Abstract
Although wild-type Hsp70 (Hsp70WT) inhibits procaspase-3 processing by preventing apoptosome formation, Hsp70WT does not block active caspase-3. Because all caspase-3 inhibitors bear canonical DXXD caspase-3 recognition motifs, we determined whether mutated Hsp70s with caspase-binding motifs act as direct caspase-3 inhibitors. Based on Hsp70 molecular modeling, the DNQP, DEVQ, and EEVD regions localized on the surface of Hsp70WT were chosen, allowing us to design mutants while trying to avoid disrupting the global fold of the molecule and losing the possibility of protein-protein interactions. We replaced DNQP with DQMD, and DEVQ and EEVD with DEVD residues that should be optimal substrates for caspase-3. The resultant Hsp70 mutants directly interacted with active caspase-3 and blocked its proteolytic activity while retaining the ability to reverse protein denaturation and disrupt the interaction between Apaf-1 and procaspase-9. The Hsp70C-terminal mutants interacted with Apaf-1 and active caspase-3 significantly longer than Hsp70WT. The Hsp70 DXXD mutants protected neuron and teratocarcinoma (NT) cells against cell death much better than Hsp70WT whether given before or after serum withdrawal. Hsp70 mutants represent a possible approach to antiapoptotic biotherapeutics. Similar rational designs could be used to engineer inhibitors of additional caspase family members.
INTRODUCTION
Hsp70 physically interacts with Apaf-1 and apoptosis-inducing factor (AIF), resulting in suppression of caspase-dependent and caspase-independent apoptosis (Beere et al 2000; Saleh et al 2000; Ravagnan et al 2001). However, Hsp70 does not block the activity of the processed caspase-3 (Mosser et al 1997). Apoptosis plays an important role in a wide variety of physiological and pathological processes in various organisms (Budihardjo et al 1999; Horvitz 1999). Activation of caspase-3 is a key event in many types of apoptosis. Inhibition of apoptosis might be an important strategy for future therapies for many diseases. The development of molecules that directly block active caspase-3 may therefore have therapeutic potential for the treatment of degenerative diseases.
The amino acid sequence of Hsp70 itself could be relevant to apoptosis because Hsp70 contains DEVQ and EEVD sequences (Freeman et al 1995), which resemble the DEVD characteristic signature cleavage motifs of the caspase-3 (Alnemri et al 1996). These sequences could function as pseudosubstrates for active caspases, thus reducing caspase availability to cleave other cell proteins essential for cell survival (Casciola-Rosen et al 1996). However, a recent report demonstrates that Hsp70 does not rescue substrate cleavage by activated caspase-3 (Mosser et al 1997), indicating that the DEVQ and EEVD sequences in Hsp70 are not caspase targets. The most potent of many natural as well as synthetic inhibitors of caspases contain a tetrapeptide recognition motif (DEVD) that is optimal for caspase-3 (Zhou et al 1997; Ekert et al 1999; Clem 2001). Based on the structural features of the caspase inhibitors mentioned here, we postulated that introducing caspase-3–binding sequences (DEVD) into the Hsp70 molecule might make Hsp70 a direct active caspase-3 inhibitor. Therefore, we engineered a series of Hsp70 variants. These mutant proteins were based on Hsp70 molecular modeling that predicted that point mutations on the surface of Hsp70 could be made that would tend to maintain the structural integrity of Hsp70 and could also convert the mutant proteins into direct caspase inhibitors.
To control the dose and timing of administration of Hsp70 and its mutants, we used a modified TAT sequence called a protein transduction domain (PTD) that facilitates protein entry into cells better than TAT fusion proteins (Ho et al 2001). Therefore, PTD-Hsp70 and PTD-Hsp70 mutant fusion proteins were made. We show that the PTD-Hsp70 mutant proteins go into cells, interact with active caspase-3, block its activity, and significantly improve cell survival compared with PTD–wild-type Hsp70 (Hsp70WT). The additional antiapoptotic properties of these Hsp70 mutants might make them eventually useful for the treatment of acute cellular injury as well as degenerative diseases.
MATERIALS AND METHODS
Construction of the pRSETB-PTD expression plasmids
Complementary oligonucleotides containing the PTD and TAT domains were synthesized, then subcloned into a NdeI- and NheI-digested pRSETB expression vector (Invitrogen, Carlsbad, CA, USA). Deoxyribonucleic acid (DNA) sequencing confirmed that these constructs were correct. The modified PTD amino acid sequence is YARAAARQARA, and the TAT sequence is YGRKKRRQRRR (Ho et al 2001).
Construction of Hsp70 mutants
The complementary DNA (cDNA) encoding human Hsp70 (ATCC, Manassas, VA, USA, pAG153-human Hsp70) was inserted in frame into the BamHI and EcoRI sites of the pRSETB-PTD vector. Hsp70 mutant constructs were made using polymerase chain reaction (PCR) site-directed mutagenesis. PCR was performed using the following primers. For Hsp70, 528DEVD531(Hsp70.Q): F 5′-CGGAGGACGAGGTGGATCGCGAGAGGGTGTC-3′ and R 5′-GACACCCTCTCGCGATCCACCTCGTCCTCCG-3′. For Hsp70, 528DEVDG532 (Hsp70.QG): R 5′-CGGAGGACGAGGTGGATGGCGAGAGGGTGTC-3′ and R 5′ GACAC CCTCTCGCCATCCACCTCGTCCTCCG-3′. For Hsp70, 637DEVD640 (Hsp70.E): F 5′-GCCCCACCATTGATGAGGTAGATTAGG-3′ and R 5′-CCTAATCTACCTCATCAATGGTGGGGC-3′. The Hsp70.Q.E (DEVQ → DEVD, EEVD → DEVD) mutant was made with Hsp70.Q as a template and using Hsp70.E primers. For Hsp70, 217DEVDG221 (Hsp70DEVDG): F 5′-GATCGACGACGGCATCGACGAGGTGGACGGTGCCACGGCCGGGGAC-3′ and R 5′-GTCCCCGGCCGTGGCACCGTCCACCTCGTCGATGCCGTCGTCGATC-3′. For Hsp70, 434DQMDF438 mutant (Hsp70D): F 5′-CACCACCTACTCCGACCAAATGGACTTCGTGCTGATCCAGG-3′ and R 5′-CCTGGATCAGCACGAAGTCCATTTGGTCGGAGTAGGTGGTG-3′. For Hsp70, 434DQMDG12438 mutant (Hsp70DG): F 5′-CACCACCTACTCCGACCAAATGGACGGGGTGCTGATCCAGG-3′ and R 5′-CCTGGATCAGCACCCCGTCCATTTGGTCGGAGTAGGTGGTG-3′. These plasmids were sequenced to confirm the desired mutations.
