | −0.45 | +10.7 | − | |||
| Empagliflozin 25 | −0.52 | +7.4 | − | |||
SGLT2, sodium-glucose co-transporter-2; HbA1c, glycated haemoglobin; DKA, diabetic ketoacidosis.
Relative to placebo.
Pooled data for both EASE-2 and EASE-3 studies.
Canagliflozin
In 2015, Henry et al. [33] conducted one of the earliest ground-breaking placebo-controlled studies evaluating SGLT2 inhibitors, focusing on the efficacy and safety of canagliflozin as an add-on to insulin therapy in adults with type 1 diabetes. Adults with type 1 diabetes, aged 25–65, who had HbA1c above the recommended values (HbA1c of 7.0–9.0% [53–75 mmol/mol]) using either multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) were included. A total of 351 participants were divided into three groups, each receiving a daily dose of either 100 mg or 300 mg of canagliflozin or a placebo for 18 weeks. Study results showed that at the end of the observational period, a greater proportion of participants had a reduction in HbA1c ≥0.4% (≥4.4 mmol/mol) and no increase in body weight with canagliflozin 100 mg and 300 mg compared to placebo (36.9%, 41.4%, and 14.5%, respectively, p < 0.001). Additionally, both doses of canagliflozin were associated with a reduction in the total daily dose of insulin at the end of the study compared to baseline. Furthermore, there was no difference in the incidence of hypoglycaemia between the study arms. On the other hand, an increased incidence of DKA was observed with canagliflozin 100 mg and 300 mg compared to placebo (4.3%, 6.0%, and 0%, respectively) [33].
Dapagliflozin
The DEPICT-1 (Dapagliflozin Evaluation in Patients With Inadequately Controlled Type 1 Diabetes) study focused on the long-term use of dapagliflozin as adjunctive therapy to insulin in adults with type 1 diabetes [34, 40]. This multicentre, double-blind, placebo-controlled study included adults with type 1 diabetes (18–75 years) with HbA1c values above recommended (7.5–10.5% [58–91 mmol/mol]), with participants receiving either dapagliflozin 5 mg, 10 mg, or a placebo once daily for a total of 52 weeks. The study consisted of a 24-week short period [40], followed by a 28-week extension period for a total of 52 weeks [34]. In total, 758 participants completed the 24-week short-term period, with results showing that both doses of dapagliflozin significantly reduced HbA1c compared with placebo at week 24 (mean difference from baseline to week 24 for dapagliflozin 5 mg vs. placebo was −0.42% [−4.6 mmol/mol] and for dapagliflozin 10 mg versus placebo was −0.45% [−4.9 mmol/mol], p < 0.0001 for both comparisons) [40]. The 28-week extension period was completed by 708 participants, but because the total 52-week efficacy analyses were exploratory, no p values were calculated. From baseline to week 52, both dapagliflozin 5 mg and 10 mg significantly reduced HbA1c (the difference vs. placebo was −0.33% [−3.6 mmol/mol] and −0.36% [−3.9 mmol/mol], respectively) [34]. In the 52-week period, the researchers also observed that the total daily dose of insulin in both dapagliflozin groups was reduced compared to the placebo group. Additionally, they reported decreased body weight in both dapagliflozin groups compared to the placebo group. Finally, although they reported similar hypoglycaemia incidents across all three study groups, the incidence of DKA was greater in the dapagliflozin 5 mg and 10 mg groups than in the placebo group (4.0%, 3.4%, and 1.9%, respectively) [34].
The DEPICT-2 study was similar in design to the DEPICT-1 study, but the participants were from different geographical regions [39]. The results from both the short 24-week period [41] as well as the extended period for a total of 52 weeks [39] were consistent with the DEPICT-1 study (Table 1). Both the DEPICT-1 and DEPICT-2 had a subgroup of participants in the short 24-week periods using CGM, which allows for daily glucose variability assessment and significantly complements HbA1c measurements [42]. The CGM results showed a significant increase in TIR (3.9–10.0 mmol/L [70–180 mg/dL]) for both doses of dapagliflozin in both studies (all p < 0.0001) (Table 1).
