Abstract
Expression of FKBP51, a large molecular weight immunophilin, is strongly enhanced by glucocorticoids, progestins, and androgens. However, the activity of a 3.4-kb fragment of the FKBP51 gene (FKBP5) promoter was only weakly increased by progestin and we show here that it is unresponsive to glucocorticoids and androgens. The entire FKBP5 was scanned for consensus hormone response elements (HREs) using MatInspector. We found that 2 regions of intron E, which are conserved in rat and mouse FKBP5, contain HRE-like sequences with high match scores. Deoxyribonucleic acid fragments (approximately 1 kb in length) containing these regions were amplified and tested in reporter gene assays for steroid responsiveness. One region of intron E of FKBP5 (pIE2) conferred both glucocorticoid and progestin responsiveness to 2 heterologous reporter genes, whereas the other, less-conserved region of intron E (pIE1) was responsive only to progestins. The inclusion of pIE1 upstream of pIE2 (pIE1IE2) enhanced progestin but not glucocorticoid responsiveness. None of the constructs containing intronic sequences was responsive to androgens. Mutation of the putative HREs within pIE1 and pIE2 eliminated hormone responsiveness. Electrophoretic mobility shift assays demonstrated that progesterone receptors (PR) bound to the HRE in pIE1, whereas both PR and glucocorticoid receptors interacted with the HRE in pIE2. These data suggest that distal intronic elements significantly contribute to transcriptional regulation of FKBP5 by glucocorticoids and progestins.
INTRODUCTION
Mammalian gene expression is regulated at multiple levels. Whereas certain genes may be regulated by posttranscriptional mechanisms (Casey et al 1989), the expression of most genes is regulated primarily at the level of initiation of transcription (Beyersmann 2000). General transcription factors and ribonucleic acid (RNA) polymerase interact at the proximal promoter to form complexes that initiate transcription. Distal sequences (response elements) bind inducible transcription factors, which in an orientation- and spatially-independent manner may either enhance or repress the formation of transcription initiation complexes.
Response elements involved in gene regulation are most often located in deoxyribonucleic acid (DNA) sequences upstream of the gene. For example, upstream elements for cyclic adenosine 5′ monophosphate-response element–binding protein, Sp1, and serum response factor regulate the early growth response factor–1 gene (Russell et al 2003). However, regulatory elements have been identified in intron and exon sequences. For example, c-Myc– binding sites in the first intron contribute to transcriptional regulation of the mouse ornithine decarboxylase gene (Auvinen et al 2003). Estrogen regulation of rat vascular endothelial growth factor and glucocorticoid regulation of human Dexras1 are mediated by hormone response elements (HREs) in the 3′-untranslated regions of the respective genes (Hyder et al 2000; Kemppainen et al 2003). On the other hand, the mouse c-Ha-ras gene contains an enhancer in exon 1 that confers estrogen responsiveness and an enhancer in intron 1 that confers glucocorticoid responsiveness (Pethe and Shekhar 1999).
Our recent work has focused on understanding how the FK506-binding protein (FKBP) immunophilin genes are regulated. Of particular interest are the genes for the large molecular weight immunophilins, FKBP51 and FKBP52, which were first identified as components of steroid receptor complexes (Pratt and Toft 1997). They contain an N-terminal peptidylprolyl cis-trans isomerase domain and a C-terminal tetratricopeptide repeat domain that participates in protein-protein interactions. In addition to modulating steroid receptor function (Denny et al 2000; Galigniana et al 2001; Hubler et al 2003; Riggs et al 2003), the large molecular weight FKBPs have been shown to play a role in a number of biochemical processes including regulation of transient receptor potential–like calcium channels (Goel et al 2001), apoptosis (Giraudier et al 2002), transduction efficiency of viral vectors (Qing et al 2001), and gene transcription (Mamane et al 2000; Guo et al 2001). Given their diverse functions, it is important to understand how the expression of these proteins is regulated. FKBP52 messenger RNA (mRNA) is increased by estrogen and heat stress (Kumar et al 2001; Mark et al 2001). On the other hand, FKBP51 is increased by glucocorticoids (Baughman et al 1997; Reynolds et al 1998; Wan and Nordeen 2002; Vermeer et al 2003), progestins (Kester et al 1997; Richer et al 2002; Wan and Nordeen 2002; Hubler et al 2003), and androgens (Amler et al 2000; Mousses et al 2001; Zhu et al 2001; Jiang and Wang 2003). The genomic organization of the human FKBP51 and FKBP52 protein genes (FKBP5 and FKBP4, respectively) has been described (Scammell et al 2001; Scammell et al 2003), but otherwise little is known of how these genes are regulated at the molecular level.
Recently, we isolated a 3.4-kb fragment of the FKBP5 promoter and examined its ability to respond to steroid hormones. We were surprised to find that this fragment exhibited only a modest response to progestin (Hubler et al 2003) and subsequently found that it was unresponsive to glucocorticoids and androgens (reported in this study). This prompted us to search for regulatory elements in other regions of FKBP5. We have identified 2 regions of intron E of FKBP5, more than 75 kb distal to the promoter. One of these confers robust glucocorticoid and progestin responsiveness to a heterologous reporter, whereas the other is only responsive to progestin. These sequences are similar to consensus HREs and are conserved in rat and mouse FKBP5. Mutational and electrophoretic mobility shift assays (EMSA) further suggested that these elements are functionally important, providing evidence that the regulation of FKBP5 by glucocorticoids and progestins occurs at least in part through distal intronic HREs.
MATERIALS AND METHODS
Cell cultures
A549 human lung carcinoma cells were grown in monolayer cultures in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS) (Hyclone Laboratories Inc, Logan, UT, USA), 2 mM l-glutamine, 50 U/mL penicillin G, and 0.05 mg/mL streptomycin. T-47D human breast cancer cells were grown in Roswell Park Memorial Institute–1640 medium with 10% FBS and antibiotics, whereas LNCaP human prostate cancer cells were grown in the same medium with 2 mM l-glutamine. Cells were grown at 37°C in a humidified atmosphere of 5% CO2-95% air. Cells were transferred to medium containing charcoal-dextran–treated FBS (CDS-FBS, Hyclone) for 18 to 24 hours before hormone treatment.