The Hsp70N (amino acids 1–430) cDNA fragment was digested with BamHI and SmaI. The Hsp70C (amino acids 420–640) was constructed with BglII and HindIII. All the above constructs were confirmed using DNA sequencing. The following constructs and reagents were also used: pET15b/caspase-3, pET21b/procaspase-9, pcDNA3/Apaf-1, pEF/caspase-3-LacZ, and poly(ADP-ribose) polymerases (PARP). pSV/β-gal was from Promega, Madison, WI, USA Cal4-Luc from Stratagene, La Jolla, CA, USA pMEP4 from Invitrogen, Carlsbad, CA, USA and the mammalian 2-hybrid system was obtained from Clontech, San Jose, CA, USA. The other plasmids were constructed by PCR or appropriate restrictive enzyme digestion methods including pET15b/caspase-3 (p17) and pET15b/caspase-3 (p12), pVP16/Apaf-1, pVP16/cytochrome c, pET21b/Apaf-1, pET21b/PARP, pM/Hsp70WT, and its mutants, pVP16/ caspase-3 (aa29–277), and pM/procaspase-9.
Expression and purification of PTD-Hsp70 fusion proteins
All PTD-Hsp70 fusion proteins were expressed and purified according to the Qiagen (Valencia, CA, USA) protocol under native conditions. The proteins were desalted on a PD-10 column (Amersham Pharmacia Biotech, Upsala, Sweden) and concentrated with a Centriplus YM-50 column (Millipore, Billerica, MA, USA). Protein concentrations were determined using a protein assay kit (Bio-Rad, Hercules, CA, USA) and protein purity was checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (purity >98% in each protein, not shown). Protein identities were confirmed by Western blots using anti-Hsp70 (Bio-Rad) and anti-histidine (His) antibodies (Cell Signaling Inc, Beverly, MA, USA).
Western blot and immunofluorescence analysis of imported proteins
NT cells (Stratagene) grown on 6-well plates were incubated with TAT-Hsp70WT or PTD-Hsp70WT proteins in Dulbecco's modified Eagle's medium (DMEM) + F12 medium plus 10% fetal calf serum under the conditions described in the figures. The cells were digested with 0.25% trypsin at 37°C for 30 minutes, washed 5 times with cold phosphate-buffered saline (PBS) to remove extracellular associated proteins, and lysed in radioimmunoprecipitation buffer. Western blotting was performed with anti-His antibody.
For protein localization, cells were grown on glass coverslips, then treated with recombinant PTD or TAT-Hsp70 fusion proteins for 30 minutes, and then washed with PBS containing 0.1% Triton X-100 at least 5 times to remove extracellular proteins. The cells were fixed, then stained with 1 μg/mL of Hoechst (Sigma, St Louis, MO, USA) and an anti-His antibody followed by a tetramethylrhodamine isothiocyanate–conjugated secondary antibody (Sigma). The cells were imaged by fluorescence microscopy.
Luciferase, β-gal activity assays
Cells were transfected with equal amounts of pRSV/Luciferase and β-galactosidase (β-gal) and the indicated amounts of the His-Hsp70 mutant constructs. After 24 hours, cells were exposed to 45°C for 30 minutes. After a 3-hour recovery at 37°C, cells were divided for Western blot for the Hsp70 mutants and for β-gal expression. Luciferease and β-gal activity were measured according to Promega protocols. Some of the transfected cells were divided for β-gal activity analysis under normal culture conditions at 37°C.
Immunoprecipitation
NT cells were cultured in serum-free medium for 6 hours, and then returned to 10% serum in DMEM + F12 medium for an additional 6 or 12 hours. They were treated with 5 μM PTD-Hsp70 and its mutants for 30 minutes, then treated with 0.25% trypsin at 37°C for 30 minutes, washed 5 times, and resuspended in lysis buffer on ice for 30 minutes. The protein (0.5 mg) from the cell lysates was immunoprecipitated with 2 μL His antibody and 10 μL protein G in 500 μL reaction buffer for 2 hours with constant agitation at 4°C. The beads were collected after 5 washes. They were resolved by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes (BioRad), and immunoblotted with anti–caspase-3 antibody (BD-Transduction Lab, San Jose, CA, USA) which recognizes active forms of caspase-3 (p17).
Hsp70 cleavage and caspase-3 inhibition assays
For cell-free experiments, 250 μg Hela cytosolic extracts (S-100) were incubated with 1 μL 35S-labeled Hsp70WT and its mutants and caspase-3 activated by the addition of cytochrome c (100 μg/mL) and deoxyadenosine triphosphate (dATP) (1 mM) at 37°C for 30 minutes in a final volume of 50 μL. The sample was then subjected to 10% and 15% SDS-PAGE. For the active caspase-3 assays, 15 ng active caspase-3 (Oncogene Inc, Cambridge, MA, USA) was added in the presence of 1 μL 35S-labeled Hsp70WT and its mutants at 37°C for 30 minutes in the reaction buffer. Twenty microliters of the sample was then subjected to 10% and 15% SDS-PAGE.
The caspase-3 inhibition assays were carried out according to a previously described method (Mosser et al 1997). Briefly, reaction mixtures (50 μL) contained 0.5 μL 35S-labeled PARP together with 15 ng active caspase-3 in reaction buffer. For cell-free experiments, Hela S-100 fractions were incubated with cytochrome c (100 μg/mL) and dATP (1 mM) to activate procaspase-3. The mixtures were incubated at 37°C for 30 minutes and then analyzed by 15% SDS-PAGE. Protease inhibition was studied by adding 15 μM Hsp70 and its mutants to the reaction mixtures for a preincubation period of 30 minutes at 37°C before addition of the substrate.
DEVDase activity was determined by adding 30 ng of recombinant active caspase-3 to 100 μL 20 μM DEVD-pNA colorimetric substrate in the presence or absence of different amounts of Hsp70WT or its mutants. The mixture was incubated at 37°C for 2 hours and the reaction was stopped by adding ice-cold water (200 μL). The OD405 values for each sample were read.
In vitro protein interaction
Three microliter 35S-labeled active caspase-3 (p17, p12), 35S-labeled Hsp70WT and its mutants, and 0.5 μM Apaf-1, Hsp70WT and its mutants previously immobilized on beads were incubated in binding buffer for 30 minutes at 4°C. The mixtures were washed 5 times and then analyzed by 10% SDS-PAGE.
For the effects of Hsp70WT and its mutants on the interaction between procaspase-9 and Apaf-1, 3 μL 35S-labeled procaspase-9 and 0.5 μM Apaf-1–conjugated beads were incubated in binding buffer for 30 minutes at 25°C. This was then incubated with or without cytochrome c (100 μg/mL) and dATP (1 mM) in the presence or absence of 10 μM Hsp70WT or its mutants for 3 hours at 4°C. The mixtures were washed 5 times and then analyzed by 10% SDS-PAGE.
Cell survival and apoptosis assays
NT cells were treated with PTD-Hsp70 and its mutants before and after serum depletion for 3 hours as described in the figures. Cell viability and apoptotic assays were performed using 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) and Hoechst staining. The pEF-caspase-3-LacZ was transfected into NT cells in medium containing 4 μM thiamine (Sigma) using a NT cell-specific transfection kit (MBS Mammalian Cell Transfection Kit, Stratagene). After a 2-day transfection, cells were cultured in DMEM + F12 medium containing 10 μg/mL puromycin (Sigma) and 4 μM thiamine for 28 days. Puromycin-resistant and caspase-3–expressing cells were selected in 96-well plates. Cells that showed regulated expression of the caspase-3-LacZ when the 4-μM thiamine was removed from the medium were selected. Cell growth assays were performed as described by Mosser et al (1997). For the apoptosis assays, NT cells were grown on coverslips. After treatment, cells were stained with 1 μg/mL of Hoechest-33258 and were analyzed by fluorescence microscopy.