Sotagliflozin
Sotagliflozin stands out among SGLT2 inhibitors because it was developed as a dual SGLT1/2 inhibitor, meaning it not only blocks SGLT2 in the kidneys but also SGLT1 in the gastrointestinal tract. This dual inhibition could provide several beneficial effects, including postprandial glucose reduction, increased urinary glucose excretion, and postprandial glucagon-like peptide 1 release [43]. The inTandem1−3 clinical trials studied the effects of sotagliflozin in adults with type 1 diabetes [35, 36, 37]. The inTandem3 study was a phase 3, multicentre, double-blind trial exploring the effects of sotagliflozin in adults with type 1 diabetes [37]. The study included 1402 participants receiving insulin treatment (CSII or MDI) randomly assigned to either receive sotagliflozin 400 mg daily or a placebo for 24 weeks. The results of the study showed that a significantly greater proportion of participants in the sotagliflozin group achieved an HbA1c <7.0% (<53 mmol/mol) at week 24, with no episodes of severe hypoglycaemia or DKA (28.6%) compared to the placebo group (15.2%) (p < 0.001). The researchers also noted a significantly greater mean change from baseline in the sotagliflozin group than in the placebo group for HbA1c, weight, systolic blood pressure, and mean daily bolus insulin dose. Furthermore, there were fewer cases of documented hypoglycaemia in the sotagliflozin group than in the placebo group, whereas the rate of severe hypoglycaemia was similar in both study groups. Finally, the results showed that the rate of DKA was higher in the sotagliflozin group than in the placebo group (3.0% vs. 0.6%, respectively). The main conclusion of this study was that sotagliflozin significantly improves glycaemic outcomes in adults with type 1 diabetes on insulin therapy, albeit increasing the risk of DKA [37].
The inTandem1 and inTandem2 studies were very similar in design, with the distinction that inTandem1 was carried out in North America [35], whereas inTandem2 was conducted in Europe and Israel [36]. Both of these studies were double-blind, placebo-controlled, phase 3 trials, focused on adults with type 1 diabetes (aged 18 years or older) who were treated with MDI or CSII and who had inadequate glycaemic control (HbA1c 7.0–11.0% [53–97 mmol/mol]). In both studies, approximately 800 participants were randomized following insulin therapy optimization and received either once-daily sotagliflozin 200 mg, sotagliflozin 400 mg, or a placebo in combination with insulin. The primary endpoint of both studies was the placebo-adjusted change in HbA1c from baseline to week 24. In the inTandem1 study, placebo-adjusted HbA1c reductions from a mean baseline of 7.57% (59 mmol/mol) at 24 weeks were −0.36% (−3.9 mmol/mol) with sotagliflozin 200 mg and −0.41% (−4.5 mmol/mol) with sotagliflozin 400 mg, and at 52 weeks, −0.25% (−2.7 mmol/mol) and −0.31% (−3.4 mmol/mol), respectively (all comparisons p < 0.001). The results also showed that among participants with a baseline HbA1c ≥7.0% (≥53 mmol/mol), an HbA1c <7% (<53 mmol/mol) at 24 weeks was achieved by 15.7% of participants receiving placebo, 27.2% of participants receiving sotagliflozin 200 mg, and 40.3% of participants receiving sotagliflozin 400 mg (p ≤ 0.003 vs. placebo) [35]. The inTandem2 study had similar results, with placebo-adjusted changes in HbA1c from a baseline of 7.8% (62 mmol/mol) being −0.37% (−4.0 mmol/mol) with sotagliflozin 200 mg and −0.35% (−3.8 mmol/mol) with sotagliflozin 400 mg at 24 weeks (p < 0.001), with the differences maintained at 52 weeks [36]. What is more, both studies showed improvements in other variables while using sotagliflozin in combination with insulin, including reduced fasting plasma glucose, weight, and daily insulin dose [35, 36]. In the CGM substudy groups of both studies, TIR (3.9–10.0 mmol/L [70–180 mg/dL]) increased significantly with sotagliflozin 400 mg compared to placebo (Table 1) (p < 0.001 in both studies). Additionally, fewer cases of hypoglycaemia were observed with sotagliflozin relative to placebo. On the other hand, sotagliflozin was associated with an increased incidence of DKA in both studies (Table 1). Other more frequent adverse effects reported were mycotic genital infections and diarrhoea [35, 36].
