Abstract
Introduction
Rosacea is a common, facial, chronic inflammatory skin disease. Due to its complex pathogenesis, adequate therapy of rosacea can be challenging. An innovative recent therapeutic tool is cold atmospheric plasma (CAP), which is already established in the treatment of chronic wounds and promising in different other skin diseases.
Methods
In a split-face pilot study we investigated dielectric-barrier-discharged CAP in erythemato-telangiectatic (ETR) and/or papulopustular rosacea (PPR). CAP treatment was applied on lesional skin of a randomized side once daily (90 s/area) for 6 weeks. The other untreated side served as control. Co-primary endpoints were ≥1 improvement of the Investigator Global Assessment (IGA) score on the treated side compared to control and a decline of the Dermatology Life Quality Index (DLQI) after 6 weeks. Secondary endpoints included inflammatory lesion count (papules and pustules), skin redness intensity and erythema size. Adverse events (AEs) were recorded constantly. Additionally, participants were weekly assessed for symptoms, skin condition, trigger factors, skin care, treatment success, and local tolerance parameters. All p values were calculated using the Wilcoxon signed-rank test.
Results
Twelve subjects (ETR, n = 3; ETR and PPR, n = 9) completed the study. DLQI was significantly improved after 6 weeks (p = 0.007). On the CAP-treated side, lesions (p = 0.007) and erythema size (p = 0.041) were significantly reduced compared to the control. IGA (p = 0.2) and skin redness intensity (p = 0.5) did not differ significantly between control and CAP-treated side. No serious AEs occurred and treatment was well tolerated.
Conclusion
CAP is a promising new treatment of rosacea, especially for PPR.
Keywords: Rosacea, Cold atmospheric plasma, Plasma medicine, Pilot study, Split-face study
Introduction
Rosacea is a highly prevalent and chronic inflammatory skin disease that mainly involves central areas of the face, such as the forehead, nose, cheeks, and chin [1]. It is estimated to affect up to 5.5% of the world’s population, with the highest prevalence in the age group of 45–60 years [2, 3]. A multifactorial etiology is assumed, involving genetics as well as environmental factors. Furthermore, exacerbation of rosacea can be caused by a variety of endogenous and exogenous triggers, e.g., stress, heat, spicy food, menstruation, and UV radiation [4–6]. Symbiotic microbiota, in particular Demodex folliculorum, have been identified as another component of the rosacea pathogenesis with an increased density of Demodex mites in lesional skin [7–9].
By activating toll-like receptors (e.g., TLR2) and the inflammasome, Demodex induces inflammation [10]. Furthermore, the acquired immune system plays a central role in the rosacea pathogenesis [11]. Mechanistically, granulocytes, macrophages, and dendritic cells are stimulated, releasing antimicrobial peptides and pro-inflammatory cytokines [12]. Additionally, other immune cells, such as T cells, mast cells, and plasma cells, are enriched in all cutaneous rosacea subtypes [13].
The clinical presentation of rosacea is fluctuating. It is traditionally classified into four subtypes, each with its own constellation of signs and symptoms: erythemato-telangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea [14]. These subtypes can appear isolated, in chronological order, or coexist. The ETR subtype is characterized by vascular features of flushing, erythema, and telangiectasia (rosacea with only vascular symptoms). PPR, on the other hand, consists of inflammatory lesions (rosacea with papulopustules) [9, 14, 15].
Rosacea treatment strategies primarily include the identification and avoidance of trigger factors. Besides, different topical agents are currently listed in European recommendations for the treatment of rosacea [16, 17]. Brimonidine is approved for symptomatic treatment of erythema. In contrast, metronidazole, azelaic acid, and ivermectin are treatment options for PPR. Due to rosacea’s high global prevalence with a partially serious burden on patients and the unmet need for treatments targeting more than one subtype, there is a great demand for optimization of treatment [18].
Cold atmospheric plasma (CAP) has emerged as a promising new tool for the treatment of diverse medical indications. CAP is well established for wound therapy and has shown promising results in the treatment of cancer, acne, psoriasis, and other immune-mediated skin diseases [19, 20]. It contains various active agents, yet reactive oxygen and nitrogen species are considered to be the most relevant [21, 22]. CAP acts as an immunomodulatory effector by its impact on inflammatory cytokines and immune cells in different skin diseases [23–25]. In addition, CAP has well-characterized antimicrobial properties, including activity against Demodex mites [26, 27].
Mechanistically, CAP devices can be classified into two different types: direct plasma (e.g., generated by dielectric barrier discharge [DBD]) or indirect plasma (jet plasma). DBD plasma devices consist of a single electrode covered with a dielectric using the skin as a counter-electrode. Hence, plasma is generated directly on the skin surface [28]. In the light of its promising properties, we utilized DBD-generated CAP to investigate in a pilot study its response and tolerability in the treatment of rosacea.
Materials and Methods
Study Design
Our study was conducted as a prospective, investigator-initiated, randomized controlled study at the Clinic for Dermatology and Venereology of the University Medical Center Rostock, Germany. Recruitment time was from February to May 2022. The study included participants with ETR and/or PPR. Exclusion criteria involved any topical or systemic treatment of rosacea, e.g., with corticosteroids, antibiotics, immunosuppressive agents, or laser therapy within the 4 weeks prior to participation; pregnancy; age below 18 years; implants (especially if electroconductive); arrhythmia; heart failure during the previous 6 months; or suffering from epileptic seizures [29]. Daily skin care routine could be continued, all other rosacea-directed treatments were prohibited during the duration of the study.
