Abstract
Background
To investigate the optimal radiotherapy plans for synchronous bilateral breast cancer (SBBC) patients receiving postmastectomy radiotherapy (PMRT), including regional lymph node irradiation (RNI).
Methods
For 10 SBBC patients who underwent bilateral mastectomy and received bilateral PMRT with RNI, 3 integrally optimized plans with a single isocenter were designed for each patient in this retrospective study: intensity-modulated radiation therapy (IMRT) with 9 fixed beams (9F-IMRT), volumetric-modulated arc therapy (VMAT) with 2 pairs of half arcs (2F-VMAT), VMAT with 2 pairs of outer tangential arcs and 1 pair of 200-degree arcs (3F-VMAT). The paired t-test (in the case of normal variables) and Friedman's test (in the case of nonnormal variables) were applied to compare the planning target volumes (PTVs) and organs at risk (OARs) values of the 3 techniques.
Results
The 3 techniques provided adequate target dose coverage and comparable results for PTVs. For OARs, 3F-VMAT yielded the lowest mean or median values of the left lung (15.02 ± 1.57 Gy) and right lung (14.91 ± 1.14 Gy), heart (6.19 (1.96) Gy), coronary artery (15.96 ± 5.76 Gy) and liver (8.10 ± 2.70 Gy) which were significantly different from those of 9F-IMRT and 2F-VMAT. The percentages of volume at various doses (V5, V10, V20, and V30) of 3F-VMAT plans were also lower than or comparable with those of 9F-IMRT and 2F-VMAT. The monitor units (MUs) of 3F-VMAT were 31% higher than those of 9F-IMRT and comparable with those of 2F-VMAT; however, there were time savings and halved beam-on times (BOTs) compared to 9F-IMRT.
Conclusions
The 3F-VMAT plan yielded comparable target coverage compared with 9F-IMRT and 2F-VMAT, was superior in dose sparing of normal tissues and enabled shorter BOTs, improving treatment efficiency. In our research, 3F-VMAT was the optimal radiotherapy technique for SBBC patients receiving PMRT including RNI.
Keywords: synchronous bilateral breast cancer, postmastectomy radiotherapy, regional lymph node, dosimetric comparison, intensity-modulated radiation therapy, volumetric-modulated arc therapy
Background
Due to increasing health consciousness in people, improvements in diagnostic imaging and the standardization of contralateral breast examination, an increasing number of breast cancer patients are diagnosed with contralateral breast cancer (CBC). Different time intervals between the diagnosis of the first breast cancer and CBC have been investigated to classify synchronous bilateral breast cancer (SBBC) and metachronous bilateral breast cancer (MBBC).1–5 The eighth edition of the American Joint Committee on Cancer (AJCC) first reported that SBBC is defined when CBC is diagnosed within 4 months of the first breast cancer. Based on the Surveillance, Epidemiology, and End Results (SEER) database, the proportion of SBBC cases has increased over time, from 1.4% of all breast cancers in 1975 to 2.9% in 2014 in the United States. 4 Li et al. 2 reported that the proportion of bilateral breast cancer (BBC) in China ranged from 0.22% to 3.08% among 33 centers in 2018, and SBBC accounted for 69.1% of BBC. Although the incidence of SBBC is not frequent, it presents poorer overall survival than unilateral breast cancer (UBC), and there are no standard treatment guidelines for SBBC.6,7 Consistently, the treatment options for SBBC are the same as those for UBC: neoadjuvant or adjuvant chemotherapy, mastectomy or breast conservative surgery (BCS) and adjuvant radiotherapy (RT). Studies have reported that bilateral mastectomy for SBBC patients is the predominant surgical management for cancer or prophylaxis, although contralateral tumors may be detected at an early stage.4,8,9 Adjuvant RT is beneficial for long-term survival. Postmastectomy RT (PMRT) is widely applied to treat breast cancer patients with tumors >5 cm or with 4 or more positive axillary lymph nodes (ALNs). Recent studies have researched the role of PMRT for early-stage patients with T1-2 tumors and 1 to 3 positive ALNs, and the results have also shown a definite reduction in locoregional recurrence and cancer mortality.10,11
However, no standard RT technique has yet been recommended for SBBC, and RT plans for SBBC become more challenging when regional lymph node irradiation (RNI) is needed, such as with ALNs, internal mammary lymph nodes (IMNs) and supraclavicular lymph nodes (SCLs). The traditional RT techniques for UBC, using tangential fields with 2-dimensional (2D) or 3-dimensional conformal RT (3D-CRT), are inadequate for SBBC with/without RNI, as the inevitable hotspot in the sternum and insatiable target coverage for breast/chest wall. Since a large and complex treatment volume is required for SBBC with/without RNI, recent trends have shown that intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT) can improve the dose distribution for the target while notably reducing the heart and lung doses compared with traditional RT techniques.12–14 Most of the previous research on SBBC13,14 has considered early-stage patients who underwent bilateral breast-conserving surgery. Different techniques were designed and studied, such as a 3D-CRT plan with 2 isocenters, an IMRT plan with 12 fixed beams and a VMAT plan with 2 half arcs or full arcs. In these studies, the 3D-CRT plan showed inadequate target coverage and an inhomogeneous dose distribution, and prolonged treatment time. IMRT and VMAT achieved acceptable planning capability, while VMAT showed better treatment efficiency. Until now, RT planning studies for SBBC, including RNI, have been rare. Narasimhulu et al. 15 studied the feasibility of hypofractionation in SBBC irradiation using 2 pairs of opposite tangential beams for each breast or chest wall with the field-in-field forward IMRT technique and a single anteroposterior beam for SCLs. Cho et al. 6 proposed a modified hybrid plan with 2 isocenters using VMAT planning for the left breast/chest wall and regional LN area (including IMNs, ALNs, and SCLs), followed by 3D-CRT for the right breast considering the background dose of VMAT planning. However, multiple isocenters for SBBC using traditional RT can also cause inaccurate positioning during daily setup and prolong the treatment time. At the same time, hybrid plans have encountered difficulty controlling hot spots and cold spots during planning. Hence, an integrally optimized plan with a single isocenter should be the first choice for SBBC. Since the irradiation beams in IMRT and VMAT plans emit from many directions, unnecessary directions crossing the lung and heart should be shielded during SBBC treatment. Therefore, physicists have attempted using IMRT or VMAT by limiting the beam direction to reduce the dose to organs at risk (OARs).
In the present investigation, we devised IMRT and VMAT treatment plans for SBBC patients undergoing bilateral mastectomy with positive ALNs to identify an efficient method that could solve outstanding dose distribution problems and be applicable to various patients in the clinic.
