Abstract
Several studies have demonstrated age-related regional differences in the magnitude of the BOLD signal using task-based fMRI. It has been suggested that functional changes reflect either compensatory or de-differentiation mechanisms, both of which assume response to a specific stimulus. Here, we have tested whether ageing affects both task-based and resting brain function, and the extent to which functional changes are mediated by reductions in grey matter (GM) volume.
Two groups, of 22 healthy younger and 22 older volunteers, underwent an imaging protocol involving structural and functional MRI, both during a memory task and at rest. The two groups had similar socio-demographical characteristics and cognitive performance. Image analysis revealed both structural and functional differences. Increased BOLD signal in older relative to younger volunteers was mainly observed in the frontal lobes, both during the task and at rest. Functional changes in the frontal lobes were largely located in brain regions spared from GM loss, and adding GM covariates to the fMRI analysis did not significantly alter the group differences. Our results are consistent with the suggestion that, during normal ageing, the brain responds to neuronal loss by fine-tuning connections between spared neurons. Longitudinal studies will be necessary to fully test this hypothesis.
Keywords: Ageing, Neuroimaging, Resting, fMRI, Frontal lobes, Memory, Structural MRI
1. Introduction
Normal ageing is associated with grey matter (GM) volume reduction, white matter (WM) changes and resting cerebral blood flow (rCBF) reduction (Raz et al., 1998; Raz et al., 2005; Stoquart-ElSankari et al., 2007). However, functional MRI (fMRI) task-based studies consistently report increased blood oxygen level dependent (BOLD) signal in the frontal lobes in older people which has been variously interpreted as being due to “compensatory” or “de-differentiation” mechanisms (Rajah and D'Esposito, 2005). The de-differentiation hypothesis argues that the increased activity in older people reflects a reduction in neural specificity, whereas the compensation hypothesis suggests that the increased activity in older people reflects additional recruitment of brain activity to counteract age-related cognitive decline (Park and Reuter-Lorenz, 2009). Compensation and de-differentiation refer to functional responses to a stimulus, and when tested using an experimental paradigm, the interpretation of results is inevitably limited to the details of that paradigm. We define functional architecture as the structure that subserves these functional responses, and we propose that the integrity of this structure is accessible via resting-state fMRI (rs-fMRI).
Rs-fMRI measures differences in spontaneous fluctuations in resting brain function without the use of any specific task paradigm. Interest in investigating brain functionality using rs-fMRI has considerably increased over the past few years (Fox and Raichle, 2007) and its potential value has been recently demonstrated in clinical studies (Greicius et al., 2007; Greicius et al., 2004; Sorg et al., 2007). The rs-fMRI approach is used to study resting state networks (RSNs), which comprise brain regions that share a common time-course of spontaneous fluctuations and appear to be associated in their activity (Raichle et al., 2001). RSNs have been consistently observed across subjects (Damoiseaux et al., 2006) and their presence has also been reported when participants were asleep (Fukunaga et al., 2008) and in anaesthetized monkeys (Vincent et al., 2007). Moreover, we have recently demonstrated a close correspondence between RSNs obtained from resting data and maps derived from summary activation images from >7000 fMRI activation experiments (Smith et al., 2009). Taken together these results suggest that RSNs represent functionally-critical neuronal networks that reflect properties of functional brain organization.
Previous neuroimaging studies investigating age-related functional changes at rest have reported reduced brain network connection in advanced ageing (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008), defined as a reduction in the correlation coefficient between brain regions belonging to specific RSNs, such as the default mode network (DMN)(Raichle et al., 2001) and the dorsal attention system. Whilst most of the differences in the BOLD signal between older and younger subjects have been reported in frontal brain regions [see (Park and Reuter-Lorenz, 2009) for a review], no particular attention has been given to RSNs that include the frontal lobes.
Here, we used a multi-modal MRI protocol to investigate structural and functional characteristics, both at rest and during a memory task, of 22 younger and 22 older healthy subjects. The aims of the current study were twofold. First, we investigated the nature of the functional difference between younger and older individuals by including both task-based and resting fMRI. Second, we tested the extent to which local differences in brain volume affect and mediate resting and task-related brain function in younger and older subjects.
2. Methods
2.1. Participant recruitment
Descriptive data for 22 healthy younger subjects aged 20 to 35 years old and 22 healthy older subjects aged 56 to 74 years old are shown in Table 1. All subjects were right-handed and were recruited in Oxfordshire, UK. Exclusion criteria were current or past history of neurological or psychiatric disorders, memory complaints, head injury, substance abuse (including alcohol), hypertension and corticosteroid therapy. Data were collected as part of a larger study investigating the effect of APOE on the brain (Filippini et al., 2010; Filippini et al., 2009). The genetic profile of each participant was determined by using a cheek swab sample and, as part of the screening procedure, they were asked whether they had family history of dementia (either 1st or 2nd degree). Participants involved in this study were selected on the basis of the ‘normal’ APOE distribution (Menzel et al., 1983). Older subjects were high-functioning community-dwelling individuals with a high educational level. Three older subjects were hypercholesterolemic and one had diabetes. Older subjects underwent a pre-screening cognitive test [Addenbrooke's Cognitive Examination-revised version (ACE-R) (Mioshi et al., 2006)] to exclude possible confounds due to cognitive complaints. The score for all subjects was greater than 95, higher than the lower limit suggested for healthy subjects. Moreover, older subjects were also screened for brain vascular insults and excluded if they had two or more hyperintense lesions equal or larger than 10 mm diameter, or more than eight hyperintense lesions with a diameter from 5 to 9 mm (Bozzali et al., 2006), on a Fluid Attenuated Inversion Recovery (FLAIR) image. The study was approved by the local Ethics Committee and informed written consent was obtained from all subjects.