Plasma Treatment and Photo Documentation
All study participants performed a randomized half-sided treatment for 6 weeks. The device used for this study was the DBD device PlasmaDerm® Flex (CINOGY Technologies GmbH, Duderstadt, Germany). The PlasmaDerm® device consists of a control unit connected to a handset. A disposable plasma applicator (spacer) is connected to the handset. The spacer is positioned directly on the skin area to be treated and ensures a gap with environmental air between spacer and skin. For safety reasons, the device can only be started by pressing the on button, thus no changes of technical parameters are possible. Via a display timer, an exact treatment duration of 90 s per area is adjusted.
Computer-based randomization was applied to allocate treatment to a CAP-treated and an untreated control half. Treatment was performed on affected skin of the chin, cheek, and forehead. The nose was omitted from the treatment to ensure an exact separation of both face sides. All participants were thoroughly instructed, how to use their CAP devices, and the first treatment was conducted on site. At home, they were asked to perform treatment in front of a mirror and correct application on curved face areas was ensured by constant swinging of the spacer.
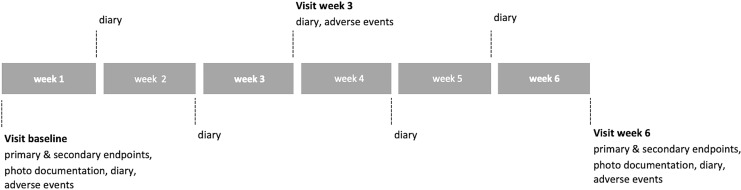
Study visits took place at baseline, after three, and after 6 weeks of CAP treatment. At baseline and after 6 weeks (shown in Fig. 1), a standardized photo documentation was performed with frontal and lateral photos, which were evaluated by two blinded investigators. In case of disagreement, a third investigator was consulted. Outside of the study, participants were offered to perform CAP treatment on both face halves for an additional 6 weeks.
Fig. 1.
Study timeline indicating visits and endpoints recorded.
Primary Endpoints: IGA Score and DLQI
As primary endpoints, the Investigator’s Global Assessment (IGA) of Rosacea severity score and the Dermatology Life Quality Index (DLQI, 0–30) were gathered at baseline and week six (shown in Fig. 1). The IGA combined erythema assessment with the amount and size of inflammatory lesions resulting in five grades (grades: 0–4) (Table 1) [30]. IGA improvement of ≥1 on the treated side compared to control side was considered as treatment success.
Table 1.
Investigator global assessment score
| Grade | Description | Amount/size of inflammatory lesions | Erythema |
|---|---|---|---|
| 0 | Clear | None | None |
| 1 | Almost clear | Very few small papules/pustules | Very mild |
| 2 | Mild | Few small papules/pustules | Mild |
| 3 | Moderate | Several small or large papules/pustules | Moderate |
| 4 | Severe | Numerous small and/or large papules/pustules | Severe |
The DLQI score is a clinical assessment questionnaire developed specifically for dermatologic conditions and measures the individual quality of life (QOL) restriction [31]. It consists of a 10-item scale and reaches scores from 0 to 30. In our study, an improvement from baseline to week six on the treated half in comparison to the control half was considered as treatment success.
Secondary Endpoints: Lesion Count, Skin Redness Intensity, and Erythema Size
All secondary endpoints were assessed at baseline and after 6 weeks. The absolute lesion count was assessed in PPR and included numbering of papules and pustules by the investigator. For evaluation of skin redness intensity and erythema size in ETR, the photos were assessed using the picture-analyzing program ImageJ® (available at https://imagej.net/ij/index.html, last accessed March 8, 2023).
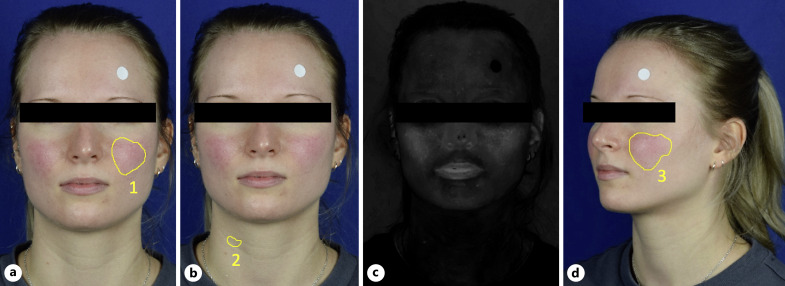
The analysis of skin redness intensity was performed as previously described by Logger et al. [32]. In short, to quantify the mean red intensity, the color space CIE method L*a*b* (L, lightness; a, green-red ratio; b, blue-yellow ratio) was utilized (shown in Fig. 2a–c). First, the most intense visible facial erythema (=lesional skin) was defined as the region of interest (ROI) 1 using frontal images. Second, a non-lesional area was defined as non-lesional ROI 2. Third, the image was converted to CIE L*a*b* color space. The proportion of red (α) of both ROIs was calculated separately and then their ratio was formed (∆α).
Fig. 2.
Photo analysis of a female participant with ETR using ImageJ. a Erythema was defined as region of interest (ROI) 1 in the frontal images. A white sticker served as size reference. b ROI 2 was selected on a non-lesional region of the neck. c CIE L*a*b color space filter was applied to analyze mean intensity of skin redness α separately for ROI 1 and ROI 2, which were then set in relation to each other (resulting in ∆α). d Erythema size (ROI 3) was calculated according to the reference point in the lateral images.
