Abstract
Heat shock protein (Hsp) 72 is a cytosolic stress protein that is highly inducible by several factors including exercise. Hsp60 is primarily mitochondrial in cellular location, plays a key role in the intracellular protein translocation and cytoprotection, is increased in skeletal muscle by exercise, and is found in the peripheral circulation of healthy humans. Glucose deprivation increases Hsp72 in cultured cells, whereas reduced glycogen availability elevates Hsp72 in contracting human skeletal muscle. To determine whether maintained blood glucose during exercise attenuates the exercise-induced increase in intramuscular and circulating Hsp72 and Hsp60, 6 males performed 120 minutes of semirecumbent cycling at ∼65% maximal oxygen uptake on 2 occasions while ingesting either a 6.4% glucose (GLU) or sweet placebo (CON) beverage throughout exercise. Muscle biopsies, obtained before and immediately after exercise, were analyzed for Hsp72 and Hsp60 protein expression. Blood samples were simultaneously obtained from a brachial artery, a femoral vein, and the hepatic vein before and during exercise for the analysis of serum Hsp72 and Hsp60. Leg and hepatosplanchnic blood flow were measured to determine Hsp72-Hsp60 flux across these tissue beds. Neither exercise nor glucose ingestion affected the Hsp72 or Hsp60 protein expression in, or their release from, contracting skeletal muscle. Arterial serum Hsp72 increased (P < 0.05) throughout exercise in both trials but was attenuated (P < 0.05) in GLU. This may have been in part because of the increased (P < 0.05) hepatosplanchnic Hsp72 release in CON, being totally abolished (P < 0.05) in GLU. Serum Hsp60 increased (P < 0.05) after 60 minutes of exercise in CON before returning to resting levels at 120 minutes. In contrast, no exercise-induced increase in serum Hsp60 was observed in GLU. We detected neither hepatosplanchnic nor contracting limb Hsp60 release in either trial. In conclusion, maintaining glucose availability during exercise attenuates the circulating Hsp response in healthy humans.
INTRODUCTION
Heat shock proteins (Hsps), highly conserved proteins found in all prokaryotes and eukaryotes, are molecular chaperones of naïve, aberrantly folded, or mutated proteins, as well as are essential to restore normal function and provide protection from disrupted cell homeostasis (Hartl 1996). The most abundant and widely studied families are the 60-kDa (Hsp60) and the 70-kDa (Hsp70) families (Bukau and Horwich 1998). Hsp60 is primarily mitochondrial in cellular location, playing a key role in the intracellular protein translocation and cytoprotection (Sigler et al 1998). Hsp70 is a cytosolic stress protein whose inducible form is Hsp72.
Exercise results in an increase in serum concentration of Hsp72 (Walsh et al 2001; Febbraio et al 2002a) in part because of a release by the hepatosplanchnic viscera (Febbraio et al 2002a). During exercise, the rise in hepatic glucose production is suppressed by glucose ingestion because the demand for glucose is met by the ingested carbohydrate (Jeukendrup et al 1999b). Hence, the metabolic stress placed on the liver may be reduced under such circumstances, ultimately resulting in a decreased hepatosplanchnic Hsp72 release. Hsp60 has been found in the circulation of healthy individuals (Pockley et al 1999; Lewthwaite et al 2002), and the concentration of Hsp60 within the serum is associated with cardiovascular disease (Pockley et al 2000), high-density lipoprotein cholesterol, and tumor necrosis factor-α (Lewthwaite et al 2002). However, to our knowledge, no studies have examined the effect of physical exercise on the circulating Hsp60 response, the potential sources of circulating Hsp60 or whether glucose ingestion blunts this Hsp60 response. Therefore, the primary aim of the present study was to determine whether glucose ingestion would decrease the exercise-induced increase in serum Hsp60 and Hsp72 levels, and whether a reduction in hepatosplanchnic Hsp release may be responsible, in part, for any attenuation in the Hsp response.
