Abstract
The present study tested the hypothesis that in response to physical stress the human brain has the capacity to release heat shock protein 72 (Hsp72) in vivo. Therefore, 6 humans (males) cycled for 180 minutes at 60% of their maximal oxygen uptake, and the cerebral Hsp72 response was determined on the basis of the internal jugular venous to arterial difference and global cerebral blood flow. At rest, there was a net balance of Hsp72 across the brain, but after 180 minutes of exercise, we were able to detect the release of Hsp72 from the brain (335 ± 182 ng/min). However, large individual differences were observed as 3 of the 6 subjects had a marked increase in the release of Hsp72, whereas exercise had little effect on the cerebral Hsp72 balance in the remaining 3 subjects. Given that cerebral blood flow was unchanged during exercise compared with values obtained at rest, it is unlikely that the cerebral Hsp72 release relates to necrosis of specific cells within the brain. These data demonstrate that the human brain is able to release Hsp72 in vivo in response to a physical stressor such as exercise. Further study is required to determine the biological significance of these observations.
INTRODUCTION
Heat shock proteins (Hsps) are quintessential intracellular proteins present in the cells of all living organisms, where their primary function is to interact with denatured and naïve proteins, preventing the aggregation of aberrantly folded proteins, and aiding naïve proteins in reaching their native state (Young et al 2003). However, in addition to these well-characterized intracellular roles, Hsps also have important extracellular functions. Accumulating evidence strongly suggests that extracellular Hsp72 has potent immunoregulatory effects. Specifically, it has been demonstrated that the interaction of Hsp72 with specific surface receptors on cells of the innate immune system results in an upregulation of the expression of costimulatory and antigen presenting molecules and various cytokines crucial to generation of adaptive immunity (Asea et al 2000, 2002; Vabulas et al 2002). Accordingly, it has been suggested that Hsp72 released from necrotic cells acts as a “danger” signal alerting the immune system to the presence of dying cells (Vabulas et al 2002).
Intriguingly, it has been demonstrated that under conditions of stress several cell types possess the capacity to release Hsp72 into the extracellular milieu, independently of cellular necrosis (Hightower and Guidon 1989; Guzhova et al 2001; Broquet et al 2003). Interestingly, detectable levels of Hsp72 are present in the systemic circulation of healthy individuals (Pockley et al 1998), and we have shown previously that the systemic concentration of Hsp72 is elevated after exercise (Walsh et al 2001). Furthermore, we have demonstrated that the hepatosplanchnic tissues—most probably the liver—are able to release Hsp72 (Febbraio et al 2002a) during exercise, whereas the contracting skeletal muscle does not appear to release Hsp72 during exercise (Febbraio et al 2002b). It is well documented that specific cells within the brain can synthesize Hsp72 in response to various stressors, eg, hyperthermia (Walters et al 1998; Leoni et al 2000), ischemia (Simon et al 1991), hypoxia (Murphy et al 1999), and energy depletion (Imuta et al 1998). In addition, it has been demonstrated that glial cells are able to actively release Hsp72 after stimulation (Guzhova et al 2001). Therefore, in the present study, we tested the hypothesis that the brain will release Hsp72 during exercise.
MATERIALS AND METHODS
Six healthy, endurance-trained males participated in the study (mean age 26 ± 2 years [±SEM], body mass 74 ± 3 kg, and maximal oxygen uptake [VO2max] 4.9 ± 0.2 L/ min). Subjects were informed as to the potential risks associated with participation in the study before obtaining their written informed consent to participate. The study was carried out in accordance with the Declaration of Helsinki and approved by the Ethical Committee of Copenhagen and Frederiksberg (KF 01-135/00).
Experimental protocol
Subjects arrived at the laboratory after an overnight (12 hours) fast and having consumed no items containing caffeine in the previous 24 hours. Catheters were then inserted into the bulb of the right internal jugular vein and the radial artery of the nondominant arm. An antecubital venous catheter for the infusion of 133Xe was inserted contralaterally to the arterial catheter. Subjects rested quietly for 1 hour and baseline measurements were obtained. Subjects then began a 180-minute bout of continuous exercise on a cycle ergometer (Monark 829E, Monark Exercise, Varberg, Sweden) at a work rate of 210 ± 13 W corresponding to 60% of the predetermined VO2max. During the exercise trial, subjects consumed 250 mL of a 6% carbohydrate (CHO)–containing solution every 15 minutes (providing 0.7 g of CHO/kg body mass/ h). The laboratory temperature during all trials was 21 ± 1°C.
