Abstract
Heat shock proteins (HSPs) are induced by various physical, chemical, and biological stresses. HSPs are known to function as molecular chaperones, and they not only regulate various processes of protein biogenesis but also function as lifeguards against proteotoxic stresses. Because it is very useful to discover nontoxic chaperone-inducing compounds, we searched for them in herbal medicines. Some herbal medicines had positive effects on the induction of HSPs (Hsp70, Hsp40, and Hsp27) in cultured mammalian cells. We next examined 2 major constituents of these herbal medicines, glycyrrhizin and paeoniflorin, with previously defined chemical structures. Glycyrrhizin had an enhancing effect on the HSP induction by heat shock but could not induce HSPs by itself. In contrast, paeoniflorin had not only an enhancing effect but also an inducing effect by itself on HSP expression. Thus, paeoniflorin might be termed a chaperone inducer and glycyrrhizin a chaperone coinducer. Treatment of cells with paeoniflorin but not glycyrrhizin resulted in enhanced phosphorylation and acquisition of the deoxyribonucleic acid–binding ability of heat shock transcription factor 1 (HSF1), as well as the formation of characteristic HSF1 granules in the nucleus, suggesting that the induction of HSPs by paeoniflorin is mediated by the activation of HSF1. Also, thermotolerance was induced by treatment with paeoniflorin but not glycyrrhizin. Paeoniflorin had no toxic effect at concentrations as high as 80 μg/ mL (166.4 μM). To our knowledge, this is the first report on the induction of HSPs by herbal medicines.
INTRODUCTION
Virtually all organisms respond to upshifts in temperature (heat shock) by synthesizing a set of proteins called heat shock proteins (HSPs). These HSPs are induced not only by heat shock but also by various other environmental stresses (Schlesinger et al 1982). Usually, HSPs are also expressed constitutively at normal growth temperatures and have basic and indispensable functions in the life cycle of proteins as molecular chaperones (Hartl 1996; Hartl and Hayer-Hartl 2002; Morimoto 2002), as well as play a role in protecting cells from environmental deleterious stresses (Parsell and Lindquist 1993). Molecular chaperones are able to inhibit the aggregation of partially denatured proteins and refold them as demonstrated in in vitro and in vivo studies (Minami et al 1996; Michels et al 1997). Therefore, molecular chaperones are considered to be endogenous cytoprotective factors, lifeguards, or guardians of the proteome (Morimoto and Santoro 1998; Jaattela 1999; Ohtsuka and Hata 2000; Ohtsuka and Suzuki 2000; Muchowshi 2002).
Many lines of study indicate that molecular chaperones have beneficial functions at both the cellular and tissue level for the organism. For example, induction of molecular chaperones in animals by whole-body hyperthermia or by gene transfer can protect the brain and heart from tissue injury induced by ischemia (Liu et al 1992; Plumier et al 1997; Brown and Sharp 1999; Carroll and Yellon 1999). A moderate overexpression of molecular chaperones resulted in an extended life span in Nematoda and the fruit fly (Smith 1958; Lithgow et al 1995; Tatar et al 1997; Yokoyama et al 2002; Hsu et al 2003; Walker and Lithgow 2003; Morley and Morimoto 2004). Also, molecular chaperones can suppress the aggregate formation of mutant proteins that cause neurodegenerative diseases, such as spinocerebellar ataxia 1 (Cummings et al 1998), spinal and bulbar muscular atrophy (Kobayashi et al 2000), familial amyotrophic lateral sclerosis (Takeuchi et al 2002), and Huntington's disease (Jana et al 2000), as demonstrated by model experimental systems. Although the substrates of the molecular chaperones are usually normal or wild-type proteins, molecular chaperones can also deal with mutant proteins in some cases, that is, they can even help mutant proteins fold correctly and maintain normal function by inhibiting nonproductive folding pathways (Jeoung et al 1991; Tang et al 1997).
To date, several chemical compounds are known to have positive effects on the induction of HSPs, such as geranylgeranylacetone (GGA) (Hirakawa et al 1996), bimoclomol (BRLP-42) (Vigh et al 1997), herbimycin-A (Morris et al 1996), rebamipide (Hahm et al 1997), and carbenoxolone (Nagayama et al 2001). GGA, an acrylic isoprenoid, is clinically used as an antiulcer drug and can induce HSPs by way of heat shock transcription factor 1 (HSF1) activation. Pretreatment of animals with GGA markedly suppresses ischemia-reperfusion injury of the liver, small intestine, and heart (Rokutan 2000). Induction of HSPs by GGA, however, is somewhat cell-type specific, occurring especially in gastrointestinal mucosal cells. Bimoclomol, a hydroxylamine derivative, is a coinducer of HSPs. Bimoclomol itself has no HSP-inducing activity, but when cells are heat shocked in the presence of bimoclomol, HSPs are induced at higher levels than when cells are only heat shocked (Vigh et al 1997). Bimoclomol, however, is reported to show protective activities against various forms of stresses at the cell, tissue, or organism level (Nanasi and Jednakovits 2001; Lubbers et al 2002). Herbimycin-A, rebamipide, and carbenoxolone have not been extensively examined with respect to the induction of HSPs. It should be very useful and beneficial to find other nontoxic chaperone inducers for the prevention and treatment of various pathological states, such as stress ulcers and ischemia-induced injuries, and of diseases associated with protein misfolding and protein aggregation.
Here, we report on a novel HSP-inducing compound, paeoniflorin, which is one of the major constituents of a herbal medicine derived from Paeonia lactiflora Pall.