Methods
Patient Selection and Planning Objectives
Ten SBBC patients with positive lymph nodes who underwent bilateral mastectomy and then received PMRT at our institution between 2017 and 2021 were referred to this retrospective study. We have deidentified all patient details while retaining useful characteristics which are shown in Table 1 and preserving the computed tomographic (CT) images for everyone. One patient had undergone bilateral mastectomy with left flap reconstruction, and the remaining patients have not undergone reconstruction. Half of the patients were treated with left and/or right IMNs. All patients were irradiated with both level II and III axillary lymph nodes (ALNs-II and ALNs-III), and one of them was irradiated with left level I axillary lymph nodes (ALNs-I). Styrofoam combined with a head and neck thermoplastic mask was used for patient immobilization. The patient setup was head first-supine with both arms raised above the head, and CT images were acquired with a thickness of 5 mm in free breathing mode, scanning from the second cervical vertebra to the second lumbar vertebra.
Table 1.
Characteristics of 10 Patients Indicating age, Mastectomy, Reconstruction and Regional LN Involvement in the Target Volume.
| Par # | Age | Mastectomy | Reconstruction | Lymph node | ||||
|---|---|---|---|---|---|---|---|---|
| ALNs-I | ALNs-II | ALNs-III | IMNs | SCLs | ||||
| 1 | 68 | Both | Both | Both | Both | |||
| 2 | 43 | Both | Both | Both | Both | Both | ||
| 3 | 40 | Both | Both | Both | Right | Both | ||
| 4 | 60 | Both | Left | Both | Both | Both | ||
| 5 | 55 | Both | Both | Both | Left | Both | ||
| 6 | 53 | Both | Both | Both | Both | |||
| 7 | 66 | Both | Left | Both | Both | Both | ||
| 8 | 55 | Both | Both | Both | Left | Both | ||
| 9 | 58 | Both | Both | Both | Both | |||
| 10 | 47 | Both | Both | Both | Both | Both | ||
Abbreviations: ALN, axillary lymph node; IMN, internal mammary lymph node; SCL, supraclavicular lymph node.
All targets and structures were delineated by the same oncology physician according to ESTRO recommendations. The clinical target volumes (CTVs) were defined as CTV-CW, encompassing the chest wall and/or IMN and/or ALN areas, and CTV-LN, encompassing the SCL area. The left and right CTVs were named CTV-CW_L/R and CTV-LN_L/R, respectively. The planning target volumes of CTV-CW_L/R (PCW_L/R) were obtained with margins of 8 mm based on CTV-CW_L/R and cropped to the skin, and the planning target volumes of CTV-LN_L/R (PLN_L/R) were obtained with margins of 5 mm based on CTV-LN_L/R and restricted to skin cropping at 5 mm from the surface (Figure 1). A 5-mm water equivelar bolus was used on the bilateral chest wall throughout the treatment to ensure the accumulated dose in the skin, which is a high-risk area for recurrence. The mean planning target volumes (PTVs) were as follows: 519.58 ± 269.44 cm3 (PCW_L), 447.10 ± 97.72 cm3 (PCW_R), 236.12 ± 35.98 cm3 (PLN_L) and 220.43 ± 64.04 cm3 (PLN_R). The standard deviation of the PCW_L volume was very large owing to the large volume of left flap reconstruction, with a value of 1194.14 cm3 for patient 4. The bilateral lungs, heart, coronary artery area, liver, esophagus, humeral heads, thyroid, and spinal cord were considered as OARs. The mean volumes were 1082.34 ± 394.96 cm3 (left lung), 1400.38 ± 340.41 cm3 (right lung), 511.91 ± 88.23 cm3 (heart), 73.08 ± 12.87 cm3 (coronary artery area), 1218.58 ± 248.84 cm3 (liver), 28.37 ± 5.54 cm3 (esophagus), 45.05 ± 7.15 cm3 (left humeral head), 45.28 ± 5.17 cm3 (right humeral head) and 18.37 ± 9.15 cm3 (thyroid).
Figure 1.
PTVs and OARs for SBBC patients. Abbreviations: SBBC, synchronous bilateral breast cancer; PTV, planning target volume; OARs, organs at risk.
The prescribed dose for all treatment plans was 50 Gy in 25 fractions to the PTVs according to NCCN guidelines. 16 Radiation boost to the SCLs evaluated as high risk was not considered in this study. Plans aimed to achieve 100% of the prescribed dose in 95% of PTVs and a maximum dose less than 107% of the prescription. Since there is no distinct treatment protocol for SBBC, the dose constraints for OARs were established based on the results of PMRT for UBC with SCLs irradiation 17 and previous SBBC studies,18,19 which are listed as follows: For lung, Dmean < 15 Gy, V5 < 70%, V20 < 30%, V30 < 20%; for heart, Dmean < 8 Gy, V30 < 15%; for coronary artery area: Dmean < 25 Gy; for liver, V20 < 20%; for esophagus, V20 < 30%; for humeral heads, Dmean < 15 Gy; for thyroid, Dmean < 42 Gy; for spinal cord, Dmax < 40 Gy; Vx representing the percentage volume of OAR receiving X Gy.
The reporting of this study conforms to STROBE guidelines. 20
Planning Techniques
The treatment plans were generated on the Monaco treatment planning system, version 5.11.03, by the same physicist. Monaco used the Monte Carlo algorithm for dose calculation with a grid spacing of 3 mm and a statistical uncertainty deviation of 1% per calculation. An auto-flash margin of 2 cm was used for both chest walls to compensate for the insufficient dose caused by the intrafraction respiratory motion. All plans were calculated by applying the data from an Elekta Infinity linear accelerator equipped with an agility multileaf collimator (MLC), which includes a 160-leaf MLC with a 5-mm leaf width. A 6-MV photon was used in all plans.
9. Fixed Beams IMRT
For the IMRT plan, a single isocenter located under the sternum was used for the whole PTV. Considering the execution efficiency, 9 coplanar beams with fixed gantries were adopted, as shown in Figure 2(a). Six beams were similar outer tangential beams starting from 100° to 130° and from 230° to 260°, spaced 15°, and the collimator angles were set parallel to the long axis of the nearby chest wall. The other 3 beams were placed at 50°, 0° and 310°, and the collimator angles were set to 0°. IMRT plans were executed using the dynamic MLC delivery method with a maximum control point of 50 per beam, a minimum segment width of 7 mm and medium fluence smoothing.
Figure 2.