Facial erythema was measured in a stepwise process (shown in Fig. 2d). First, the erythema was identified as ROI 3 in the lateral photos. Second, a white sticker of determined size was used as size reference. Third, the size of ROI 3 was calculated in relation to the reference point.
Rosacea Diary
The diary was obtained from the German awareness campaign “Aktiv gegen Rosacea,” which is sponsored by Galderma Laboratorium GmbH, modified for the purposes of our study and answered weekly [33] (shown in online suppl. Tables 1, 2; for all online suppl. material, see https://doi.org/10.1159/000533190). It enabled our participants to rate on a five-point scale the subjective expression of main (erythema and/or papules/pustules) and additional symptoms (burning, stinging, dry appearance) from “not affected” to “very severe,” and their current skin condition from “very good” to “severe.” Furthermore, it assessed the appearance of predefined triggers (weather, spicy foods, alcohol, stress), application of specific skin care products, notification of CAP treatment success, and occurrence of local discomfort (irritation or pain). Furthermore, regular CAP utilization was monitored. In addition, participants were able to comment on their subjective experience with CAP in a free-text field.
Adverse Events
Adverse events (AEs) were recorded separately for both face sides adhering to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 and their relation to CAP treatment was assessed [34]. Accordingly, appearance only and/or with a higher intensity on the CAP-treated face side was regarded as treatment-related.
Statistics
IBM SPSS Statistics for Windows (version 25, IBM Corp., Armonk, NY, USA) was used for statistical analysis. All p values were derived from Wilcoxon signed-rank tests. A p value of p < 0.05 was regarded as statistically significant. The p values were not adjusted for multiple testing.
Results
Baseline Characteristics
Twelve participants were included. Their median age was 50.5 years. Each participant had used topical and/or systemic guideline medication for rosacea prior to the study. Baseline characteristics are summarized in Table 2.
Table 2.
Baseline characteristics
| Characteristic | Value |
|---|---|
| Total patients, n | 12 |
| Female/male | 10/2 |
| Age, years | |
| Median (min-max) | 50.5 (27–77) |
| Subtype, n | |
| ETR | 3 |
| ETR + PPR | 9 |
| DLQI score, 0–30 points | |
| Median (min-max) | 2.5 (1–6) |
| Baseline medians for both face sides | Control | Treated | p value (control vs. treated) |
|---|---|---|---|
| IGA score, 0–4 grades | 3 | 3 | 0.6 |
| Lesion count, n | 7 | 6 | 0.3 |
| Intensity of skin redness Δα | 13.0 | 11.9 | 0.5 |
| Erythema size, cm2 | 10.3 | 11.7 | 0.1 |
DLQI, Dermatology Life Quality Index; ETR, erythemato-telangiectatic rosacea; IGA, Investigator Global Assessment; PPR, papulopustular rosacea.
p value <0.05 was considered as statistically significant.
Primary Endpoints
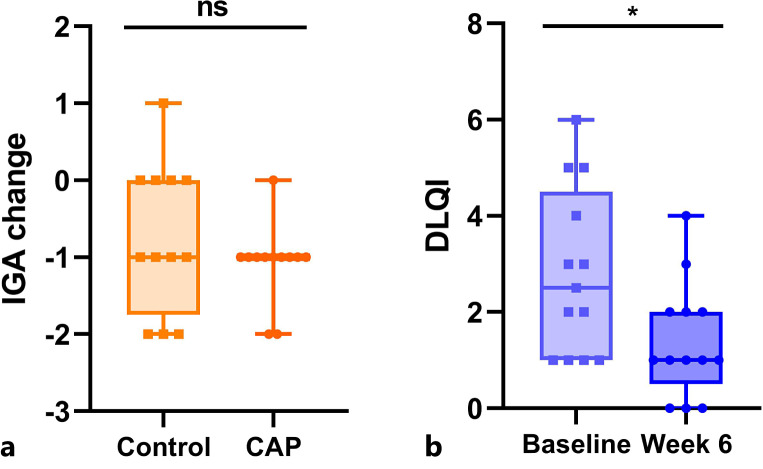
Both, in the treated (median change = −1.0) as well as the untreated (median change = −1.0) areas, the IGA scores reduced from baseline to week six (p = 0.2 for the difference of treatment vs. control (mediant-c) = 0.0) (shown in Fig. 3a). The median DLQI at baseline was 2.5 and decreased to 1.0 (p = 0.007) (shown in Fig. 3b).
Fig. 3.
Boxplots of primary endpoints. a IGA change from baseline to week six. b Proband-specific DLQI at baseline and week six. CAP, cold atmospheric plasma; ns, not significant; *p < 0.05.
Secondary Endpoints
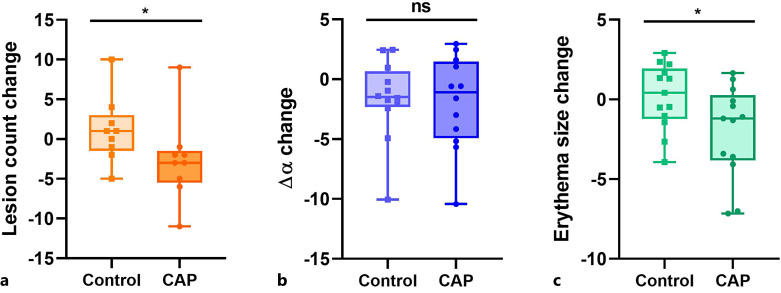
A decrease of lesion count was observed on the treated side (median change = −3.0), however, lesions increased on the control side (median change = +1.0) (p = 0.007; mediant-c = −4.0) (shown in Fig. 4a). The intensity of skin redness (∆α) declined on both face sides (median changes control = −1.5; CAP = −1.1; p = 0.5; mediant-c = −0.4) (shown in Fig. 4b).