Plasmid construction and luciferase assay
The FKBP5 sequence was scanned for the canonical HRE, GGTACAnnnTGTTCT (Nordeen et al 1990; Nelson et al 1999), using the MatInspector transcription factor program (Quandt et al 1995). DNA fragments of intron E (approximately 1 kb in length) containing sequence with high match scores were polymerase chain reaction amplified from human genomic DNA (CLONTECH Laboratories Inc, Palo Alto, CA, USA) and cloned upstream of the SV40 promoter in MluI- and BglII-digested pGL3-Promoter (Promega Corp, Madison, WI, USA). The hormone responsiveness of 2 constructs (pIE1Luc and pIE2Luc) was evaluated in T-47D, A549, and LNCaP cells. T-47D and LNCaP cells were chosen because FKBP51 is robustly regulated in these cell types by progestins (Kester et al 1997; Richer et al 2002; Wan and Nordeen 2002; Hubler et al 2003) and androgens (Zhu et al 2001; Jiang and Wang 2003), respectively. A549 cells were chosen because we have shown that, as in many other glucocorticoid-responsive cell types (Baughman et al 1997; Reynolds et al 1998; Wan and Nordeen 2002; Vermeer et al 2003), FKBP51 expression is enhanced by glucocorticoids in these cells (data not shown). Also tested in T-47D and A549 cells was p3540Luc, a reporter gene containing 3.4 kb of the FKBP5 promoter (Hubler et al 2003), whereas in A549 cells, the activity of αENaC-Luc, a reporter gene containing the glucocorticoid-responsive promoter for the α-subunit of the human epithelial sodium channel (Sayegh et al 1999; Mick et al 2001) was also examined. Also tested in LNCaP cells were p3540Luc and prostate-specific antigen enhancer (PSE)-Luc, a reporter gene containing the androgen-responsive enhancer for human prostate-specific antigen (Huang et al 1999).
In addition, intron E DNA fragments contained in pIE1Luc or pIE2Luc were cloned upstream of MluI- and KpnI-digested p531Luc, a reporter gene containing 0.4 kb of the FKBP5 promoter (Hubler et al 2003), producing the constructs pIE1h51Luc and pIE2h51Luc, respectively. The hormone responsiveness of pIE1h51Luc and pIE2h51Luc was evaluated in T-47D and A549 cells. Another construct, pIE1IE2Luc, that contained intron E DNA fragments from both pIE1 and pIE2, was prepared by cloning pIE1 into KpnI- and SacI-digested pIE2Luc. The progestin and glucocorticoid responsiveness of pIE1IE2Luc was evaluated in T47-D and A549 cells, respectively.
We also generated and tested reporter plasmids in which mutations were introduced into the HREs. Mutant pIE1Luc (pIE1mut) was constructed by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA), pIE1Luc as the template, and primers GAG GAC TTA AGC AGA GGT ATA CAC TAC TCT TCT CAA CAG GCT TAG (sense) and CTA AGC CTG TTG AGA AGA GTA GTG TAT ACC TCT GCT TAA GTC CTC (antisense), corresponding to positions 629–673 in the progestin-responsive FKBP5 intron E sequence (GenBank accession number AY362696, nucleotide mutations underlined). Mutant pIE2Luc (pIE2mut) was constructed using pIE2Luc as the template and primers GTG CCA GCC ACA TTC AGA GTA GGG TAT TCT GTG CTC TTC AAA AC (sense) and GTT TTG AAG AGC ACA GAA TAC CCT ACT CTG AAT GTG GCT GGC AC (antisense), corresponding to positions 684–727 in the glucocorticoid- and progestin-responsive FKBP5 intron E sequence (GenBank accession number AY362697, nucleotide mutations underlined). Plasmid mutations were confirmed by sequencing across both strands.
Cells were plated in 6-well tissue culture dishes at 1 × 105 cells per well and transfected with 2 μg DNA/well of the indicated plasmids using Superfect (QIAGEN, Valencia, CA, USA) as described (Hubler et al 2003). The medium was replaced, and 18 to 24 hours later, A549 cells were treated with 10-nM dexamethasone (Sigma, St Louis, MO, USA), T-47D cells were treated with 10-nM R5020 (promegestone, NEN Life Science Products Inc, Boston, MA, USA), and LNCaP cells were treated with 10-nM dihydrotestosterone (Steraloids Inc, Wilton, NH, USA). After 24 hours, cells were lysed and assayed for luciferase activity as described (Jones et al 1996).
Electrophoretic mobility shift assays
EMSAs were performed using the LightShift Chemiluminescent EMSA Kit (Pierce Biotechnology Inc, Rockford, IL, USA) according to protocol. Briefly, A549 or T-47D cells were grown in media supplemented with 10% CDS-FBS for 24 hours, after which the medium was replaced with fresh medium containing 10-nM dexamethasone (A549 cells) or 10-nM R5020 (T-47D cells) for 3 hours. Nuclear extracts were prepared using Pierce NE-PER nuclear and cytoplasmic extraction reagents. Binding reactions of 20 μL (containing 60 fmol double-stranded, biotin-labeled probe, 50 ng/μL poly(dI-dC), 10 mM Tris, 50 mM KCl, 1 mM dithiothreitol, and 3 μL nuclear extract) were conducted at room temperature for 20 minutes. In some experiments, the binding reaction also contained 200-fold excess of unlabeled competitor DNA, 5 μL anti– glucocorticoid receptor (GR) antibody (PA1-510A, Affinity Bioreagents, Golden, CO, USA), or 0.25 μL anti–progesterone receptor (PR) antibody (B-30, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), which were added 15 minutes before the labeled probe. The pIE1 HRE probe was TTA AGC AGA GGT ACA CAC TGT TCT TCT CAA CAG (sense strand, corresponding to nucleotides 635–667 in GenBank accession number AY362696), whereas the pIE2 HRE probe was ACA TTC AGA ACA GGG TGT TCT GTG CTC (sense strand, corresponding to nucleotides 693–719 in GenBank accession number AY362697). Reaction mixtures were separated on a 6% polyacrylamide gel, transferred to Nytran (Schleicher and Schuell, Keene, NH, USA), and cross-linked to the membrane in a GS Gene-Linker Chamber (Bio-Rad, Richmond, CA, USA). The signals were then observed using the streptavidin–horseradish peroxidase conjugate and LightShift Chemiluminescent Substrate (Pierce).
RESULTS
DNA sequences in intron E confer glucocorticoid and progestin responsiveness
We recently isolated a 3.4-kb fragment of the FKBP5 promoter with the goal of identifying cis-acting elements that mediate responsiveness to a variety of steroid hormones (Hubler et al 2003). However, in reporter gene assays, this fragment (p3540Luc) is only weakly stimulated (2.1- ± 0.4-fold) by 10-nM R5020 in progesterone-responsive T-47D cells (Fig 1A), as we demonstrated previously (Hubler et al 2003), and is unresponsive to stimulation with 10-nM dexamethasone in glucocorticoid-responsive A549 cells and stimulation with 10-nM dihydrotestosterone in androgen-responsive LNCaP cells (Fig 1B,C, respectively). These results suggest that HREs must reside elsewhere in FKBP5. We therefore scanned the full 115 kb of FKBP5 for the canonical HRE sequence (GGT ACA nnn TGT TCT) (Nordeen et al 1990; Nelson et al 1999) using MatInspector. Two sequences with the highest match scores were identified within the 21.7-kb intron E of FKBP5 (Scammell et al 2001). DNA fragments (approximately 1 kb in length) containing each of these sequences were amplified, placed upstream of the SV40 promoter in pGL3-Promoter, and transfected into hormone-responsive cell lines.