Beam arrangements and isocenter positions (yellow star) for (a) 9F-IMRT; (b) 2F-VMAT; and (c) 3F-VMAT. Abbreviations: 9F-IMRT, 9 fixed beams intensity-modulated radiation therapy; 2F-VMAT, 2 pairs of half arcs volumetric-modulated arc therapy; 3F-VMAT, VMAT with 2 pairs of outer tangential arcs and 1 pair of 200-degree arcs.
2. Pairs of Half Arcs VMAT
For 2 pairs of half arcs VMAT plans (2F-VMAT), the single isocenter was applied in the same location as IMRT. Two pairs of 180-degree arcs focused on left or right PTVs were adopted, as shown in Figure 2(b). For the left chest wall and supraclavicular area, the arcs started at a gantry of 240° with clockwise travel to 60° and then returned to the initial 240°. For the right chest wall and supraclavicular area, the arcs started at a gantry of 300° with clockwise travel to 120° and then returned to the initial 300°. All collimator angles were set to 0°. The sequencing parameters for optimization were set as follows: maximum control point of 200 per arc, minimum segment width of 7 mm and medium fluence smoothing.
VMAT with 2 Pairs of Outer Tangential Arcs and 1 Pair of 200-degree Arcs
VMAT with 2 pairs of outer tangential arcs and 1 pair of 200-degree arcs (3F-VMAT) used 2 pairs of 40° to 50° outer tangent arcs and 1 pair of 200° arcs, as shown in Figure 2(c). The pairs (clockwise and counterclockwise) of outer tangent arcs were fixed to each nearby chest wall, as 90° to 130° for PCW_L and 230° to 270° for PCW_R, and the collimator angles were set parallel to the long axis of the focused chest wall. The 200° arcs were chosen to give the optimizer the necessary freedom of modulation for irradiating the supraclavicular area and compensating the chest wall. The collimator was set to 0°. The sequencing parameters for optimization were the same as those of the 2F-VMAT plans.
Dosimetric Evaluation
All treatment plans were evaluated based on dose-volume histogram (DVH) analysis. For every PTV, the mean dose (Dmean), minimum dose (Dmin), V100%, and V107% (the PTV volumes receiving at least 100% and 107% of the prescribed dose, respectively) were analyzed. Concurrently, the homogeneity index (HI) for each PTV and conformity index (CI) for whole PTVs were also analyzed. The HI was defined as follows: 21
where D2%, D50%, and D98% represent the corresponding doses for 2%, 50%, and 98% of the PTV volume, respectively. Lower HI values indicate more homogeneous target doses, and 0 is the ideal value.
The CI was defined as follows: 22
This CI simultaneously considers the irradiation of the target and healthy tissues. is the volume covered by the prescribed isodose in whole PTVs, represents the total volume of all PTVs, and represents the volume receiving the prescribed dose. CI values close to 1 indicate a high degree of conformity. For the bilateral lungs, heart, and coronary artery area, the values of Dmean, V5, V10, V20, and V30 were evaluated. For esophagus and liver, the values of Dmean and V20 were evaluated. For spinal cord, humeral heads and thyroid, the values of Dmax or Dmean were evaluated.
Additionally, the terms of monitor units (MUs) and beam-on times (BOTs) were recorded and compared as delivery parameters for each plan. Patient-specific quality assurance (QA) for plans was performed using ArcCHECK Phantom (Sun Nuclear Corporation) with the cavity plug present equipped with a Semifex 31010 ionization chamber (PTW-Freiburg) in the center. The measurements were analyzed with 3%/2-mm gamma criteria with a low dose threshold of 10% and a tolerance level of pixel passing of 95%.
Statistical Analysis
All data were analyzed using IBM Statistical Package for Social Sciences software, version 24.0 (IBM Corporation). Variables conforming to a normal distribution are expressed as the mean ± standard deviation, and nonnormal variables are reported as the median (interquartile range). The paired t-test (in case of normality) and Friedman's test (in case of nonnormality) were applied to compare the PTVs and OARs values of the 3 techniques, and a P value of ≤ .05 was considered statistically significant.
Results
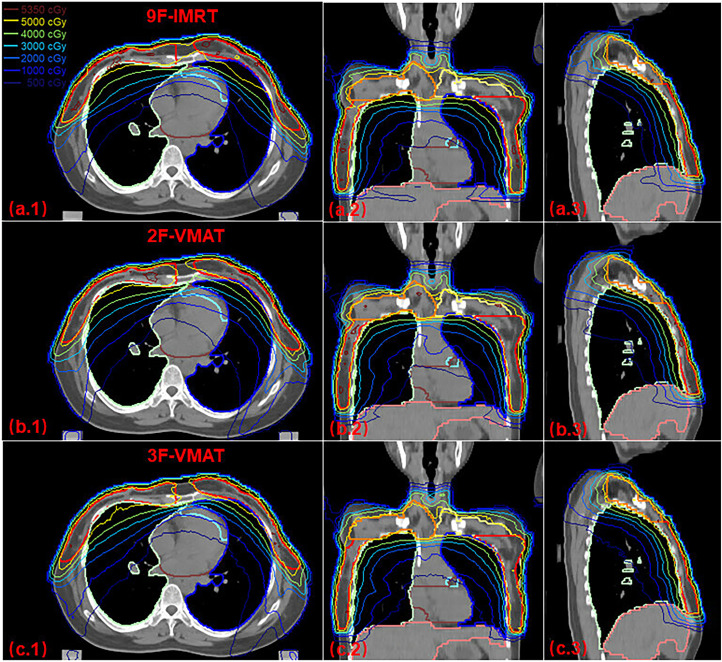
The evaluation parameters of PTVs coverage and OARs irradiation dose with the 9F-IMRT, 2F-VMAT, and 3F-VMAT techniques are summarized in Table 2 and Table 3, respectively. The average DVHs of PTVs and OARs for the 3 techniques are demonstrated in Figure 4. Figure 3 shows the transverse, coronal, and sagittal dose distributions with the 3 plans from 1 case.
Table 2.