Fig. 4.
Boxplots for the change of secondary endpoints from baseline to week six. a Lesion count, b intensity of skin redness ∆α, c erythema size. CAP, cold atmospheric plasma; ns, not significant; *p < 0.05.
Furthermore, a favorable change of erythema size was detected. It decreased on the CAP-treated side (median change = −1.2), while it increased on the control side (median change = +0.4) (p = 0.041; mediant-c = −1.0) (shown in Fig. 4c; online suppl. Fig. 1 exemplifies assessment of endpoints).
Rosacea Diary
CAP was administered regularly. The participants’ perception of main and additional symptoms, as well as their general skin condition did not change significantly from baseline to week six. Also, the occurrence of triggering factors and application of skin care products were constant throughout the whole treatment period. Moreover, CAP did not cause irritation or pain. Most importantly, ten (83%) participants agreed that after 6 weeks, their CAP treatment has been successful. The treatment was subjectively assessed as comfortable, skin smoothing and effective in mitigating exacerbation due to triggering factors.
Additional Treatment
Participants were offered to use CAP on both sides of the face for an additional 6 weeks, which was realized by eight participants (ETR, n = 1; ETR and PPR, n = 7). Notably, inflammatory lesions now also declined on the former control side (median change from week six to week twelve = −6.0, p = 0.03). On the contralateral side, which was CAP treated for 12 weeks, lesions could be reduced even further (median change = −2.0; p = 0.04). All other endpoints were not significantly changed on both sides.
Adverse Events
In total, five participants noticed AEs of five different categories (online suppl. Table 3): paresthesia (n = 2), tension of the skin (n = 1), dry skin (n = 1), papulopustular rash (n = 1), and erythema (n = 1). All AEs were mild and only in three cases (paresthesia, tension of the skin, dry skin) AEs were regarded as treatment-related. None of the enrolled participants terminated the study prematurely due to AEs or because of other reasons.
Discussion
CAP is used in a variety of clinical settings with great success [35]. Well-established applications include chronic wound therapy and disinfection [36]. Furthermore, different studies have suggested CAP for the treatment of cancer [37, 38]. Atopic dermatitis, psoriasis, and herpes zoster, among others, are further skin diseases in which CAP has been successfully applied [23, 39–42]. Recently, Karrer et al. [43] used a jet plasma device in a prospective, randomized, controlled, split-face trial to assess its safety and efficacy in the treatment of acne vulgaris. Reduction of particularly inflammatory lesions after ten CAP treatments within 4 to 6 weeks was significantly higher on the CAP treated than on the untreated side. Also, percentage of patients reporting improved aesthetics was higher for the treated than for the untreated side after treatment completion and at the 2- and 4-week follow-up [43].
Accordingly, in our pilot study we observed a decrease of papular and pustular lesions only on the CAP-treated side (CAP vs. control, p = 0.007). Just recently, the concept of PPR as a chronic demodex infection probably associated with T-cell exhaustion has been reasoned extensively [9]. CAP was shown to be capable of both, reduction of demodex count on the face comparable to topical ivermectin and modulation of immune cells [24, 44]. Hence, the positive effects of CAP on PPR detected in our study are plausible.
Transient or persistent facial erythema is the most common feature of the different cutaneous rosacea subtypes and was present in all participants of our study [45]. We analyzed erythema size with ImageJ, and only in the CAP-treated side a decrease was detected (CAP vs. control; p = 0.041). Importantly, we did not differentiate between transient or persistent erythema. Transient flushes are produced by neurogenic inflammation through various triggers [9, 46, 47]. Furthermore, in persistent erythema a perilesional redness may be distinguished, which can be based on the sustained vasodilatation and plasma extravasation induced by the inflammatory infiltrates [45, 48]. Hence, the immune-modulating properties of CAP might have been associated with the reduction of erythema size. However, the complex vicious circle of vascular and inflammatory changes in rosacea is characterized by a persistent dilation of vessels, neoangiogenesis, telangiectasias, and derma matrix degradation, which are often insufficiently cured by anti-inflammatory treatments [45]. Likewise, daily CAP treatment over 6 weeks was not able to diminish median skin redness intensity ∆α more than the control (comparison of ∆α for control and CAP-treated side not significant). Of note, α correlates with hemoglobin, skin blood flow, and vascularization [32]. Accordingly, in a former study of acute wound healing, we revealed that DBD-based CAP increased hemoglobin distribution in the microcirculatory system and oxygen saturation in deeper tissue layers [49]. A stimulation of microcirculation of the skin has also been shown by others [50–54]. This phenomenon might have negatively influenced the effect on skin redness intensity despite CAP’s immune-modulating efficacy. Nevertheless, absolute ∆α-values decreased on both sides, control and treatment. Hence, further experiments are necessary to elucidate the detailed implication of CAP on the rosacea pathogenesis, in particular its immune-modulating activity.