Physical exercise may induce an increase in both Hsp72 and Hsp60 protein expression in a variety of tissues and mammalian species (Khassaf et al 2001; Kregel 2002). Interestingly, increased synthesis of glucose-regulated proteins (members of the Hsp family of proteins) occurs in Chinese hamster ovary cells deprived of glucose (Sciandra and Subjeck 1983). In addition, reduced glycogen availability is associated with elevated Hsp72 messenger ribonucleic acid (mRNA) and protein levels in contracting human skeletal muscle (Febbraio et al 2002c). It has been previously shown that 2 hours of bicycle exercise can deplete muscle glycogen (Febbraio et al 2000b), and because skeletal muscle glucose uptake and oxidation is increased by glucose ingestion (Febbraio et al 2000b) potentially sparing muscle glycogen use, another aim was to test the hypothesis that glucose ingestion would be capable of attenuating the exercise-induced increase in contracting muscle Hsp expression by decreasing the reliance on intramuscular glycogen.
MATERIALS AND METHODS
Subjects
Six healthy, active men (23.7 ± 6.3 years; 180 ± 5 cm; 74.9 ± 8.8 kg; maximal oxygen uptake [VO2max] = 4.06 ± 0.10 L/min; mean ± SD) participated in the study, which was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark, and performed according to the Declaration of Helsinki. Subjects were informed about the possible risks and discomfort involved before their written consent was obtained.
Preliminary testing
After the medical screening, each subject underwent a VO2max test on a semirecumbent cycle ergometer. Semirecumbent cycling was chosen to allow for the determination of leg blood flow (LBF) using the thermodilution technique during the experimental trials. From this test, a workload was calculated, which would elicit ∼65% of each individual's VO2max. At least 3 days after the VO2max test and 48 hours before the experimental trials, subjects reported to the laboratory and completed 45 minutes of upright cycling exercise at a workload corresponding to 65% of maximal heart rate. Thereafter, the subjects were provided with food packages, which they consumed for the next 2 days (15.6 MJ/day, ∼70% CHO, 15% protein, 15% fat). During this period, the subjects were asked to adhere to the diet and to refrain from strenuous exercise and intake of alcohol, tobacco, and caffeine. This protocol was adopted to minimize any differences in the metabolic and hormonal status of the subjects (intersubject variability) before each trial.
Experimental procedures
Subjects participated in 2 experimental trials separated by at least 10 days and conducted in random order. During each trial, the subjects exercised on a semirecumbent cycle ergometer for 120 minutes. They commenced exercise for 5 minutes at 50% VO2max and subsequently cycled for 115 minutes at ∼65% VO2max. Trials were conducted in a room maintained at 22°C, and a circulating fan was placed in front of the subjects during exercise to minimize thermal stress. Each trial was identical except that in one trial (GLU) subjects ingested 250 mL of a 6.4% carbohydrate beverage (Lucozade Sport; Glaxo Smith Kline, UK) at the onset of and at 15 minutes intervals throughout exercise, whereas in the other they consumed an artificially flavored placebo (CON). On the day of each experiment, the subjects reported to the laboratory at 0730 hours after a 12–14 hours overnight fast. They voided, changed into appropriate exercise attire, and rested in a supine position for 10 minutes.
A hepatic venous catheter was subsequently inserted (Febbraio et al 2002a). During experiments on the first 3 subjects, the hepatic venous catheter was introduced via the right median cubital vein and was guided with the subject supine. The position of the catheter was confirmed with fluoroscopy in the body position used during cycling. To ensure that ventilation (Ve) did not displace the catheter, the position was also confirmed after maximal voluntary Ve. Despite these efforts, the catheter dislodged during exercise in 2 of the 6 experiments, and therefore, we could only obtain data in both trials for 1 subject. Hence, we introduced the catheter via the right femoral vein in the subsequent experiments in the 3 subjects, and in these trials, the catheter remained in the hepatic vein. As a result, data presented for hepatosplanchnic Hsp72-Hsp60 flux are presented as n = 4. After this procedure, a catheter was placed in the left brachial artery (1.0 mm inner diameter; 20 gauge) and a third catheter (7 Fr diameter Cook, Denmark) was inserted into the left femoral vein ∼1–2 cm distal to the inguinal ligament (Febbraio et al 2002a). Hepatosplanchnic blood flow (HBF) and LBF was measured at rest and at 60 and 120 minutes during exercise using the indocyanine green dye and the constant infusion thermodilution techniques, respectively (Febbraio et al 2002a).