Comparison of PTVs Coverage for 9F-IMRT, 2F-VMAT, and 3F-VMAT.
| 9F-IMRT | 2F-VMAT | 3F-VMAT | P | ||
|---|---|---|---|---|---|
| PCW_L | Dmean (Gy) | 51.92 ± 0.16 | 51.82 ± 0.13 | 51.54 ± 0.15 | -a, **b, **c |
| Dmin (Gy) | 40.79 ± 2.56 | 41.07 ± 3.50 | 41.12 ± 4.15 | -a, -b, -c | |
| V100% (%) | 94.98 ± 1.11 | 95.73 ± 0.95 | 94.54 ± 1.02 | -a, -b, -c | |
| V107% (%) | 1.27 ± 1.18 | 1.31 ± 0.93 | 1.27 ± 0.84 | -a, -b, -c | |
| HI | 0.0852 ± 0.0098 | 0.0782 ± 0.0063 | 0.0778 ± 0.0095 | -a, -b, -c | |
| PLN_L | Dmean (Gy) | 51.72 ± 0.34 | 51.68 ± 0.27 | 51.56 ± 0.12 | -a, -b, -c |
| Dmin (Gy) | 37.96 ± 5.67 | 38.49 ± 6.24 | 38.07 ± 6.78 | -a, -b, -c | |
| V100% (%) | 97.40 (2.63) | 95.00 (0.42) | 95.00 (2.04) | -a, *b, -c | |
| V107% (%) | 0.08 (0.46) | 1.21 (2.86) | 0.94 (0.52) | **a, *b, -c | |
| HI | 0.0639 (0.0295) | 0.0770 (0.0179) | 0.0773 (0.0152) | -a, *b, -c | |
| PCW_R | Dmean (Gy) | 51.85 ± 0.22 | 51.78 ± 0.13 | 51.55 ± 0.16 | -a, **b, **c |
| Dmin (Gy) | 42.88 ± 2.01 | 43.87 ± 2.00 | 43.75 ± 2.91 | -a, -b, -c | |
| V100% (%) | 95.73 ± 0.88 | 96.20 ± 1.68 | 96.03 ± 0.89 | -a, -b, -c | |
| V107% (%) | 0.98 ± 1.11 | 1.45 ± 1.04 | 0.58 ± 0.41 | -a, -b, -c | |
| HI | 0.0772 ± 0.0126 | 0.0729 ± 0.0138 | 0.0669 ± 0.0066 | -a, *b, -c | |
| PLN_R | Dmean (Gy) | 51.75 ± 0.35 | 51.67 ± 0.27 | 51.53 ± 0.16 | -a, -b, -c |
| Dmin (Gy) | 40.25 (6.50) | 40.69 (5.13) | 42.47 (4.71) | -a, -b, -c | |
| V100% (%) | 96.94 ± 1.17 | 95.30 ± 1.32 | 94.66 ± 2.34 | *a, *b, -c | |
| V107% (%) | 0.09 (0.57) | 0.89 (1.94) | 0.86 (0.65) | -a, -b, -c | |
| HI | 0.0623 (0.0126) | 0.0777 (0.0225) | 0.0701 (0.0159) | *a, *b, -c | |
| CI | 0.7694 ± 0.0201 | 0.8012 ± 0.0229 | 0.7947 ± 0.0172 | **a, **b, -c | |
| MUs | 1023.52 ± 166.03 | 1328.76 ± 120.84 | 1342.31 ± 112.06 | **a, **b, -c | |
| BOTs (s) | 529.59 ± 94.76 | 302.37 ± 27.19 | 259.00 ± 14.69 | **a, **b, **c | |
| QA (%) | 97.50 ± 0.95 | 96.80 ± 1.03 | 97.80 ± 0.99 | -a, -b, -c | |
a = 9F-IMRT versus 2F-VMAT; b = 9F-IMRT versus 3F-VMAT; c = 2F-VMAT versus 3F-VMAT. *P < .05; **P < .01; -P > .05. Abbreviations: 9F-IMRT, 9 fixed beams intensity-modulated radiation therapy; 2F-VMAT, 2 pairs of half arcs volumetric-modulated arc therapy; 3F-VMAT, VMAT with 2 pairs of outer tangential arcs and 1 pair of 200-degree arcs; PTV, planning target volume; BOTs, beam-on times; HI, homogeneity index; CI, conformity index; QA, quality assurance.
Table 3.
Comparison of OARs Dose for 9F-IMRT, 2F-VMAT, and 3F-VMAT.
| 9F-IMRT | 2F-VMAT | 3F-VMAT | P | ||
|---|---|---|---|---|---|
| Lung_L | Dmean (Gy) | 15.90 ± 1.61 | 16.17 ± 1.61 | 15.02 ± 1.57 | -a, **b, **c |
| V5 (%) | 64.55 (17.03) | 71.17 (8.15) | 65.05 (9.79) | **a, -b, *c | |
| V10 (%) | 44.72 (4.06) | 47.66 (2.44) | 42.50 (8.29) | **a, -b, **c | |
| V20 (%) | 29.19 ± 3.58 | 30.41 ± 3.86 | 28.43 ± 3.69 | -a, -b, -c | |
| V30 (%) | 21.20 ± 3.03 | 20.09 ± 3.44 | 18.95 ± 3.33 | -a, **b, -c | |
| Lung_R | Dmean (Gy) | 15.56 ± 0.09 | 15.84 ± 0.89 | 14.91 ± 1.14 | -a, *b, **c |
| V5 (%) | 63.44 ± 4.07 | 71.97 ± 3.87 | 62.36 ± 3.90 | **a, -b, **c | |
| V10 (%) | 42.99 ± 2.29 | 47.07 ± 2.45 | 40.98 ± 3.39 | **a, *b, **c | |
| V20 (%) | 29.72 ± 2.64 | 29.74 ± 2.38 | 27.47 ± 3.42 | -a, **b, *c | |
| V30 (%) | 21.09 ± 2.35 | 19.61 ± 2.61 | 19.64 ± 2.75 | *a, *b, -c | |
| Heart | Dmean (Gy) | 7.75 (1.18) | 7.70 (2.25) | 6.19 (1.96) | -a, **b, *c |
| V5 (%) | 44.35 (18.74) | 47.20 (26.73) | 42.64 (16.83) | -a, -b, -c | |
| V10 (%) | 21.620 ± 5.49 | 20.69 ± 7.88 | 14.25 ± 8.45 | -a, **b, *c | |
| V20 (%) | 8.67 (5.02) | 7.11 (9.52) | 3.14 (4.66) | -a, **b, -c | |
| V30 (%) | 3.45 (3.56) | 1.58 (2.46) | 0.96 (1.91) | *a, **b, -c | |
| Coronary artery area | Dmean (Gy) | 22.30 ± 4.96 | 19.53 ± 5.40 | 15.96 ± 5.76 | *a, **b, *c |
| V5 (%) | 97.49 (4.47) | 99.00 (5.82) | 96.25 (7.56) | -a, -b, -c | |
| V10 (%) | 82.62 ± 10.05 | 78.92 ± 13.55 | 62.68 ± 18.82 | -a, **b, *c | |
| V20 (%) | 52.45 ± 16.42 | 43.10 ± 19.32 | 27.82 ± 20.25 | -a, **b, *c | |
| V30 (%) | 34.27 (24.56) | 12.26 (15.91) | 7.48 (13.79) | -a, *b, -c | |
| Esophagus | Dmean (Gy) | 13.61 ± 2.68 | 14.27 ± 2.63 | 12.50 ± 2.12 | **a, -b, **c |