Rosacea is often associated with a considerable burden for patients. Thus, it can negatively affect QOL, as well as social and psychological well-being [55–58]. In our study, we assessed the individual QOL using the common DLQI questionnaire. Although our study participants did not have a high strain at baseline, we observed a significantly improved DLQI after 6 weeks (p = 0.007; Fig. 3b). Accordingly, participants evaluated CAP treatment very positively in their weekly diary. Consistent with the excellent tolerability of CAP in other studies, AEs in our pilot trial were rare, mild, and only partly related to CAP [49, 59, 60].
An ≥1 IGA improvement of CAP-treated compared to control side after 6 weeks was another co-primary endpoint. On both sides, the IGA score declined by a median of 1.0 grade, and no significant differences were detected between the two sides (p = 0.2). Importantly, IGA scoring included a combined assessment of erythema and inflammatory lesions and could therefore not distinguish between relief of vascular symptoms or improvement of inflammatory lesions. The rosacea area and severity index is a newly developed scoring system for clinical assessment of rosacea severity that allows for a more nuanced evaluation of rosacea subtypes/phenotypes, and its use might be favorable in future trials [61].
In our pilot study, CAP was used as a monotherapy. However, different studies have revealed a great potential of CAP as a combination partner with other, in particular oncological, treatments [62–64]. In rosacea, using CAP in conjunction with other treatments might also be advantageous due to different aspects: On the one hand, CAP combination with topical drugs might increase their efficacy due to its enhancement of stratum corneum permeabilization [65, 66]. On the other hand, combined systemic and local treatment is the common standard for difficult-to-treat rosacea. CAP is particularly useful for combination with systemic treatment due to its antimicrobial properties, which are neglectable for systemic low-dose antibiotics like doxycycline [17, 67].
Taieb et al. [68] evaluated the costs of ivermectin, metronidazole, and azelaic acid for the topical treatment of PPR in the USA in 2014, which varied from $5.08–$6.60 per day. At present, CAP treatment is not reimbursed by the statutory health insurance in Germany and cost estimations derived from the current configuration of the utilized PlasmaDerm® device would be without reliable accuracy. Nevertheless, a once purchased device, which can be repetitively utilized, might be an attractive and cost-effective treatment alternative, in particular considering the long-term therapy of rosacea.
Our study has several limitations. First, because of its design as a monocentric pilot study, the number of participants was low. Second, we utilized the “old” rosacea classification into subtypes, although the National Rosacea Society Expert Committee now recommends the use of phenotypes and the differentiation according to diagnostic, major, and minor (secondary) symptoms [1]. This updated classification reflects the clinical reality better. However, the simple distinction of ETR and PPR was more convenient for our study since some of the phenotypes were either not present (e.g., phymatous changes) or not specifically considered (e.g., transient vs. persistent erythema). Third, DLQI is a general dermatological QOL questionnaire, which did not distinguish between the two sides of this split-face study. That is why a diary was additionally filled by participants that consistently revealed a positive perception of CAP treatment. The complementary use of the rosacea-specific QOL questionnaire RosaQoL might be beneficial in the future [69, 70].
Despite all these limitations, our study provides significant new findings: CAP was effective in the reduction of papulopustular lesions and erythema size in rosacea. Furthermore, we observed an improved QOL and the half-sided CAP treatment was well tolerated. Further studies with a larger sample size are needed to confirm our results and should put a special focus on treatment of inflammatory lesions and combination with other agents.
Acknowledgments
We first would like to thank all participants. Furthermore, we thank Dr. Miriam Mann and Gesine Bandow for participant acquisition as well as Birka Stroth for assistance with the photo documentation. Lastly, we would like to thank Laurits Hofmeyer, who was consulted for photo evaluation if the two other independent investigators disagreed.
Statement of Ethics
This study has been approved by the Ethics Committee of the Medical Faculty, University of Rostock (study reference: A 2021-0275) and was conducted adhering to the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant before study participation for publication of their images.
Conflict of Interest Statement
SE received research funding and speakers’ honoraria from CINOGY GmbH and research funding from Teion Medizinprodukte GmbH. The other authors have no conflicts of interest to declare.
Funding Sources
This work was supported by CINOGY System GmbH, Duderstadt, Germany. The funder had no role in the study design, collection, analysis, or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication.
Author Contributions
The study was concepted and designed by S.H. and A.T.; S.H. performed the study. S.G. and A.T. evaluated the photos. S.H. and A.T. were responsible for the data analysis and interpretation. S.H. performed the statistical analysis, with methodological support by F.W. Administrative, technical, and material support was provided by S.E. The first manuscript draft was provided by S.H.; A.T. reviewed and edited the script. All authors have participated in the critical revision of the manuscript regarding important intellectual content.