Table 1.
Socio-demographic and memory features of the two study groups. Values denote mean (±Standard Deviation) or numbers of subjects.
| Younger | Older | p | |
|---|---|---|---|
| N=22 | N=22 | ||
| Socio-demographics | |||
| Age, years | 27.9 (±4.2) | 64.7 (±5.5) | <0.001 |
| Education, years | 19.1 (±1.7) | 18.1 (±2.1) | 0.1 |
| Sex (male/female) | 10/12 | 8/14 | 0.8 |
| APOE ε4-carriers | 5 | 5 | 1 |
| Family history of dementia | 4 | 6 | 0.7 |
| Memory test (% of corrected responses) | |||
| Global performance | 86.4% | 84.3% | 0.4 |
| Familiar images | 98.9% | 98.3% | 0.6 |
| Novel images | 79.3% | 77.3% | 0.6 |
| Distractors | 95.3% | 92.4% | 0.1 |
| Reaction time (expressed in seconds) | |||
| “Familiar” blocks | 0.75 (±0.14) | 0.73 (±0.11) | 0.6 |
| “Novel” blocks | 0.99 (±0.29) | 0.97 (±0.16) | 0.7 |
2.2. Neuroimaging protocol
Scanning was performed at the University of Oxford, Centre for Clinical Magnetic Resonance Research (OCMR) using a 3 Tesla Siemens Trio scanner with a 12 channel head-coil. The neuroimaging protocol comprised functional and structural sequences as follows.
2.2.1. Functional MRI (task)
Encoding memory was assessed from a single run using a single gradient echo EPI sequence covering the whole brain (TR=3000 ms, TE=28 ms, flip angle=89°, field of view=192 mm, voxel dimension=3 mm isotropic, acquisition time=9 min 6 s.). The experimental task was a “Novel vs. Familiar” memory encoding paradigm carried out using Presentation software and previously described (Filippini et al., 2010; Filippini et al., 2009). Briefly, a set of colour images of animals and landscapes (no humans), similar in complexity, brightness and contrast and emotionally neutral was presented in a “blocked” design. Prior to the imaging session, subjects were presented 8 times with 8 images (“familiar”) and tested to ensure images had been successfully encoded. During scanning, images were displayed in pseudorandom order in a blocked design fashion with 6 blocks each of 8 familiar and novel images. Familiar images were presented in a different order each time, and novel (never seen before) images comprised a total of 48 images (image presentation=3250 ms, interstimulus interval=500 ms, block duration=30,000 ms). Between each block of images was 15,000 ms of rest, during which subjects passively viewed a fixation cross (a total of 12 rest blocks). Subjects were asked to respond as to whether each image contained an animal or not, and were told to try to remember the images. Outside the scanner, approximately 50 min after initial presentation, novel and familiar images as well as distractors were presented on a PC screen for 4000 ms each (inter-stimulus interval=1000 ms), and subjects had to select between two buttons according to whether the images had been seen inside the scanner or not.
2.2.2. Functional MRI (rest)
Whole-brain functional imaging was performed using a gradient echo EPI sequence (TR=2000 ms, TE=28 ms, flip angle=89°, field of view=224 mm, voxel dimension=3×3×3.5 mm, acquisition time=6 min 4 s.). For the resting-state scan, subjects were instructed to lie in dimmed light with their eyes open, think of nothing in particular, and not to fall asleep.
2.2.3. Structural MRI
3D high-resolution T1-weighted MR images were acquired using a MPRAGE sequence (TR=2040 ms, TE=4.7 ms, flip angle=8°, field of view=192 mm, voxel dimension=1 mm isotropic, acquisition time=12 min). Structural MRI was acquired between the two functional MRI sequences.
2.2.4. Flair
Whole-brain T2-weighted imaging was performed using a spin echo sequence (TR=9000 ms, TE=89 ms, field of view=220 mm, voxel dimension=1.1 × 0.9 × 3 mm, acquisition time=5 min 8 s).
2.3. Image analysis
Data analysis was carried out using FSL tools (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004).