MATERIALS AND METHODS
Herbal medicines and chemicals
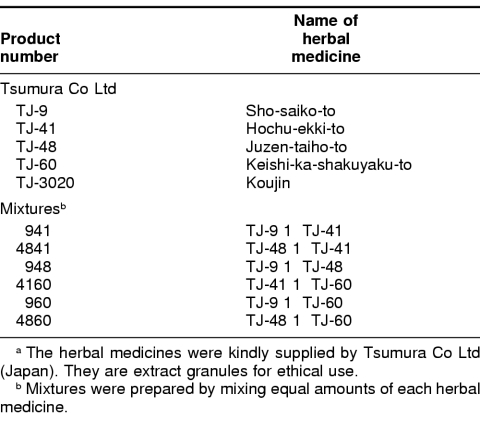
The herbal medicines used in this study were kindly supplied by Tsumura Co Ltd (Tokyo, Japan) and are listed in Table 1. Several mixtures were prepared by mixing equal amounts of 2 herbal medicines (Table 1). These herbal medicines or mixtures (0.5 g) were dissolved in 25 mL of phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4) and autoclaved at 120°C for 10 minutes. Insoluble materials were removed by centrifugation at 1000 rpm for 10 minutes, and the supernatant was stocked (20 mg/mL) and diluted for use in culture medium. Representative constituents in these herbal medicines, such as saikosaponin, liquiritin, ginsenoside, baicalein, zingerol, paeoniflorin, atractylodin, atragalosidie, and wogonin (listed in Table 2), were purchased from Wako Pure Chemical (Osaka, Japan). Paeoniflorin (molecular weight [MW]: 480.45, 10 mg) was dissolved in 1 mL of PBS and filtered with a 20-μm filter (Corning, Corning, NY, USA). Other constituents (20 mg) were dissolved in 0.1 mL of dimethyl sulfoxide, diluted in 1.9 mL of PBS or culture medium, and then filtered. Glycyrrhizin (MW: 822.94) was obtained from Minophagen Pharmaceutical Co Ltd (Tokyo, Japan) as Glycyron (20 mg/mL) and diluted for use in culture medium. Other reagents and chemicals were of the highest purity available from Wako Pure Chemical and Nakalai Tesque (Kyoto, Japan).
Table 1.
Herbal medicines (Kampo medicines) and their mixtures used in this studya
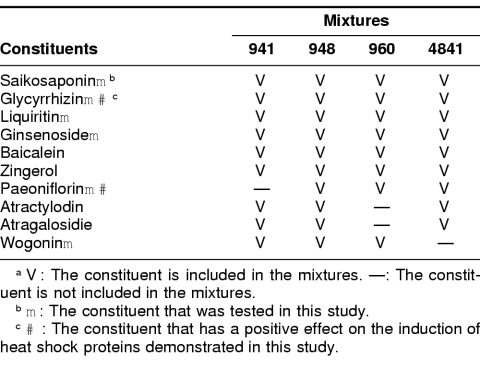
Table 2.
Representative constituents in herbal medicinesa
Cells and cell culture
In this study, we used HeLa cells, IMR-32 cells (human neuroblastoma), and normal rat kidney (NRK) cells. Cells were cultured in Dulbecco modified Eagle minimal essential medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Sigma, St Louis, MO, USA) and incubated in a CO2 incubator with 5% CO2 and 95% air at 37°C. When cells were heated, culture dishes or flasks were sealed with Parafilm and immersed in a water bath, the temperature of which was controlled to within ±0.1°C.
Cell survival assay
A clonogenic cell survival assay was performed as described previously (Hayashi et al 1991). In brief, exponentially growing HeLa cells were trypsinized, and the number of cells were counted with a hemocytometer and diluted in culture medium. An appropriate number of cells to yield 50–200 colonies per flask were inoculated into 25-cm2 T flasks (Nunc, Rochester, NY, USA). The flasks were incubated at 37°C for 5 hours to allow cells to attach to the substrate of the flasks. The cells were then heated at 42°C for 2 hours or treated with herbal medicines for 24 hours as pretreatment. After pretreatment, cells were heated at 45°C for the indicated period by immersing the flasks in a water bath, after which they were cultured again at 37°C for colony formation for 10 days. The cells were fixed and stained with methylene blue and basic fuchsin in 100% methanol, and colonies of 50 or more cells were scored.
Immunological methods
For Western blotting, cells were lysed in sodium dodecyl sulfate (SDS)–sample buffer (2.3% SDS, 62.5 mM Tris-HCl [pH 6.8], 5% β-mercaptoethanol, 10% glycerol) and boiled for 5 minutes. The protein concentration of each cell lysate was determined with a Pierce (Rockford, IL, USA) protein assay kit. A 10-μg sample was electrophoresed on a standard SDS–polyacrylamide gel and transferred to a nitrocellulose membrane (Schleicher & Schell, Dassel, Germany). The blotted membranes were probed with mouse monoclonal anti-Hsp70 (MBL, Nagoya, Japan, SR-B810), rabbit polyclonal anti-Hsp40 (Hattori et al 1992), rabbit polyclonal anti-Hsp27 (Stressgen, Victoria, Canada, SPA-800), or rabbit polyclonal anti-HSF1 (Stressgen, SPA-910) antibodies (1:500 to 1:1000 dilution) and then with horseradish peroxidase–conjugated corresponding immunoglobulin G (IgG, Zymed, San Francisco, CA, USA, 1:500 dilution). They were then treated with enhanced chemiluminescence reagent (Amersham, Piscataway, NJ, USA), and the signals were detected by exposure of the membranes to X-ray films (Kodak, Rochester, NY, USA). The relative signal intensity was quantified by densitometry with NIH Image software on a personal computer.
To determine the intracellular localization of HSF1, cells grown on a glass coverslip were fixed in 100% methanol at −20°C for 10 minutes, then treated with 10% normal goat serum to inhibit nonspecific binding. They were then processed for immunofluorescence staining by using anti-HSF1 antibody (Stressgen, SPA-910, 1:200 dilution) as the first antibody and fluorescein isothiocyanate–conjugated anti-rabbit IgG (Zymed, 1:100 dilution) as the second antibody. The cells were observed through a Fluorophoto microscope (Nikon, Tokyo, Japan).
Gel mobility shift assay
Preparation of whole-cell extracts from HeLa cells, gel mobility shift assay, and antibody supershift experiments were carried out according to the methods described by Ito et al (2001). The oligonucleotide sequence of the heat shock element (HSE) probe was as follows:
HSE of Drosophila HSP70 (+): 5′-gcctcgaatgttcgcgaagtttcg-3′,
HSE of Drosophila HSP70 (−): 5′-cgaaacttcgcgaacattcgaggc-3′.
HeLa cell extract (15 μg) was mixed with or without 1 μL of 1:100 diluted anti-HSF1 antibody (StressGen, SPA-910) in PBS in a total volume of 10 μL. After incubation on ice for 20 minutes, 1 × 104 cpm of [γ32P]adenosine triphosphate end–labeled, double-stranded HSE probe and 1 μg of poly(dI-dC) in 25 μL of binding buffer were added and incubated on ice for 20 minutes. The binding reaction mixture was then subjected to electrophoresis on a 4% polyacrylamide gel in 0.5× TBE buffer and autoradiographed with an X-ray film.