The mammalian 2-hybrid system
The Apaf-1cDNA, caspase-3 amino acids 29–277, and cytochrome c were in-frame inserted into a pVP16 vector, and the procaspase-9, Hsp70WT and its mutants were in-frame inserted into the pM plasmid. Cos-1 cells were cotransfected with the above constructs, the Gal4-reporter and β-gal vector using the SuperFect TM kit (Qiagen). After 24- or 72-hour transfections, transfected cells were treated with staurosporine (STS). Luciferase activity was measured according to the manufacturer's instructions.
Molecular modeling
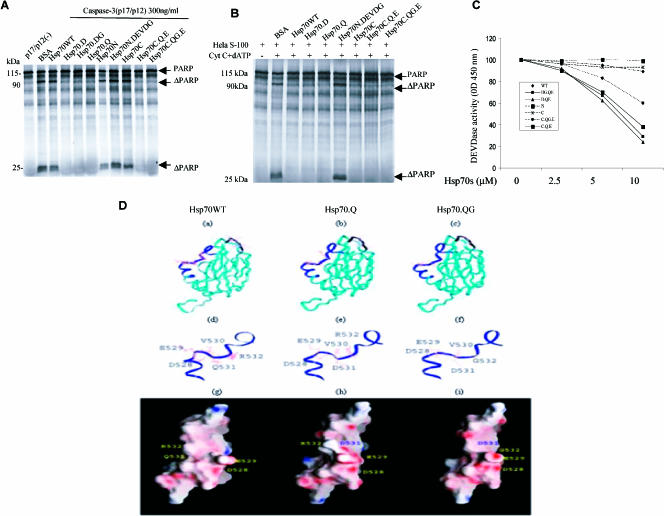
We modeled 3 proteins, Hsp70WT, Hsp70.Q, and Hsp70.QG using the nuclear magnetic resonance (NMR) structure of Hsp70WT (Pdb code 1CKR) as a template (Morshauser et al 1999). Threading of the Hsp70WT, Hsp70.Q, and Hsp70.QG sequences on the sequence (521–540) of the Hsp70WT were optimized using the PDB-BLAST search and then modeled using INSIGHT 2000 (Accelyrus, San Diego, CA). After homology replacement of these specific amino acids, the structure was further energy minimized using the DISCOVER module of INSIGHT. Each model was subjected to 500 cycles of steepest descent followed by 500 cycles of conjugate gradient minimization. The figure (see Fig 5E) illustrating the surface of these molecules was created using GRASP according to the electrostatic potential scaled from electropositive (red) to electronegative (blue) (Nicholls et al 1991).
Fig 5.
Inhibitory effects of Hsp70 and its mutants on caspase-3 activity. (A) 35S-labeled PARP was incubated with recombinant active caspase-3 either with or without 15 μM PTD–wild-type Hsp70 protein (Hsp70WT) or its mutant proteins. (B) The Hela S-100 fraction, cytochrome c, and deoxyadenosine triphosphate (dATP) were incubated with recombinant Hsp70WT and its mutants (15 μM) along with 35S-labeled PARP. (C) Inhibition of DEVDase by Hsp70s. The tetrapeptide DEVD-pN (A) was used as a substrate. Fifteen nanograms of active caspase-3 and purified Hsp70WT and its mutants were added at concentrations ranging from 0 to 10 μM for the DEVD-pN assay. (D) Hsp70 protein models for Hsp70.WT (a, d, g), Hsp70.Q (b, e, h), and Hsp70.QG (c, f, i) are shown with amino acids 528–531 represented as a black ribbon in Hsp70.WT (a) and amino acids 521–540 represented as a blue ribbon in Hsp70.Q (b) and Hsp70.QG (c). The detailed models of these amino acid regions (521–540) are also shown for each protein (d, e, f) along with a representation of the surface electrostatic charge for each protein (g, h, i)
Statistics
Error bars in all figures represent standard errors of the mean, and the differences between wild-type and mutant proteins are significantly different (P ≤ 0.05, t-test) unless otherwise stated. At least 3 replicates of at least 3 separate experiments were performed for the quantitative data shown in each figure.
RESULTS
Generation of Hsp70 mutants and transduction of PTD-Hsp70 fusion proteins into cells
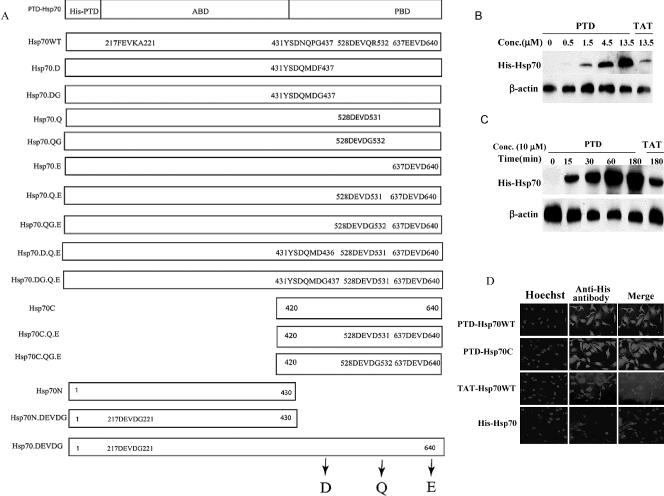
We genetically engineered the TAT or the modified TAT (PTD) into Hsp70s N-terminal. Mutant proteins were produced in which the 217FEVK220 sequence in the adenosine triphosphate (ATP)-binding domain and the 433DNQP436, 528DEVQ531, and 637EEVD640 sequences in the peptide-binding domain of Hsp70 were replaced with the caspase-3 recognition sequence DEVD or with the 4 residues (DQMD) from the cleavage site of the baculovirus protein p35. These included mutation of DNQP to DQMD (Hsp70.D), DEVQ to DEVD (Hsp70.Q), and EEVD to DEVD (Hsp70.E). Hsp70N and Hsp70C were also constructed along with the DXXD mutants. Additional mutants were constructed in which a glycine (G) was inserted after the aspartic acid (Asp) or glutamine residue: (Hsp70.DG; Hsp70.DG.Q.E; Hsp70.QG; Hsp70.QG.E; Hsp70C.QG.E, and Hsp70N.DEVDG). All proteins had His and PTD domains (Fig 1A). Molecular modeling predicted that the mutated regions of these proteins would not be buried internally.
Fig 1.