Funding Statement
This work was supported by CINOGY System GmbH, Duderstadt, Germany. The funder had no role in the study design, collection, analysis, or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the national rosacea society expert committee. J Am Acad Dermatol. 2018 Jan;78(1):148–55. 10.1016/j.jaad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 2. Augustin M, Herberger K, Hintzen S, Heigel H, Franzke N, Schafer I. Prevalence of skin lesions and need for treatment in a cohort of 90 880 workers. Br J Dermatol. 2011 Oct;165(4):865–73. 10.1111/j.1365-2133.2011.10436.x. [DOI] [PubMed] [Google Scholar]
- 3. Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018 Aug;179(2):282–9. 10.1111/bjd.16481. [DOI] [PubMed] [Google Scholar]
- 4. Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013 Dec;69(6 Suppl 1):S15–26. 10.1016/j.jaad.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 5. Wu WH, Geng H, Cho E, Eliassen AH, Drucker AM, Li TY, et al. Reproductive and hormonal factors and risk of incident rosacea among US White women. J Am Acad Dermatol. 2022 Jul;87(1):138–40. 10.1016/j.jaad.2021.06.865. [DOI] [PubMed] [Google Scholar]
- 6. Yamasaki K, Miyachi Y. Perspectives on rosacea patient characteristics and quality of life using baseline data from a phase 3 clinical study conducted in Japan. J Dermatol. 2022 Dec;49(12):1221–7. 10.1111/1346-8138.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casas C, Paul C, Lahfa M, Livideanu B, Lejeune O, Alvarez-Georges S, et al. Quantification of demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol. 2012 Dec;21(12):906–10. 10.1111/exd.12030. [DOI] [PubMed] [Google Scholar]
- 8. Holmes AD. Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013 Dec;69(6):1025–32. 10.1016/j.jaad.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 9. Forton FMN. Rosacea, an infectious disease: why rosacea with papulopustules should be considered a demodicosis. A narrative review. J Eur Acad Dermatol Venereol. 2022 Jul;36(7):987–1002. 10.1111/jdv.18049. [DOI] [PubMed] [Google Scholar]
- 10. Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885. 10.12688/f1000research.16537.1. [DOI] [Google Scholar]
- 11. Steinhoff M, Buddenkotte J, Aubert J, Sulk M, Novak P, Schwab VD, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011 Dec;15(1):2–11. 10.1038/jidsymp.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holmes AD, Steinhoff M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp Dermatol. 2017 Aug;26(8):659–67. 10.1111/exd.13143. [DOI] [PubMed] [Google Scholar]
- 13. Buhl T, Sulk M, Nowak P, Buddenkotte J, McDonald I, Aubert J, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015 Sep;135(9):2198–208. 10.1038/jid.2015.141. [DOI] [PubMed] [Google Scholar]
- 14. Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, et al. Standard grading system for rosacea: report of the national rosacea society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004 Jun;50(6):907–12. 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 15. Forton FMN. The pathogenic role of demodex mites in rosacea: a potential therapeutic target already in erythematotelangiectatic rosacea? Dermatol Ther. 2020 Dec;10(6):1229–53. 10.1007/s13555-020-00458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hampton PJ, Berth-Jones J, Duarte Williamson CE, Hay R, Leslie TA, Porter I, et al. British association of dermatologists guidelines for the management of people with rosacea 2021. Br J Dermatol. 2021 Oct;185(4):725–35. 10.1111/bjd.20485. [DOI] [PubMed] [Google Scholar]
- 17. Clanner-Engelshofen BM, Bernhard D, Dargatz S, Flaig MJ, Gieler U, Kinberger M, et al. S2k-Leitlinie: rosacea. J Dtsch Dermatol Ges. 2022 Aug;20(8):1147–67. 10.1111/ddg.14849_g. [DOI] [PubMed] [Google Scholar]
- 18. Jackson JM, Coulon R, Arbiser JL. Evaluation of a first-in-class proteasome inhibitor in patients with moderate to severe rosacea. J Drugs Dermatol. 2021 Jun 1;20(6):660–4. 10.36849/JDD.2021.5925. [DOI] [PubMed] [Google Scholar]
- 19. Busco G, Robert E, Chettouh-Hammas N, Pouvesle JM, Grillon C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic Biol Med. 2020 Dec;161:290–304. 10.1016/j.freeradbiomed.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Deutsche Gesellschaft für Mund-, Kiefer- und Gesichtschirurgie e.V. (DGMKG). Rationaler therapeutischer Einsatz von kaltem physikalischem Plasma. Available from: https://register.awmf.org/de/leitlinien/detail/007-107 (Accessed at Mar 5, 2023).
- 21. Heinlin J, Morfill G, Landthaler M, Stolz W, Isbary G, Zimmermann JL, et al. Plasma medicine: possible applications in dermatology. J Dtsch Dermatol Ges. 2010 Dec;8(12):968–76. 10.1111/j.1610-0387.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- 22. Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys. 2012;45(26):263001. 10.1088/0022-3727/45/26/263001. [DOI] [Google Scholar]
- 23. Lee YS, Lee MH, Kim HJ, Won HR, Kim CH. Non-thermal atmospheric plasma ameliorates imiquimod-induced psoriasis-like skin inflammation in mice through inhibition of immune responses and up-regulation of PD-L1 expression. Sci Rep. 2017 Nov 14;7(1):15564. 10.1038/s41598-017-15725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smolkova B, Frtus A, Uzhytchak M, Lunova M, Kubinová Š, Dejneka A, et al. Critical analysis of non-thermal plasma-driven modulation of immune cells from clinical perspective. Int J Mol Sci. 2020 Aug 28;21(17):6226. 10.3390/ijms21176226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seebauer C, Freund E, Hasse S, Miller V, Segebarth M, Lucas C, et al. Effects of cold physical plasma on oral lichen planus: an in vitro study (effects of CAP on OLP). Oral Dis. 2021 Oct;27(7):1728–37. 10.1111/odi.13697. [DOI] [PubMed] [Google Scholar]
- 26. Daeschlein G, Scholz S, Arnold A, von Woedtke T, Kindel E, Niggemeier M, et al. In vitro activity of atmospheric pressure plasma jet (APPJ) plasma against clinical isolates of demodex folliculorum. IEEE Trans Plasma Sci. 2010;38(10):2969–73. 10.1109/tps.2010.2061870. [DOI] [Google Scholar]
- 27. Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010 Jul;163(1):78–82. 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- 28. Metelmann HR, von Woedtke T, Weltmann KD. Plasmamedizin. 1 ed. Heidelberg: Springer Berlin; 2016. [Google Scholar]
- 29. Cinogy System GmbH . Instructions for use plasmaderm® flex plasmaderm® cutan. Duderstadt: CINOGY System GmbH; 2021. [Google Scholar]
- 30. Schaller M, Dirschka T, Kemeny L, Briantais P, Jacovella J. Superior efficacy with ivermectin 1% cream compared to metronidazole 0.75% cream contributes to a better quality of life in patients with severe papulopustular rosacea: a subanalysis of the randomized, investigator-blinded attract study. Dermatol Ther. 2016 Sep;6(3):427–36. 10.1007/s13555-016-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994 May;19(3):210–6. 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 32. Logger JGM, de Jong E, Driessen RJB, van Erp PEJ. Evaluation of a simple image-based tool to quantify facial erythema in rosacea during treatment. Skin Res Technol. 2020 Nov;26(6):804–12. 10.1111/srt.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aktiv gegen Rosazea . Ihr personliches Rosacea-Tagebuch. Available from: https://www.rosacea-info.de/pdf-download/rosacea-tagebuch.pdf (Accessed at Mar 5, 2023). [Google Scholar]
- 34.National Cancer Institute; National Institutes of Health; U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (Accessed at Mar 8, 2023).