Cell-cycle phase analysis by flow cytometry
HeLa cells were trypsinized and washed with PBS 3 times. A pellet of 2 × 106 cells was resuspended in 1 mL of hypotonic propidium iodide solution (50 μg/mL of propidium iodide, 0.1% sodium citrate, 0.2% Nonidet P-40, 0.25 mg/mL of ribonuclease), incubated at 4°C for 30 minutes in the dark, and then mixed and further incubated at 37°C for 30 minutes. The sample was filtered through a cell-strainer cap (Falcon) to remove cell aggregates before analysis by flow cytometry (Beckman-Coulter, Fullerton, CA, USA, Cytomics FC500).
RESULTS
Induction of HSPs by herbal medicines
We obtained 5 herbal medicines (TJ-9, TJ-41, TJ-48, TJ-60, and TJ-3020) from Tsumura Co Ltd and prepared 6 mixtures by mixing equal amounts of 2 herbal medicines (941 = TJ-9 + TJ-41, 4841 = TJ-48 + TJ-41, 948 = TJ-9 + TJ-48, 4160 = TJ-41 + TJ-60, 960 = TJ-9 + TJ-60, and 4860 = TJ-48 + TJ-60) (Table 1). Each herbal medicine and the 6 mixtures were tested for the induction of HSPs. In the first stage of screening, we asked whether these herbal medicines could enhance the induction of HSPs in the presence or absence of mild heat shock. Expression levels of HSPs in HeLa cells treated with herbal medicines for 24 hours with or without mild heat shock (42°C for 2 hours) were monitored by Western blotting with anti-Hsp70 antibody. As shown in Figure 1, 4 mixtures of herbal medicines (941, 948, 960, and 4841) had enhancing effects on the induction of Hsp70 by mild heat shock; the expression level of Hsp70 with the 4 mixtures was higher than that with heat shock alone (50–400 μg of herbal medicine + heat(+) in Fig 1 A–D). On the other hand, 3 mixtures (948, 960, and 4841) each had a positive effect on the induction of Hsp70 by itself; the expression level of Hsp70 was higher than that of the nontreated control (50–400 μg of herbal medicine + heat(−) in Fig 1 B–D). There was no obvious dose dependency in the induction of Hsp70 under our present experimental conditions at this stage. TJ-48 alone or TJ-60 alone had some positive effect on the induction of Hsp70 (data not shown).
Fig 1.
Induction of heat shock protein 70 (Hsp70) by mixtures of herbal medicines. HeLa cells were treated with 941 (A), 948 (B), 960 (C), or 4841 (D) mixtures at 37°C for 24 hours at the concentrations indicated (μg/mL), with or without mild heat shock. Heat shock (42°C for 2 hours) was given for the final 2 hours of herbal medicine treatment, and cells were cultured for further 3 hours at 37°C. Hsp70 was detected by Western blotting, and the relative intensity of each band was measured by densitometry. Each bar indicates the mean ± standard deviation of the relative amount of Hsp70 from at least 3 independent experiments. The relative amount of Hsp70 in nontreated control cells was taken as 100% (Control, open bar). Typical Western blot data are shown under each graph. All 4 mixtures of herbal medicines had enhancing effects on the induction of Hsp70 (heat(+)); the relative amount of Hsp70 is much more than that of heat shock alone (Heat, closed bar). Three mixtures (B, 948; C, 960; D, 4841) could induce Hsp70 without heat shock (heat(−)); the relative amount of Hsp70 is much more than that of the nontreated control (Control, open bar)
Fig 4.
Gel mobility shift assay. (A) Effect of paeoniflorin (PF). Lane 1, extract from control cells; lane 2, extract from heat-shocked (42°C for 2 hours) cells; lanes 3, 4, 5, 6, and 7, extracts from cells treated with 80 μg/mL of paeoniflorin for 4, 8, 12, 16, and 24 hours, respectively; lane 8, extract from cells heat shocked (42°C for 2 hours) and then incubated with anti–heat shock transcription factor 1 (HSF1) antibody; lane 9, extract from cells treated with 80 μg/mL of paeoniflorin for 4 hours and then incubated with anti-HSF1 antibody; lane 10, extract from cells treated with 80 μg/mL of paeoniflorin for 8 hours and then incubated with anti-HSF1 antibody. (B) Effect of glycyrrhizin (GL). Lane 1, extract from control cells; lane 2, extract from heat-shocked (42°C for 2 hours) cells; lanes 3, 4, 5, 6, and 7, extracts from cells treated with 80 μg/mL glycyrrhizin for 4, 8, 12, 16, and 24 hours, respectively; lane 8, extract from cells heat shocked (42°C for 2 hours) and then incubated with anti-HSF1 antibody; lane 9, extract from cells treated with 80 μg/mL of glycyrrhizin for 4 hours and then incubated with anti-HSF1 antibody; lane 10, extract from cells treated with 80 μg/mL of glycyrrhizin for 8 hours and then incubated with anti-HSF1 antibody. Open arrowheads indicate nonspecific bands. Filled arrowheads indicate heat shock element–protein complexes. Arrows indicate supershifted complexes with anti-HSF1 antibody
Because the results shown in Figure 1 appear to be attributable to the specific constituents in these herbal medicines, we chose 10 representative constituents, as listed in Table 2, having previously defined chemical structures. The difference between 941 and the other 3 mixtures (948, 960, and 4841) is that paeoniflorin is not included in mixture 941. Therefore, we expected that paeoniflorin would be able to induce HSPs by itself. We examined the effect of 6 constituents including paeoniflorin on Hsp70 induction (indicated by closed triangles in Table 2). Among these 6 constituents, paeoniflorin and glycyrrhizin had positive effects on the induction of Hsp70 (Fig 2). Both paeoniflorin and glycyrrhizin had an enhancing effect on the Hsp70 induction by mild heat shock; the expression level of Hsp70 was higher than that of heat shock alone (10–80 μg of paeoniflorin + heat(+) in Fig 2A, and 10–80 μg of glycyrrhizin + heat(+) in Fig 2B). According to our expectation, paeoniflorin alone could induce Hsp70 without the necessity of added heat shock (10–80 μg of paeoniflorin + heat(−) in Fig 2A), but glycyrrhizin alone could not induce Hsp70 (10–80 μg of glycyrrhizin + heat(−) in Fig 2B). In this case, again, we could not obtain obvious dose-dependent induction of Hsp70 in the range of 10–80 μg/mL of paeoniflorin. Paeoniflorin was able to induce Hsp40 and Hsp27 as well as Hsp70 (Fig 3 A,B, lanes 4–7). Similar results were also obtained in NRK cells with respect to the induction of HSPs by these herbal medicines (data not shown).