Fig 1.
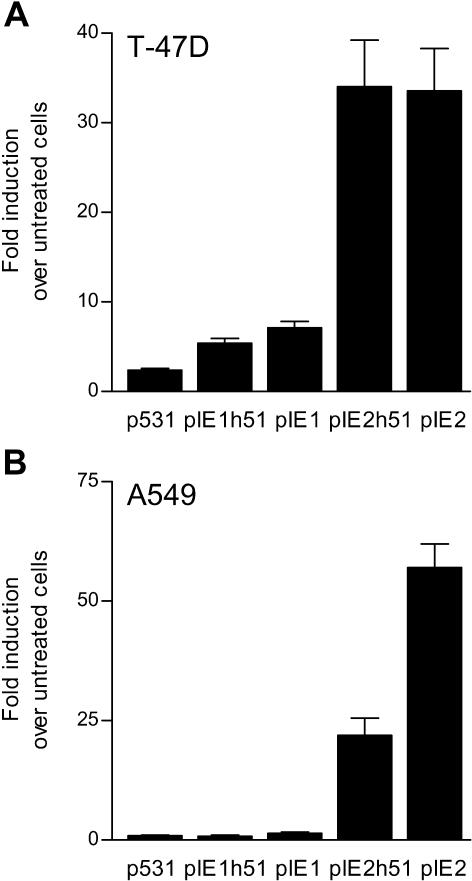
HREs in intron E of FKBP5 confer glucocorticoid and progestin, but not androgen, responsiveness. (A) T-47D cells were transfected with a luciferase-reporter plasmid driven by the FKBP5 promoter (p3540) or 1 of 2 fragments of intron E of FKBP5 fused upstream of the SV40 promoter in pGL3-Promoter (pIE1 and pIE2) and treated with vehicle or 10-nM R5020. (B) A549 cells were transfected with the luciferase-reporter plasmids p3540, pIE1, pIE2, or a luciferase-reporter plasmid driven by the human αENaC promoter (ENaC), and treated with vehicle or 10-nM dexamethasone. (C) LNCaP cells were transfected with luciferase-reporter plasmids p3540Luc, pIE1, pIE2, or a luciferase-reporter plasmid driven by the human prostate-specific antigen enhancer, and treated with vehicle or 10-nM dihydrotestosterone. After 24 hours, cells were collected for assay of luciferase activity, and the data are expressed as fold induction over untreated cells. Each bar represents the mean ± SEM of 3 independent experiments. HRE, hormone response element; PSE, prostate-specific antigen enhancer
One of these constructs, pIE1Luc, contains the sequence GGT ACA CAC TGT TCT (consensus HRE GGT ACA nnn TGT TCT, half-sites underlined) and was stimulated 7.1- ± 0.6-fold by R5020 in T-47D cells (Fig 1A). However, it failed to respond to 10-nM dexamethasone stimulation in A549 cells or to 10-nM dihydrotestesterone stimulation in LNCaP cells (Fig 1 B,C, respectively). Higher concentrations of dexamethasone or dihydrotestosterone (up to 1 μM) were also ineffective in regulating pIE1Luc (data not shown). αENaC-Luc and PSE-Luc were used as positive controls (Huang et al 1999; Mick et al 2001) and gave robust responses to dexamethasone and dihydrotestosterone in A549 and LNCaP cells, respectively. These results suggest that the HRE(s) within pIE1 is preferentially responsive to activation of PR. The nucleotide sequence of pIE1 has been deposited in GenBank with accession number AY362696.
A different pattern of regulation was observed with the second construct, pIE2Luc, which contains a DNA fragment of intron E located 9 kb downstream of the fragment in pIE1Luc and includes the sequence AGA ACA GGG TGT TCT (half-sites underlined). pIE2Luc was robustly stimulated by 10-nM R5020 (18- ± 1.8-fold) in T-47D cells and by 10-nM dexamethasone (49- ± 6.1-fold) in A549 cells (Fig 1A,B, respectively). However, similar to pIE1Luc, pIE2Luc failed to respond to 10-nM dihydrotestosterone in LNCaP cells (Fig 1C). The nucleotide sequence of pIE2 has been deposited in GenBank with accession number AY362697.
Thus, fragments of 2 regions of intron E of FKBP5 displayed hormone responsiveness when cloned upstream of the SV40 promoter in the pGL3-Promoter vector. We also tested the activities of these enhancers when placed upstream of their natural promoter. pIE1 and pIE2 were cloned into p531Luc, which contains approximately 0.4 kb of the basal FKBP5 promoter in pGL3-Basic (Hubler et al 2003). The resulting constructs, pIE1h51Luc and pIE2h51Luc, were tested for hormone responsiveness in T-47D and A549 cells. In T-47D cells, the activity of pIE1h51Luc was enhanced 5.4- ± 0.6-fold by 10-nM R5020 treatment, which was greater than that seen with p531Luc (2.4- ± 0.2-fold above untreated) but less than the stimulation of pIE1Luc (7.1- ± 0.7-fold) (Fig 2A). The activity of pIE2h51Luc was enhanced as robustly by R5020 as pIE2Luc (34- ± 5.2-fold vs 34- ± 4.7-fold above untreated). In A549 cells, pIE2h51Luc activity was strongly enhanced by 10-nM dexamethasone (22- ± 3.6-fold above untreated), although the stimulation was not as dramatic as that seen with the pIE2Luc plasmid (57- ± 5.0-fold) (Fig 2B). The activities of p531Luc, pIE1h51Luc, and again pIE1Luc were not affected by 10-nM dexamethasone. These results show that pIE1 and pIE2 contain enhancer sequences that are hormone-responsive regardless of the promoter.
Fig 2.