| V20 (%) | 25.02 ± 5.50 | 26.27 ± 5.79 | 23.66 ± 3.95 | **a, -b, *c | |
| Liver | Dmean (Gy) | 10.37 ± 3.14 | 10.66 ± 3.21 | 8.10 ± 2.70 | -a, **b, **c |
| V20 (%) | 16.47 ± 6.98 | 15.63 ± 6.67 | 12.63 ± 5.9 | -a, -b, *c | |
| Spinal cord | Dmax (Gy) | 34.20 ± 4.48 | 36.88 ± 2.36 | 35.79 ± 4.51 | -a, -b, -c |
| Humeral head_L | Dmean (Gy) | 15.29 ± 2.66 | 15.65 ± 2.29 | 14.07 ± 2.47 | -a, -b, **c |
| Humeral head_R | Dmean (Gy) | 16.20 ± 5.55 | 14.41 ± 3.79 | 14.23 ± 2.64 | -a, -b, -c |
| Thyroid | Dmean (Gy) | 43.77 ± 3.47 | 44.80 ± 2.37 | 42.18 ± 3.69 | -a, *b, *c |
a = 9F-IMRT versus 2F-VMAT; b = 9F-IMRT versus 3F-VMAT; c = 2F-VMAT versus 3F-VMAT. *P < .05; **p < .01; -P > .05. Abbreviations: 9F-IMRT, 9 fixed beams intensity-modulated radiation therapy; 2F-VMAT, 2 pairs of half arcs volumetric-modulated arc therapy; 3F-VMAT, VMAT with 2 pairs of outer tangential arcs and 1 pair of 200-degree arcs; OARs, organs at risk.
Figure 4.
Averaged DVHs of ten patients for PTVs and OARs. Abbreviations: PTV, planning target volume; OARs, organs at risk; DVH, dose-volume histogram.
Figure 3.
Case of isodose distribution on transverse, coronal and sagittal views of 9F-IMRT, 2F-VMAT and 3F-VMAT plans. Abbreviations: 9F-IMRT, 9 fixed beams intensity-modulated radiation therapy; 2F-VMAT, 2 pairs of half arcs volumetric-modulated arc therapy; 3F-VMAT, VMAT with 2 pairs of outer tangential arcs and 1 pair of 200-degree arcs.
Target Coverage
Table 2 illustrates the detailed characteristics of the 4 PTVs (PCW_L, PLN_L, PCW_R, and PLN_R). All the plans achieved satisfactory coverage of the PTVs (V100%>94.5%), but the values of V107% presented a minor violation. Specifically, 2F-VMAT showed higher mean values of V107% for PCWs (1.31 ± 0.93% for PCW_L and 1.45 ± 1.04% for PCW_R) than 9F-IMRT (1.27 ± 1.18% for PCW_L and 0.98 ± 1.11% for PCW_R) and 3F-VMAT (1.27 ± 0.84% for PCW_L and 0.58 ± 0.41% for PCW_R), but the difference was not significant. For the PLNs, 2F-VMAT showed a significantly higher median V107% with 1.21 (2.86) % for PLN_L and 0.89 (1.94) % for PLN_R than 9F-IMRT with 0.08 (0.46) % for PLN_L (P < .01) and 0.09 (0.57) % for PLN_R (P < .05) and 3F-VMAT with 0.94 (0.52) % for PLN_L (P < .05) and 0.86 (0.65) % for PLN_R (P < .05).
For all the PTVs, 3F-VMAT yielded a lower Dmean than the other plans. Furthermore, the difference was significant for PCWs (P < .01). Nevertheless, the differences in Dmean among the 3 plans were less than 0.8%.
All HI values were small and less than 0.1. For PCWs, 3F-VMAT plans achieved the lowest mean HI, followed by 2F-VMAT. For PLNs, 9F-IMRT achieved the lowest median HI. Concerning the CI values, the conformity around the whole PTVs was evaluated, and 2F-VMAT showed a higher mean CI, followed by 3F-VMAT. There was no significant difference between 2F-VMAT (0.8012 ± 0.0229) and 3F-VMAT (0.7947 ± 0.0172). The CI value of 9F-IMRT (0.7694 ± 0.0201) was significantly different from those of 2F-VMAT (P < .05) and 3F-VMAT (P < .05). In general, the 3 techniques provided adequate target dose coverage and comparable results for PTVs.
Table 2 also demonstrates the MUs, BOTs, and QA parameters for evaluating treatment efficiency. The MUs of the 9F-IMRT plans were 1023.52 ± 166.03, significantly less than those of 2F-VMAT (1328.76 ± 120.84, P < .01) and 3F-VMAT (1342.31 ± 112.06, P < .01). The difference between VMAT plans was insignificant. However, 9F-IMRT plans were time-consuming, as the BOTs were 75% and 104% longer than those of 2F-VMAT and 3F-VMAT, respectively. The 2F-VMAT had a 17% longer irradiation time than the 3F-VMAT, and the differences in BOTs among the 3 plans were reported to be statistically significant (P < .01). All QA passing rates met the criterion, indicating that the plans were safe and feasible. Comparatively, the 3F-VMAT plan provided a nonnegligible advantage in shortening the treatment time to decrease patient discomfort and treatment uncertainty.
OAR Dose Distribution
The average DVHs of the bilateral lungs, heart, and coronary artery are displayed in Figure 4, and the detailed mean or median values of all OARs are shown in Table 3.