2.3.1. Functional MRI (task)
fMRI analysis was carried out using FEAT (FMRI Expert Analysis Tool v. 5.98, http://www.fmrib.ox.ac.uk/fsl/feat5/). Pre-processing consisted of head motion correction, brain extraction, spatial smoothing using a Gaussian kernel of FWHM (full width at half maximum) 5 mm, and high pass temporal filtering equivalent to 130 s. Because age-related effects that are not task-related have been reported to influence the BOLD signal (most importantly, differences in coil sensitivity) (Aizenstein et al., 2004; D'Esposito et al., 2003) each individual fMRI statistical image, for both task and rest studies, was normalised on a voxel-by-voxel basis by each individual whole-brain mean after pre-processing. After running the first level analysis each individual fMRI statistical image was divided by the individual mean functional image and then fed into the higher group analysis. Time-series statistical analysis was carried out with local autocorrelation correction (Woolrich et al., 2001). A boxcar convolved with a gamma hemodynamic response function (HRF) and its temporal derivative was used to model the activation time-course. The main contrast of interest for the encoding memory paradigm was “novel vs. familiar”.
FMRI volumes were registered to the individual's structural scan and standard space images using a non-linear registration tool (FNIRT). These transformations into standard space were applied to contrast images and their variances. Higher-level (group level) analysis was carried out using FMRIB's Local Analysis of Mixed Effects (FLAME) (Woolrich et al., 2004). The cross-subject general linear model (GLM) included the two groups (younger and older). We tested for group means and differences for each of the contrasts of interest. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a family-wise-error (FWE) corrected cluster significance threshold of p<0.05 was applied to the suprathreshold clusters.
2.3.2. Functional MRI at rest (rs-fMRI)
fMRI analysis of resting state data was carried out using MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components, part of FSL http://www.fmrib.ox.ac.uk/fsl/melodic/) (Beckmann et al., 2005). Individual pre-statistical processing consisted of motion correction, brain extraction, spatial smoothing using a Gaussian kernel of FWHM (full width at half maximum) 5 mm, and high pass temporal filtering with a cut-off of 150 s. (0.007 Hz). FMRI volumes were registered to the individual's structural scan and standard space images using FMRIB's Nonlinear Image Registration Tool (FNIRT).
Pre-processed functional data containing 180 time-points for each subject were temporally concatenated across subjects in order to create a single 4D dataset. The number of components was fixed to 25 based on an initial analysis of the population using model order estimation. The subject dependent effect sizes identified in the initial analysis suggested that only 25 components were significantly non-zero on average. RSNs of interest covered the entire brain and were selected using spatial correlation against a set of previously defined maps (Beckmann et al., 2005).
The between-subject analysis of the resting data was carried out using a regression technique (‘dual regression’), which allows for voxel-wise comparisons of resting functional connectivity. This approach proceeds in three stages: first, the (groupwise) concatenated multiple FMRI data sets are decomposed using Independent Component Analysis (ICA) in order to identify large-scale patterns of functional connectivity in the population of subjects. Second, the dual regression approach is used to identify, within each subject's FMRI data set, subject-specific temporal dynamics and spatial maps that are associated with each group IC map. This involves (A) using the full set of group-ICA spatial maps in a linear model fit (spatial regression) against the separate FMRI data sets, resulting in matrices describing temporal dynamics for each component for each subject and (B) using these subject-specific time-course matrices in a linear model fit (temporal regression) against the associated FMRI data set to estimate subject-specific spatial maps. Partial correlation coefficients (PCCs) are calculated from the spatial maps output from the GLM as a measure of coherence of a voxel's timecourse with the RSN. PCCs were converted to z-values using a Fisher z-transformation. Finally, the different component maps are collected across subjects into single 4D files (one per original ICA map, with the 4th dimension being subject ID). A voxelwise GLM to assess group differences was applied to the spatial maps of z-transformed PCCs using permutation-based non-parametric testing (5000 permutations) (Nichols and Holmes, 2002) with cluster-based thresholding (clusters determined by Z>2.3) and a family-wise-error (FWE) corrected cluster significance threshold of p<0.01 applied to the suprathreshold clusters to account for multiple (voxel-wise) comparisons across the RSNs employed in this study. This results in spatial maps characterizing the between-subject/group differences.
Non-parametric tests were used to safeguard against the possibility that the between-subjects effects were non-Gaussian, and because such non-parametric inference has greater robustness against spatial non-stationarity than commonly used parametric methods (Hayasaka et al., 2004).
Moreover, because previous studies reported a reduction in functional correlation between the anterior and posterior cingulate components of the DMN (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008), we ran a separate analysis to specifically test this hypothesis. We used the dual-regression approach in our sample by first increasing the dimensionality of the group ICA to 100 components to separate the DMN that was obtained (as a single component) with a 25 component ICA into the anterior and posterior sub-components, then evaluating the strength of the correlation between the anterior and posterior cingulate networks for each subject using the subject-specific time courses output from the first stage of the dual regression. A two-tailed two-group t-test of the correlations between the subject-specific time-courses extracted from the two sub-components of the DMN was done to assess group differences in functional connectivity between the two networks.
Acquiring resting fMRI data after a task may potentially introduce a confound (Barnes et al., 2009), therefore we acquired a 10 minute sequence (ASL — data corrupted, thus not reported here) in between the task and rest EPI acquisitions to ensure that a reasonable amount of time elapsed for the resting-state dynamics to return to baseline.