Regulation of pIE1 and pIE2 enhancers is independent of the basal promoter. T-47D (A) or A549 (B) cells were transfected with luciferase-reporter plasmids driven by the FKBP5 promoter (p531), the pIE1 enhancer upstream of the FKBP5 promoter (pIE1h51), the pIE1 enhancer in pGL3-Promoter (pIE1), the pIE2 enhancer upstream of the FKBP5 promoter (pIE2h51), or the pIE2 enhancer in pGL3-Promoter (pIE2). T-47D cells (A) were treated with vehicle or 10-nM R5020, whereas A549 cells (B) were treated with vehicle or 10-nM dexamethasone for 24 hours, after which the cells were collected for assay of luciferase activity. The data are expressed as fold induction over untreated cells. Each bar represents the mean ± SEM of at least 3 independent experiments
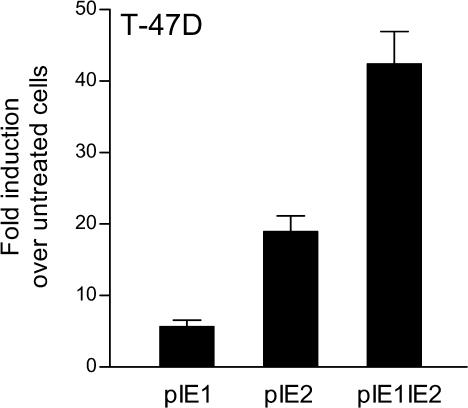
Because progestin responsiveness was exhibited by both pIE1 and pIE2 constructs, we asked whether they can act in concert to regulate FKBP5. We cloned pIE1 upstream of pIE2 in pGL3-Promoter, creating pIE1IE2Luc, and compared its activity to pIE1Luc and pIE2Luc in T47-D cells treated with R5020. The activity of pIE1IE2Luc was enhanced 42- ± 4.6-fold above control by 10-nM R5020 treatment, whereas the activities of pIE1Luc and pIE2Luc were increased by 5.7- ± 0.9-fold and 19- ± 2.1-fold, respectively (Fig 3). These results suggest that enhancer elements in both pIE1 and pIE2 act together to achieve robust regulation of FKBP5 by progestins. Because pIE1IE2Luc had no greater activity than pIE2Luc in A549 cells treated with dexamethasone (data not shown), we may also conclude that glucocorticoid regulation of FKBP5 is mediated at least in part by an enhancer in pIE2. Similar to pIE1Luc and pIE2Luc, pIE1IE2Luc was unresponsive to 10-nM dihydrotestosterone in LNCaP cells (data not shown), suggesting that elements elsewhere in FKBP5 must be responsible for regulation of the gene by androgens.
Fig 3.
Elements in pIE1 and pIE2 together mediate robust regulation of FKBP5 by progestin. T-47D cells were transfected with luciferase-reporter plasmids driven by either the pIE1 enhancer (pIE1), the pIE2 enhancer (pIE2), or pIE1 placed upstream of pIE2 (pIE1pIE2) in pGL3-Promoter, and treated with vehicle or 10-nM R5020. After 24 hours, the cells were collected for assay of luciferase activity. The data are expressed as fold induction over untreated cells. Each bar represents the mean ± SEM of 4 independent experiments
Conservation of HREs in intron E of FKBP5 of different species
The fragments pIE1 and pIE2 include DNA sequences that are hormone responsive. To establish that the putative HREs we identified in intron E of FKBP5 using MatInspector indeed mediate hormone responsiveness, we evaluated these elements using several criteria. First, regulatory elements in noncoding regions tend to be conserved among species (Pennacchio and Rubin 2001). Therefore, we asked whether the HRE-like sequences in pIE1 and pIE2 in intron E of FKBP5 are conserved in the FKBP51 genes of 2 other species, rat and mouse. We obtained the genomic organization of rat and mouse FKBP5 by BLAST alignment (Tatusova and Madden 1999) of rat chromosome 20 (GenBank accession number NW_047597) and mouse chromosome 17 (NT_039649) with the mouse FKBP51 mRNA sequence (NM_010220). The exon-intron boundary positions in rat and mouse FKBP5 were found to be identical to those in human FKBP5 (Scammell et al 2001), although the size of intron E differed between the genes (rat, 11.3 kb; mouse, 12.4 kb; humans 21.7 kb). The sequences within intron E of rat, mouse, and human FKBP5 were then aligned using BLAST (Tatusova and Madden 1999). Two areas of similarity included the HRE-like sequences first identified in human FKBP5. The 2 half-sites of the HRE within pIE1 were conserved in the rat and human genes and exhibited only 1 difference in mouse FKBP5 (Fig 4, upper panel). The adjacent sequence was less well conserved. On the other hand, not only were the half-sites of the putative HRE within pIE2 perfectly conserved in the rat, mouse, and human genes but the surrounding sequence was also conserved (Fig 4, lower panel). Thus, these hormone-responsive regions of intron E are highly conserved among species.
Fig 4.
Relative positions and nucleotide sequences of putative hormone response elements (HREs) within intron E of FKBP5. (A) Solid boxes represent exons 1 to 11 and horizontal lines represent the 5′ promoter and introns A to J of FKBP5. The positions of pIE1 and pIE2 within intron E are indicated by arrows. FKBP5 spans approximately 115 kb; introns are drawn to scale, but exons are not (average size of exons 1 to 10 is 0.1 kb, whereas exon 11 is >1.2 kb). (B) The consensus HRE is shown at top in bold with half-sites underlined. HRE half-sites within pIE1 and pIE2 are shown in upper case. The sequences of HRE-like regions in pIE1 and pIE2 of intron E of human FKBP5 are derived from human chromosome 6 (GenBank accession number NT_007592, nucleotides 26375999 to 26375965 and 26367010 to 26366976, respectively), of rat FKBP5 from rat chromosome 20 (NW_047597, nucleotides 3272063 to 3272029 and 3267890 to 3267856, respectively), and of mouse FKBP5 from mouse chromosome 17 (NT_039649, nucleotides 5002533 to 5002499 and 4998218 to 4998184, respectively). Bases that are conserved between human, rat, and mouse sequences are indicated with dashed lines
Mutational analysis of HREs in pIE1 and pIE2
We next determined whether mutations of critical nucleotides within HREs in pIE1 and pIE2 reduce enhancer activities. Nordeen et al (1990) have shown that critical positions within the half-sites of a bone fide HRE are positions −4, −3, +3, +4, and position +6 (underlined in the consensus HRE, −7GGT ACA nnn TGT TCT+7) (Nordeen et al 1990). Indeed, substitutions at only 1 or 2 of these sites can eliminate hormone responsiveness (Itani et al 2002; Kemppainen et al 2003). We introduced substitutions at positions −3, +3, and +4 into the HRE-like sequence in pIE1Luc and at positions −4, −3, and +3 into the putative HRE in pIE2Luc (Fig 5A). The hormone responsiveness of these constructs was tested in T-47D and A549 cells. We found that the mutation in pIE1 rendered this construct (pIE1mut) completely insensitive to stimulation by 10-nM R5020 in T-47D cells (Fig 5B). Mutation of the putative HRE in pIE2 abolished its responsiveness to R5020 in T-47D cells and to 10-nM dexamethasone in A549 cells (Fig 5B,C, respectively). These results suggest that the HRE-like sequences identified in intron E of FKBP5 are functional in progestin- and glucocorticoid-responsive cells.
Fig 5.