Immediately before exercise and at 60 and 120 minutes during exercise, blood samples were simultaneously collected from the brachial artery and the femoral and hepatic veins for the measurement of Hsp72 and Hsp60 as described previously (Febbraio et al 2002a). Muscle biopsy samples were obtained from the vastus lateralis muscle before and immediately after exercise and analyzed for Hsp72 (#EKS-700, Stressgen Biotechnologies, Victoria, BC, Canada) and Hsp60 (#EKS-600, Stressgen Biotechnologies) proteins by an enzyme-linked immunosorbent assay using methods described in detail elsewhere (Walsh et al 2001). Muscle glycogen was also measured by enzymatic analyses with fluorometric detection as described previously (Febbraio et al 2002c). Care was taken to sample muscle from different regions of the muscle before and after exercise. We have demonstrated previously that this biopsy procedure does not result in increased Hsp72 mRNA in noncontracting muscle (Febbraio et al 2002b).
Calculations and statistics
Net leg Hsp72 and Hsp60 balances were calculated by multiplying the femoral vein-arterial Hsp72 and Hsp60 differences by the net LBF. Similarly, the net hepatosplanchnic Hsp72 and Hsp60 balances were calculated by multiplying the hepatosplanchnic vein-arterial Hsp72 and Hsp60 differences by the net HBF. Comparative data are expressed as means ± SEM. A 2-way (trial × time) analysis of variance with repeated measures on the time factor was used to compute the statistics (Statistica®, Tulsa, OK, USA), for all measures. Significance was accepted with a P value of ≤0.05. If analyses revealed a significant interaction, a Newman–Keuls post hoc test was used to locate specific differences.
RESULTS
LBF increased (P < 0.05) from 0.54 ± 0.07 and 0.56 ± 0.06 L/min at rest to an average of 6.12 ± 0.52 and 6.14 ± 0.38 L/min during exercise for CON and GLU, respectively. HBF was neither affected by exercise nor glucose ingestion averaging 1.26 ± 0.18 and 1.29 ± 0.20 L/min for CON and GLU, respectively (n = 4).
Glucose ingestion attenuates the exercise-induced increase in circulating Hsp72 and Hsp60
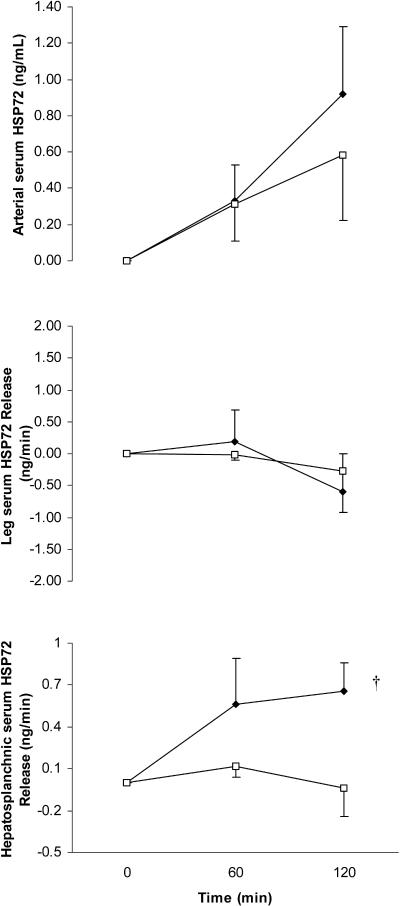
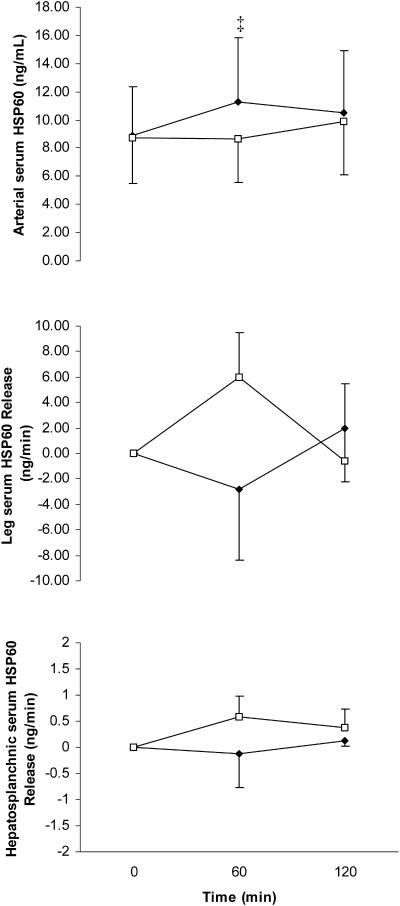
The arterial serum Hsp72 response increased (P < 0.05) throughout exercise in both GLU and CON; however, the increase during the final 60 minutes of exercise was attenuated (P < 0.05) in GLU (Fig 1). The large standard error bars at 120 minutes of exercise when comparing arterial serum Hsp72 values between trials was because of a differential Hsp72 response between subjects rather than between trials because all 6 subjects displayed markedly lower concentrations at 120 minutes in GLU compared with CON. As a result, the difference when comparing trials