Concerning the lungs, 3F-VMAT achieved the lowest Dmean (15.02 ± 1.57 Gy and 14.91 ± 1.14 Gy for the left and right lungs, respectively) and showed significant differences with those of 9F-IMRT (15.90 ± 1.61 Gy for the left lung, P < .01 and 15.56 ± 0.09 Gy for the right lung, P < .05) and 2F-VMAT (16.17 ± 1.61 Gy for the left lung, P < .01 and 15.84 ± 0.89 Gy for the right lung, P < .01). For the left lung, in the low-dose (V5, V10) and medium-dose areas (V20), 3F-VMAT was superior to 2F-VMAT and similar to 9F-IMRT, while the values of the 2F-VMAT plans reached the maximum tolerance value. In the high-dose area (V30), 3F-VMAT achieved the lowest value, followed by 2F-VMAT. For the right lung, 3F-VMAT was significantly superior to 2F-VMAT in the low-dose and medium-dose areas and comparable in the high-dose area. Simultaneously, compared with 9F-IMRT, 3F-VMAT achieved slightly lower values in all dose areas. 2F-VMAT displayed the worst results in the low-dose area; however, it achieved a comparable value to 9F-IMRT in the medium-dose area and a superior value in the high-dose area. The dose distribution of lungs between 9F-IMRT and 2F-VMAT was consistent with our previous studies: 19 the plan with the IMRT technique had a dosimetric advantage at the low-dose level, and the plan with the VMAT technique was superior at the high-dose level. The distribution of low doses in the lungs was related to irradiation from the anterior and posterior directions. To decrease the low doses in the lungs using the VMAT technique, 2 pairs of outer tangential partial arcs in the 3F-VMAT plan were designed to irradiate the near chest wall (shown in Figure 2(c)), and the other arcs were designed to irradiate bilateral supraclavicular targets and compensate for the chest wall.
For the heart, 3F-VMAT showed a significant reduction in terms of Dmean with 6.19 (1.96) Gy, which was approximately 20% less than those of 9F-IMRT with 7.75 (1.18) Gy (P < .01) and 2F-VMAT with 7.70 (2.25) Gy (P < .05). 3F-VMAT achieved the lowest values in all evaluation terms compared with the other plans and had significant differences from 9F-IMRT at the medium- and high-dose levels. 2F-VMAT had lower values than 9F-IMRT, except for the terms of V5.
For the coronary artery, 3F-VMAT showed a significant reduction in terms of Dmean (15.96 ± 5.76 Gy), which was approximately 28% less than that of 9F-IMRT (22.30 ± 4.96 Gy, P < .01) and 18% less than that of 2F-VMAT (19.53 ± 5.40 Gy, P < .05). In addition, 2F-VMAT had significantly less Dmean (12%) than 9F-IMRT. In terms of V5, 3 plans displayed much coverage of the coronary artery (> 95%). In terms of V10, V20, and V30, 3F-VMAT showed the lowest values, followed by 2F-VMAT. The differences between 3F-VMAT and 9F-IMRT were dramatic and significant.
For esophagus, liver and thyroid, 3F-VMAT showed little advantage in terms of Dmean. For spinal cord and humeral heads, the 3 techniques showed comparable results in Table 3.
Discussion
Most SBBC patients with positive lymph nodes undergo bilateral mastectomy and receive PMRT with RNI to improve overall survival; however, PMRT is challenging in breast cancer to balance adequate target coverage and normal tissue sparing owing to the enormous and complex target volume surrounding important OARs, such as the lungs, heart, coronary artery, liver, esophagus and so on. In this study, IMRT and VMAT with different beam arrangements were investigated. Considering plan quality, treatment efficiency, and patient comfort, 3 integrally optimized plans with a single isocenter were designed. All plans showed comparable target coverage and acceptable normal tissue sparing. Two pairs of outer tangential partial arcs fixed to each chest wall and 1 pair of partial arcs focusing on the bilateral supraclavicular areas and compensating chest wall in the 3F-VMAT plan showed the best results to reduce the OARs doses while maintaining the target coverage. Simultaneously, 3F-VMAT plans shortened the irradiation time compared with the other plans.
In our previous study, 19 the difference in OARs between the IMRT and VMAT techniques was obvious: IMRT demonstrated a reduction in low-dose exposure, and VMAT showed a reduction in high-dose exposure. The superabundant low-dose distribution in OARs for the VMAT technique was due to vast exposure from anterior and posterior beams. Although strict constraints for dose-volume parameters were provided to the optimizer, it was not sufficiently smart to optimize the best beam angles to achieve the constraints. Therefore, we designed 2 pairs of outer tangential arcs in the 3F-VMAT plan intently irradiating each chest wall to decrease exposure from anterior beams, and the results showed a significant reduction at the low-dose level. During VMAT planning, it was difficult to control the values of V5 and V10 for bilateral lungs to constraints in 2F-VMAT, while lower values were easily achieved in 3F-VMAT (left lung: V5 = 71.17 (8.15)% versus 65.05 (9.79)%, V10 = 47.66 (2.44)% versus 42.50 (8.29)%; right lung: V5 = 71.97 ± 3.87% versus 62.36 ± 3.90%, V10 = 47.07 ± 2.45% versus 40.98 ± 3.39% for 2F-VMAT and 3F-IMRT, respectively). Furthermore, the pair of 200°arcs (clockwise and counterclockwise) easily spared the dose to the internal area between the left and right PTVs since dual arcs focused on the left PTVs or right PTVs for each arc to minimize the leaf opening over normal tissues. In the 2F-VMAT plan, overmuch inter tangential beams transmit to the middle area, and the cumulative dose is difficult to weaken.
For breast cancer patients with positive lymph nodes, PMRT with RNI reduces the risks of both local recurrence and breast cancer mortality.10,23 However, the use of comprehensive RT increases the risk of long-term cardiopulmonary toxicity, in turn decreasing survival. Therefore, the cardiovascular toxicity caused by RT has been studied extensively.24–26 Darby et al. 25 reported that the incidence of major coronary events increases linearly by 7.4% per Gy of the mean heart dose (MHD). Some studies have focused on important heart substructures, such as the left anterior descending artery and the left ventricle, and they found that the irradiation dose of heart substructures is also closely related to cardiotoxicity.27,28 Therefore, the MHD and the mean dose of cardiac substructures should be strictly limited during RT planning, which could be beneficial for breast cancer patients. In our study, we found a dosimetric advantage of 3F-VMAT for sparing the heart and coronary artery doses in SBBC patients. Compared to 9F-IMRT and 2F-VMAT, 3F-VMAT reduced the MHD from 7.75 (1.18) Gy and 7.70 (2.25) Gy, respectively, to 6.19 (1.96) Gy. For the mean dose of the coronary artery area, 3F-VMAT showed approximately 28% less than 9F-IMRT and 18% less than 2F-VMAT. Cho et al. 6 designed a hybrid plan using VMAT for the left breast or chest wall and left regional LN area (internal mammary, axillary, and supraclavicular), followed by 3D-CRT for the right breast considering the background dose of VMAT planning for SBBC patients with left RNI. The patients referred in their study were not irradiated to the right LN area, and 5 of 10 patients received left breast total mastectomy. The PTV coverage V95% in their proposed plan was 95.3%, which was loose compared with our present study. The MHD was 8.0 ± 2.6 Gy higher than the values of 3F-VMAT plan (6.8 ± 2.0 Gy). In our study, no breath-holding technique was applied. The deep-inspiration breath hold (DIBH) has been a common technique to reduce the dose to the heart in left-side breast cancer RT.29,30 Gaudino et al. 31 reported that VMAT with DIBH showed better heart and LAD sparing and a lower mean dose to the whole lungs compared to the free-breathing (FB) modality for SBBC patients treated with 50.4 Gy to the bilateral breast and 64.4 Gy to the tumor bed in 28 fractions. In that study, the MHD was reduced in the DIBH modality, with an average of 6.5 Gy versus 8 Gy in FB. To further reduce the cardiac dose, DIBH could be applied for our SBBC patients with RNI.