Empagliflozin
The EASE1-3 (Empagliflozin as Adjunctive to inSulin thErapy) studies focused on the effects of add-on empagliflozin to insulin therapy in adults with type 1 diabetes. EASE-1 was a short, 4-week, placebo-controlled pilot study that included 75 adults with type 1 diabetes and showed promising results; empagliflozin significantly increased urinary glucose excretion and improved HbA1c [44]. The EASE-2 and EASE-3 studies were two multicentre, double-blind, placebo-controlled clinical trials conducted over 52 weeks (EASE-2) and 26 weeks (EASE-3) that included a total of 1,707 participants [38]. Both trials were similar by design and assessed the effect of empagliflozin 10 mg and 25 mg versus placebo with the following differences; the EASE-3 study had a shorter treatment duration, assessed CGM only in a substudy of participants (compared to all participants in EASE-2), and studied an additional lower dose of empagliflozin 2.5 mg. Overall, the most significant observed mean placebo-subtracted HbA1c reductions were −0.28% (−3.1 mmol/mol) for empagliflozin 2.5 mg, −0.54% (−5.9 mmol/mol) for empagliflozin 10 mg, and −0.53% (−5.8 mmol/mol) for empagliflozin 25 mg (all p < 0.0001). Importantly, CGM results showed significant increases in TIR (3.9–10.0 mmol/L [70–180 mg/dL]) with empagliflozin 10 and 25 mg in EASE-2 (+12.2%% and +12.5%, respectively, both p < 0.0001) and in EASE-3 (+10.7% [p < 0.0001] and 7.4% [p < 0.01], respectively), while empagliflozin 2.5 mg did not significantly change TIR (Table 1). The results also showed significant reductions in mean weight, total daily insulin dose, and systolic blood pressure for all three doses of empagliflozin. DKA occurred more frequently with empagliflozin 10 mg (4.3%) and 25 mg (3.3%) but not with empagliflozin 2.5 mg (0.8%) compared to placebo (1.2%). One fatal case occurred in the empagliflozin 25 mg group, which was mainly related to delayed DKA diagnosis and treatment [38]. In contrast to previously mentioned studies, the EASE-3 study also investigated the effect of small SGLT2 inhibitor dosing in addition to insulin in adults with type 1 diabetes. Small doses of empagliflozin importantly did not result in a more frequent DKA rate while at the same time providing small, albeit important, improvements in some glycaemic outcomes.
SGLT2 Inhibitors in Youth with Type 1 Diabetes
All of the studies mentioned earlier focused on adults with type 1 diabetes, and there are limited data regarding the adjunct use of SGLT2 inhibitors in youth with type 1 diabetes. Therefore, during our primary search, we also screened for pilot studies of SGLT2 inhibitor use in youth with type 1 diabetes (Fig. 1). Up to date, two pilot studies of SGLT2 inhibitor use in youth with type 1 diabetes have been conducted. The most relevant data from both studies are summarized in Table 2.
Table 2.
Pilot placebo-controlled studies of SGLT2 inhibitors as an adjunct therapy to insulin in youth with type 1 diabetes
| Study | SGLT2 inhibitor (mg) | Insulin dosea (%) | Urinary glucose excretiona (%) | Time in rangea 3.9–10.0 mmol/L (70–180 mg/dL) (%) | β-Hydroxybutyrate level (mmol/L) |
|
|---|---|---|---|---|---|---|
| SGLT2 inhibitor | placebo | |||||
| Biester et al. [45] | Dapagliflozin 10 | −13.6 | +610 | − | 0.17 | 0.11 |
|
| ||||||
| DAPADream [46] | Dapagliflozin 20 | −22 | +212 | +18 | 0.29 | 0.16 |
SGLT2, sodium-glucose co-transporter-2.
Relative to placebo.
A pilot placebo-controlled randomized clinical trial evaluated the effects of a single dose of dapagliflozin 10 mg as an add-on to insulin in 33 youth (median age 16 years) with type 1 diabetes, divided into three categories based on their baseline HbA1c: <7.5%, 7.5–9.0%, or >9.0% (<58, 58–75, >75 mmol/mol) [45]. The study period was 24 h, during which intravenous insulin and glucose infusion maintained steady glucose levels. Dapagliflozin reduced the mean insulin dose by 13.6% and increased urinary glucose excretion by 610%, both effects not related to baseline HbA1c. However, 5 episodes of β-hydroxybutyrate levels between ≥0.6 and <1.0 mmol/L were observed in the dapagliflozin group compared to 1 episode in the placebo group [45].