Hormone responsiveness of pIE1 and pIE2 enhancers is abrogated by mutation of the hormone response elements (HREs). (A) Substitutions were introduced into each of the half-sites (underlined) in wild-type pIE1 and pIE2 HREs. The sites of these mutations are shown in bold. (B) T-47D cells were transfected with pGL3-Promoter luciferase-reporter plasmids driven by either the wild-type pIE1 enhancer (pIE1), pIE1 containing mutation of HRE1 (pIE1mut), the wild-type pIE2 enhancer (pIE2), or pIE2 containing mutation of HRE2 (pIE2mut). Cells were treated with vehicle or 10-nM R5020. (C) A549 cells were transfected with pGL3-Promoter luciferase-reporter plasmids driven by either the wild-type pIE2 enhancer (pIE2) or pIE2 containing mutation of HRE2 (pIE2mut) and treated with either vehicle or 10-nM dexamethasone. After 24 hours, cells were collected for assay of luciferase activity. The data are expressed as fold induction over untreated cells. Each bar represents the mean ± SEM of 3 independent experiments
Interaction of steroid receptors with HRE core sequences
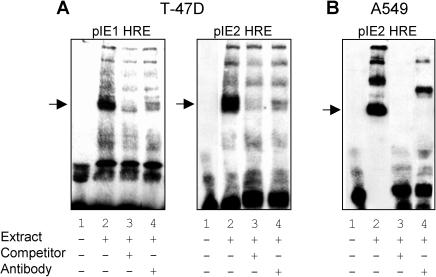
Lastly, it was important to establish specific interactions of steroid receptors with these enhancer elements. To this end, we performed gel shift assays using nuclear extracts from T-47D and A549 cells, as a source of PR and GR, respectively, and biotin-labeled probes encompassing the core HRE sequences in pIE1 and pIE2. Incubation of nuclear extract from T-47D cells with pIE1 or pIE2 HRE probes resulted in the appearance of DNA-protein complexes (Fig 6A). The intensities of these bands were dramatically reduced when either excess unlabelled probe or antibody to PR was added to the incubation mixture. These results suggest that PR in T-47D nuclear extract forms complexes with the HREs in both pIE1 and pIE2. Incubation of nuclear extract from A549 cells also resulted in complex formation, although in this case there was more than 1 dominant form (Fig 6B). However, the formation of these complexes was blocked when the incubation was performed in the presence of excess unlabelled probe. Furthermore, coincubation with GR antibody supershifted or reduced the intensity of each of the bands, suggesting that these protein-DNA complexes contain GR. Others have also observed multiple protein-DNA complexes containing GR that have been ascribed to monomeric and dimeric GR complexes as well as higher-order complexes involving other nuclear factors (Freedman and Alroy 1993; Chen et al 2000; Flick et al 2002).
Fig 6.
Specific interaction of progesterone receptor (PR) and glucocorticoid receptor (GR) with hormone response element (HRE) sequences in FKBP5 intron E. (A) T47-D cells were treated with 10-nM R5020 for 3 hours and nuclear extracts were prepared. Binding reactions were performed at room temperature for 20 minutes with biotin-labeled oligonucleotide probes encompassing the HREs in pIE1 (pIE1 HRE) or pIE2 (pIE2 HRE) either alone (lane 1), or with T-47D cell nuclear extract in the absence (lane 2) or presence of 200-fold excess unlabeled probe (lane 3) or in the presence of PR antibody (lane 4). (B) A549 cells were treated for 3 hours with 10-nM dexamethasone and nuclear extracts were prepared. Binding reactions were performed at room temperature for 20 minutes with biotin-labeled oligonucleotide probes encompassing the HRE in pIE2 (pIE2 HRE) either alone (lane 1), or with A549 nuclear extract in the absence (lane 2) or presence of 200-fold excess unlabeled probe (lane 3) or in the presence of GR antibody (lane 4). After gel electrophoresis, the reaction mixtures were transferred to nytran membranes, and signals were detected by chemiluminescence. PR and GR binding to HRE sequences are indicated by arrows
DISCUSSION
The expression of FKBP51 is increased by glucocorticoids, progestins, and androgens. In this study, we demonstrate that glucocorticoid and progestin regulation of this gene is mediated at least in part by distal intronic HREs. Several lines of evidence support this mechanism. First, whereas 5′-flanking sequence of FKBP5 was modestly responsive to progestins and unresponsive to dexamethasone or dihydrotestosterone, HRE-like sequences in intron E of FKBP5 conferred progestin and glucocorticoid responsiveness to heterologous reporter genes. Second, these HREs are conserved in human, rat, and mouse FKBP5. Third, mutation of the HREs abolished responsiveness to progestins and glucocorticoids. Fourth, gel shift analyses demonstrated that PR and GR interact with these enhancer elements.
There are a number of examples of independently regulated elements in intron sequences. These include genes that are regulated by hormones (Slater et al 1985; Tan et al 1992; Qi et al 1999; Choi et al 2000). They also include regulation of chaperone proteins such as heat shock proteins (Hsp). For example, a heat shock element in intron A of hsp90β is essential for constitutive and heat shock– induced expression of hsp90β (Shen et al 1997). On the other hand, sequences in the first intron of hsp90α mediate inhibition of hsp90α expression (Zhang et al 1999). There are also examples of regulatory elements in introns that alone are inactive but are necessary for maximum activation of gene expression through HREs in the promoter (Hovring et al 1999; Jackson-Hayes et al 2003). As described above, the majority of intronic regulatory elements are found in the first intron (intron A). However, regulatory elements in downstream introns or other regions of the gene distant from the transcription start site, although unusual, are not unprecedented. For example, the human ciliary neurotrophic factor receptor gene is regulated by the TR4 orphan receptor through an enhancer in intron 5 (intron E) (Young et al 1997), and sequences in introns 7 (G) and 9 (I) contribute to expression of the human myeloperoxidase gene (Yamada et al 1993). Furthermore, steroid regulation of the human neutral endopeptidase gene is mediated by an HRE in the 3′-untranslated region of the gene, more than 70 kb from the transcription start site (Shen et al 2000). Robust responsiveness to progestins and glucocorticoids was observed here with intronic regulatory elements in intron E of FKBP5, more than 75 kb from the transcription start site. To our knowledge, this is 1 of the most distal HREs identified in an intron of a steroid-responsive gene. However, recent sequence alignment analyses predict that downstream introns harbor more regulatory elements than previously recognized (Levy et al 2001; Hare and Palumbi 2003). Such computational methods will require functional confirmation by experimental approaches similar to those used here.
One of the intronic fragments (pIE1) was only responsive to progestin, whereas the activity of the other (pIE2) was stimulated by both progestin and corticosteroid. Thus, 2 elements within intron E likely contribute to regulation of FKBP5 by progestin. The selective responsiveness of pIE1 is somewhat surprising because pIE1 contains the canonical HRE GGTACAnnnTGTTCT that has high binding affinity for PR and GR (Nordeen et al 1990; Lieberman et al 1993). However, a number of factors may contribute to steroid-specific responsiveness including sequences both distal and adjacent to the half-sites, chromatin context, and cell type–specific factors (Guido et al 1996; Thackray et al 1998; Nelson et al 1999; Huynh et al 2002; Lambert and Nordeen 2003). On the other hand, the HRE in pIE2 is a variant of the idealized HRE but mediates robust stimulation by both hormones. This HRE closely resembles (1 base mismatch) the HRE of the glucocorticoid-responsive human αENaC promoter (Sayegh et al 1999; Mick et al 2001).