Lung cancer data have demonstrated that the percent volume of the lungs receiving at least X Gy (Vx) was associated with radiation pneumonitis (RP). Jiang et al. 32 introduced limiting V5 < 65%, V20 < 40%, and mean lung dose (MLD) < 20 to 22 Gy, and the results showed a reduction in the incidence of RP. According to the National Comprehensive Cancer Network guidelines for non-small-cell lung cancer, the dose constraints were suggested as follows: V5 < 65%, V20 < 35% and MLD < 20 Gy. Most previous SBBC studies13,14,33,34 have reported RT without supraclavicular LNs which resulted in a significant increase in lung dose, and the MLD ranged from 6 to 16 Gy. Cho et al. 6 reported V5 of 56.7 to 67.5%, V20 of 19.0% to 27.5% and MLD of 11 to 14.4 Gy for SBBC patients with left supraclavicular LN irradiation. Unfortunately, Cho et al. did not demonstrate the dosimetric parameters of separate left and right lungs, while the lower values of the right lung pulled down that of the whole lung. Boman et al. 35 reported the whole lung dosimetric parameters of eleven SBBC patients with both supraclavicular areas irradiation (V5: 54.4-59.1%, V20: 18.5-18.9%, MLD: 10.9-11.3 Gy) while compromising PTV coverage (V95%: 90.6-91.2%). In their study, 8 of 11 patients were treated with DIBH, which could spare the dose to both lungs. In the present study, SBBC patients with positive lymph nodes underwent bilateral mastectomy and received PMRT including RNI in the FB modality. The V5, V20 and MLD were 62.36% to 71.97%, 27.47% to 30.41%, and 14.91 to 16.17 Gy, respectively, and 3F-VMAT showed the lowest values. In our previous study, 18 the VMAT technique using flattening filter-free (FFF) beams reduced the dose to the lungs. In future clinical practice, DIBH and FFF beams may be used for these patients to decrease the dose to the lungs.
This study has limitations. In the present study, only ten patients were enrolled. SBBC patients with positive lymph nodes undergo bilateral mastectomy and receive PMRT with RNI in our institution that were rare. In our future studies, we will accumulate more patients and conduct more researches. In addition, no more other advanced techniques, as helicoidal tomotherapy which superiorly spare OARs were applied in our study. In the future, we may compare much more different techniques.
Conclusion
For SBBC patients with PMRT, including RNI, the IMRT, and VMAT techniques with different beam arrangements were conducted, and each plan was integrally optimized with a single isocenter. Based on dosimetric comparison, 3F-VMAT provided comparable target coverage and superior dose sparing of normal tissues compared with 9F-IMRT and 2F-VMAT. Moreover, 3F-VMAT enabled shorter BOTs, decreasing patient discomfort and treatment uncertainty. In our research, 3F-VMAT may be the optimal RT technique for SBBC patients with PMRT, including RNI. In future work, DIBH and FFF beams could be combined with 3F-VMAT to reduce the dose to the heart and lungs.
Abbreviations
- ALNs
axillary lymph nodes
- BOTs
beam-on times
- CTV
clinical target volume
- CI
conformity index
- Dx %
the corresponding doses for X% of the PTV volume
- D mean
mean dose
- D min
minimum dose
- DIBH
deep-inspiration breath hold
- FB
free-breathing
- FFF
flattening filter-free
- HI
homogeneity index
- IMNs
internal mammary lymph nodes
- IMRT
intensity-modulated radiation therapy
- MUs
monitor units
- OARs
organs at risk
- PMRT
postmastectomy radiotherapy
- PTV
planning target volume
- PCW_L/R PTV
encompassing left or right chest wall and/or IMN and/or AXL areas
- PLN_L/R PTV
encompassing left or right SCAL area
- QA
quality assurance
- RNI
regional lymph node irradiation
- RT
adjuvant radiotherapy
- SCLs
supraclavicular lymph nodes
- SBBC
synchronous bilateral breast cancer
- VMAT
volumetric-modulated arc therapy
- V x%
percentage volume receiving at least X% of the prescribed dose
- V x
percentage volume receiving a dose of X Gy
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Sun Yat-sen Clinical Research 5010 Program (grant number SYS-5010-202303) and Guangzhou Science and Technology Program (grant number 2023A03J0723).
Ethical Approval: This study was approved by the Medical Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China (Approval number: SYSKY-2023-968-01; Approval Date: September 18, 2023).
Availability of Data and Materials: The data and materials related to patients’ information are not readily available, but can be inquired from the authors.