Protein transduction domain (PTD)–Hsp70, TAT-Hsp70 proteins, and their mutants. (A) Diagram of Hsp70 and its mutant proteins used in this study. The wild-type Hsp70 protein (Hsp70WT) had a 6.His domain followed by the protein transduction domain (PTD or TAT) fused to the N-terminal of the human Hsp70cDNA. (B,C) Concentration- (B) and time (C)-dependence of the cellular import of PTD-Hsp70WT and TAT-Hsp70WT proteins. NT cells were treated with PTD-Hsp70WT or TAT-Hsp70WT at the concentrations indicated for 0.5 hours at 37°C (B); or the cells were exposed to 10 μM PTD-Hsp70WT or TAT-Hsp70WT proteins at 37°C for the times indicated (C). The imported proteins were immunoblotted with histidine (His) antibody. (D) Immunofluorescent staining of NT cells treated with PTD-Hsp70s or TAT-Hsp70WT. Cells were incubated for 30 minutes with 10 μM of each protein. Left panels show nuclear staining with Hoechst, the right panels show anti-His antibody staining for each of the imported proteins
To examine uptake of the TAT- and PTD-Hsp70s into cells, the purified Hsp70 proteins were added directly to cultured cells at various concentrations, and levels of transduced proteins were analyzed by Western blot. We also incubated cells with PTD- or TAT-Hsp70 at a final concentration of 10 μM for the indicated times. PTD-Hsp70 entered NT cells in a dose-dependent (Fig 1B) and time-dependent fashion (Fig 1C). PTD-Hsp70 uptake was greater than TAT-Hsp70 (Fig 1 B,C). Immunostaining showed the PTD-Hsp70, PTD-Hsp70C, and TAT-Hsp70WT proteins throughout the cytoplasm and nuclei (Fig 1D). In addition, the data show that TAT- and PTD-Hsp70 fusion proteins and Hsp70 cross the cell membranes with different efficiencies, which suggests that TAT- and PTD-Hsp70s do not stick to cell membranes nonspecifically. In addition, detailed studies of other PTD proteins shows entry of the PTD proteins into cells both in vitro and in vivo (Schwarze et al 1999, 2000; Ho et al 2001; Morris et al 2001).
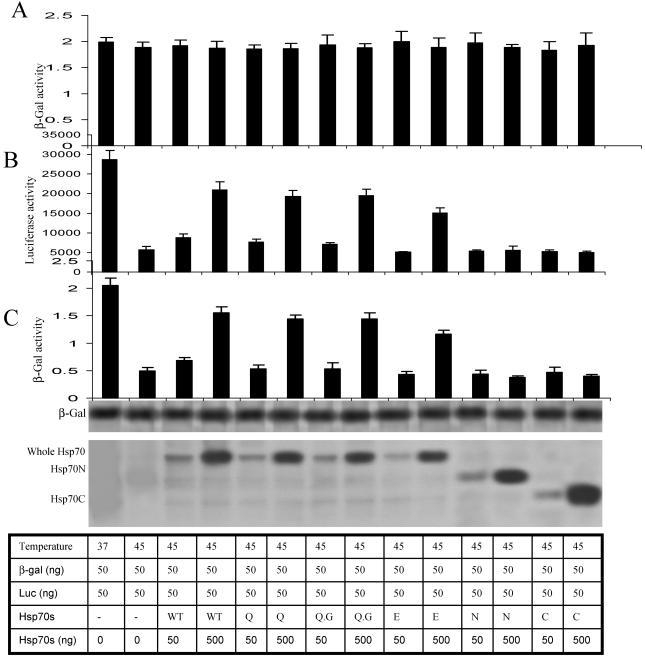
Hsp70 point mutants except Hsp70N and Hsp70C retain chaperone function equivalent to Hsp70WT
Previous studies have shown that transient overexpression of Hsp70 protected cellular luciferase activity from heat inactivation (Nicholls et al 1991; Nollen et al 1999). We, therefore, determined whether mutations of Hsp70 altered its chaperone function. For these experiments, Cos-1 cells were transfected with plasmids encoding luciferease or β-galactosidase (β-gal) and the indicated His-Hsp70 (Fig 2). After a 24-hour transfection, the cells were exposed at 45°C for 30 minutes and luciferase and β-gal enzyme activities were measured. A similar refolding function can also be obtained when cells are transfected with different Hsp70 point mutants. The refolding activity of the Hsp70E mutant was marginally changed. In contrast, Hsp70N and Hsp70C both lost their refolding abilities (Fig 2 B,C). We then determined whether Hsp70WT or the mutants would affect native β-gal activity. Therefore, after transfection, Cos-1 cells were exposed to 37°C for 24 hours and β-gal enzyme activity was measured. These results showed that none of the molecules had any effect on native β-gal activity (Fig 2A). We also demonstrated that Hsp70 and its mutants did not alter the expression of β-gal. Therefore, β-gal can be used as an internal control in all the experiments.
Fig 2.
Effects of wild-type Hsp70 protein (Hsp70WT) and its mutants on thermal (45°C) inactivation of luciferase and β-galactosidase (β-gal). Cos-1 cells were transfected with luciferase, β-gal and Hsp70 constructs. Twenty-four hours after transfection, some of these cells then had β-galactosidase enzyme activity measured as shown in Panel A. Other cells were then exposed to 45°C for 30 minutes (Panels B and C) and after a 3 hours recovery at 37°C, the luciferease (Panel B) and β-galactosidase (Panel C) enzyme activities were measured. Expression of β-Gal (fourth panel down), Hsp70WT, and mutant Hsp70s were analyzed by Western blot using appropriate antibodies
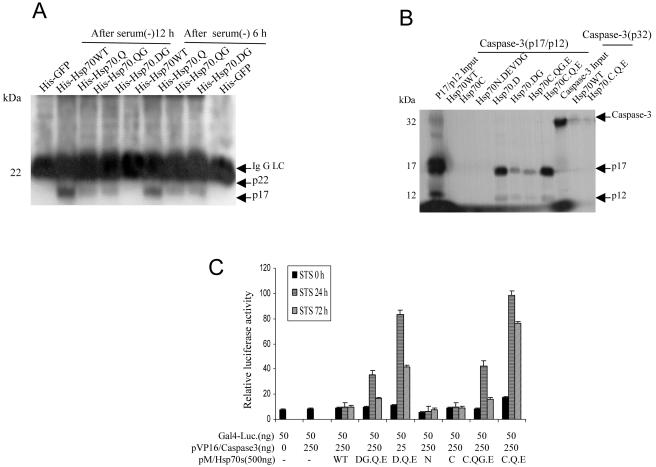
Hsp70 mutants interact with active caspase-3
We performed an immunoprecipitation assay to demonstrate that the transduced Hsp70 mutant proteins interact physiologically with active caspase-3 in intact cells. Serum-deprived NT cells were treated with PTD-Hsp70 and its mutants. The cell lysates were immunoprecipitated with His antibody and then immunoblotted with caspase-3 antibody. The results showed that the Hsp70 mutant proteins, except Hsp70WT, interacted with active caspase-3 (Fig 3A). To determine if Hsp70 mutants directly interacted with active caspase-3, we mixed Affigel-bound PTD-Hsp70 and its mutants with 35S-labeled active caspase-3 (p17/p12). The mutant Hsp70s except Hsp70WT, Hsp70C, and Hsp70N.DEVDG bound active caspase-3 (Fig 3B).
Fig 3.