Blood samples and calculations
Paired samples of arterial and cerebral venous blood were collected in heparinized syringes and kept on ice until analysis for hematocrit on ABL 700 apparatus (Radiometer, Copenhagen, Denmark). To determine the serum Hsp72 protein concentration, additional arterial and cerebral venous blood samples were obtained and placed in a tube containing a clot-inducing plug (Vacutainer Systems Europe, Meylan, Cedex, France). This tube was inverted 6 times, left on ice for 30 minutes, then spun in a centrifuge at 1200 × g at 4°C. A highly sensitive, enzyme-linked immunosorbent assay method (EKS-700 Stressgen, Victoria, BC, Canada) was used to determine the concentration of Hsp72 protein in serum as described previously (Walsh et al 2001). All samples were tested in duplicate, and the coefficient of variation between duplicate samples was <7%.
Cerebral blood flow (CBF) was measured in milliliters per minute per gram of cerebral tissue by the Kety-Schmidt technique in the desaturation mode using 133Xe as the radioactive tracer (Kety and Schmidt 1948; Madsen et al 1993), and global CBF was estimated assuming an average brain mass of 1400 g (Miller and Corsellis 1977; Dekaban 1978). Cerebral plasma flow was calculated on the basis of global CBF and the corresponding hematocrit value, and the cerebral balance of Hsp72 was calculated as cerebral plasma flow multiplied by the arteriovenous difference.
Statistics
A paired samples Student's t-test was used to compute the statistics using the SPSS computer software program. All data are expressed as means ± SEM.
RESULTS
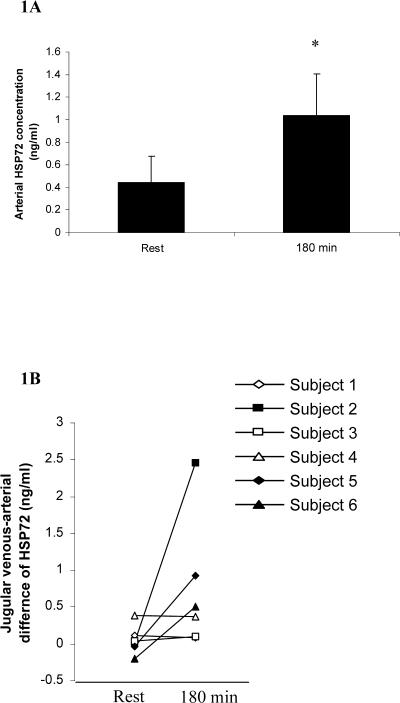
The arterial serum Hsp72 protein concentration was below the detection limit of the assay in 2 subjects at rest. After 180 minutes of exercise, the arterial serum Hsp72 concentration was elevated in 4 of the 6 subjects, averaging 1.00 ± 0.38 ng/mL (P > 0.05) (Fig 1A). The cerebral venous-arterial difference for Hsp72 was negligible in all subjects at rest, but after 180 minutes of exercise, an increase in the cerebral venous-arterial difference for Hsp72 was observed (Fig 1B); however, this increase did reach statistical significance compared with resting values.
Fig 1.
Arterial heat shock protein 72 (Hsp72) concentration (A) and internal jugular venous-arterial Hsp72 difference (B), before (rest) and after 180 minutes of exercise (180 minutes) at 60% maximal oxygen uptake. Data expressed as means ± SEM (n = 6). *P < 0.05 as determined by paired samples Student's t-test
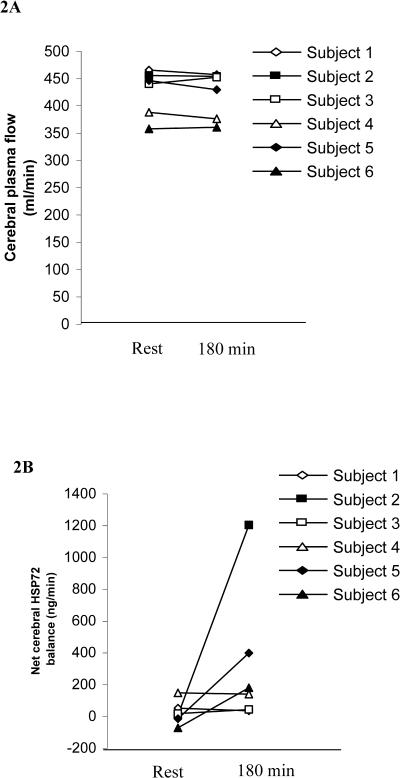
Cerebral plasma flow was unchanged by exercise compared with values obtained at rest (Fig 2A). Therefore, although cerebral Hsp72 release was negligible at rest, an increase in cerebral Hsp72 release after 180 minutes of exercise was observed (Fig 2B); however, as with the venous-arterial difference for Hsp72, this increase was not statistically significant. Importantly, when individual values are examined, although 3 of the 6 subjects show no change in cerebral Hsp72 release after 180 minutes of exercise, the remaining subjects show a large increase in cerebral Hsp72 release after exercise.
Fig 2.