Fig 2.
Effect of paeoniflorin and glycyrrhizin on the induction of heat shock protein 70 (Hsp70). HeLa cells were treated with paeoniflorin (A) and glycyrrhizin (B) at 37°C for 24 hours at the concentrations indicated (μg/mL), with or without mild heat shock. Heat shock (42°C for 2 hours) was given in the final 2 hours of paeoniflorin or glycyrrhizin treatment, and cells were cultured for further 3 hours at 37°C. Hsp70 was detected by Western blotting, and the relative intensity of each band was measured by densitometry. Each bar indicates the mean ± standard deviation of the relative amount of Hsp70 from at least 3 independent experiments. The relative amount of Hsp70 in nontreated control cells was taken as 100% (Control, open bar). Typical Western blot data are shown under each graph. The structures of paeoniflorin and glycyrrhizin are shown over each graph. Peoniflorin is included in 948, 960, and 4841 mixtures but not in the 941 mixture. Glycyrrhizin is included in all 4 mixtures. Both paeoniflorin and glycyrrhizin had enhancing effects on the induction of Hsp70 (heat(+)); the relative amount of Hsp70 is much more than that of heat shock alone (Heat, closed bar). Paeoniflorin but not glycyrrhizin could induce Hsp70 without heat shock (heat(−)); the relative amount of Hsp70 is much more than that of the nontreated control (Control, open bar)
Fig 3.
Phosphorylation of heat shock transcription factor 1 (HSF1) and induction of heat shock proteins (HSPs) in HeLa cells (A and C) and IMR-32 cells (B and D) by paeoniflorin. (A and B) Lane 1, cells were heated at 42°C for 2 hours; lane 2, nontreated control cells. Cells were treated with 80 μg/mL of paeoniflorin (PF) for 4, 8, 12, 16, and 24 hours (lanes 3, 4, 5, 6, and 7, respectively). After treatments, the cell lysate was analyzed by Western blotting with anti-HSF1 antibody (top of panels A and B) or with anti-Hsp70, anti-Hsp40, or anti-Hsp27 antibodies. (C and D) Lane 1, cells were heated at 42°C for 2 hours; lane 2, nontreated control cells; cells were treated with 80 μg/mL of paeoniflorin (PF) for 4 and 8 hours (lanes 3 and 4, respectively) or with 80 μg/mL of glycyrrhizin (GL) for 4 and 8 hours (lanes 5 and 6, respectively). After treatments, cell lysate was analyzed by Western blotting with anti-HSF1 antibody. Treatment of cells with heat shock (lane 1 in panels A–D) or paeoniflorin (lanes 3 and 4 in panels A–D) resulted in clear mobility shift of the HSF1 signal as compared with that of nontreated control cells (lane 2 in panels A–D) for both HeLa and IMR-32 cells. The mobility shift of HSF1 implies phosphorylation of HSF1. Treatment of cells with paeoniflorin for 16 and 24 hours resulted in disappearance of the phosphorylated form of HSF1 (lanes 6 and 7 in panels A and B), indicating attenuation of HSF1 activity. Glycyrrhizin treatment had no effect on the mobility shift of HSF1 (lanes 5 and 6 in panels C and D). Hsp70, Hsp40 and Hsp27 were induced by paeoniflorin treatment (lanes 4–7 in panels A and B)
The concentration of paeoniflorin required for the induction of HSPs was relatively high (more than 10 μg/ mL = 20.8 μM) under our experimental conditions (10% fetal bovine serum). When cells were incubated in serum-free medium, however, 1–5 μg/mL of paeoniflorin could induce HSPs (data not shown). Therefore, it seems that paeoniflorin might bind to or be inactivated by serum proteins.
HSF1 activation by paeoniflorin
Induction of HSPs by heat shock is usually regulated by the trans-acting HSF1 and cis-acting HSE present at the promoter region of each heat shock gene (Wu et al 1994). Activation of HSF1 is a multistep process, including relocalization into the nucleus, oligomerization, acquisition of a deoxyribonucleic acid (DNA)–binding competent state, and phosphorylation, until it finally accumulates in the characteristic structures to form HSF1 granules in the nucleus (Sarge et al 1993; Cotto et al 1997; Morimoto 1998). Although the phosphorylation of HSF1 is not always necessary for its activation, in some cases, its phosphorylation can be used as a good indicator of cells being under stressful conditions. Phosphorylation of HSF1 is usually detected as an upward band shift by Western blotting (Sarge et al 1993). As shown in Figure 3, treatment of HeLa cells or IMR-32 cells with paeoniflorin (80 μg/mL) for 4–8 hours resulted in the clear-cut upshift of the HSF1 band (Fig 3 A–D, lanes 3 and 4). On the other hand, glycyrrhizin could not induce the upshift of the HSF1 (Fig 3 C,D, lanes 5 and 6). These results clearly indicate that paeoniflorin but not glycyrrhizin is able to promote the phosphorylation of HSF1. After the treatment of cells with paeoniflorin for 16–24 hours, the phosphorylated form of HSF1 disappeared (Fig 3 A,B, lanes 6 and 7), indicating dephosphorylation of HSF1.
The acquisition of the DNA-binding ability of HSF1 by paeoniflorin treatment was demonstrated by a gel mobility shift assay using labeled HSE oligonucleotide. Specific DNA-protein complexes were detected in cells treated with paeoniflorin for 4 and 8 hours (Fig 4A, lanes 3 and 4) as well as in heat-shocked cells (42°C for 2 hours, Fig 4A, lane 2). The bands of DNA-protein complexes were supershifted in the presence of anti-HSF1 antibody (Fig 4A, lanes 8–10), indicating that the protein bound to HSE was HSF1. The specific DNA-protein complex disappeared when cells were treated with paeoniflorin for 12– 24 hours (Fig 4A, lanes 5–7). In contrast, glycyrrhizin could not induce DNA-binding activity of HSF1 (Fig 4B, lanes 3–7).