2.3.3. Structural MRI
Whole brain analysis was carried out using a voxel-based morphometry-style analysis (FSL-VBM) (Douaud et al., 2007), using default settings as described at www.fmrib.ox.ac.uk/fsl/fslvbm/. In brief, brain extraction and tissue-type segmentation were performed and resulting GM partial volume images were aligned to standard space using first linear (FLIRT) and then non-linear (FNIRT) registration tools. The resulting images were averaged, modulated and smoothed with an isotropic Gaussian kernel of 5 mm FWHM to create a study-specific template, and the GM images were re-registered to this, including modulation by the warp field Jacobian. Finally, voxel-wise GLM was applied using permutation-based non-parametric testing (5000 permutations), clusters were determined by Z>2.3 and then a family-wise-error (FWE) corrected cluster significance threshold of p<0.05 was applied to the suprathreshold clusters.
2.3.4. Flair
In the older group volumetric analysis of WM hyperintensities in FLAIR images was performed manually using the Jim 4.0 software (Xinapse Medical Systems, Thorpe Waterville, UK). All axial slices of each subject were investigated. After marking all hyperintensities as region of interests (ROIs), total ROI volume was calculated for each subject.
2.4. Covariates
Structural images were used as additional covariates on a voxel-by-voxel basis to interrogate fMRI data. GM images of each subject were extracted using FMRIB's Automated Segmentation Tool (FAST, http://www.fmrib.ox.ac.uk/fsl/fast4/), registered to standard space, smoothed to match the intrinsic smoothness of the fMRI data, voxel-wise demeaned across all subjects in both groups together and added as a confound regressor (nuisance) to the GLM design matrix used to analyse fMRI data.
2.5. Statistics
Statistical analyses of non-imaging variables were carried out using SPSS software (SPSS, Inc., Chicago IL). Independent-Samples T-tests were used for socio-demographic variables, brain structure volumes, memory performance, and reaction times. Exact Fisher's and Yates continuity correction were used for categorical variables (sex, family history of dementia and APOE prevalence). Threshold for statistical significance was set to p <0.05.
3. Results
3.1. Participants
Younger and older participants did not differ for sex, years of education, memory recognition performance or reaction times (both recorded during fMRI task-related acquisition) (Table 1). Family history of dementia and the presence of the APOE ε4 allele, both known to influence fMRI studies (Bassett et al., 2006; Bookheimer et al., 2000; Xu et al., 2008) and ageing mechanisms (Filippini et al., 2010), were equally distributed in the two groups (Table 1) and reflected the proportion expected in the normal population.
3.2. Memory task FMRI
The encoding task adopted here activated areas associated with the memory encoding process. BOLD fMRI signal intensity changes were found in the hippocampus, temporal fusiform cortex, and parahippocampal gyrus bilaterally, and in association areas in the frontal, parietal and occipital lobes, as previously described (Fleisher et al., 2005; Golby et al., 2005).
Fig. 1 shows group differences for the novel vs. familiar contrast. In general, increased activation in younger participants was found in temporal and posterior brain regions, and in the frontal cortex for older participants. Specifically, increased BOLD signal change in younger relative to older participants was observed bilaterally in occipito-temporal regions, extending into the fusiform cortex and the parahippocampal gyrus [reporting here and below, maximum z score, cluster size in voxels, and (peak MNI coordinates in standard space): 3.93, 3761, and (14, −88, −8)] (Fig. 1A). Increased BOLD signal change in older relative to younger participants was found in three clusters located in the frontal lobes including anterior cingulate and paracingulate regions, inferior, middle and superior frontal gyri and the frontal pole [5.03, 953, and (12, 60, 22); −4.15, 509, and (−44, 36, −10); −3.85, 1195, and (18, 28, 58)] (Fig. 1B). No differences in hippocampal activation were observed between the two groups. Motion parameters were similar between the 2 groups [mean values (SD) in mm, younger: 0.27(0.25) – older: 0.32(0.22), t=−0.81, df=42, p=0.42).
Fig. 1.
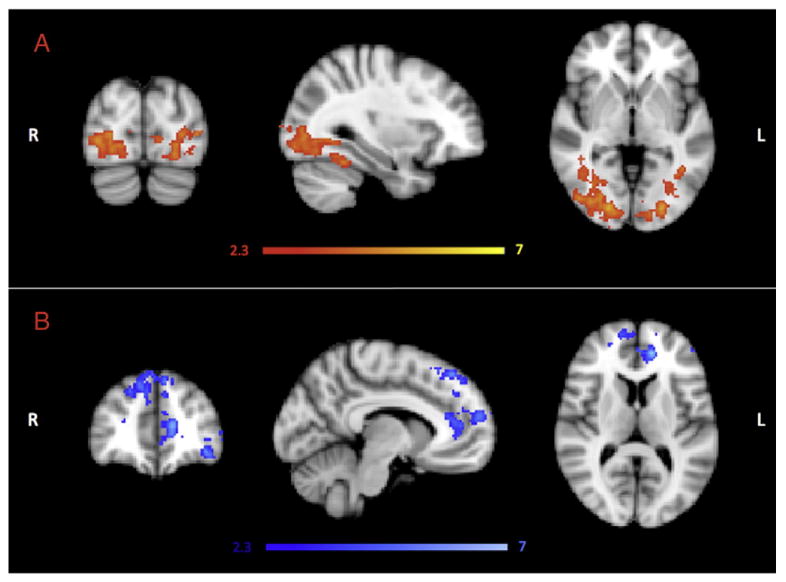
fMRI results for the novel vs. familiar contrast in the encoding memory task. (A) Red colour defines brain regions where the BOLD signal change was greater in the younger compared with the older group, whereas (B) blue colour defines brain regions where the BOLD signal change was greater in the older compared with the younger participants. For both p < 0.05 corrected for multiple comparisons.