Recently, a single-centre, double-blind, randomized, crossover, placebo-controlled trial (DAPADream study) evaluated the efficacy of fully closed-loop insulin therapy with SGLT2 inhibitor (dapagliflozin 10 mg twice a day) compared to placebo [46]. The primary endpoint was TIR (3.9–10.0 mmol/L [70–180 mg/dL]), and 15 adolescents and 15 young adults with type 1 diabetes were included. The participants were admitted into the hospital twice for 27 h, with a 30-day washout period between admissions. TIR was significantly higher in the dapagliflozin group (68% ± 6%) compared to placebo (50% ± 13%) (p < 0.001). Furthermore, dapagliflozin was associated with a threefold increase in urinary glucose excretion and a total insulin reduction of 22%. Importantly, no abnormal elevations in β-hydroxybutyrate levels were observed [46].
In summary, different SGLT2 inhibitors improve glycaemic outcomes in adults with type 1 diabetes when used as add-on therapy to insulin. However, a profound side effect of SGLT2 inhibitor use is the development of DKA, which has a higher incidence rate among patients using SGLT2 inhibitors compared to placebo. These findings were omnipresent in all the adult clinical trials presented in this scoping review, with the notable exception of low-dose empagliflozin treatment, which did not increase DKA incidence but still resulted in a slight improvement in glycaemic outcomes (Table 1). The pilot studies in youth with type 1 diabetes presented in this review demonstrated that add-on SGLT2 inhibitor therapy reduced the required insulin dose and significantly increased urinary glucose excretion. While episodes of higher β-hydroxybutyrate levels were observed in one youth study, the use of a fully closed-loop insulin delivery system was not associated with abnormally elevated β-hydroxybutyrate levels.
Discussion
Our search identified eight of the most relevant placebo-controlled clinical trials of add-on SGLT2 inhibitor use in adults with type 1 diabetes. All of the adult studies mentioned above followed a similar design while researching different SGLT2 inhibitors. The study by Henry et al. [33] demonstrated the potential of canagliflozin in complementing insulin therapy in type 1 diabetes, including its ability to lower the daily insulin dose and thereby possibly reduce insulin-associated weight gain. The results of the DEPICT-1 and DEPICT-2 studies accord with the results from the initial trial, with dapagliflozin proving beneficial in treating adults with type 1 diabetes. However, parallel with the observed benefits in improved glycaemic control, weight reduction, and decreased daily insulin, there was an increased rate of DKA. Sotagliflozin, standing out as a dual SGLT1/2 inhibitor, also proved beneficial as an add-on therapy to insulin in the inTandem1−3 trials, improving glycaemic outcomes in adults with type 1 diabetes, albeit increasing the risk of DKA. Similarly, empagliflozin proved beneficial in achieving better glycaemic outcomes in adults with type 1 diabetes while increasing the risk of DKA at higher doses, even resulting in one fatality during the EASE studies. Noteworthy, smaller doses of empagliflozin improved specific glycaemic outcomes while not increasing the risk of DKA.
A distinct pattern can be observed concerning the effect of SGLT2 inhibitors on adults with type 1 diabetes on insulin therapy; SGLT2 inhibitors improve glycaemic outcomes when used as an adjunct therapy to insulin while at the same time increasing the risk of adverse effects, the most severe being DKA. As DKA is a potentially life-threatening event, the risk of developing DKA while taking SGLT2 inhibitors limits their use in adults with type 1 diabetes. Interestingly, DKA is considered a rare adverse effect in individuals with T2D on SGLT2 inhibitor therapy, albeit a severe complication [20]. The mechanism behind DKA development with SGLT2 inhibitor use is not fully understood. However, it is hypothesized that dehydration and insulinopenia (“the two-hit hypothesis”) play a crucial role in the mechanism. It is believed that dehydration increases glucocorticoid and catecholamine release, leading to white adipose tissue lipolysis and ketogenesis in the background of insulinopenia as a result of lower plasma glucose levels due to SGLT2 inhibitor-induced glucosuria [18]. A recent extensive meta-analysis assessed the risk of DKA due to SGLT2 inhibitor treatment in adults with type 1 diabetes [47]. It analysed 18 placebo-controlled randomized controlled trials (including 7,396 adult participants) and evaluated five different SGLT2 inhibitors. The results showed that baseline body mass index (BMI), insulin resistance, total insulin dose reduction-to-baseline insulin sensitivity ratio, and volume depletion modified the risk of DKA and therapeutic response to SGLT2 inhibitors. The researchers concluded that this information might enable the targeted use of SGLT2 inhibitors in individuals with type 1 diabetes with the most significant benefit and the lowest risk of DKA [47].