Cerebral plasma flow (A) and net cerebral heat shock protein 72 release (B), before (rest) and after 180 minutes of exercise (180 minutes) at 60% maximal oxygen uptake. Data expressed as means ± SEM (n = 6)
The cerebral respiratory quotient was unchanged between rest and 180 minutes of exercise. In contrast, cerebral lactate release was greater (P < 0.05) after 180 minutes of exercise compared with rest (Table 1).
Table 1.
Cerebral lactate release and respiratory exchange ratio before (rest) and after 180 min of exercise (180 min) at 60% maximal oxygen uptakea
DISCUSSION
Although not a ubiquitous response, the results of this study demonstrate that the brain is capable of releasing large amounts of Hsp72 after prolonged exercise. Although several investigators have demonstrated the induction of Hsp72 synthesis in specific regions of the brain in response to hyperthermia (Walters et al 1998; Leoni et al 2000), ischemia (Simon et al 1991), hypoxia (Murphy et al 1999), and energy depletion (Imuta et al 1998), this is the first study, in any species, to demonstrate that the brain is capable of releasing Hsp72 in vivo. Given that the exercise was moderate in nature and that the increased cerebral Hsp72 release occurred in the absence of any changes in cerebral blood flow, it is unlikely that necrosis of specific cells within the brain contributed to the elevated cerebral Hsp72 release. Therefore, it is possible that specific cells within the brain possess an exocytotic pathway for the release of Hsp72 into the extracellular environment, allowing it to perform specific functions.
Although Hsps are quintessentially viewed as intracellular proteins with a vital role in maintaining cellular homeostasis, important extracellular roles for Hsps have been identified. In particular, accumulating evidence supports the hypothesis that Hsp72 released from necrotic cells into the extracellular environment may act as a “danger” signal alerting the immune system to the presence of dying cells (Asea et al 2000, 2002; Vabulas et al 2002). However, the results presented in this study, a previous study demonstrating that the hepatosplanchnic tissues release Hsp72 during exercise (Febbraio et al 2002a), and a recent study showing an elevation in the plasma concentration of Hsp72 in rats subjected to tail shock, suggest that Hsp72 release into the systemic circulation can occur independently of cell necrosis. Importantly, it has been demonstrated that cultured rat embryo cells (Hightower and Guidon 1989) and glial cells (Guzhova et al 2001) subjected to transient heat shock are able to release Hsp72 independently of cell necrosis in vitro; furthermore, a protein transport mechanism used by the cell to release Hsp72 has been identified recently (Broquet et al 2003). Taken together, the in vitro and in vivo evidence suggests that in addition to the well-established intracellular “housekeeping” functions of Hsp72 and the immunoregulatory role of Hsp72 released from necrotic cells, cells under stress are able to actively release Hsp72 into the extracellular milieu through a specific protein secretion pathway. Regarding the possible biological role of actively released Hsp72, it has been demonstrated that Hsp72 released from glia are taken up by neuronal cells which may enhance neuronal stress tolerance, and it has been hypothesized that an elevation in the extracellular Hsp72 concentration may facilitate recovery from bacterial inflammation (Campisi et al 2003). However, the elucidation of the biological role of Hsp72 released from the brain and hepatosplanchnic tissues during exercise awaits further investigation.
It is important to note that in the present study we observed an increase in brain Hsp72 release after exercise in only 3 of 6 subjects; however, the reasons for the subject specificity of this response are unclear. Nonetheless, and importantly, our data clearly demonstrate that the brain is capable of releasing Hsp72 in vivo in response to a stressor. It was not possible in the present study to address the nature of the stimulus by which exercise increases brain Hsp72 release. As discussed above, necrosis of specific cells within the brain is unlikely to have contributed to the elevated cerebral Hsp72 release. The depletion of energy stores, hypoxia, and ischemia have been shown to induce the synthesis of Hsp72 within specific cells of the brain (Simon et al 1991; Imuta et al 1998; Murphy et al 1999). However, we have previously published data from the present study (Nybo et al 2003) showing that cerebral lactate release and the cerebral respiratory exchange ratio are unchanged during exercise compared with during rest. This would suggest that neither energy depletion nor a relative hypoxia or ischemia within specific cells of the brain are likely to account for the augmented brain Hsp72 release after exercise. However, given that prolonged exercise causes an elevation in brain temperature (Nybo et al 2002) and that hyperthermia induces the synthesis of Hsp72 within the brain (Walters et al 1998; Leoni et al 2000), it is possible that an elevation in brain temperature may contribute to the increased release of Hsp72 from the brain after exercise.