3.3. Resting state FMRI (rs-fMRI)
ICA defined twenty-five components representing group-averaged networks (RSNs) of brain regions with BOLD fMRI signals that were temporally correlated (Beckmann et al., 2005).
A total of 5 components were identified as RSNs (covering the entire brain) and were evaluated further, whereas the remaining components mainly reflected motion artefacts, BOLD signal drift out and were therefore discarded. In detail, the 5 RSNs of interest included the visual cortex (VC), default mode network (DMN), sensorimotor (SM), auditory (Au) and a prefrontal RSN including brain regions involved in executive functions (EF) (Supplementary figure). EF comprised the inferior, middle and superior prefrontal cortices, anterior and paracingulate gyri and frontal pole (Beckmann et al., 2005; De Luca et al., 2006). VC included the occipital pole extending laterally towards the occipito-temporal junction and superior parietal regions and primary visual areas located in the calcarine sulcus bilaterally as well as medial extra striate regions (Beckmann et al., 2005; De Luca et al., 2006). The DMN included prefrontal cortical areas, anterior and posterior cingulate, lateral parietal and inferior/middle temporal gyri, cerebellar areas, and thalamic nuclei extending to medial–temporal regions (Beckmann et al., 2005; De Luca et al., 2006; Raichle et al., 2001). SM included brain regions located in the pre- and post-central gyri extending to the supplementary motor area (Beckmann et al., 2005; De Luca et al., 2006). Au included auditory cortices, planum polare and planum temporale and the lateral superior temporal gyrus (Beckmann et al., 2005; De Luca et al., 2006).
Significantly increased temporal correlation (coherence) within the EF RSN was observed in older relative to younger subjects in a cluster of regions including prefrontal brain regions, extending from the paracingulate gyrus into the inferior, middle and superior frontal gyri, and smaller clusters located in the cerebellum, occipital lobe and in the precuneus (Fig. 2). There were no frontal regions in which younger subjects had increased correlation compared with older participants. No significant differences between younger and older subjects were found in the DMN, VC, Au and SM RSNs.
Fig. 2.
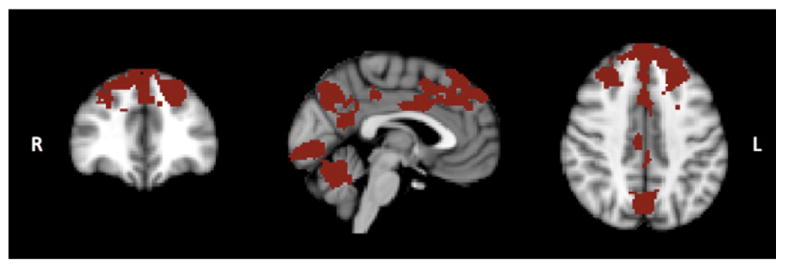
Group differences in resting fMRI data are shown. Increased coherence in older relative to younger was found in a cluster of regions including the anterior cingulate and paracingulate, inferior, middle and superior frontal gyri and in smaller clusters located in the cerebellum, occipital lobe and precuneus (p < 0.01 corrected for multiple comparisons).
The two-tailed two-group t-test to assess group differences in the correlations between the subject-specific timecourses extracted from the two sub-components of the DMN revealed a significant reduction in the correlation coefficient between the anterior and posterior cingulate regions in the older relative to the younger group [mean values(SD), younger: 3.1(2.1)–older: 1.3(2.7), t=2.41, df=42, p=0.02] (Fig. 3). Motion parameters were not different between the 2 groups [mean values(SD) in mm, younger: 0.19(0.12), older: 0.24(0.09), t=−1.42, df=42, p=0.16)].
Fig. 3.
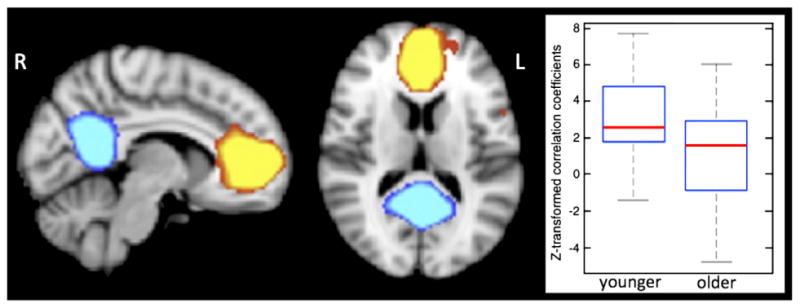
Anterior (red-to-yellow) and posterior (blue-to-light blue) portion of the DMN have been separated using a higher dimensionality (100 components) analysis. Box plot shows an age-related reduction in the correlation coefficient between the anterior and posterior cingulate regions.