at this point was highly significant (P = 0.006). Arterial serum Hsp60 increased (P < 0.05) after 60 minutes of the control trial before returning to resting levels at the end (120 minutes) of exertion. Such increase was totally abolished with glucose ingestion (P < 0.05) (Fig 2). The large standard error bars in arterial serum Hsp60 values was because of a high intersubject variability in basal Hsp60 levels rather than different Hsp60 responses with exercise or treatment. In fact, all 6 subjects displayed markedly lower concentrations at 60 minutes in GLU compared with CON. As a result, the difference when comparing trials at this point was highly significant (P = 0.003).
Fig 1.
Arterial serum heat shock protein 72 (Hsp72) (top), leg Hsp72 release (middle), and hepatosplanchnic Hsp72 release (bottom) before (0 minute) and during 120 minutes of semirecumbent cycling at ∼65% of maximal oxygen uptake with the ingestion of a placebo (CON, filled diamonds) or glucose (GLU, open squares) beverage throughout exercise. * denotes difference when comparing CON with GLU, † denotes main effect for time (P < 0.05) in CON. Data expressed as mean ± SEM (n = 6 for top and middle panel, n = 4 for bottom panel)
Fig 2.
Arterial serum heat shock protein 60 (Hsp60) (top), leg Hsp60 release (middle), and hepatosplanchnic Hsp60 release (bottom) before (0 minute) and during 120 minutes of semirecumbent cycling at ∼65% of maximal oxygen uptake with the ingestion of a placebo (CON, filled diamonds) or glucose (GLU, open squares) beverage throughout exercise. ‡ denotes difference when comparing CON with GLU. Data expressed as mean ± SEM (n = 6 for top and middle panel, n = 4 for bottom panel)
The reduction with glucose ingestion of the exercise-induced increase in circulating Hsp72 was because of a blunting in hepatosplanchnic Hsp72 release
To test the hypothesis that the attenuation of the exercise-induced increase in circulating Hsps by glucose ingestion was because of a reduction in the hepatosplanchnic rather than leg Hsps release, we calculated both leg and hepatosplanchnic Hsps releases. There was no measurable leg serum Hsp72 or Hsp60 release in either GLU or CON. Although we were only able to analyze hepatosplanchnic Hsp72 release from 4 subjects, hepatosplanchnic Hsp72 release was markedly attenuated in GLU compared with CON in all subjects (Fig 1). Although we had a low subject number, statistical analyses revealed hepatosplanchnic Hsp72 release in CON (P < 0.05) but not in GLU. Therefore, the attenuation of the exercise-induced increase in circulating Hsp72 was in part because of the abolished hepatosplanchnic Hsp72 release with glucose ingestion. We did not detect hepatosplanchnic Hsp60 release in either trial (Fig 2).