Intracellular localization of HSF1 was examined by indirect immunofluorescence staining. HSF1 was localized both in the cytoplasm and the nucleus at normal growth temperature (Fig 5B). Upon heat shock (42°C for 2 hours), HSF1 was relocalized into the nucleus to form typical HSF1 granules (Fig 5D). The HSF1 granules were clearly different from the phase dense nucleolus (compare Fig 5C with Fig 5D). When cells were treated with paeoniflorin (80 μg/mL) for 4–8 hours, evident HSF1 granules were formed (Fig 5 E,F). Interestingly, the HSF1 granules disappeared during continuous treatment of cells with paeoniflorin for 16 and 24 hours (Fig 5 G,H). The time course of the DNA-binding activity of HSF1 (Fig 4) and the formation and disappearance of HSF1 granules (Fig 5) well corresponds to that of phosphorylation and dephosphorylation of HSF1 (Fig 3). This might imply the activation of HSF1 and attenuation of stress response. A similar phenomenon has been reported when cells were continuously heated at 42°C for more than 6 hours (Sarge et al 1993). Thus, the activation of HSF1 and its attenuation during continuous paeoniflorin treatment indicate that cells are being exposed to a mild level of stress. On the other hand, when cells were treated with glycyrrhizin (80 μg/mL), HSF1 appeared to be gradually relocalized into the nucleus but HSF1 granules were not formed (Fig 5 I–L). As shown in Figure 2B, glycyrrhizin had an enhancing effect on the induction of HSPs by heat shock. This might be explained, in part, by the gradual relocalization of HSF1 by glycyrrhizin treatment. Thus, glycyrrhizin may be called a coinducer of HSPs. Because HSF1 is not phosphorylated and its DNA-binding activity is not induced by the treatment with glycyrrhizin (Figs 3 and 4), HSF1 might be relocalized into the nucleus irrespective of its phosphorylation and DNA-binding competency. In other words, HSF1 might be first relocalized into the nucleus after which the subsequent HSF1 activation steps (oligomerization, acquisition of the DNA-binding ability, and phosphorylation) might occur in the nucleus under the stress conditions.
Fig 5.
Intracellular localization of heat shock transcription factor 1 (HSF1) in HeLa cells. (A and B) Nontreated control cells; (C and D) cells heated at 42°C for 2 hours; (E, F, G, and H) cells treated with paeoniflorin (PF) for 4, 8, 16, and 24 hours, respectively; (I, J, K, and L) cells treated with glycyrrhizin (GL) for 4, 8, 16, and 24 hours, respectively. HSF1 is localized both in the cytoplasm and the nucleus in nontreated control cells (B). Treatment of cells with heat shock (D) or with paeoniflorin for 4 and 8 hours (E and F) induced relocalization of HSF1 into the nucleus to form typical HSF1 granules. Continuous treatment of cells with paeoniflorin for 16 and 24 hours resulted in the disappearance of HSF1 granules, indicating attenuation of HSF1 activity. Treatment of cells with glycyrrhizin resulted in the gradual relocalization of HSF1 into the nucleus (I–L). The phase-contrast micrographs are shown in A and C; the HSF1-specific fluorescence micrographs of each corresponding field are shown in B and D
From these results, it is strongly suggested that induction of HSPs by the treatment with paeoniflorin is mediated by the activation of HSF1.
Induction of thermotolerance by paeoniflorin and paeoniflorin-containing herbal medicine
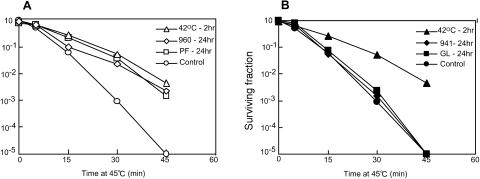
When living cells are exposed to nonlethal heat shock, they acquire a transient resistance to an otherwise lethal heat challenge as determined by the increase in clonogenic cell survival (Gerner and Schneider 1975). This phenomenon is termed acquired thermotolerance. As shown in Figure 6A, thermotolerance was clearly induced by the treatment with paeoniflorin (80 μg/mL for 24 hours) and the 960 mixture (400 μg/mL for 24 hours), which contains paeoniflorin. Surviving fractions of these cells were almost the same as those of cells treated with mild heat shock (42°C for 2 hours). In contrast, glycyrrhizin or the 941 mixture (not containing paeoniflorin) could not induce thermotolerance at the same concentrations (Fig 6B). Thus, the development of thermotolerance is well correlated with the induction of HSPs.
Fig 6.
(A) Development of thermotolerance in HeLa cells treated with paeoniflorin and the 960 mixture (containing paeoniflorin). Cells were treated with heat shock (42°C for 2 hours, ▵), 960 mixture (400 μg/mL for 24 hours, ⋄), or paeoniflorin (PF, 80 μg/mL for 24 hours, □) and then heated at 45°C for the indicated period. After 45°C heating, cells were subjected to the clonogenic survival assay. (B) Effect of glycyrrhizin and the 941 mixture (not containing paeoniflorin) on the development of thermotolerance in HeLa cells. Cells were treated with heat shock (42°C for 2 hours, ▴), 941 mixture (400 μg/mL for 24 hours, ♦), or glycyrrhizin (GL, 80 μg/mL for 24 hours, ▪) and then heated at 45°C for the indicated period. After 45°C heating, cells were subjected to the clonogenic survival assay. Survival curves of nontreated control cells are indicated by open and closed circles in panel A and panel B, respectively. All data points represent the means of 3 independent experiments
Paeoniflorin and glycyrrhizin are not toxic
For the purpose of clinical use of chaperone-inducing compounds, they should not be toxic or deleterious. When HeLa cells were cultured in the presence of a mixture of herbal medicines (960 and 941) at a concentration of 400 μg/mL and in the presence of paeoniflorin or glycyrrhizin at a concentration of 80 μg/mL, no appreciable growth inhibition was observed (Fig 7A). Also, the cell-cycle phase distributions of HeLa cells treated with these herbal medicines or constituents (24 hours at 37°C) were not significantly different from those of nontreated control cells (Fig 7B). Of course, these herbal medicines and constituents had no apparent effect on the morphology of HeLa cells under the same conditions as described in Figure 7B (data not shown).