The idea of limiting SGLT2 inhibitor use to a select population of individuals with type 1 diabetes on insulin therapy was an exciting prospect. In accordance with this thought, in February 2019, the European Medicines Agency (EMA) released positive opinions for two SGLT2 inhibitors, namely, dapagliflozin and sotagliflozin, to be used as complementary treatments in individuals with type 1 diabetes with a BMI ≥27 kg/m2 [48, 49]. Furthermore, in August 2019, the UK's National Institute for Health and Care Excellence (NICE) recommended using dapagliflozin for treating adults with type 1 diabetes with a BMI ≥27 kg/m2, provided that optimal insulin therapy alone did not provide adequate glycaemic control [50]. However, both the EMA and NICE recommendations for dapagliflozin have recently been revoked on demand from the company due to concerns over increased risk for DKA [51, 52], leaving sotagliflozin as the sole EMA-approved SGLT2 inhibitor to be used in adjunct with insulin in type 1 diabetes. On the other hand, the US Food and Drug Administration (FDA) has not yet approved any SGLT2 inhibitor to be used in type 1 diabetes [28]. In 2018, the Advanced Technologies & Treatment for Diabetes (ATTD) Congress convened an international consensus conference to provide strategies to mitigate DKA and euglycaemic DKA risk in individuals with type 1 diabetes using SGLT2 inhibitors [53]. These strategies include appropriate patient selection for SGLT2 inhibitor therapy (presentation with normal ketone levels, willingness to follow prescribed regimens for ketone monitoring, ability to respond appropriately to elevated ketone levels), insulin dose adjustments (cautious reduction of insulin dose when initiating SGLT2 inhibitor therapy), careful dosing of SGLT2 inhibitors (lowest possible dose at initiation), instructions on discontinuing SGLT2 inhibitor therapy (in case of nausea or vomiting), DKA prevention and treatment guidelines, and patient and clinician education.
Our search also revealed two relevant pilot studies of add-on SGLT2 inhibitor use (dapagliflozin) in youth with type 1 diabetes. The study performed by Biester et al. indicates that dapagliflozin can help reduce the required insulin dose while significantly increasing urinary glucose excretion. This is noteworthy since insulin-associated weight gain is an important complication of intensive therapy for type 1 diabetes [54]. However, the researchers also observed a greater incidence of higher β-hydroxybutyrate levels in the study group compared to the placebo group. This result could indicate increased DKA risk, which, as mentioned, is present in adults with type 1 diabetes using add-on SGLT2 inhibitors. Although performed on a smaller scale than the studies discussed above, this study provides insight into the possibility of using SGLT2 inhibitors in small doses in the paediatric population. However, this study was conducted in a controlled environment with intravenous insulin administration.
The DAPADream study was unique in that it focused on the use of add-on SGLT2 inhibitor therapy to a fully closed-loop system. The results of this study showed great promise, with dapagliflozin increasing TIR and reducing the insulin dose while at the same time not abnormally increasing β-hydroxybutyrate levels. This result is exciting primarily because the researchers observed improvements in glycaemic targets without apparent increased risk for dangerously high ketone levels, thereby possibly suggesting that SGLT2 inhibitor add-on therapy to a fully closed-loop insulin system could prove effective and, most importantly, safe. In a recent study on adult patients with type 1 diabetes, empagliflozin was used as add-on therapy to closed-loop insulin delivery for 4 weeks [55]. The results of this study show that empagliflozin add-on therapy to closed-loop systems significantly increased TIR compared to placebo, with the researchers observing no DKA or severe hypoglycaemia events. Notably, higher ketone concentrations were more common on empagliflozin compared to placebo. Further investigations are needed on a broader scale and with a longer study duration to fully assess the potential of SGLT2 inhibitors in conjunction with modern closed-loop systems. An exciting prospect for a future study might be the use of low-dose empagliflozin, as reported in the EASE-3 trial, in conjunction with a fully closed-loop system over a more extended period. Perhaps, this could prove effective in achieving glycaemic goals with no increased risk of higher ketone levels or DKA. In any case, future research on SGLT2 inhibitor use in type 1 diabetes, in adults or youth, should establish rigorous ketone monitoring protocols to minimize the risk of patients developing DKA.