Hsp70 mutants interact with active caspase-3. (A) NT cells were cultured in serum-free conditions for 6 hours and then returned to 10% serum in Dulbecco's modified Eagle's medium (DMEM) + F12 medium for an additional 6 and 12 hours. The cells were treated with 5 μM protein transduction domain (PTD)–Hsp70 or its mutants for 30 minutes. Cells were resuspended in lysis buffer. Equal amounts of protein from cell lysates were immunoprecipitated with histidine (His) antibody and then immunoblotted with an anti–caspase-3 antibody that recognized the active 17 kDa (p17) subunit of caspase-3. His-GFP served as a negative control. (B) Direct interaction of Hsp70 mutants with caspase-3 p17/12 active subunits. Beads pull-down experiments were performed as described in Materials and Methods. The signal generated by 10% of the total input amount of each 35S-labeled protein is shown. (C) In vivo analysis of the interaction of Hsp70 mutants and active caspase-3. The mammalian 2-hybrid assays were performed using the indicated plasmids that were cotransfected into Cos-1 cells, together with the Gal4-dependent luciferase reporter. For each transfection, cells were also transfected with a constant amount of pSv/β-gal for monitoring transfection efficiency and normalization of the luciferase activity. Cells were treated with staurosporine (STS) for 0, 24, or 72 hours
We then examined the interaction between these Hsp70 mutants and caspase-3 in vivo using a mammalian 2-hybrid model. Hsp70WT and its mutant cDNAs were in frame cloned into the pM vector containing the Gal4 DNA-binding domain. Similarly, the caspase-3 cDNA, composed of residues 29–277, was inserted into the pVP16 plasmid containing the activation domain. The resulting constructs were cotransfected into Cos-1 cells with a Gal4-Luciferase. Transfection of pM/ Hsp70WT, Hsp70 mutants, or pVP16/caspase-3 alone failed to activate reporter activity. However, reporter activity was dramatically enhanced when pM/Hsp70 mutant-transfected cells were treated with STS as compared with Hsp70WT, Hsp70C (C), or Hsp70N (N) (Fig 3C). All these mutants tested behaved indistinguishably from Hsp70WT in their ability to interact with active caspase-3.
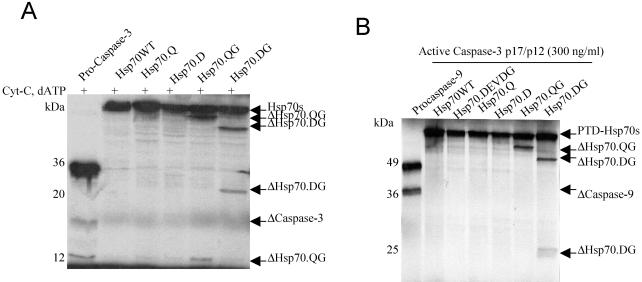
Some Hsp70 mutants are cleaved by caspase-3
Because Hsp70 mutants contained caspase-3 binding sites, we examined the potential of these Hsp70 mutants to function as pseudosubstrates for caspase-3. A cell-free system containing 35S-labeled Hsp70 and its mutants was incubated with cytochrome c, dATP, and Hela S-100 fraction. The results showed that Hsp70.QG and Hsp70.DG mutations were cleaved (Fig 4A). The cleaved forms of Hsp70.QG and Hsp70.DG were resolved into 2 major bands migrating at 62 and 13 kDa and 52 and 23 kDa, respectively. These matched the cleavage sites where we introduced the point mutations at positions 531 (528DEVDG) and 436 (433DQMDG) in Hsp70. To test whether the cleavages are specific for caspase-3, 35S-labeled Hsp70 and its mutants were incubated with purified recombinant active caspase-3. The cleavage patterns (Fig 4B) were similar to the cell-free system.
Fig 4.
Cleavage of Hsp70 mutants. (A) Cytochrome c initiated proteolysis of Hsp70 mutants. The 35S-labeled Hsp70 proteins were incubated with the Hela S-100 fraction, cytochrome c, and deoxyadenosine triphosphate (dATP) for 30 minutes. The samples were resolved on 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). (B) Active caspase-3 cleaved Hsp70 mutants. 35S-labeled Hsp70 proteins were incubated with recombinant active caspase-3 for 30 minutes at 37°C. The samples were resolved on 10% SDS-PAGE
Hsp70 mutants inhibit caspase-3 activity
Because Hsp70 mutants directly interacted with active caspase-3, we tested whether Hsp70 mutants would antagonize activated caspase-3. We used an in vitro cleavage assay containing purified activated caspase-3 and 35S-labeled PARP to monitor caspase-3 activity in the presence of Hsp70 mutant proteins. PARP protein (120 kDa) was cleaved into 95- and 25-kDa fragments when incubated with active caspase-3 for 30 minutes at 37°C, whereas the addition of Hsp70 mutant proteins inhibited this cleavage (Fig 5A). In contrast, Hsp70WT, Hsp70N, Hsp70C, and Hsp70N.DEVDG had little or no effect on PARP cleavage (Fig 5A). However, when Hsp70WT, Hsp70C, Hsp70N.DEVDG, and Hsp70 mutants were added to the Hela S-100 fraction system, all these Hsp70 proteins (except Hsp70N.DEVDG) blocked PARP cleavage (Fig 5B). This is because Hsp70WT and most mutant Hsp70s would block the apoptosome activation of caspase-3.
Because not all the Hsp70 mutants can be cleaved by active caspase-3 and these mutants had different structural features, we hypothesized that these Hsp70 mutants may have different effects on caspase-3 activity. Therefore, we quantitatively analyzed the inhibitory effects of Hsp70 mutants on caspase-3 activity using a DEVDase assay. As expected, Hsp70WT had no effect on caspase-3 activity, whereas the Hsp70 mutants demonstrated different inhibitory effects on caspase-3 (Fig 5C). In a time course experiment, cleavable Hsp70.QG and Hsp70.DG no longer blocked caspase activity after 3-hour incubations whereas noncleavable Hsp70.Q and Hsp70.D still did (data not shown).
A Blast alignment showed that amino acids 521–540 are highly conserved in all Hsp70 molecules. To better understand the importance of these residues, we compared the energy-minimized structures of Hsp70WT and these mutant proteins (Fig 5D). Similarly, to further investigate the importance of hydrogen bonds formed between the arginine (Arg) in the S1 pocket and the Asp found in the P1 position (Asp531), we also mutated Arg532 of Hsp70 to ensure that there was no competition or steric repulsion between Arg532 in the S1 pocket. This mutation also provided insight into the role of this amino acid in binding and inhibiting active caspase-3 and to the cleavage of the mutant Hsp70s by caspase-3.
Using the NMR solution structure of the Hsp70WT substrate-binding domain as a homology model template, we created the models for the mutant proteins (Fig 5D, a–c): Hsp70WT (amino acids; 521–540, represented as a black ribbon) and Hsp70.Q and Hsp70.QG (amino acids; 521–540, represented as a blue ribbon and provided as an inset (Fig 5D, d–f)). Amino acids 528–531, which resemble the canonical DEVD sequence, are exposed on the surface of Hsp70WT allowing us to generate these homology mutants while trying to avoid disrupting the global fold of the molecule and losing the possibility of protein-protein interactions. The electrostatic surface potential (Fig 5D, g– i) illustrates the charge distribution on the surfaces of Hsp70WT, Hsp70.Q, and Hsp70.QG. It is notable that the negatively charged residues D528, E529, and D531 are displayed on a common face of the Hsp70.Q molecule (Fig 5D, h). These 3 residues as well as the bulky hydrophobic residue, V530, are all conformationally positioned to form hydrogen bonds or salt bridges with active caspase-3 (Wei et al 2000). Other mutant molecular models are not shown.