Fig 7.
Effect of herbal medicines and constituents on cell growth and cell cycle. (A) HeLa cells (1 × 105) were inoculated in triplicate on day 0 and incubated at 37°C in the presence of paeoniflorin (PF, 80 μg/mL, □), glycyrrhizin (GL, 80 μg/mL, ○), 960 mixture (400 μg/mL, ▵), or 941 mixture (400 μg/mL, •) for 12 days. Every 2 days, the number of cells was counted with a hemocytometer. Each data point represents the mean of 3 independent experiments. No appreciable growth inhibition was observed in cells treated with herbal medicines and constituents. (B) HeLa cells were treated with heat shock (42°C for 2 hours), 960 mixture (400 μg/mL for 24 hours), 941 mixture (400 μg/mL for 24 hours), paeoniflorin (PF, 80 μg/mL for 24 hours), or glycyrrhizin (GL, 80 μg/mL for 24 hours). Then, cell-cycle phase distribution was analyzed by flow cytometry. ○, G1 phase; □, S phase; ▵, G2/M phase. Each data point represents the mean of 3 independent experiments. There are no apparent differences between nontreated control cells and cells treated with herbal medicines and constituents
DISCUSSION
In this study, we demonstrated that treatment of cells with paeoniflorin alone resulted in phosphorylation and acquisition of the DNA-binding ability of HSF1, relocalization of HSF1 to form characteristic HSF1 granules in the nucleus, and finally induction of HSPs. These results strongly suggest that the induction of HSPs by paeoniflorin is mediated by the activation of HSF1. Although glycyrrhizin alone could not induce HSPs, it promoted relocalization of HSF1 into the nucleus. This might explain why glycyrrhizin could enhance the induction of HSPs in combination with mild heat shock. Also, development of thermotolerance is well correlated with the expression of HSPs. These results clearly indicate that paeoniflorin is a chaperone inducer and glycyrrhizin a chaperone coinducer. Precise molecular mechanisms of the positive effect of these compounds on the induction of HSPs, however, remain to be elucidated.
It has been reported that several chemical compounds can induce or enhance the expression of HSPs. GGA is shown to induce HSPs in a primary culture of gastric mucosal cells under the condition of low serum concentration (Hirakawa 1996). GGA, however, could not induce HSPs in cultured rat hepatocytes (Ikeyama et al 2001). Therefore, the positive effect of GGA on the induction of HSPs might be cell-type specific. When orally administered, however, GGA enhanced the induction of HSPs in the rat liver in combination with heat shock and protected the liver from the injury caused by ischemia-reperfusion (Yamagami et al 2000). Bimoclomol (BRLP-42), a hydroxylamine derivative, acts as a coinducer of HSPs, enhancing the amount of HSPs after heat shock compared with heat shock alone (Vigh et al 1997). Bimoclomol is shown to bind directly to HSF1 and induce a prolonged binding of HSF1 to HSE (Hargitai et al 2003). It has been reported from results in experimental animal models that bimoclomol has potential therapeutic use in the treatment of diabetic peripheral neuropathy (Biro et al 1997), cardiac dysfunction (Lubbers et al 2002), and cerebrovascular disorders (F. Erdo and S.L. Erdo 1998). Because paeoniflorin and glycyrrhizin had no appreciable toxicity at the concentrations used in this study, they might be used for the prevention and treatment of various pathological states, such as stress ulcers and ischemia-induced injuries, and of diseases associated with protein misfolding and protein aggregation.
Peony plants, such as P suffruticosa, P lactiflora, P veitchii, and P obovata, have been used in traditional Chinese medicines or herbal medicines in China and Japan. Paeoniflorin, isolated from P lactiflora, is one of the major constituents of peony plants. Peony extracts and their constituents have been shown to have various biological and biomodulating activities, including improvement of memory (Ohta et al 1993), antioxidant activity (Okubo et al 2000), antiepileptic activity (Tsuda et al 1997), antimutagenic properties (Sakai et al 1990), and antihyperglycemia (Hsu et al 1997). Glycyrrhizin, tripertenoid saponin composed of 1 molecule of glycyrrhetinic acid and 2 of glucuronic acid, is a main constituent of the hydrophilic fraction of licorice (Glycyrrhiza glabra) extracts, which have also been used as herbal medicines. Glycyrrhizin is known to have a wide range of pharmacological actions, such as antiviral (Pompei et al 1979), anticarcinogenic (Nishino et al 1984), antiallergic (Takeda et al 1991; Park et al 2004), and anti-inflammatory activities (Inoue et al 1986). Although the molecular mechanisms of these pharmacological functions of peoniflorin and glycyrrhizin are not yet fully understood, these activities might be ascribed in part to their positive effect on the induction of molecular chaperones. In the future, a wide variety of pharmacological activities of peoniflorin and glycyrrhizin ought to be studied in relation to their properties of chaperone inducer and coinducer. Also, paeoniflorin and paeoniflorin-containing herbal medicines might be used clinically as chaperone inducers and glycyrrhizin and glycyrrhizin-containing herbal medicines as chaperone coinducers. We are now studying whether paeoniflorin and glycyrrhizin can induce or enhance the expression of HSPs in the whole organism.
In this study, we tested a limited number of herbal medicines and constituents. It may be of value to search for other chaperone inducers and coinducers in herbal medicines.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Priority Area (12217171) and for the High-Tech Research Center Establishment Project from the Japanese Ministry of Education, Culture, Sports, Science, and Technology. We thank Drs Yutaka Inaguma and Hidenori Ito, Institute for Developmental Research, Aichi Human Service Center, for their support in gel mobility shift assay and Yasunori Ichihashi and Tatsuhiko Itoh for their technical assistance.