3.4. Structural MRI
Fig. 4 shows widespread reduction in grey matter (GM) volume in older relative to younger participants. Brain regions with group differences included the anterior cingulate, precuneus, superior, middle and inferior frontal gyri, supramarginal gyrus, insular cortex, temporal, parietal and lateral occipital regions bilaterally. Subcortical regions including the caudate, thalamus and hippocampus also showed age-related GM reduction. There were no brain regions in which older subjects had increased GM density volume compared with younger participants. As expected, WM hyperintensities in older subjects were largely periventricular. The group mean volume was equal to 777.59(±578.56) mm3.
Fig. 4.
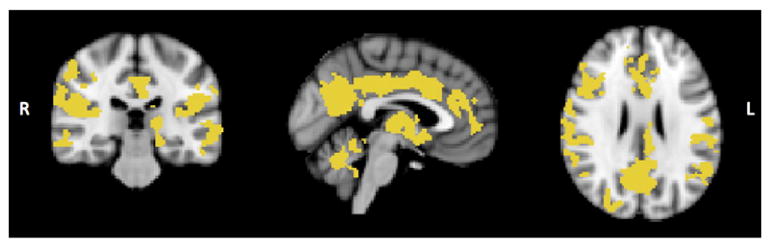
Brain regions where older participants had a reduction in GM volume compared with younger participants using a whole brain analysis approach (p < 0.05 corrected for multiple comparisons).
3.5. GM maps as covariates
To determine whether BOLD differences, both at task and at rest, between the younger and older group were influenced by structural differences, GM maps were added as covariates (nuisance variables) in the memory-encoding and in the RSN fMRI analysis models. In both cases, the BOLD-related group differences (reported above) survived largely unchanged. Comparison of the results when adding vs. not adding GM as a covariate revealed an 85% overlap of significant voxels. In general adding the covariate reduced the spatial extent of results, but there were negligible differences in the frontal lobes. Both task and rest BOLD signal (particularly in the frontal lobes) increases in older relative to younger were located in brain regions where GM was relatively spared (Fig. 5). Specifically, 93% of the voxels displaying significant group differences during the task and 90% of the voxels displaying significant group differences in the EF RSN were found in GM regions without significant relative GM loss in the older subjects (Fig. 5). Moreover, 757 voxels (28.5% of the significant voxels during the task) overlapped in the frontal lobes between significant clusters of increased task-related and resting fMRI signal in older compared to younger adults.
Fig. 5.
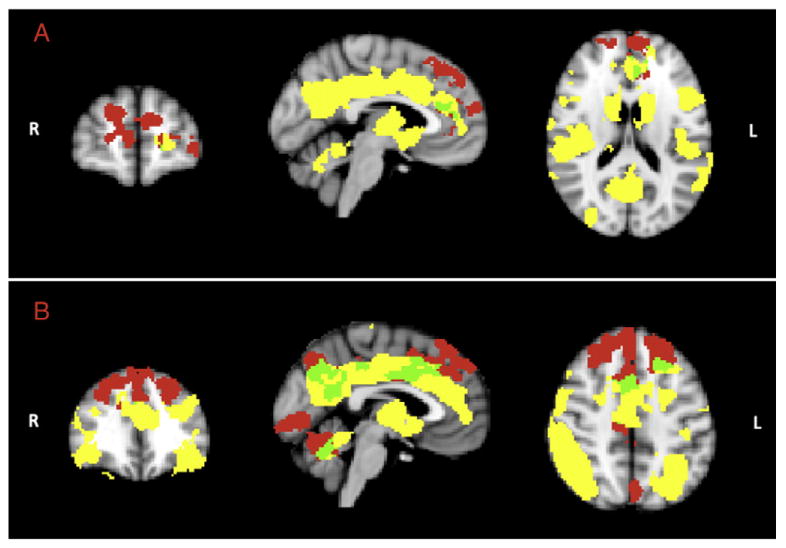
Areas of overlap between task-based difference and GM volume reduction (A) and frontal RSN difference and brain morphology reduction (B). Yellow denotes GM volume difference between the two study groups. Red denotes, task-based or RSN difference between younger and older group not overlapping with GM volume reduction. Green denotes areas of overlap between group-related task or RSN differences and GM volume changes.
4. Discussion
We have demonstrated that increased activation in the frontal lobes in older relative to younger participants, previously described using task fMRI, can also be found with rs-fMRI. This phenomenon may reflect age-related adaptation in the functional architecture of the brain that is not exclusively caused by a response to an external stimulus.
4.1. Age-related changes in task-based fMRI data
We were able to replicate previous reports of BOLD signal differences in task-related brain regions during normal ageing. In particular, the posterior-to-anterior shift (PASA) emerges as a reliable feature for functional imaging studies during normal ageing. The PASA model had been previously reported during a variety of cognitive tasks, including memory-related paradigms, but also those evaluating attention and perception cognitive domains (Davis et al., 2008).