- 35. Bernhardt T, Semmler ML, Schafer M, Bekeschus S, Emmert S, Boeckmann L. Plasma medicine: applications of cold atmospheric pressure plasma in dermatology. Oxid Med Cel Longev. 2019;2019:3873928. 10.1155/2019/3873928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gan L, Zhang S, Poorun D, Liu D, Lu X, He M, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018 Jan;16(1):7–13. 10.1111/ddg.13373. [DOI] [PubMed] [Google Scholar]
- 37. Semmler ML, Bekeschus S, Schafer M, Bernhardt T, Fischer T, Witzke K, et al. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (CAP) in cancer treatment. Cancers. 2020 Jan 22;12(2):269. 10.3390/cancers12020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miebach L, Freund E, Clemen R, Kersting S, Partecke LI, Bekeschus S. Gas plasma-oxidized sodium chloride acts via hydrogen peroxide in a model of peritoneal carcinomatosis. Proc Natl Acad Sci USA. 2022 Aug 2;119(31):e2200708119. 10.1073/pnas.2200708119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Woedtke T, Emmert S, Metelmann HR, Rupf S, Weltmann KD. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Phys Plasmas. 2020;27(7):070601. 10.1063/5.0008093. [DOI] [Google Scholar]
- 40. Moon IJ, Yun MR, Yoon HK, Lee KH, Choi SY, Lee WJ, et al. Treatment of atopic dermatitis using non-thermal atmospheric plasma in an animal model. Sci Rep. 2021 Aug 9;11(1):16091. 10.1038/s41598-021-95471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim N, Lee S, Lee S, Kang J, Choi YA, Park J, et al. Portable cold atmospheric plasma patch-mediated skin anti-inflammatory therapy. Adv Sci. 2022 Dec;9(34):e2202800. 10.1002/advs.202202800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan F, Wang Y, Zhang S, Shui R, Chen J. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. 10.3389/fonc.2022.918484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karrer S, Berneburg M, Zeman F, Koller M, Müller K. A prospective, randomised, controlled, split-face clinical trial to assess the safety and the efficacy of cold atmospheric plasma in the treatment of acne vulgaris. Appl Sci. 2021;11(23):11181. 10.3390/app112311181. [DOI] [Google Scholar]
- 44. Malik S, Gill M, Fridman G, Fridman A, Friedman PC. Cold atmospheric plasma reduces demodex count on the face comparably to topical ivermectin, as measured by reflectance confocal microscopy. Exp Dermatol. 2022 Sep;31(9):1352–4. 10.1111/exd.14584. [DOI] [PubMed] [Google Scholar]
- 45. Steinhoff M, Schmelz M, Schauber J. Facial erythema of rosacea: aetiology, different pathophysiologies and treatment options. Acta Derm Venereol. 2016 Jun 15;96(5):579–86. 10.2340/00015555-2335. [DOI] [PubMed] [Google Scholar]
- 46. Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014 Jan;94(1):265–301. 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reinholz M, Ruzicka T, Steinhoff M, Schaller M, Gieler U, Schofer H, et al. Pathogenese und Klinik der Rosazea als Schlüssel für eine symptomorientierte Therapie. J Dtsch Dermatol Ges. 2016 Dec;14(S6):4–16. 10.1111/ddg.13139_g. [DOI] [PubMed] [Google Scholar]
- 48. Schwab VD, Sulk M, Seeliger S, Nowak P, Aubert J, Mess C, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011 Dec;15(1):53–62. 10.1038/jidsymp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Welzen A, Hoch M, Wahl P, Weber F, Rode S, Tietze JK, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021 Nov;34(6):328–36. 10.1159/000517524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fluhr JW, Sassning S, Lademann O, Darvin ME, Schanzer S, Kramer A, et al. In vivo skin treatment with tissue-tolerable plasma influences skin physiology and antioxidant profile in human stratum corneum. Exp Dermatol. 2012 Feb;21(2):130–4. 10.1111/j.1600-0625.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- 51. Heuer K, Hoffmanns MA, Demir E, Baldus S, Volkmar CM, Rohle M, et al. The topical use of non-thermal dielectric barrier discharge (DBD): nitric oxide related effects on human skin. Nitric Oxide. 2015 Jan 30;44:52–60. 10.1016/j.niox.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 52. Kisch T, Schleusser S, Helmke A, Mauss KL, Wenzel ET, Hasemann B, et al. The repetitive use of non-thermal dielectric barrier discharge plasma boosts cutaneous microcirculatory effects. Microvasc Res. 2016 Jul;106:8–13. 10.1016/j.mvr.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 53. Borchardt T, Ernst J, Helmke A, Tanyeli M, Schilling AF, Felmerer G, et al. Effect of direct cold atmospheric plasma (diCAP) on microcirculation of intact skin in a controlled mechanical environment. Microcirculation. 2017 Nov;24(8):e12399. 