REFERENCES
- Biro K, Jednakovits A, Kukorelli T, Hegedus E, Koranyi L. Bimoclomol (BRLP-42) ameliorates peripheral neuropathy in streptozotocin-induced diabetic rats. Brain Res Bull. 1997;44:259–263. doi: 10.1016/s0361-9230(97)00118-4.0361-9230(1997)044<0259:BBAPNI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brown IR, Sharp FR 1999 The cellular stress gene response in brain. In: Stress Proteins, ed Latchman DS. Springer-Verlag, Berlin, 243–263. [Google Scholar]
- Carroll R, Yellon DM 1999 Heat stress proteins and their relationship to myocardial protection. In: Stress Proteins, ed Latchman DS. Springer-Verlag, Berlin, 265–279. [Google Scholar]
- Cotto JJ, Fox SG, Morimoto RI. HSF1 granules: a novel stress-induced nuclear compartment of human cells. J Cell Sci. 1997;110:2925–2934. doi: 10.1242/jcs.110.23.2925.0021-9533(1997)110<2925:HGANSN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet. 1998;19:148–154. doi: 10.1038/502.1061-4036(1998)019<0148:CSOAAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Erdo F, Erdo SL. Bimoclomol protects against vascular consequences of experimental subarachnoid hemorrhage in rats. Brain Res Bull. 1998;45:163–166. doi: 10.1016/s0361-9230(97)00333-x.0361-9230(1998)045<0163:BPAVCO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gerner EW, Schneider MJ. Induced thermal resistance in HeLa cells. Nature. 1975;256:500–502. doi: 10.1038/256500a0.0028-0836(1975)256<0500:ITRIHC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hahm KB, Park IS, Kim YS, Kim JH, Cho SW, Lee SI, Youn JK. Role of rebamipide on induction of heat-shock proteins and protection against reactive oxygen metabolite-mediated cell damage in cultured gastric mucosal cells. Free Radic Biol Med. 1997;22:711–716. doi: 10.1016/s0891-5849(96)00406-6.0891-5849(1997)022<0711:ROROIO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hargitai J, Lewis H, and Boros I. et al. 2003 Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem Biophys Res Commun. 307:689–695. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0.0028-0836(1996)381<0571:MCICPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408.0193-4511(2002)295<1852:MCITCF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hattori H, Liu YC, and Tohnai I. et al. 1992 Intracellular localization and partial amino acid sequence of a stress-inducible 40-kDa protein in HeLa cells. Cell Struct Funct. 17:77–86. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Tohnai I, Kaneda T, Kobayashi T, Ohtsuka K. Translocation of hsp-70 and protein synthesis during continuous heating at mild temperatures in HeLa cells. Radiat Res. 1991;125:80–88.0033-7587(1991)125<0080:TOHAPS>2.0.CO;2 [PubMed] [Google Scholar]
- Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996;111:345–357. doi: 10.1053/gast.1996.v111.pm8690199.0016-5085(1996)111<0345:GIHSPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701.0193-4511(2003)300<1142:ROAAAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hsu FL, Lai CW, Cheng JT. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of. Paeonia lactiflora. Planta Med. 1997;63:323–325. doi: 10.1055/s-2006-957692. [DOI] [PubMed] [Google Scholar]
- Ikeyama S, Kusumoto K, Miyake H, Rokutan K, Tashiro S. A non-toxic heat shock protein 70 inducer, geranylgeranylacetone, suppresses apoptosis of cultured rat hepatocytes caused by hydrogen peroxide and ethanol. J Hepatol. 2001;35:53–61. doi: 10.1016/s0168-8278(01)00053-8.0168-8278(2001)035<0053:ANHSPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Inoue H, Saito H, Koshihara Y, Murota S. Inhibitory effect of glycyrrhetinic acid derivatives on lipoxygenase and prostaglandin synthetase. Chem Pharm Bull. 1986;34:897–901. doi: 10.1248/cpb.34.897.0009-2363(1986)034<0897:IEOGAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp Cell Res. 2001;266:213–221. doi: 10.1006/excr.2001.5220.0014-4827(2001)266<0213:ROTLOS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–271. doi: 10.3109/07853899908995889.0785-3890(1999)031<0261:HSPACL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jana NR, Tanaka M, Wang GH, Nukina N. Polyglutamine length-dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum Mol Genet. 2000;9:2009–2018. doi: 10.1093/hmg/9.13.2009.0964-6906(2000)009<2009:PLIOHA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jeoung DI, Chen S, Windsor J, Pollack RE. Human major HSP70 protein complements the localization and functional defects of cytoplasmic mutant SV40 T antigen in Swiss 3T3 mouse fibroblast cells. Genes Dev. 1991;5:2235–2244. doi: 10.1101/gad.5.12a.2235.0890-9369(1991)005<2235:HMHPCT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kume A, Li M, Doyu M, Hata M, Ohtsuka K, Sobue G. Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J Biol Chem. 2000;275:8772–8778. doi: 10.1074/jbc.275.12.8772.0021-9258(2000)275<8772:CHAHSA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540.0027-8424(1995)092<7540:TAELCB>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Engelman RM, and Moraru II. et al. 1992 Heat shock. A new approach for myocardial preservation in cardiac surgery. Circulation. 86:II358–II363. [PubMed] [Google Scholar]
- Lubbers NL, Polakowski JS, Wegner CD, Burke SE, Diaz GJ, Daniell KM, Cox BF. Oral bimoclomol elevates heat shock protein 70 and reduces myocardial infarct size in rats. Eur J Pharmacol. 2002;435:79–83. doi: 10.1016/s0014-2999(01)01552-7.0014-2999(2002)435<0079:OBEHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Michels AA, Kanon B, Konings AWT, Ohtsuka K, Bensaude O, Kampinga HH. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J Biol Chem. 1997;272:33283–33289. doi: 10.1074/jbc.272.52.33283.0021-9258(1997)272<33283:HAHCAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Minami Y, Hohfeld J, Ohtsuka K, Hartl FU. Regulation of the heat-shock protein 70 reaction cycle by the mammalian dnaj homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617.0021-9258(1996)271<19617:ROTHPR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788.0890-9369(1998)012<3788:ROTHST>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Dynamic remodeling of transcription complexes by molecular chaperones. Cell. 2002;110:281–284. doi: 10.1016/s0092-8674(02)00860-7.0092-8674(2002)110<0281:DROTCB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833.1087-0156(1998)016<0833:SRAHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532.1059-1524(2004)015<0657:ROLICE>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SD, Cumming DVE, Latchman DS, Yellon DM. Specific induction of the 70-kD heat stress proteins by the tyrosine kinase inhibitor herbimycin-A protects rat neonatal cardiomyocytes. A new pharmacological route to stress protein expression? J Clin Investig. 1996;97:706–712. doi: 10.1172/JCI118468.0021-9738(1996)097<0706:SIOTKH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowshi PJ. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35:9–12. doi: 10.1016/s0896-6273(02)00761-4.0896-6273(2002)035<0009:PMAFAN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nagayama S, Jono H, and Suzaki H. et al. 2001 Carbenoxolone, a new inducer of heat shock protein 70. Life Sci. 24:2867–2873. [DOI] [PubMed] [Google Scholar]