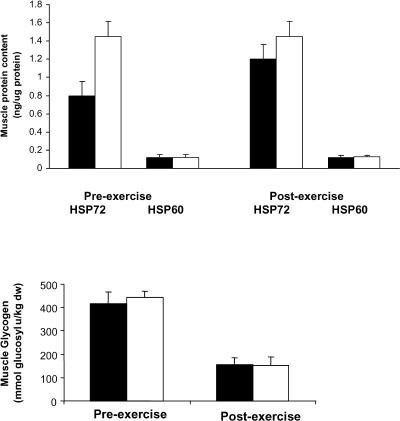
Glucose ingestion does not affect intramuscular Hsp72 and Hsp60 protein expression or muscle glycogen use
Neither muscle Hsp72 nor Hsp60 protein expression was affected by exercise or glucose ingestion (Fig 3). Although Hsp72 appeared elevated before exercise in GLU compared with CON, this was largely because of the results from 1 subject. Hence, even when the data from this subject were included, analysis by a paired t-test on the preexercise data results was not significant (P = 0.13). Of note, muscle Hsp72 expression was much higher than Hsp60 protein expression. Muscle glycogen content was reduced (P < 0.05) during exercise but was not different when comparing GLU with CON either before or after exercise (Fig 3).
Fig 3.
Heat shock protein (Hsp) 72 and Hsp60 in skeletal muscle (top) and glycogen (bottom) obtained before (preexercise) and immediately after 120 minutes of semirecumbent cycling at ∼65% of maximal oxygen uptake with the ingestion of a placebo (CON, filled bars) or glucose (GLU, open bars) beverage throughout exercise. Data expressed as mean ± SEM (n = 6)
DISCUSSION
The results from the present study demonstrate that glucose ingestion during exercise attenuates the exercise-induced increase in serum Hsp72 and Hsp60. However, 120 minutes of moderate intensity cycling does not affect the Hsp72 or Hsp60 protein expression within contracting skeletal muscle.
It has been reported that Hsp60 is expressed in the serum of healthy and hypertensive subjects (Pockley et al 2002), with decreased values in aging (Rea et al 2001). Interestingly, we report in this study that physical exertion induces a small increase in circulating Hsp60 during the first 60 minutes of submaximal exercise, returning to basal values after 120 minutes of exercise. To our knowledge, this is the first report that similar to Hsp72 (Walsh et al 2001; Febbraio et al 2002a), Hsp60 is also induced in humans during exercise. Of note, neither the contracting limb nor the liver contributed to the increase in circulating Hsp60. It appeared that leg and hepatosplanchnic serum Hsp60 release was higher in GLU at 60 minutes, when arterial serum Hsp60 was lower (P < 0.05) at this time point compared with CON. However, this was largely because of the results from 1 subject, with all others showing little if any change. This was contrary to the consistently higher arterial serum Hsp60 displayed by all subjects in CON compared with GLU. It is also noteworthy, that the absolute release of Hsp60 peaked at 4 ng/ min for the leg and 0.6 ng/min for the hepatosplanchnic viscera, whereas the arterial concentrations ranged between 8 and 12 ng/mL (Fig 2). This is in contrast with the Hsp72 data, where the peak hepatosplanchnic release matched the arterial concentration more closely (Fig 1). Hence, the data suggest that other tissues release Hsp60 in blood during the exercise. In this regard, it has been reported that exercise induces Hsp60 expression in the cytoplasm of leukocytes (Fehrenbach et al 2000), and that hydrogen peroxide, a common oxidant induced by exercise, leads to Hsp60 expression in lymphocytes (Khassaf et al 2003). Furthermore, Hsp60 proteins have been found at unexpected locations, such as the lymphocyte cell surface (Soltys and Gupta 1996; Khan et al 1998), suggesting the possibility that they may be released into the blood (Soltys and Gupta 1999). Therefore, our data suggest that rather than contracting muscle fibers or hepatocytes, other cells may contribute to the exercise-induced increase in circulating Hsp60, which is quickly returned to basal levels after 120 minutes of exercise, denoting a rapid Hsp60 turnover.