We were unable to identify an androgen response element (ARE) in either intronic pIE1 or pIE2 constructs or in a 3.4-kb fragment of the FKBP5 promoter. Although these and other constructs tested contained HRE-like sequences to which the androgen receptor (AR) binds with high affinity (Roche et al 1992; Nelson et al 1999), elements that differ significantly from the consensus HRE may be responsible for the regulation of FKBP5 by androgen. In nature, AREs can exhibit significant variation in sequence, and individual elements in isolation often have low transcriptional activity (Verrijdt et al 2003). For example, androgen regulation of the rat probasin promoter occurs through the interaction of AR with 2 distinct classes of ARE that act in synergy to increase DNA-binding activity, hormone sensitivity, and transcriptional activation (Reid et al 2001). The PSE, used here as a positive control, is made up of at least 4 tandem, nonconsensus AREs (Huang et al 1999). The diversity of AREs and the possibility that this gene may also be regulated by androgen through distal elements may make identifying the FKBP5 ARE(s) quite challenging.
Increased expression of FKBP51 may have important physiological and biochemical implications. We have shown that FKBP51 from squirrel monkey is a potent inhibitor of GR and PR activities (Reynolds et al 1999; Denny et al 2000; Hubler et al 2003). Indeed, elevated FKBP51 is likely the major cause of glucocorticoid and progesterone resistance observed in squirrel monkeys and other New World primates (Chrousos et al 1982a, 1982b; Scammell 2000). Although less potent than squirrel monkey FKBP51, human FKBP51 can also modify steroid receptor activity (Denny et al 2000; Hubler et al 2003) and may normally play a role in regulating steroid responsiveness. Thus, the induction of FKBP51 by glucocorticoids, progestins, and androgens may be part of a short feedback loop resulting in partial desensitization subsequent to initial exposure to hormone (Cheung and Smith 2000).
Acknowledgments
We are grateful to Dr C. P. Thomas (University of Iowa College of Medicine, Iowa City, IA, USA) and Dr M. F. Carey (UCLA School of Medicine, Los Angeles, CA, USA), who provided αENaC-Luc and PSE-Luc constructs, respectively. We thank Dr Kathy Ault-Ziel for technical help with the EMSA analysis and Dr Vermeer (University Medical Center Utrecht, Utrecht, The Netherlands) for helpful comments. This work was supported by grants 13200 and 01254 from the National Center for Research Resources. T. R. H. was supported by a predoctoral fellowship from the American Heart Association, Southeast affiliate.
REFERENCES
- Amler LC, Agus DB, and LeDuc C. et al. 2000 Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 60:6134–6141. [PubMed] [Google Scholar]
- Auvinen M, Jarvinen K, and Hotti A. et al. 2003 Transcriptional regulation of the ornithine decarboxylase gene by c-Myc/Max/Mad network and retinoblastoma protein interacting with c-Myc. Int J Biochem Cell Biol. 35:496–521. [DOI] [PubMed] [Google Scholar]
- Baughman G, Wiederrecht GJ, Chang F, Martin MM, Bourgeois S. Tissue distribution and abundance of human FKBP51, and FK506-binding protein that can mediate calcineurin inhibition. Biochem Biophys Res Commun. 1997;232:437–443. doi: 10.1006/bbrc.1997.6307.0006-291X(1997)232<0437:TDAAOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Beyersmann D. Regulation of mammalian gene expression. EXS. 2000;89:11–28. doi: 10.1007/978-3-0348-8393-1_2.0071-335X(2000)089<0011:ROMGE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Casey JL, Koeller DM, Ramin VC, Klausner RD, Harford JB. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3′ untranslated region of the mRNA. EMBO J. 1989;8:3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x.0261-4189(1989)008<3693:IROTRM>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Burke TF, Cumberland JE, Brummet M, Beck LA, Casolaro V, Georas SN. Glucocorticoids inhibit calcium- and calcineurin-dependent activation of the human IL-4 promoter. J Immunol. 2000;164:825–832. doi: 10.4049/jimmunol.164.2.825.0022-1767(2000)164<0825:GICACA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: an update. Mol Endocrinol. 2000;14:939–946. doi: 10.1210/mend.14.7.0489.0888-8809(2000)014<0939:MCIWSR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Choi I, Gudas LJ, Katzenellenbogen BS. Regulation of keratin 19 gene expression by estrogen in human breast cancer cells and identification of the estrogen responsive gene region. Mol Cell Endocrinol. 2000;164:225–237. doi: 10.1016/s0303-7207(00)00197-0.0303-7207(2000)164<0225:ROKGEB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Renquist D, Brandon D, Barnard D, Fowler D, Loriaux DL, Lipsett MB. The squirrel monkey: receptor-mediated end-organ resistance to progesterone? J Clin Endocrinol Metab. 1982a;55:364–368. doi: 10.1210/jcem-55-2-364.0021-972X(1982)055<0364:TSMRER>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Renquist D, and Brandon D. et al. 1982b Glucocorticoid hormone resistance during primate evolution: receptor-mediated mechanisms. Proc Natl Acad Sci U S A. 79:2036–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785.0013-7227(2000)141<4107:SMIFIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Flick MB, Sapi E, Kacinski BM. Hormonal regulation of the c-fms proto-oncogene in breast cancer cells is mediated by a composite glucocorticoid response element. J Cell Biochem. 2002;85:10–23.0730-2312(2002)085<0010:HROTCP>2.0.CO;2 [PubMed] [Google Scholar]
- Freedman LP, Alroy I. Gel mobility shift assay to study nuclear hormone receptor-DNA interactions. Methods Mol Genet. 1993;1:280–299.1067-2389(1993)001<0280:GMSATS>2.0.CO;2 [Google Scholar]
- Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–14889. doi: 10.1074/jbc.M010809200.0021-9258(2001)276<14884:ETTPID>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Giraudier S, Chagraoui H, and Komura E. et al. 2002 Overexpression of FKBP51 in idiopathic myelofibrosis regulates the growth factor independence of megakaryocyte progenitors. Blood. 100:2932–2940. [DOI] [PubMed] [Google Scholar]
- Goel M, Garcia R, Estacion M, Schilling WP. Regulation of Drosophila TRPL channels by immunophilin FKBP59. J Biol Chem. 2001;276:38762–38773. doi: 10.1074/jbc.M104125200.0021-9258(2001)276<38762:RODTCB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Guido EC, Delorme EO, Clemm DL, Stein RB, Rosen J, Miner JN. Determinants of promoter-specific activity by glucocorticoid receptor. Mol Endocrinol. 1996;10:1178–1190. doi: 10.1210/mend.10.10.9121486.0888-8809(1996)010<1178:DOPABG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Guo Y, Guettouche T, and Fenna M. et al. 2001 Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex. J Biol Chem. 276:45791–45799. [DOI] [PubMed] [Google Scholar]