- Nanasi PP, Jednakovits A. Multilateral in vivo and in vitro protective effects of the novel heat shock protein coinducer, bimoclomol results of preclinical studies. Cardiovasc Drug Rev. 2001;19:133–151. doi: 10.1111/j.1527-3466.2001.tb00060.x.0897-5957(2001)019<0133:MIVAIV>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nishino H, Kitagawa K, Iwashima A. Antitumor-promoting activity of glycyrrhetic acid in mouse skin tumor formation induced by 7,12-dimethylbenz[a]anthracene plus teleocidin. Carcinogenesis. 1984;5:1529–1530. doi: 10.1093/carcin/5.11.1529.0143-3334(1984)005<1529:AAOGAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ohta H, Ni JW, Matsumoto K, Watanabe H, Shimizu M. Peony and its major constituent, paeoniflorin, improve radial maze performance impaired by scopolamine in rats. Pharmacol Biochem Behav. 1993;45:719–723. doi: 10.1016/0091-3057(93)90530-7.0091-3057(1993)045<0719:PAIMCP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Hata M. Molecular chaperone function of mammalian Hsp70 and Hsp40—a review. Int J Hyperth. 2000;16:231–245. doi: 10.1080/026567300285259.0265-6736(2000)016<0231:MCFOMH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Suzuki T. Roles of molecular chaperones in the nervous system. Brain Res Bull. 2000;53:141–146. doi: 10.1016/s0361-9230(00)00325-7.0361-9230(2000)053<0141:ROMCIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Okubo T, Nagai F, Seto T, Satoh K, Ushiyama K, Kano I. The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of Moutan Cortex and Paeoniae Radix. Biol Pharm Bull. 2000;23:199–203. doi: 10.1248/bpb.23.199.0918-6158(2000)023<0199:TIOPOD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Park HY, Park SH, Yoon HK, Han MJ, Kim DH. Anti-allergic activity of 18beta-glycyrrhetinic acid-3-O-beta-d-glucuronide. Arch Pharm Res. 2004;27:57–60. doi: 10.1007/BF02980047.0253-6269(2004)027<0057:AAOBA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253.0066-4197(1993)027<0437:TFOHPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Plumier JCL, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2.1466-1268(1997)002<0162:TMETHI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei R, Flore O, Marccialis MA, Pani A, Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281:689–690. doi: 10.1038/281689a0.0028-0836(1979)281<0689:GAIVGA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rokutan K. Role of heat shock proteins in gastric mucosal protection. J Gastroenterol Hepatol. 2000;15:D12–D19. doi: 10.1046/j.1440-1746.2000.02144.x.0815-9319(2000)015<D12:ROHSPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Nagase H, Ose Y, Kito H, Sato T, Kawai M, Mizuno M. Inhibitory action of peony root extract on the mutagenicity of benzo[a]pyrene. Mutat Res. 1990;244:129–134. doi: 10.1016/0165-7992(90)90061-n.0027-5107(1990)244<0129:IAOPRE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392.0270-7306(1993)013<1392:AOHSGT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger MJ, Ashburner M, and Tissieres A ed. 1982 Heat Shock from Bacteria to Man. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Smith JM. Prolongation of the life of Drosophila subobscura by a brief exposure of adults to a high temperature. Nature. 1958;181:496–497. doi: 10.1038/181496a0.0028-0836(1958)181<0496:POTLOD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takeda H, Ohta K, and Niki H. et al. 1991 Eosinophilic peritonitis responding to treatment with glycyrrhizin. Tokai J Exp Clin Med. 16:183–186. [PubMed] [Google Scholar]
- Takeuchi H, Kobayashi Y, Yoshihara T, Niwa JI, Doyu M, Ohtsuka K, Sobue G. Hsp70 and Hsp40 improve neurite outgrowth and suppress intracytoplasmic aggregate formation in cultured neuronal cells expressing mutant SOD1. Brain Res. 2002;949:11–22. doi: 10.1016/s0006-8993(02)02568-4.0006-8993(2002)949<0011:HAHINO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tang Y, Ramakrishnan C, Thomas J, DeFranco DB. A role for HDJ-2/HSDJ in correcting subnuclear trafficking, transactivation, and transrepression defects of a glucocorticoid receptor zinc finger mutant. Mol Biol Cell. 1997;8:795–809. doi: 10.1091/mbc.8.5.795.1059-1524(1997)008<0795:ARFHIC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997;390:30. doi: 10.1038/36237.0028-0836(1997)390<0030:CEL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tsuda T, Sugaya A, Ohguchi H, Kishida N, Sugaya E. Protective effects of peony root extract and its components on neuron damage in the hippocampus induced by the cobalt focus epilepsy model. Exp Neurol. 1997;146:518–525. doi: 10.1006/exnr.1997.6570.0014-4886(1997)146<0518:PEOPRE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vigh L, Literati PN, and Horvath I. et al. 1997 Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 3:1150–1154. [DOI] [PubMed] [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x.1474-9718(2003)002<0131:LEICEB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wu C, Clos J, and Giorgi G. et al. 1994 Structure and regulation of heat shock transcription factor. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 395–416. [Google Scholar]
- Yamagami K, Yamamoto Y, Ishikawa Y, Yonezawa K, Toyokuni S, Yamaoka Y. Effects of geranyl-geranyl-acetone administration before heat shock preconditioning for conferring tolerance against ischemia-reperfusion injury in rat livers. J Lab Clin Med. 2000;135:465–475. doi: 10.1067/mlc.2000.106806.0022-2143(2000)135<0465:EOGABH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Fukumoto K, and Murakami T. et al. 2002 Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 516:53–57. [DOI] [PubMed] [Google Scholar]