We show that glucose ingestion attenuates the exercise-induced increase in circulating Hsp60, suggesting that Hsp60 is released into or expressed in the blood (by other tissues than contracting limb or liver) in absence of exogenous glucose, when the hepatic glucose production is increased (Jeukendrup et al 1999a). Whether there is any causal relationship between liver glycogenolysis or gluconeogenesis (or both) and circulating Hsp60 remains to be elucidated. Because fat oxidation is increased in absence of exogenous glucose (Coyle et al 1997; Angus et al 2000; Spriet and Watt 2003), another question to be answered is whether there is any causal relationship between serum Hsp60 levels and fat oxidation. Further experiments in isolated hepatocytes, myocytes, and adipocytes are required to answer these questions.
It has recently been reported that stress such as physical trauma (Pittet et al 2002) and exercise (Walsh et al 2001; Febbraio et al 2002a) increases the serum levels of Hsp72 in humans. Furthermore, during exercise, the hepatosplanchnic viscera can account for part of this increase (Febbraio et al 2002a), whereas the contracting limb does not contribute to the increase in circulating Hsp72 (Febbraio et al 2002a, 2002c). The results from the present study demonstrate that glucose ingestion can attenuate the exercise-induced increase in arterial serum Hsp72 (Fig 2). It appears that this is at least in part because of a reduction in hepatosplanchnic Hsp72 release. Although we could only collect data in 4 subjects, all 4 demonstrated a total blunting in the hepatosplanchnic Hsp72 release, making it clear that glucose ingestion blunts this response. We are unable to provide a mechanism for such an observation; however, it is possible that the ingestion of glucose reduced hepatic stress because hepatic glucose production during exercise is reduced to basal levels when glucose is ingested (Jeukendrup et al 1999b). Further experiments in isolated hepatocytes are required to provide a mechanism for our observations.
Although hepatosplanchnic serum Hsp72 was totally attenuated in GLU, the arterial systemic Hsp72 was elevated after 120 minutes of exercise (Fig 2). This indicates that tissues other than the hepatosplanchnic viscera contributed to the systemic increase in Hsp72 in this trial. Campisi et al (2003) demonstrated that physical activity increased the Hsp72 content in a number of tissues including the brain, the liver, the heart, the spleen, and the lymph nodes, albeit in a rodent model. In the present study, Hsp72 must have been released from some of these tissues giving rise to the elevated serum Hsp72 response in the presence of a totally attenuated hepatosplanchnic Hsp72 release in GLU. Indeed, we have recently demonstrated that Hsp72 is released from the human brain during 180 minutes of exercise (Lancaster et al 2004).
The observation of a failure for Hsp72 to be increased immediately after an acute bout of exercise supports some (Puntschart et al 1996; Walsh et al 2001) but not all (Febbraio et al 2002c) previous studies. Of note, in the present and previous (Puntschart et al 1996; Walsh et al 2001) studies, the exercise duration ranged from 30 to 120 minutes. In contrast, in our study (Febbraio et al 2002c) showing that a single bout of exercise resulted in an increase in Hsp72 protein expression, such a phenomenon was only observed when exercise was performed for a period of 4–5 hours in the presence of a lower than normal glycogen content at the onset of exercise. When exercise was performed for 4–5 hours with adequate preexercise glycogen stores, no increase in Hsp72 protein was observed (Febbraio et al 2002c). Hence, it appears that only in circumstances where the muscle cells are under a great deal of metabolic stress for prolonged periods does “nondamaging” exercise result in an acute increase in Hsp72 expression.