Given the scarcity of data regarding the use of SGLT2 inhibitors in youth with type 1 diabetes, we could not set strict inclusion criteria for study selection, which limits the generalizability of this section of our scoping review. Even though we conducted a scoping review, we limited our search to clinical studies and trials in order to summarize the most relevant data at the possible cost of not including expert opinions or other sources of evidence.
Conclusions
SGLT2 inhibitors are glucose-lowering drugs of great significance and are demonstrating promising results beyond diabetes. However, their role in the treatment of type 1 diabetes remains uncertain. Many extensive studies have confirmed the efficacy of SGLT2 inhibitors as an adjunct to insulin in adults with type 1 diabetes, with improvements in several clinical outcomes, including reduction of HbA1c, decrease in total required insulin dose, and increased TIR. On the other hand, the risk of developing euglycaemic DKA remains a significant limiting factor. Strategies that would mitigate the risk of developing DKA while at the same time maximizing the benefits of add-on SGLT2 inhibitor use need to be further investigated. Finally, combining modern glucose-responsive insulin therapy with add-on SGLT2 inhibitor therapy, especially in youth with type 1 diabetes, might further improve glycaemic control, and additional studies are warranted.
Conflict of Interest Statement
Tadej Battelino served on advisory boards of Novo Nordisk, Sanofi, Eli Lilly, Boehringer, Medtronic, Indigo, and DreaMed Diabetes. Tadej Battelino received honoraria for participating in the speaker's bureaux of Eli Lilly, Novo Nordisk, Medtronic, Abbott, Sanofi, Aventis, Astra Zeneca, and Roche. Tadej Battelino owns stocks of DreamMed Diabetes. Tadej Battelino's institution received research grant support from Abbott, Medtronic, Novo Nordisk, GluSense, Sanofi, Novartis, Sandoz, and Zealand Pharma. Klemen Dovč received honoraria for participation in the speaker's bureau of Abbott, Pfizer, Novo Nordisk, and Eli Lilly. Klemen Dovč is a member of the European Commission Expert panel for Medical Devices for Endocrinology and Diabetes. Tim Hropot declares no conflicts of interest.
Funding Sources
Klemen Dovč and Tadej Battelino were supported in part by the Slovenian National Research Agency Grants No. J3–6798, V3–1505, and P3–0343.
Author Contributions
Klemen Dovč and Tim Hropot contributed to the study design and writing of the manuscript. Tim Hropot conducted the study search and was responsible for figure and table development. Tadej Battelino supervised the entire project and provided support throughout.
Funding Statement
Klemen Dovč and Tadej Battelino were supported in part by the Slovenian National Research Agency Grants No. J3–6798, V3–1505, and P3–0343.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383((9911)):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The DCCT Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: diabetes control and complications trial. J Pediatr. 1994;125((2)):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 3.Lind M, Svensson AM, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371((21)):1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 4.Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392((10146)):477–486. doi: 10.1016/S0140-6736(18)31506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, et al. Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes 2018 ISPAD clinical practice consensus guidelines. Pediatric Diabetes. 2018;19((Suppl 27)):105–114. doi: 10.1111/pedi.12737. [DOI] [PubMed] [Google Scholar]
- 6.Gerhardsson P, Schwandt A, Witsch M, Kordonouri O, Svensson J, Forsander G, et al. The sweet project: 10-year benchmarking in 19 countries worldwide is associated with improved HbA1c and increased use of diabetes technology in youth with type 1 diabetes. Diabetes Technol Ther. 2021;23((7)):491–499. doi: 10.1089/dia.2020.0618. [DOI] [PubMed] [Google Scholar]
- 7.Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318((14)):1358–1366. doi: 10.1001/jama.2017.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. American Diabetes Association Professional Practice Committee 14. children and adolescents: standards of medical care in diabetes − 2022. Diabetes Care. 2022;45((Suppl 1)):S208–31. doi: 10.2337/dc22-S014. [DOI] [PubMed] [Google Scholar]