10.1111/micc.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borchardt T, Helmke A, Ernst J, Emmert S, Schilling AF, Felmerer G, et al. Topically confined enhancement of cutaneous microcirculation by cold plasma. Skin Pharmacol Physiol. 2022;35(6):343–53. 10.1159/000527700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014 Nov;71(5):973–80. 10.1016/j.jaad.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 56. Bewley A, Fowler J, Schofer H, Kerrouche N, Rives V. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis. Dermatol Ther. 2016 Jun;6(2):237–47. 10.1007/s13555-016-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oussedik E, Bourcier M, Tan J. Psychosocial burden and other impacts of rosacea on patients’ quality of life. Dermatol Clin. 2018 Apr;36(2):103–13. 10.1016/j.det.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 58. Huang Y, Yan S, Xie H, Wang B, Zhao Z, Huang Y, et al. Health related quality of life of rosacea patients in China assessed by dermatology life quality index and willingness to pay. Patient Prefer Adherence. 2022 Mar;16:659–70. 10.2147/PPA.S345258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Daeschlein G, Scholz S, Ahmed R, Majumdar A, von Woedtke T, Haase H, et al. Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture. J Dtsch Dermatol Ges. 2012 Jul;10(7):509–15. 10.1111/j.1610-0387.2012.07857.x. [DOI] [PubMed] [Google Scholar]
- 60. Rutkowski R, Daeschlein G, von Woedtke T, Smeets R, Gosau M, Metelmann HR. Long-term risk assessment for medical application of cold atmospheric pressure plasma. Diagnostics. 2020 Apr 11;10(4):210. 10.3390/diagnostics10040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wienholtz NKF, Thyssen JP, Christensen CE, Thomsen SF, Karmisholt KE, Jemec GBE, et al. Validity and reliability of the rosacea area and severity index: a novel scoring system for clinical assessment of rosacea severity. J Eur Acad Dermatol Venereol. 2023 Mar;37(3):573–80. 10.1111/jdv.18721. [DOI] [PubMed] [Google Scholar]
- 62. Chen G, Chen Z, Wen D, Wang Z, Li H, Zeng Y, et al. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc Natl Acad Sci USA. 2020 Feb 18;117(7):3687–92. 10.1073/pnas.1917891117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gjika E, Pal-Ghosh S, Kirschner ME, Lin L, Sherman JH, Stepp MA, et al. Combination therapy of cold atmospheric plasma (CAP) with temozolomide in the treatment of U87MG glioblastoma cells. Sci Rep. 2020 Oct 5;10(1):16495. 10.1038/s41598-020-73457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ha JH, Kim YJ. Photodynamic and cold atmospheric plasma combination therapy using polymeric nanoparticles for the synergistic treatment of cervical cancer. Int J Mol Sci. 2021 Jan 25;22(3):1172. 10.3390/ijms22031172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gelker M, Mrotzek J, Ichter A, Muller-Goymann CC, Viol W. Influence of pulse characteristics and power density on stratum corneum permeabilization by dielectric barrier discharge. Biochim Biophys Acta Gen Subj. 2019 Oct;1863(10):1513–23. 10.1016/j.bbagen.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 66. Wen X, Xin Y, Hamblin MR, Jiang X. Applications of cold atmospheric plasma for transdermal drug delivery: a review. Drug Deliv Transl Res. 2021 Jun;11(3):741–7. 10.1007/s13346-020-00808-2. [DOI] [PubMed] [Google Scholar]
- 67. Steinhoff M, Vocanson M, Voegel JJ, Hacini-Rachinel F, Schafer G. Topical ivermectin 10 mg/g and oral doxycycline 40 mg modified-release: current evidence on the complementary use of anti-inflammatory rosacea treatments. Adv Ther. 2016 Sep;33(9):1481–501. 10.1007/s12325-016-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Taieb A, Stein Gold L, Feldman SR, Dansk V, Bertranou E. Cost-effectiveness of ivermectin 1% cream in adults with papulopustular rosacea in the United States. J Manag Care Spec Pharm. 2016 Jun;22(6):654–65. 10.18553/jmcp.2016.15210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nicholson K, Abramova L, Chren MM, Yeung J, Chon SY, Chen SC. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007 Aug;57(2):213–21. 10.1016/j.jaad.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 70. Chernyshov PV, Finlay AY, Tomas-Aragones L, Steinhoff M, Manolache L, Pustisek N, et al. Quality of life measurement in rosacea. Position statement of the European academy of dermatology and Venereology task forces on quality of life and patient oriented outcomes and acne, rosacea and hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2023 May;37(5):954–64. 10.1111/jdv.18918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.