To our knowledge, only 2 previous studies from the same group (Khassaf et al 2001, 2003) have measured Hsp60 in human skeletal muscle. In these studies, Hsp60 was increased (Khassaf et al 2001) or unaffected (Khassaf et al 2003) 2 days after exercise. In the present study, there was no indication that exercise increased Hsp60 protein expression immediately after exercise, irrespective of glucose ingestion. However, we did observe a much lower basal expression of Hsp60 protein compared with Hsp72. This is not surprising because Hsp72 is cytosolically expressed, whereas Hsp60 resides in the mitochondria. We cannot compare our results with those reported previously (Khassaf et al 2001, 2003) because in these previous studies, the expression of each protein was expressed as a percent change from basal. We were not surprised that glucose ingestion did not affect intramuscular expression of these stress proteins because in this study such a practice did not alter the rate of glycogen use in the contracting muscle. As demonstrated previously, Hsp72 protein expression is influenced by glycogen availability (Febbraio et al 2002c).
In summary, maintaining glucose availability during exercise attenuates the circulating Hsp72-Hsp60 response in healthy humans. The decrease in the Hsp72 systemic response is probably at least in part because of a decrease in the hepatosplanchnic Hsp72 release. Further studies clarifying the role of Hsp72 and Hsp60 in glucose and fat metabolism are warranted.
Acknowledgments
We would like to thank the subjects for their participation. We would also like to thank Ruth Rousing, Peter Nissen, Nina Schjerling, Ellen Dawson, Dr Rebecca Starkie, Natalie Hiscock, Graeme Lancaster, and Hanne Willumsen for their excellent technical assistance. This study was supported by grants from The Danish National Research Foundation (504-14) and The Australian Research Council (DP0209570).
REFERENCES
- Angus DJ, Hargreaves M, Dancey J, Febbraio MA. Effect of carbohydrate or carbohydrate plus medium-chain triglyceride ingestion on cycling time trial performance. J Appl Physiol. 2000;88:113–119. doi: 10.1152/jappl.2000.88.1.113.8750-7587(2000)088<0113:EOCOCP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9.0092-8674(1998)092<0351:THAHCM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, Smith TP, Fleshner M. Habitual physical activity facilitates stress-induced HSP72 induction in the brain, peripheral and immune tissues. Am J Physiol. 2003;284:R520–R530. doi: 10.1152/ajpregu.00513.2002.0002-9513(2003)284<R520:HPAFSH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Coyle EF, Jeukendrup AE, Wagenmakers AJ, Saris WH. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am J Physiol. 1997;273:E268–E275. doi: 10.1152/ajpendo.1997.273.2.E268.0002-9513(1997)273<E268:FAOIDR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Chui A, Angus DJ, Arkinstall MJ, Hawley JA. Effects of carbohydrate ingestion before and during exercise on glucose kinetics and performance. J Appl Physiol. 2000a;89:2220–2226. doi: 10.1152/jappl.2000.89.6.2220.8750-7587(2000)089<2220:EOCIBA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Kennan J, Angus DJ, Campbell SE, Garnham AP. Pre-exercise carbohydrate ingestion, glucose kinetics and muscle glycogen use: effect of the glycemic index. J Appl Physiol. 2000b;89:1845–1852. doi: 10.1152/jappl.2000.89.5.1845.8750-7587(2000)089<1845:PCIGKA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatoplanchnic release of heat shock protein 72 in humans. J Physiol (Lond) 2002a;544:957–962. doi: 10.1113/jphysiol.2002.025148.0022-3751(2002)544<0957:EIHROH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Fischer CP, Keller C, Hiscock N, Pedersen BK. IL-6 activates HSP72 gene expression in human skeletal muscle. Biochem Biophys Res Commun. 2002b;296:1264–1266. doi: 10.1016/s0006-291x(02)02079-x.0006-291X(2002)296<1264:IAHGEI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Walsh R, Koukoulas I, van Hall G, Saltin B, Pedersen BK. Reduced muscle glycogen availability elevates HSP72 in contracting human skeletal muscle. J Physiol (Lond) 2002c;538:911–917. doi: 10.1113/jphysiol.2001.013145.0022-3751(2002)538<0911:RMGAEH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Passek F, Niess AM, Pohla H, Weinstock C, Dickhuth HH, Northoff H. HSP expression in human leukocytes is modulated by endurance exercise. Med Sci Sports Exerc. 