- 9.Miller KM, Hermann J, Foster N, Hofer SE, Rickels MR, Danne T, et al. Longitudinal changes in continuous glucose monitoring use among individuals with type 1 diabetes: International comparison in the German and Austrian DPV and U.S. T1D exchange registries. Diabetes Care. 2020;43((1)):E1–E2. doi: 10.2337/dc19-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dovc K, Battelino T. Evolution of diabetes technology. Endocrinol Metab Clin North Am. 2020;49((1)):1–18. doi: 10.1016/j.ecl.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Prattichizzo F, Phillip M, Hirsch IB, Mathieu C, Battelino T. Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. 2022;10((1)):75–84. doi: 10.1016/S2213-8587(21)00245-X. [DOI] [PubMed] [Google Scholar]
- 12.Biester T, Tauschmann M, Chobot A, Kordonouri O, Danne T, Kapellen T, et al. The automated pancreas: a review of technologies and clinical practice. Diabetes Obes Metab. 2021;24((Suppl 1)):1–15. doi: 10.1111/dom.14576. [DOI] [PubMed] [Google Scholar]
- 13.Ware J, Allen JM, Boughton CK, Wilinska ME, Hartnell S, Thankamony A, et al. Randomized trial of closed: loop control in very young children with type 1 diabetes. N Engl J Med. 2022;386((3)):209–219. doi: 10.1056/NEJMoa2111673. [DOI] [PubMed] [Google Scholar]
- 14.Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397((10270)):208–219. doi: 10.1016/S0140-6736(20)32514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381((18)):1707–1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, Dimeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21((2)):66–72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61((10)):2087–2097. doi: 10.1007/s00125-018-4656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry RJ, Shulman GI. Sodium-glucose cotransporter-2 inhibitors: understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem. 2020;295((42)):14379–90. doi: 10.1074/jbc.REV120.008387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivian EM. Dapagliflozin: a new sodium-glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. Am J Health Syst Pharm. 2015;72((5)):361–372. doi: 10.2146/ajhp140168. [DOI] [PubMed] [Google Scholar]
- 20.Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. American Diabetes Association Professional Practice Committee 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes − 2022. Diabetes Care. 2022;45((Suppl 1)):S125–S143. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]
- 21.Steiner S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. Zeitschrift fur Gefassmedizin. N Engl J Med. 2016;13:17–18. [Google Scholar]
- 22.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380((4)):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 23.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375((4)):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 24.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021 Oct 14;385((16)):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 25.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020 Oct 8;383((15)):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 26.Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Bělohlávek J, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323((14)):1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattarai M, Salih M, Regmi M, Al-Akchar M, Deshpande R, Niaz Z, et al. Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in patients with type 2 diabetes and other risk factors for cardiovascular disease: a meta-analysis. JAMA Netw Open. 2022;5((1)):e2142078–12. doi: 10.1001/jamanetworkopen.2021.42078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor SI, Blau JE, Rother KI, Beitelshees AL. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol. 2019;7((12)):949–958. doi: 10.1016/S2213-8587(19)30154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169((7)):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 30.Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapaglif lozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38((3)):412–419. doi: 10.2337/dc13-2955. [DOI] [PubMed] [Google Scholar]
- 31.Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care. 2015;38((7)):1181–1188. doi: 10.2337/dc14-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins BA, Cherney DZI, Partridge H, Soleymanlou N, Tschirhart H, Zinman B, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37((5)):1480–1483. doi: 10.2337/dc13-2338. [DOI] [PubMed] [Google Scholar]
- 33.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38((12)):2258–2265. doi: 10.2337/dc15-1730. [DOI] [PubMed] [Google Scholar]
- 34.Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care. 2018;41((12)):2552–2559. doi: 10.2337/dc18-1087. [DOI] [PubMed] [Google Scholar]