In summary, we have demonstrated that the brain is capable of releasing Hsp72 in response to prolonged exercise; however, this response appears to be subject dependent because we observed an increase in brain Hsp72 release in only 3 of 6 subjects. Importantly, it seems very unlikely that the release of Hsp72 from the brain was due to cell necrosis because cerebral blood flow and oxygen consumption (Walters et al 1998) were unchanged during exercise compared with values obtained at rest. These data support previous findings (Dekaban 1978; Imuta et al 1998; Febbraio et al 2002a) and suggest that the brain possesses an exocytotic pathway for the release of Hsp72. Future studies will be required to determine the biological significance of this release.
Acknowledgments
We thank the subjects for participating in this demanding study, and we acknowledge the technical assistance of Winnie Taagerup. This study was supported by the Australian Research Council (DP0209570; M.A.F.), the Danish Medical Research Council (N.H.S.), and Danish Elite Sports Assocation, Team Danmark. M.A.F. is supported by a Senior Research Fellowship from The National Health and Medical Research Council of Australia.
REFERENCES
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697.1078-8956(2000)006<0435:HSCPTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200.0021-9258(2002)277<15028:NSTPUB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–21606. doi: 10.1074/jbc.M302326200.0021-9258(2003)278<21601:EOTMCH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Campisi JL, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2.1466-1268(2003)008<0272:SEHIAF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410.0364-5134(1978)004<0345:CIBWDT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002a;544:957–962. doi: 10.1113/jphysiol.2002.025148.0022-3751(2002)544<0957:EIHROH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Walsh R, Koukoulas I, van Hall G, Saltin B, Pedersen BK. Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol. 2002b;538:911–917. doi: 10.1113/jphysiol.2001.013145.0022-3751(2002)538<0911:RGAIAW>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3.0006-8993(2001)914<0066:IVSSTH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT Jr.. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206.0021-9541(1989)138<0257:SRFCMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Imuta N, Ogawa S, Maeda Y, Kuwabara K, Hori O, Ueda H, Yanagihara T, Tohyama M. Induction of 72-kDa inducible heat shock protein (HSP72) in cultured rat astrocytes after energy depletion. J Neurochem. 1998;70:550–557. doi: 10.1046/j.1471-4159.1998.70020550.x.0022-3042(1998)070<0550:IOKIHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Investig. 1948;27:476–483. doi: 10.1172/JCI101994.0021-9738(1948)027<0476:TNOMFT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni S, Brambilla D, Risuleo G, de Feo G, Scarsella G. Effect of different whole body hyperthermic sessions on the heat shock response in mice liver and brain. Mol Cell Biochem. 2000;204:41–47. doi: 10.1023/a:1007053504960.0300-8177(2000)204<0041:EODWBH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Madsen PL, Sperling BK, Warming T, Schmidt JF, Secher NH, Wildschiodtz G, Holm S, Lassen NA. Middle cerebral artery blood velocity and cerebral blood flow and O2 uptake during dynamic exercise. J Appl Physiol. 1993;74:245–250. doi: 10.1152/jappl.1993.74.1.245.8750-7587(1993)074<0245:MCABVA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Miller AK, Corsellis JA. Evidence for a secular increase in human brain weight during the past century. Ann Hum Biol. 1977;4:253–257. doi: 10.1080/03014467700007142.0301-4460(1977)004<0253:EFASII>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Song D, Welsh FA, Wilson DF, Pastuszko A. Regional expression of heat shock protein 72 mRNA following mild and severe hypoxia in neonatal piglet brain. Adv Exp Med Biol. 1999;471:155–163. doi: 10.1007/978-1-4615-4717-4_19.0065-2598(1999)471<0155:REOHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nybo L, Moller K, Pedersen BK, Nielsen B, Secher NH. Association between fatigue and failure to preserve cerebral energy turnover during prolonged exercise. Acta Physiol Scand. 2003;179:67–74. doi: 10.1046/j.1365-201X.2003.01175.x.0001-6772(2003)179<0067:ABFAFT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023.0022-3751(2002)545<0697:IHRFTH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Investig. 1998;27:367–377. doi: 10.3109/08820139809022710.0882-0139(1998)027<0367:DOHSPH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Simon RP, Cho H, Gwinn R, Lowenstein DH. The temporal profile of 72-kDa heat-shock protein expression following global ischemia. J Neurosci. 1991;11:881–889. doi: 10.1523/JNEUROSCI.11-03-00881.1991.0270-6474(1991)011<0881:TTPOKH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/ interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200.0021-9258(2002)277<15107:HAESOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2.1466-1268(2001)006<0386:EISHIH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters TJ, Ryan KL, Tehrany MR, Jones MB, Paulus LA, Mason PA. HSP70 expression in the CNS in response to exercise and heat stress in rats. J Appl Physiol. 1998;84:1269–1277. doi: 10.1152/jappl.1998.84.4.1269.8750-7587(1998)084<1269:HEITCI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009.0376-5067(2003)028<0541:MTFLFO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]