The increased BOLD signal detected in the frontal lobe in older relative to younger participants was mainly located in the anterior cingulate and superior frontal gyrus, regions known to be involved in executive control functions (Rushworth et al., 2004), whereas the increased BOLD signal in younger relative to older subjects was located in occipito-temporal regions, known to be involved in perceptual processing (Likova and Tyler, 2008). We did not detect any difference between younger and older participants in the BOLD signal within hippocampal regions using an intentional memory-encoding paradigm. Our results are consistent with previous studies that used a similar approach (intentional encoding) and that showed no age-related differences in hippocampal BOLD signal (van der Veen et al., 2006). Conversely, previous studies using an incidental memory-encoding paradigm reported BOLD signal reduction in older relative to younger subjects in hippocampal areas (Gutchess et al., 2005). These results may suggest that hippocampal-related differences may be influenced by the specific task demand and/or differences in subjects' characteristics.
4.2. Age-related changes in resting fMRI data
Increased task-related activation in the frontal lobes in ageing has been widely and consistently reported as the most typical task-related functional neuroimaging feature in studies investigating normal ageing mechanisms (Grady, 2002, 2008). Here, we have shown that patterns of age-related functional brain changes occurring within frontal regions can be detected not only using a task-based approach, but also using resting fMRI data. We interpret this as evidence that age-related differences occurring in these brain regions may reflect changes in the functional architecture of the brain rather than “compensatory” or “de-differentiation” processes in response to an external stimulus.
Previous studies evaluating the effect of ageing on resting state brain function reported reduction in the temporal correlation between frontal and posterior regions of the brain within specific RSNs, such as the DMN or the dorsal attention system (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). Andrews-Hanna et al., observed a reduction in the correlation coefficient between distinct brain regions belonging to the DMN, particularly between the medial prefrontal and the retrosplenial cortices. Similarly, Damoiseaux et al., reported a reduction of brain activity in the anterior and posterior components of the DMN in older compared with younger participants. We specifically tested for a reduction in the temporal correlation between the anterior and posterior component of the DMN and we replicated previous results (i.e. an age-related reduction of the correlation between the anterior and posterior component of the DMN). However, no significant difference was observed when we tested the coherence of the DMN network as a whole.
A greater understanding of the effects of the normal ageing process in the DMN certainly warrants further investigation. In particular, changes in the DMN have been observed during pathological ageing (Greicius et al., 2004; Sorg et al., 2007), which suggests that this network may be particularly useful in detecting individuals at risk of developing neurodegenerative disorders.
4.3. Effect of structural changes on BOLD signal differences
Age-related GM volume reduction has been widely reported (Raz et al., 1998; Raz et al., 2005), but to date the potential effect of GM changes on BOLD signal has not been tested. Here, we demonstrated that BOLD differences between the two groups, both task-based and at rest, survived adding GM maps as covariates. Therefore, the specific regions of the frontal lobe that exhibit BOLD signal differences between the older and younger groups are not confounded by anatomical differences.
We find GM reductions and increased connectivity at rest and increased task-related activation in the frontal lobes of older relative to younger participants. However, closer inspection reveals that the areas of significant GM reductions and fMRI increases are largely non-overlapping, suggesting that those brain regions in the frontal lobes that are less affected by age-related GM loss become more functionally active during the task and more coherent at rest. This is in line with the hypothesis that age-related brain atrophy may lead to adaptive functional brain reorganisation during healthy ageing (Greenwood, 2007). To formally test this hypothesis (i.e. whether the brain responds to neuronal loss by fine-tuning connections between spared neurons) it would be necessary to conduct a longitudinal study to assess the relationship between decreasing GM (structural changes) and increasing BOLD signal (functional changes) in the frontal lobes for each individual. Indeed, individual variability in the plasticity of the brain's response to ageing defines those who age most and least successfully.
4.4. Study limitations
Limitations in studying age-related specific brain changes have to be considered in interpreting our findings. Differences in younger and older participants may be partially accounted for by cohort effects in cross-sectional designs. Moreover, it is not possible to exclude the use of different cognitive strategies during the task between younger and older subjects. Aine et al., suggested that most of the age-related functional differences observed may reflect the use of different techniques, such as subvocalizations or inner speech, in order to improve and facilitate memory processes (Aine et al., 2006). However, results from resting data can be more safely interpreted because no specific strategies are required when subjects are at rest. Furthermore, the BOLD signal measured by fMRI is dependent on vascular processes (i.e. cerebral blood flow, cerebral blood volume and vascular compliance) and vascular alterations in older subjects are known to affect the BOLD response (Riecker et al., 2003). Further studies specifically investigating age changes on individual vascular components and how they affect task-based and resting fMRI data are warranted. Finally, all participants recruited in this study are highly-educated and are therefore not representative of the entire population. It is possible that the brain differences we observe are particular to higher-functioning individuals. Future studies should specifically address this point by including subjects with a wider range of educational level.