2000;32:592–600. doi: 10.1097/00005768-200003000-00007.0195-9131(2000)032<0592:HEIHLI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0.0028-0836(1996)381<0571:MCICPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Raben A, Gijsen A, Stegen JHCH, Brouns F, Saris WHM, Wagenmakers AJM. Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. J Physiol (Lond) 1999a;515:579–589. doi: 10.1111/j.1469-7793.1999.579ac.x.0022-3751(1999)515<0579:GKDPEI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeukendrup AE, Wagenmakers AJ, Stegen JH, Gijsen AP, Brouns F, Saris WH. Carbohydrate ingestion can completely suppress endogenous glucose production during exercise. Am J Physiol Endocrinol Metab. 1999b;276:E672–E683. doi: 10.1152/ajpendo.1999.276.4.E672.0193-1849(1999)276<E672:CICCSE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Khan IU, Wallin R, Gupta RS, Kammer GM. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc Natl Acad Sci U S A. 1998;95:10425–10430. doi: 10.1073/pnas.95.18.10425.0027-8424(1998)095<10425:PKAPOH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90:1031–1035. doi: 10.1152/jappl.2001.90.3.1031.8750-7587(2001)090<1031:TCOROH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol (Lond) 2003;549:645–652. doi: 10.1113/jphysiol.2003.040303.0022-3751(2003)549<0645:EOVCSO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001.8750-7587(2002)092<2177:HSPMFI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Møller K, Nielsen B, Secher NH, Febbraio MA, Nybø L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–280. doi: 10.1379/CSC-18R.1.1466-1268(2004)009<0276:EITROH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewthwaite J, Owen N, Coates A, Henderson B, Steptoe A. Circulating human heat shock protein 60 in the plasma of British civil servants: relationship to physiological and psychosocial stress. Circulation. 2002;106:196–201. doi: 10.1161/01.cir.0000021121.26290.2c.0009-7322(2002)106<0196:CHHSPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie C. Serum levels of Hsp72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–617. doi: 10.1097/00005373-200204000-00001.0022-5282(2002)052<0611:SLOHME>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121.1466-1268(1999)004<0029:IOHHSP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815–1820. doi: 10.1097/00004872-200209000-00027.0263-6352(2002)020<1815:CHSPAH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303.0194-911X(2000)036<0303:CHSPIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Puntschart A, Vogt M, Widmer HR, Hoppeler H, Billeter R. HSP70 expression in human skeletal muscle after exercise. Acta Physiol Scand. 1996;57:411–417. doi: 10.1046/j.1365-201X.1996.512270000.x.0001-6772(1996)057<0411:HEIHSM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36:341–352. doi: 10.1016/s0531-5565(00)00215-1.0531-5565(2001)036<0341:SHSPAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sciandra JJ, Subjeck JR. The effect of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem. 1983;258:12091–12093.0021-9258(1983)258<12091:TEOGOP>2.0.CO;2 [PubMed] [Google Scholar]
- Sigler P, Zhaohui X, Rye HS, Burston SG, Fenton WA, Horwich AL. Structure and function in GroEL-mediated protein folding. Annu Rev Biochem. 1998;67:581–608. doi: 10.1146/annurev.biochem.67.1.581.0066-4154(1998)067<0581:SAFIGP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003.0014-4827(1996)222<0016:IMLOTK>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Mitochondrial matrix proteins at unexpected locations: are they exported? Trends Biochem Sci. 1999;24:174–177. doi: 10.1016/s0968-0004(99)01390-0.0376-5067(1999)024<0174:MMPAUL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Spriet LL, Watt MJ. Regulatory mechanisms in the interaction between carbohydrate and lipid oxidation during exercise. Acta Physiol Scand. 2003;178:443–452. doi: 10.1046/j.1365-201X.2003.01152.x.0001-6772(2003)178<0443:RMITIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum HSP72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2.1466-1268(2001)006<0386:EISHIH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]