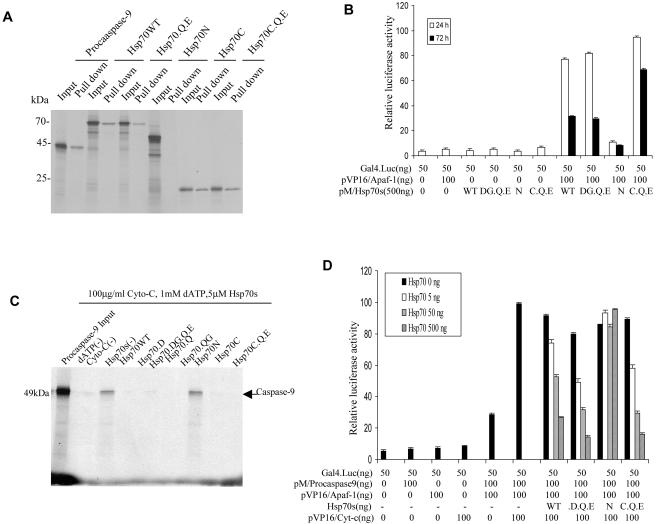
Hsp70 mutants retain the ability to interact with Apaf-1 and inhibit the interaction between procaspase-9 and Apaf-1
Hsp70WT protein decreases caspase-3 activation at least in part by directly binding to Apaf-1. To test whether Hsp70 mutants retain a similar function, a direct interaction between the Hsp70 mutants and Apaf-1 was ascertained using a beads pull-down assay. All Hsp70 mutants except Hsp70N were efficiently retained on Apaf-1– conjugated beads (Fig 6A). Evidence of in vivo interactions was obtained by the mammalian 2-hybrid experiment. These data showed that Hsp70WT, Hsp70DG.Q.E, and Hsp70C.Q.E (but not Hsp70N) interacted with Apaf-1. Notably, interaction of Hsp70C.Q.E (C.Q.E) with Apaf-1 lasted for a longer period than Hsp70WT or the cleavable mutant Hsp70DG.Q.E (DG.Q.E) (Fig 6B).
Fig 6.
Hsp70 mutants retain the ability to intact with Apaf-1 and inhibit the interaction between procaspase-9 and Apaf-1. (A) Hsp70 and its mutants interact with Apaf-1 in vitro. Equal amounts of Apaf-1, previously immobilized on Ni2+ beads, were incubated with 3 μL of 35S-labeled wild-type Hsp70 protein (Hsp70WT) and its mutants in binding buffer for 30 minutes at 25°C. 35S-labeled procaspase-9 was used as a positive control (first 2 lanes). The mixtures were washed and analyzed by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). (B) In vivo assays: mammalian 2-hybrid experiments were performed in Cos-1 cells cotransfected with the indicated plasmids. Luciferase activity was measured as described. (C) In vitro assays: three μL of 35S-labeled procaspase-9 and 0.5 μM Apaf-1 previously immobilized on Ni2+ beads were incubated in binding buffer for 30 minutes at 25°C and then incubated with or without cytochrome c and deoxyadenosine triphosphate (dATP) in the presence or absence of Hsp70WT or its mutants for 3 hours at 4°C. The mixtures were washed and then analyzed by 10% SDS-PAGE. (D) Hsp70 and its mutants interact with Apaf-1 in vivo. Cos-1 cells were cotransfected with Gal 4-Luc and the indicated plasmids. Luciferase activity was measured as described
To further test whether Hsp70 mutants disrupt the interaction between Apaf-1 and procaspase-9, Apaf-1 was bound to beads, incubated with 35S-labeled procaspase-9, cytochrome c, and dATP, followed by incubation in the presence and absence of Hsp70WT and its mutants. Hsp70WT as well as Hsp70 mutants, except Hsp70N, disrupted the interaction between Apaf-1 and procaspase-9 (Fig 6C). We next confirmed whether Hsp70 mutants would disrupt this interaction in cells. Cos-1 cells were cotransfected with pVP16/Apaf-1, pVP16/cytochrome c, pM/procaspase-9, pcDNA3/Hsp70WT, Hsp70 mutants, and Gal4-Luciferase. Hsp70WT and its mutants disrupted the interaction between Apaf-1 and procaspase-9 (Fig 6D).
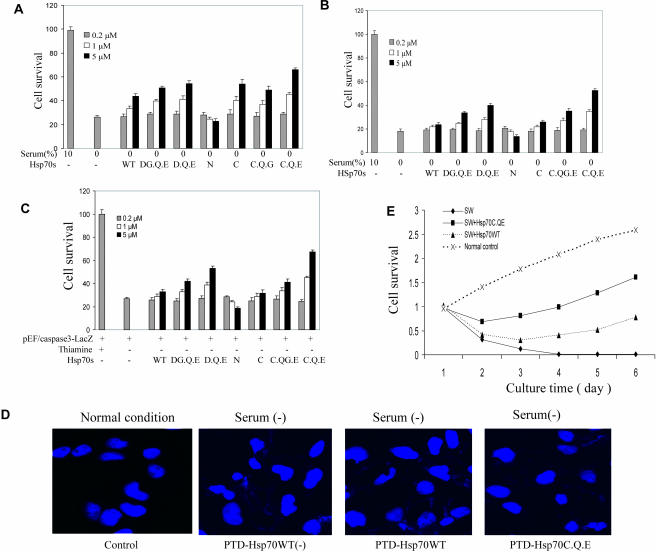
Hsp70 mutants decrease NT cell death
Because the Hsp70 mutants directly inhibited active caspase-3 activity, we determined whether they protect against serum depletion–mediated cell death better than Hsp70WT. PTD-Hsp70WT and its mutants were added 3 hours before serum withdrawal (SW). Though cell survival was improved 48 hours later by Hsp70WT, better survival was obtained with Hsp70 mutants with caspase binding sites (Fig 7A). The greatest protection was obtained with the Hsp70C.Q.E compared with Hsp70WT. In the second experiment, Hsp70WT and its mutants were added 3 hours after SW, and cell survival assessed 48 hours later. The mutant Hsp70s provided the greatest dose-dependent protection compared with Hsp70WT (Fig 7B).
Fig 7.
Wild-type Hsp70 protein (Hsp70WT) and its mutants and cell protection. (A) NT cells were treated with recombinant protein transduction domain (PTD)–Hsp70 and its mutants for 3 hours. Serum was removed for 24 hours and cell survival was measured using 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) assays. (B) NT cells had serum removed. PTD-Hsp70 and its mutants were added 3 hours later. Cell survival was quantified 24 hours later using MTT assays. (C) NT cells were stably transfected with the catalytically active form of caspase-3. Removal of thiamine from the growth media induced active caspase-3. At the same time, cells were treated with PTD-Hsp70 and its mutants. Cell survival was measured at 24 hours using MTT assays. (D) NT cells were treated with PTD-Hsp70WT and Hsp70C.Q.E, then exposed to serum-free medium for 4 hours, and then cultured in normal conditions for an additional 8 hours. The cells were stained with Hoechst and observed by immunofluorescence microscopy. (E) Cells rescued from serum depletion by Hsp70WT and Hsp70C.Q.E retained their proliferative ability. Approximately 1200 NT cells/plate were plated in 96-well plates. One group of cells was untreated and served as the normal control. Three other groups of cells had serum withdrawn (SW) and 2 of these groups of cells were treated with Hsp70C.Q.E (5 μM) or Hsp70WT (5 μM). After being in serum-free medium for 12 hours, the SW groups of cells were placed back in normal culture medium for a period of 5 days. The numbers of viable cells were measured at the indicated times using MTT assays
The next experiment determined whether Hsp70 mutants could protect against cell apoptosis by specifically blocking activated caspase-3 activity. Therefore, active caspase-3 was produced using the procaspase-3-LacZ fusion construct for transfection because overexpression of wild-type caspase-3 alone is not toxic to mammalian cells (Ekert et al 1999). Hsp70WT and its mutants were added to cultured caspase-3-LacZ NT-inducible cells, and cell survival was assessed 48 hours later. The Hsp70 mutants, particularly Hsp70D. Q.E and Hsp70 C.Q.E provided greater protection against activated caspase-3–mediated cell death than Hsp70WT, Hsp70N, or Hsp70C (Fig 7C).