- 35.Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care. 2018;41((9)):1970–1980. doi: 10.2337/dc18-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danne T, Cariou B, Banks P, Brandle M, Brath H, Franek E, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care. 2018;41((9)):1981–1990. doi: 10.2337/dc18-0342. [DOI] [PubMed] [Google Scholar]
- 37.Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377((24)):2337–2348. doi: 10.1056/NEJMoa1708337. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapyin type 1 diabetes: the EASE trials. Diabetes Care. 2018;41((12)):2560–2569. doi: 10.2337/dc18-1749. [DOI] [PubMed] [Google Scholar]
- 39.Mathieu C, Rudofsky G, Phillip M, Araki E, Lind M, Arya N, et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 52-week results from a randomized controlled trial. Diabetes Obes Metab. 2020;22((9)):1516–1526. doi: 10.1111/dom.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschöpe D, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5((11)):864–876. doi: 10.1016/S2213-8587(17)30308-X. [DOI] [PubMed] [Google Scholar]
- 41.Mathieu C, Dandona P, Gillard P, Senior P, Hasslacher C, Araki E, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care. 2018;41((9)):1938–1946. doi: 10.2337/dc18-0623. [DOI] [PubMed] [Google Scholar]
- 42.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42((8)):1593–603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapuerta P, Zambrowicz B, Strumph P, Sands A. Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor. Diab Vasc Dis Res. 2015;12((2)):101–110. doi: 10.1177/1479164114563304. [DOI] [PubMed] [Google Scholar]
- 44.Pieber TR, Famulla S, Eilbracht J, Cescutti J, Soleymanlou N, Johansen OE, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1) Diabetes Obes Metab. 2015;17((10)):928–935. doi: 10.1111/dom.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biester T, Aschemeier B, Fath M, Frey M, Scheerer MF, Kordonouri O, et al. Effects of dapagliflozin on insulin-requirement, glucose excretion and ß-hydroxybutyrate levels are not related to baseline HbA1c in youth with type 1 diabetes. Diabetes Obes Metab. 2017;19((11)):1635–1639. doi: 10.1111/dom.12975. [DOI] [PubMed] [Google Scholar]
- 46.Biester T, Muller I, von dem Berge T, Atlas E, Nimri R, Phillip M, et al. Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: the DAPADream study. Diabetes Obes Metab. 2021;23((2)):599–608. doi: 10.1111/dom.14258. [DOI] [PubMed] [Google Scholar]
- 47.Musso G, Sircana A, Saba F, Cassader M, Gambino R. Assessing the risk of ketoacidosis due to sodium-glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: a meta-analysis and meta-regression. Plos Med. 2020;17((12)):e1003461. doi: 10.1371/journal.pmed.1003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.European Medicines Agency New add-on treatment to insulin for treatment of certain patients with type 1 diabetes. 2022. Available from: https://www.ema.europa.eu/en/news/new-add-treatment-insulin-treatment-certain-patients-type-1-diabetes.
- 49.European Medicines Agency First oral add-on treatment to insulin for treatment of certain patients with type 1 diabetes. 2022. Available from: https://www.ema.europa.eu/en/news/first-oral-add-treatment-insulin-treatment-certain-patients-type-1-diabetes.
- 50.Adler AI, Ting S, Dent R, Latimer N. NICE guidance on dapagliflozin with insulin for type 1 diabetes. Lancet Diabetes Endocrinol. 2019;7((10)):750–751. doi: 10.1016/S2213-8587(19)30265-7. [DOI] [PubMed] [Google Scholar]
- 51.European Medicines Agency AstraZeneca. Forxiga (dapagliflozin) 5mg should no longer be used for the treatment of Type 1 Diabetes Mellitus. 2021. Available from: https://www.ema.europa.eu/en/medicines/dhpc/forxiga-dapagliflozin-5mg-should-no-longer-be-used-treatment-type-1-diabetes-mellitus#documents-section.
- 52.NICE Dapagliflozin with insulin for treating type 1 diabetes. Guidance. 2022. Available from: https://www.nice.org.uk/guidance/ta597.
- 53.Danne T, Garg S, Peters AL, Buse JB, Mathieu C, Pettus JH, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42((6)):1147–1154. doi: 10.2337/dc18-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purnell JQ, Zinman B, Brunzell JD, DCCT/EDIC Research Group The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the diabetes control and complications trial/epidemiology of diabetes interven. Circulation. 2013;127((2)):180–187. doi: 10.1161/CIRCULATIONAHA.111.077487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haidar A, Lovblom LE, Cardinez N, Gouchie-Provencher N, Orszag A, Tsoukas MA, et al. Empagliflozin add-on therapy to closed-loop insulin delivery in type 1 diabetes: a 2 × 2 factorial randomized crossover trial. Nat Med. 2022;28((6)):1269–1276. doi: 10.1038/s41591-022-01805-3. [DOI] [PubMed] [Google Scholar]