- Hare MP, Palumbi SR. High intron sequence conservation across three mammalian orders suggests functional constraints. Mol Biol Evol. 2003;20:969–978. doi: 10.1093/molbev/msg111.0737-4038(2003)020<0969:HISCAT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hovring PI, Matre V, Fjeldheim AK, Loseth OP, Gautvik KM. Transcription of the human thyrotropin-releasing hormone receptor gene-analysis of basal promoter elements and glucocorticoid response elements. Biochem Biophys Res Commun. 1999;257:829–834. doi: 10.1006/bbrc.1999.0545.0006-291X(1999)257<0829:TOTHTH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huang W, Shostak Y, Tarr P, Sawyers C, Carey M. Cooperative assembly of androgen receptor into a nucleoprotein complex that regulates the prostate-specific antigen enhancer. J Biol Chem. 1999;274:25756–25768. doi: 10.1074/jbc.274.36.25756.0021-9258(1999)274<25756:CAOARI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092.0013-7227(2003)144<2380:TFIFIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huynh TT, Ray DW, Brogan IJ, Stevens A, Davis JR, White A. Failure of steroid regulation of the MMTV promoter in a small cell lung cancer cell line is caused by a DNA sequence flanking the glucocorticoid response element. J Endocrinol. 2002;172:295–302. doi: 10.1677/joe.0.1720295.0022-0795(2002)172<0295:FOSROT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 2000;60:3183–3190.0008-5472(2000)060<3183:IOFERE>2.0.CO;2 [PubMed] [Google Scholar]
- Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5′-flanking region. Am J Physiol Endocrinol Metab. 2002;283:E971–E979. doi: 10.1152/ajpendo.00021.2002.0193-1849(2002)283<E971:GSHSGE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jackson-Hayes L, Song S, and Lavrentyev EN. et al. 2003 A thyroid hormone response unit formed between the promoter and first intron of the carnitine palmitoyltransferase-Ialpha gene mediates the liver-specific induction by thyroid hormone. J Biol Chem. 278:7964–7972. [DOI] [PubMed] [Google Scholar]
- Jiang F, Wang Z. Identification of androgen-responsive genes in the rat ventral prostate by complementary deoxyribonucleic acid subtraction and microarray. Endocrinology. 2003;144:1257–1265. doi: 10.1210/en.2002-220718.0013-7227(2003)144<1257:IOAGIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jones LC, Day RN, Pittler SJ, Valentine DL, Scammell JG. Cell-specific expression of the rat secretogranin II promoter. Endocrinology. 1996;137:3815–3822. doi: 10.1210/endo.137.9.8756552.0013-7227(1996)137<3815:CEOTRS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kemppainen RJ, Cox E, Behrend EN, Brogan MD, Ammons JM. Identification of a glucocorticoid response element in the 3′-flanking region of the human Dexras1 gene. Biochim Biophys Acta. 2003;1627:85–89. doi: 10.1016/s0167-4781(03)00079-4.0006-3002(2003)1627<0085:IOAGRE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kester HA, van der Leede BM, van der Saag PT, van der Burg B. Novel progesterone target genes identified by an improved differential display technique suggest that progestin-induced growth inhibition of breast cancer cells coincides with enhancement of differentiation. J Biol Chem. 1997;272:16637–16643. doi: 10.1074/jbc.272.26.16637.0021-9258(1997)272<16637:NPTGIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kumar P, Mark PJ, Ward BK, Minchin RF, Ratajczak T. Estradiol-regulated expression of the immunophilins cyclophilin 40 and FKBP52 in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2001;284:219–225. doi: 10.1006/bbrc.2001.4952.0006-291X(2001)284<0219:EEOTIC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lambert JR, Nordeen SK. CBP recruitment and histone acetylation in differential gene induction by glucocorticoids and progestins. Mol Endocrinol. 2003;17:1085–1094. doi: 10.1210/me.2001-0183.0888-8809(2003)017<1085:CRAHAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Levy S, Hannenhalli S, Workman C. Enrichment of regulatory signals in conserved non-coding genomic sequence. Bioinformatics. 2001;17:871–877. doi: 10.1093/bioinformatics/17.10.871.1367-4803(2001)017<0871:EORSIC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lieberman BA, Bona BJ, Edwards DP, Nordeen SK. The constitution of a progesterone response element. Mol Endocrinol. 1993;7:515–527. doi: 10.1210/mend.7.4.8388996.0888-8809(1993)007<0515:TCOAPR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mamane Y, Sharma S, Petropoulos L, Lin R, Hiscott J. Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity. 2000;12:129–140. doi: 10.1016/s1074-7613(00)80166-1.1074-7613(2000)012<0129:PROIAB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mark PJ, Ward BK, Kumar P, Lahooti H, Minchin RF, Ratajczak T. Human cyclophilin 40 is a heat shock protein that exhibits altered intracellular localization following heat shock. Cell Stress Chaperones. 2001;6:59–70. doi: 10.1379/1466-1268(2001)006<0059:hciahs>2.0.co;2.1466-1268(2001)006<0059:HCIAHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick VE, Itani OA, Loftus RW, Husted RF, Schmidt TJ, Thomas CP. The alpha-subunit of the epithelial sodium channel is an aldosterone-induced transcript in mammalian collecting ducts, and this transcriptional response is mediated via distinct cis-elements in the 5′-flanking region of the gene. Mol Endocrinol. 2001;15:575–588. doi: 10.1210/mend.15.4.0620.0888-8809(2001)015<0575:TAOTES>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mousses S, Wagner U, and Chen Y. et al. 2001 Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene. 20:6718–6723. [DOI] [PubMed] [Google Scholar]