4.5. Conclusions
We have shown that normal ageing is associated with functional brain changes both during task and at rest. In particular, the BOLD signal reduction in occipito-temporal brain regions observed during task-related fMRI, but not at rest, may reflect age-related stimuli-based perceptual decline, as previously reported (Park et al., 2004). Conversely, the increase in the BOLD signal in the frontal lobes in both conditions and the close similarity in the spatial location between task-related and RSNs-related differences suggest that the frontal lobes are particularly susceptible to age-related functional reorganisation. This may be explained by the fact that the frontal cortex is the most versatile integrative associative cortex and acts as a relay centre for higher cognitive function. Indeed, the frontal lobes are widely connected with other brain regions, are involved in many cognitive functions (i.e. learning, memory, attention and motivation) and are therefore the most likely to show adaptive brain mechanisms to counteract normal ageing processes, either neuronally or cognitively (Park and Reuter-Lorenz, 2009; Tamminga and Buchsbaum, 2004). We find both GM volume reduction and increased BOLD signal in the frontal lobes of older relative to younger subjects. The specific areas of change in GM and BOLD are largely non-overlapping, and indeed adding GM covariates to the fMRI analysis did not alter the result. Moreover, our results lead to the conclusion that age-related adaptive changes in the frontal lobes of the brain are not merely a response to an external stimulus.
Supplementary Material
Acknowledgments
The authors acknowledge academic collaborative contribution from GSK to fund data acquisition. PMM is a full-time employee of GSK. NF is funded by the Gordon Edward Small's Charitable Trust (Scottish Charity Register: SC008962). LDN is funded by NIDA K25 DA017712.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.neuroimage.2011.11.063.
References
- Aine CJ, Woodruff CC, Knoefel JE, Adair JC, Hudson D, Qualls C, Bockholt J, Best E, Kovacevic S, Cobb W, Padilla D, Hart B, Stephen JM. Aging: compensation or maturation? Neuroimage. 2006;32:1891–1904. doi: 10.1016/j.neuroimage.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Clark KA, Butters MA, Cochran J, Stenger VA, Meltzer CC, Reynolds CF, Carter CS. The BOLD hemodynamic response in healthy aging. J Cogn Neurosci. 2004;16:786–793. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One. 2009;4:e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain. 2006;129:1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Filippi M, Magnani G, Cercignani M, Franceschi M, Schiatti E, Castiglioni S, Mossini R, Falautano M, Scotti G, Comi G, Falini A. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology. 2006;67:453–460. doi: 10.1212/01.wnl.0000228243.56665.c2. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior–anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Filippini N, Ebmeier KP, Macintosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, Beckmann CF, Smith SM, Matthews PM, Mackay CE. Differential Effects of the APOE Genotype on Brain Function Across the Lifespan. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct Patterns of Brain Activity in Young Carriers of the APOE-{varepsilon} 4. Allele Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol. 2005;62:1881–1888. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, de Zwart JA, van Gelderen P, Balkin TJ, Braun AR, Duyn JH. Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J Cereb Blood Flow Metab. 2008;28:1377–1387. doi: 10.1038/jcbfm.2008.25. [DOI] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E, Gabrieli S, O'Shea J, Knierim K, Stebbins G, Gabrieli J. Memory encoding in Alzheimer's disease: an fMRI study of explicit and implicit memory. Brain. 2005;128:773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- Grady CL. Introduction to the special section on aging, cognition, and neuroimaging. Psychol Aging. 2002;17:3–6. doi: 10.1037//0882-7974.17.1.3. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21:657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Likova LT, Tyler CW. Occipital network for figure/ground organization. Exp Brain Res. 2008;189:257–267. doi: 10.1007/s00221-008-1417-6. [DOI] [PubMed] [Google Scholar]
- Menzel HJ, Kladetzky RG, Assmann G. Apolipoprotein E polymorphism and coronary artery disease. Arteriosclerosis. 1983;3:310–315. doi: 10.1161/01.atv.3.4.310. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Riecker A, Grodd W, Klose U, Schulz JB, Groschel K, Erb M, Ackermann H, Kastrup A. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab. 2003;23:565–573. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoquart-ElSankari S, Baledent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27:1563–1572. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Buchsbaum MS. Frontal cortex function. Am J Psychiatry. 2004;161:2178. doi: 10.1176/appi.ajp.161.12.2178. [DOI] [PubMed] [Google Scholar]
- van der Veen FM, Nijhuis FA, Tisserand DJ, Backes WH, Jolles J. Effects of aging on recognition of intentionally and incidentally stored words: an fMRI study. Neuropsychologia. 2006;44:2477–2486. doi: 10.1016/j.neuropsychologia.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Xu G, McLaren DG, Ries ML, Fitzgerald ME, Bendlin BB, Rowley HA, Sager MA, Atwood C, Asthana S, Johnson SC. The Influence of Parental History of Alzheimer's Disease and Apolipoprotein E {varepsilon} on the BOLD Signal during Recognition Memory. Brain. 2008 doi: 10.1093/brain/awn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