ORCID iD: Xiuxiu Wu https://orcid.org/0000-0003-2504-6892
References
- 1.Kheirelseid EA, Jumustafa H, Miller N, et al. Bilateral breast cancer: Analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res Treat. 2011;126(1):131‐140. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Wang Y, Pan B., et al. Clinical characteristics and clinicopathological correlations of bilateral breast cancer in China: A multicenter study from Chinese Society of Breast Surgery (CSBrS-006). Chin J Cancer Res. 2021;33(1):27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckmann KR, Buckingham J, Craft P, et al. Clinical characteristics and outcomes of bilateral breast cancer in an Australian cohort. Breast. 2011;20(2):158‐164. [DOI] [PubMed] [Google Scholar]
- 4.Sakai T, Ozkurt E, DeSantis S, et al. National trends of synchronous bilateral breast cancer incidence in the United States. Breast Cancer Res Treat. 2019;178(1):161‐167. [DOI] [PubMed] [Google Scholar]
- 5.Holm M, Tjønneland A, Balslev E, Kroman N. Prognosis of synchronous bilateral breast cancer: A review and meta-analysis of observational studies. Breast Cancer Res Treat. 2014;146(3):461‐475. [DOI] [PubMed] [Google Scholar]
- 6.Cho Y, Cho YJ, Chang WS, Kim JW, Choi WH, Lee IJ. Evaluation of optimal treatment planning for radiotherapy of synchronous bilateral breast cancer including regional lymph node irradiation. Radiat Oncol. 2019;14(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jobsen JJ, van der Palen J, Ong F, Riemersma S, Struikmans H. Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res Treat. 2015;153(2):277‐283. [DOI] [PubMed] [Google Scholar]
- 8.Chen JJ, Huang NS, Xue JY, et al. Surgical management for early-stage bilateral breast cancer patients in China. PLoS One. 2015;10(4):e0122692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moffat FL, Jr., Yakoub D. Bilateral mastectomy and the retreat from breast-conserving surgery. Breast Cancer Res Treat. 2016;159(1):15‐30. [DOI] [PubMed] [Google Scholar]
- 10.EBCTCG. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poortmans PM, Weltens C, Fortpied C, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020;21(12):1602‐1610. [DOI] [PubMed] [Google Scholar]
- 12.Yusoff S, Chia D, Tang J, et al. Bilateral breast and regional nodal irradiation in early stage breast cancer — A dosimetric comparison of IMRT and 3D conformal radiation therapy. Int J Radiat Oncol*Biol*Phys. 2012;84(3):S223. [Google Scholar]
- 13.Kim SJ, Lee MJ, Youn SM. Radiation therapy of synchronous bilateral breast carcinoma (SBBC) using multiple techniques. Med Dosim. 2018;43(1):55‐68. [DOI] [PubMed] [Google Scholar]
- 14.Nicolini G, Clivio A, Fogliata A, et al. Simultaneous integrated boost radiotherapy for bilateral breast: A treatment planning and dosimetric comparison for volumetric modulated arc and fixed field intensity modulated therapy. Radiat Oncol. 2009;4(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narasimhulu BC, Valiyaveettil D, Joseph D, Ahmed SF, Vijayakrishna E, Malik M. Synchronous bilateral breast cancer patients treated with hypofractionated bilateral breast irradiation: A dosimetric and clinical study. J Cancer Res Ther. 2020;16(6):1309‐1313. [DOI] [PubMed] [Google Scholar]
- 16.Gradishar WJ, Moran MS, Abraham J, et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J Natl Compr Canc Netw. 2023;21(6):594‐608. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Center/National Cancer Quality Control, C. Guideline of target delineation and treatment planning of adjuvant radiotherapy for breast cancer. Chin J Radiat Oncol. 2022;31(10):863‐878. [Google Scholar]
- 18.Duan S, Li C, Shi J, et al. Synchronous bilateral breast carcinoma irradiation: A comparative investigation between flattened and unflattened beams. Appl Radiat Isot. 2022;181:110079. [DOI] [PubMed] [Google Scholar]
- 19.Huang JH, Wu X-X, Lin X, et al. Evaluation of fixed-jaw IMRT and tangential partial-VMAT radiotherapy plans for synchronous bilateral breast cancer irradiation based on a dosimetric study. J Appl Clin Med Phys. 2019;20(9):31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐577. [DOI] [PubMed] [Google Scholar]
- 21.Rohrer Bley C, Meier VS, Besserer J, Schneider U. Intensity-modulated radiation therapy dose prescription and reporting: Sum and substance of the international commission on radiation units and measurements report 83 for veterinary medicine. Vet Radiol Ultrasound. 2019;60(3):255‐264. [DOI] [PubMed] [Google Scholar]
- 22.Nolan MW, Kogan L, Griffin LR, et al. Intensity-Modulated and image-guided radiation therapy for treatment of genitourinary carcinomas in dogs. J Vet Intern Med. 2012;26(4):987‐995. [DOI] [PubMed] [Google Scholar]
- 23.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366(9503):2087‐2106. [DOI] [PubMed] [Google Scholar]
- 24.Shilkrut M, Belkacemi Y, Kuten A, et al. Secondary malignancies in survivors of breast cancer: How to overcome the risk. Crit Rev Oncol Hematol. 2012;84(Suppl 1):e86‐e89. [DOI] [PubMed] [Google Scholar]
- 25.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987‐998. [DOI] [PubMed] [Google Scholar]
- 26.Lee MS, Finch W, Mahmud E. Cardiovascular complications of radiotherapy. Am J Cardiol. 2013;112(10):1688‐1696. [DOI] [PubMed] [Google Scholar]
- 27.Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys. 2007;69(5):1484‐1495. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30(4):380‐386. [DOI] [PubMed] [Google Scholar]
- 29.Misra S, Mishra A, Lal P, et al. Cardiac dose reduction using deep inspiratory breath hold (DIBH) in radiation treatment of left sided breast cancer patients with breast conservation surgery and modified radical mastectomy. J Med Imaging Radiat Sci. 2021;52(1):57‐67. [DOI] [PubMed] [Google Scholar]
- 30.Ferdinand S, Mondal M, Mallik S, et al. Dosimetric analysis of deep inspiratory breath-hold technique (DIBH) in left-sided breast cancer radiotherapy and evaluation of pre-treatment predictors of cardiac doses for guiding patient selection for DIBH. Tech Innov Patient Support Radiat Oncol. 2021;17:25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudino D, Cima S, Frapolli M, et al. Volumetric modulated arc therapy applied to synchronous bilateral breast cancer radiotherapy: Dosimetric study on deep inspiration breath hold versus free breathing set up. Biomed Phys Eng Express. 2018;4(4):045007. [Google Scholar]
- 32.Jiang ZQ, Yang K, Komaki R, et al. Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: The MD Anderson experience. Int J Radiat Oncol Biol Phys. 2012;83(1):332‐339. [DOI] [PubMed] [Google Scholar]
- 33.Fiorentino A, Mazzola R, Naccarato S, et al. Synchronous bilateral breast cancer irradiation: Clinical and dosimetrical issues using volumetric modulated arc therapy and simultaneous integrated boost. Radiol Med. 2017;122(6):464‐471. [DOI] [PubMed] [Google Scholar]
- 34.Seppala J, Heikkilä J, Myllyoja K, Koskela K. Volumetric modulated arc therapy for synchronous bilateral whole breast irradiation - A case study. Rep Pract Oncol Radiother. 2015;20(5):398‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boman E, Rossi M, Kapanen M. The robustness of dual isocenter VMAT radiation therapy for bilateral lymph node positive breast cancer. Phys Med. 2017;44:11‐17. [DOI] [PubMed] [Google Scholar]