The effects of the Hsp70WT and Hsp70C.Q.E mutant on 4-hour serum depletion–induced apoptotic morphology (nuclei) were examined using Hoechst staining. Both Hsp70WT and Hsp70C.Q.E efficiently inhibited SW-induced apoptosis, as judged by counting cells with apoptotic morphology (condensed nuclei). The effects of Hsp70WT and Hsp70C.Q.E on SW-induced apoptosis differed significantly (Fig 7D).
Although NT cells were protected from apoptosis, as measured by viability assays performed up to 48 hours after serum depletion, it was possible that the cells still might succumb to a nonapoptotic death (Mosser et al 1997). This led us to determine whether the cells rescued by Hsp70WT and its mutants were able to proliferate. We, therefore, measured the relative cell survival of control and depleted cells for 6 days after SW. The surviving cells proliferated at a rate similar (days 4 to 6) to that of the cells in normal growth conditions (Fig 7E). In addition, the mutant Hsp70C.Q.E promoted cell survival better than Hsp70WT at all times after SW (Fig 7E).
DISCUSSION
The Hsp70 DXXD mutants, designed based on Hsp70 molecular modeling, directly block activated caspase-3 in vitro and in vivo, and greatly improve cell survival compared with Hsp70WT. Administration of Hsp70 mutants even after serum depletion is still effective in reducing cell death. The results suggest that the interval between damage and treatment with Hsp70 mutants could range up to many hours and still halt the progression of apoptosis (Fig 7C). This postinjury protective effect means that PTD-Hsp70 mutants might have promise clinically in the treatment of acute cellular injury. The data are important for showing that even though Hsp70WT blocks apoptosome activation of caspase-3 and inhibits caspase-independent apoptosis by binding AIF (Beere et al 2000; Saleh et al 2000; Ravagnan et al 2001), significant additional cellular protection is afforded by Hsp70 DXXD mutants that also block activated caspase-3. These mutants would protect against death receptor, or caspase-8–mediated apoptosis, because they antagonize activated caspase-3 activity.
Because inappropriate apoptosis is implicated in a number of human diseases, cell death inhibitors might be useful for treating these diseases. Drugs that block caspase activity would be the most direct way of inhibiting cell death. However, most peptide inhibitors of caspases just delay the morphological changes of apoptosis and may not prevent the eventual cell death (Xiang et al 1996; McCarthy et al 1997). Therefore, more efficient genetic inhibitors of caspases may be a better way to block apoptosis. Bcl-2 protein not only decreases apoptotic cell death, but the rescued cells retain clonogenic activity (Ekert et al 1999). However, Bcl-2 has to act at steps before caspase-3 activation. Moreover, once Bcl-2 is cleaved by caspase during apoptosis, the truncated Bcl-2 fragment (C-terminal of Bcl-2) becomes a proapoptotic protein that accelerates cell death, converting Bcl-2 from a protective to a lethal protein (Cheng et al 1997; Clem 2001). Although some viral proteins, such as CrmA and p35 can block caspase activation, they are insoluble, tend to aggregate, and might have significant side effects. Moreover, there are many examples of models of apoptosis that are CrmA-insensitive (Garcia-Calvo et al 1998). In whole-cell models of apoptosis and in animal models of disease, the peptide-based inhibitors are not sufficient for caspase inhibition, presumably because of rapid clearance, instability, and poor cell penetration (Enari et al 1995; Garcia-Calvo et al 1998). Inhibitor-of-apoptosis protein (IAP)– bound apoptosome would serve to immediately inhibit caspase-9 and -3 or prevent release of caspase-3 from the apoptosome. However, IAPs would just prevent a modest stress in which only a small portion of caspases are activated (Bratton et al 2001). Like Bcl-2, c-IAP1 is cleaved by caspase-3 to produce a proapoptotic C-terminal fragment (Clem 2001). Though Hsp70WT cannot block active caspase-3, the Hsp70 mutants are potent and direct inhibitors of active caspase-3. Inhibition is mediated by binding of active caspase-3 to an introduced consensus recognition site at P4-P1 ↓ P1′ residues (528DEVD ↓ R532) in Hsp70, which efficiently blocks the catalytic activity of caspase-3. Asp531 is a critical residue because Hsp70WT (528DEVQ ↓ R532) cannot inhibit active caspase-3. Interestingly, the Hsp70 mutant (528DEVD ↓ G532) does not stably associate with active caspase-3 because substitution of the 532Arg residue for 532Gly significantly abrogated the ability of the molecule to inhibit caspase activity. This data provides evidence that residues outside the P4-P1 recognition site also participate in caspase inhibition. These results open the door for designing other novel molecules that might be even more optimal anticaspase inhibitors.
Our models of these modified Hsp70 proteins suggested that a single point mutation, Gln531 to Asp531, would be sufficient to create an Hsp70 analog that bound to and inhibited active caspase-3. This hypothesis was based on the premise that the presence of a nitrogen-bearing side chain at this position in Hsp70WT could sterically hinder critical interactions between Arg64, Gln285 and Arg355 of caspase-3 or provide an alternative hydrogen bond donor that could disrupt Hsp70WT interaction with caspase-3 (Lazebnik et al 1994; Nicholson et al 1995; Nicholson 1996). Using homology modeling and energy minimization we observed that this mutation only modestly perturbed the backbone conformation of the Hsp70.Q analog in comparison with the Hsp70WT structure. This model also suggested that the conformation of the Asp531 side chain, which facilitates the formation of critical hydrogen bonds that are needed between this P1 residue and the caspase-3 S1 pocket, remained exposed on the surface of the structure (Fig 5D). Although Hsp70.QG and Hsp70.Q possess identical caspase-3 recognition sequences, an electronegative patch that is localized around Asp531 on the surface of Hsp70.QG is notably absent on the surface of Hsp70.Q (light red area, Fig 5D, i). This is most likely due to a modest conformational change that is localized around these residues after the Gly532 for Arg532 mutation. Interestingly, Hsp70.QG but not Hsp70.Q is a cleavable pseudosubstrate for caspase-3. These data suggest that a substitution of arginine for glycine at this position in Hsp70.QG increases the conformational flexibility around this residue facilitating greater accessibility to the canonical caspase-3 sequence, which would permit cleavage, whereas Hsp70.Q remains more sterically hindered (Fig 5D, f). Taken together these data support the critical necessity of an Asp in the P1 position of caspase-3 inhibitors and suggest an interesting regulatory role for R532 in mediating functional HSP70–caspase-3 interactions. Our data (Fig 3C) show that the substitution of arginine at the p1′ site with glycine significantly decreases the ability to bind active caspase-3. It indicates that the Hsp70 mutant containing optimal substrate sequences in their pseudosubstrate sites are not the optimal caspase inhibitors. It also demonstrates that P1 residues of substrate determine the caspases binding ability, whereas p1′ residues control the caspase cleavage property. This would be important in various disease processes where endogenous Hsp70 would block apoptosome activation of caspase-3, but it would not block death receptor–induced cell death. Therefore, the Hsp70 DXXD mutants expand the Hsp70 protective potential by blocking death receptor–induced cell death and would improve cell survival in acute disease processes by blocking already activated caspase-3.