- Nelson CC, Hendy SC, Shukin RJ, Cheng H, Bruchovsky N, Koop BF, Rennie PS. Determinants of DNA sequence specificity of the androgen, progesterone, and glucocorticoid receptors: evidence for differential steroid receptor response elements. Mol Endocrinol. 1999;13:2090–2107. doi: 10.1210/mend.13.12.0396.0888-8809(1999)013<2090:DODSSO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nordeen SK, Suh BJ, Kuhnel B, Hutchison CD. Structural determinants of a glucocorticoid receptor recognition element. Mol Endocrinol. 1990;4:1866–1873. doi: 10.1210/mend-4-12-1866.0888-8809(1990)004<1866:SDOAGR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pennacchio LA, Rubin EM. Genomic strategies to identify mammalian regulatory sequences. Nat Rev Genet. 2001;2:100–109. doi: 10.1038/35052548.1471-0056(2001)002<0100:GSTIMR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pethe V, Shekhar PV. Estrogen inducibility of c-Ha-ras transcription in breast cancer cells. Identification of functional estrogen-responsive transcriptional regulatory elements in exon 1/intron 1 of the c-Ha-ras gene. J Biol Chem. 1999;274:30969–30978. doi: 10.1074/jbc.274.43.30969.0021-9258(1999)274<30969:EIOCTI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303.0163-769X(1997)018<0306:SRIWHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Qi JS, Yuan Y, Desai-Yajnik V, Samuels HH. Regulation of the mdm2 oncogene by thyroid hormone receptor. Mol Cell Biol. 1999;19:864–872. doi: 10.1128/mcb.19.1.864.0270-7306(1999)019<0864:ROTMOB>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001.0022-538X(2001)075<8968:AVTMGT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878.0305-1048(1995)023<4878:MAMNFA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KJ, Hendy SC, Saito J, Sorensen P, Nelson CC. Two classes of androgen receptor elements mediate cooperativity through allosteric interactions. J Biol Chem. 2001;276:2943–2952. doi: 10.1074/jbc.M009170200.0021-9258(2001)276<2943:TCOARE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Roveda KP, Tucker JA, Moore CM, Valentine DL, Scammell JG. Glucocorticoid-resistant B-lymphoblast cell line derived from the Bolivian squirrel monkey (Saimiri boliviensis boliviensis) Lab Anim Sci. 1998;48:364–370.0023-6764(1998)048<0364:GBCLDF>2.0.CO;2 [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429.0021-972X(1999)084<0663:GRITSM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200.0021-9258(2002)277<5209:DGRBTT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, and Chirillo SC. et al. 2003 The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 22:1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche PJ, Hoare SA, Parker MG. A consensus DNA-binding site for the androgen receptor. Mol Endocrinol. 1992;6:2229–2235. doi: 10.1210/mend.6.12.1491700.0888-8809(1992)006<2229:ACDSFT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Gonzales-Robayna I, Pipaon C, Richards JS. Egr-1 induction in rat granulosa cells by follicle-stimulating hormone and luteinizing hormone: combinatorial regulation by transcription factors cyclic adenosine 3′,5′-monophosphate regulatory element binding protein, serum response factor, sp1, and early growth response factor-1. Mol Endocrinol. 2003;17:520–533. doi: 10.1210/me.2002-0066.0888-8809(2003)017<0520:EIIRGC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sayegh R, Auerbach SD, Li X, Loftus RW, Husted RF, Stokes JB, Thomas CP. Glucocorticoid induction of epithelial sodium channel expression in lung and renal epithelia occurs via trans-activation of a hormone response element in the 5′-flanking region of the human epithelial sodium channel alpha subunit gene. J Biol Chem. 1999;274:12431–12437. doi: 10.1074/jbc.274.18.12431.0021-9258(1999)274<12431:GIOESC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scammell JG. Steroid resistance in the squirrel monkey: an old subject revisited. ILAR J. 2000;41:19–25. doi: 10.1093/ilar.41.1.19. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696.1095-6840(2001)124<0152:OOTFIF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scammell JG, Hubler TR, Denny WB, Valentine DL. Organization of the human FK506-binding immunophilin FKBP52 protein gene (FKBP4) Genomics. 2003;81:640–643. doi: 10.1016/s0888-7543(03)00090-9.0888-7543(2003)081<0640:OOTHFI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shen R, Sumitomo M, and Dai J. et al. 2000 Identification and characterization of two androgen response regions in the human neutral endopeptidase gene. Mol Cell Endocrinol. 170:131–142. [DOI] [PubMed] [Google Scholar]
- Shen Y, Liu J, Wang X, Cheng X, Wang Y, Wu N. Essential role of the first intron in the transcription of hsp90beta gene. FEBS Lett. 1997;413:92–98. doi: 10.1016/s0014-5793(97)00883-1.0014-5793(1997)413<0092:EROTFI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Slater EP, Rabenau O, Karin M, Baxter JD, Beato M. Glucocorticoid receptor binding and activation of a heterologous promoter by dexamethasone by the first intron of the human growth hormone gene. Mol Cell Biol. 1985;5:2984–2992. doi: 10.1128/mcb.5.11.2984.0270-7306(1985)005<2984:GRBAAO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JA, Marschke KB, Ho KC, Perry ST, Wilson EM, French FS. Response elements of the androgen-regulated C3 gene. J Biol Chem. 1992;267:4456–4466.0021-9258(1992)267<4456:REOTAC>2.0.CO;2 [PubMed] [Google Scholar]
- Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x.0378-1097(1999)174<0247:BSANTF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thackray VG, Lieberman BA, Nordeen SK. Differential gene induction by glucocorticoid and progesterone receptors. J Steroid Biochem Mol Biol. 1998;66:171–178. doi: 10.1016/s0960-0760(98)00044-2.0960-0760(1998)066<0171:DGIBGA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab. 2003;88:277–284. doi: 10.1210/jc.2002-020354.0021-972X(2003)088<0277:GIILFM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Verrijdt G, Haelens A, Claessens F. Selective DNA recognition by the androgen receptor as a mechanism for hormone-specific regulation of gene expression. Mol Genet Metab. 2003;78:175–185. doi: 10.1016/s1096-7192(03)00003-9.1096-7192(2003)078<0175:SDRBTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wan Y, Nordeen SK. Overlapping but distinct gene regulation profiles by glucocorticoids and progestins in human breast cancer cells. Mol Endocrinol. 2002;16:1204–1214. doi: 10.1210/mend.16.6.0848.0888-8809(2002)016<1204:OBDGRP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yamada M, Yoshida M, Hashinaka K. Identification of transcriptional cis-elements in introns 7 and 9 of the myeloperoxidase gene. J Biol Chem. 1993;268:13479–13485.0021-9258(1993)268<13479:IOTCII>2.0.CO;2 [PubMed] [Google Scholar]
- Young WJ, Smith SM, Chang C. Induction of the intronic enhancer of the human ciliary neurotrophic factor receptor (CNTFRalpha) gene by the TR4 orphan receptor. A member of steroid receptor superfamily. J Biol Chem. 1997;272:3109–3116. doi: 10.1074/jbc.272.5.3109.0021-9258(1997)272<3109:IOTIEO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu J, Cheng XK, Ding L, Heng FY, Wu NH, Shen YF. Regulation of human hsp90alpha gene expression. FEBS Lett. 1999;444:130–135. doi: 10.1016/s0014-5793(99)00044-7.0014-5793(1999)444<0130:ROHHGE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22:1399–1403. doi: 10.1093/carcin/22.9.1399.0143-3334(2001)022<1399:SIFOTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]