Another striking finding of this study is that Hsp70C protects NT cells against serum depletion to a greater degree than Hsp70WT, which is consistent with a previous study (Li et al 1992). A similar Hsp70C construct (Hsp70 with the adenosine triphosphatase [ATPase] domain deleted), when constitutively expressed in Rat-1 cells, protected the cells from extreme hyperthermia (Li et al 1992; Stege et al 1994; Volloch et al 1999; Yaglom et al 1999). Hsp70 mutants with only the last 57 amino acids in the carboxy terminus of Hsp70 abrogate procaspase-3 activation (Li et al 2000). This inhibition of the mitochondrial apoptotic pathway by this Hsp70C mutant (Li et al 2000) is identical to our results. Taken together, these results suggest that the ATP-binding domain of Hsp70 is dispensable for its antiapoptotic function in some cases. Interestingly, we also show that the Hsp70C and its mutants interact with Apaf-1 and active caspase-3, and these interactions last longer than Hsp70WT. The explanation for this finding may be that ATP binding to the ATPase domain lowers the affinity of Hsp70 for its substrates (Bukau and Horwich 1998; Brehmer et al 2001). Moreover, the Hsp70 mutant lacking the ATPase domain is able to bind its substrates but cannot dissociate from them (Yaglom et al 1999).
Acknowledgments
We are grateful to Dr X.D. Wang for providing us with Apaf-1, procaspase-9, and caspase-3 constructs; Dr D.W. Nicholson for the PARP construct; Dr D.L. Vaux for the pEF/Caspase-3-Lacz plasmid; and Dr Q.X. Kong for the Cos-1 cells. This work was supported by grants from the National Institutes of Health and the American Heart Association (FRS).
REFERENCES
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3.0092-8674(1996)087<0171:HCPN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, and Cain K. et al. 2000 Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Walker G, Srinivasula SM, Sun XM, Butterworth M, Alnemri ES, Cohen GM. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. Embo J. 2001;20:998–1009. doi: 10.1093/emboj/20.5.998.0261-4189(2001)020<0998:RAAROC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer D, Rudiger S, Gassler CS, Klostermeier D, Packschies L, Reinstein J, Mayer MP, Bukau B. Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat Struct Biol. 2001;8:427–432. doi: 10.1038/87588.1072-8368(2001)008<0427:TOCAOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269.1081-0706(1999)015<0269:BPOCAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9.0092-8674(1998)092<0351:THAHCM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957.0022-1007(1996)183<1957:CCPTAE>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966.0193-4511(1997)278<1966:COBTAB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clem RJ. Baculoviruses and apoptosis: the good, the bad, and the ugly. Cell Death Differ. 2001;8:137–143. doi: 10.1038/sj.cdd.4400821.1350-9047(2001)008<0137:BAATGT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ekert PG, Silke J, Vaux DL. Inhibition of apoptosis and clonogenic survival of cells expressing crmA variants: optimal caspase substrates are not necessarily optimal inhibitors. Embo J. 1999;18:330–338. doi: 10.1093/emboj/18.2.330.0261-4189(1999)018<0330:IOAACS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0.0028-0836(1995)375<0078:IOAIPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. Embo J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x.0261-4189(1995)014<2281:IOARMI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608.0021-9258(1998)273<32608:IOHCBP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61:474–477.0008-5472(2001)061<0474:SPTDET>2.0.CO;2 [PubMed] [Google Scholar]
- Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s–1706s.0008-5472(1999)059<1701s:GCOPCD>2.0.CO;2 [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0.0028-0836(1994)371<0346:COPPBA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199.0021-9258(2000)275<25665:HSPIAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Li GC, Li L, Liu RY, Rehman M, Lee WM. Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A. 1992;89:2036–2040. doi: 10.1073/pnas.89.6.2036.0027-8424(1992)089<2036:HSPHPC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy NJ, Whyte MK, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215.0021-9525(1997)136<0215:IOIPDN>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173.1087-0156(2001)019<1173:APCFTD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morshauser RC, Hu W, Wang H, Pang Y, Flynn GC, Zuiderweg ER. High-resolution solution structure of the 18 kDa substrate-binding domain of the mammalian chaperone protein Hsc70. J Mol Biol. 1999;289:1387–1403. doi: 10.1006/jmbi.1999.2776.0022-2836(1999)289<1387:HSSOTK>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317.0270-7306(1997)017<5317:ROTHHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls A, Sharp KA, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407.1097-0134(1991)011<0281:PFAAIF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nicholson DW. ICE/CED3-like proteases as therapeutic targets for the control of inappropriate apoptosis. Nat Biotechnol. 1996;14:297–301. doi: 10.1038/nbt0396-297.1087-0156(1996)014<0297:CPATTF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, and Thornberry NA. et al. 1995 Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 376:37–43. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069.0270-7306(1999)019<2069:IVCAOH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, and Susin SA. et al. 2001 Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 3:839–843. [DOI] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510.1465-7392(2000)002<0476:NROTAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569.0193-4511(1999)285<1569:IVPTDO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2.0962-8924(2000)010<0290:PTUDIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stege GJ, Li L, Kampinga HH, Konings AW, Li GC. Importance of the ATP-binding domain and nucleolar localization domain of HSP72 in the protection of nuclear proteins against heat-induced aggregation. Exp Cell Res. 1994;214:279–284. doi: 10.1006/excr.1994.1259.0014-4827(1994)214<0279:IOTADA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Volloch V, Gabai VL, Rits S, Sherman MY. ATPase activity of the heat shock protein hsp72 is dispensable for its effects on dephosphorylation of stress kinase JNK and on heat-induced apoptosis. FEBS Lett. 1999;461:73–76. doi: 10.1016/s0014-5793(99)01428-3.0014-5793(1999)461<0073:AAOTHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wei Y, Fox T, and Chambers SP. et al. 2000 The structures of caspases-1, -3, -7 and -8 reveal the basis for substrate and inhibitor selectivity. Chem Biol. 7:423–432. [DOI] [PubMed] [Google Scholar]
- Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci U S A. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559.0027-8424(1996)093<14559:BCDMNR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaglom JA, Gabai VL, Meriin AB, Mosser DD, Sherman MY. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem. 1999;274:20223–20228. doi: 10.1074/jbc.274.29.20223.0021-9258(1999)274<20223:TFOHIS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad Sci U S A. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131.0027-8424(1997)094<5131:CFOTRA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]