Abstract
Heat stress results in cardiac dysfunction and even cardiac failure. To elucidate the cellular and molecular mechanism of cardiomyocyte injury induced by heat stress, the changes of structure and function in cardiac mitochondria of heat-exposed Wistar rats and its role in cardiomyocyte injury were investigated. Heat stress induced apoptosis and necrosis of cardiomyocytes in a time- and dose-dependent fashion. In the mitochondria of heat-stressed cardiomyocytes, the respiratory control rate and oxidative phosphorylation efficiency (P:O) were decreased gradually with the rise of rectal temperature. The Ca2+-adenosine triphosphatase activity and Ca2+ content were also reduced. Exposing isolated mitochondria to the heat stress induced special internal environmental states including Ca2+ overload, oxidative stress, and altered mitochondrial membrane permeability transition (MPT). In vivo, the heat stress–induced mitochondrial MPT alteration was also found. The changes of mitochondrial MPT resulted in the release of cytochrome c from mitochondria into the cytosol, and in turn, caspase-3 was activated. Transfection of bcl-2 caused Bcl-2 overexpression in cardiomyocyte, which protected the mitochondria and reduced the heat stress–induced cardiomyocyte injury. In conclusion, it appears that the destruction of mitochondrial structure and function not only resulted in the impairment of physiological function of cardiomyocytes under heat stress but may also further lead to severe cellular injury and even cell death. These findings underline the contribution of mitochondria to the injury process in cardiomyocytes under heat stress.
INTRODUCTION
Heat stress causes serious physiological dysfunction that may result in heat-related diseases, including heat stroke, heat cramp, heat exhaustion, and even death. The cardiovascular system has been considered the primary target of heat stress (Zhang et al 1989; Steenland 1996; Chien 2000). Generally, cardiac dysfunction and failure is the main cause of heat-related death. For many years, the pathological mechanism of the heat stress–induced cardiac dysfunction has been explored. Although several reports (Zhang et al 1989; Qian et al 1999; Song et al 2000) have shown that the heat stress–induced dysfunction of cardiovascular system was accompanied with severe cardiomyocyte injury, the pathway of heat stress–induced injury of cardiomyocyte remains unclear.
Necrosis and apoptosis are 2 forms of cell death. Necrosis is caused by acute and severe pathological factors and acts as the pathological cellular basis of many diseases. Apoptosis normally counterbalances the effect of cell proliferation by mitotic division (Steller 1995; Jacobson et al 1997), but apoptosis also happens under a variety experimental and clinical conditions. Excessive apoptosis causes organ dysfunction, atrophy, or failure that result in many human diseases (Thompson 1995). In fact, deregulated apoptosis has been implicated as an important cellular pathogenetic mechanism. Compelling evidence has accumulated indicating that apoptotic cell death may also play a critical role in cardiovascular diseases, such as myocardial infarction, cardiac dysfunction, heart failure, and atherosclerosis (Edward 1997; Chien 2000). Apoptosis can be triggered by many signals through either the mitochondrial pathway (Green and Reed 1998; Mignotte and Vayssiere 1998) or the nonmitochondrial Fas pathway (Nagata 1997; Li et al 1998). Although activation of the caspase cascade is the final step of cardiomyocyte apoptosis, there are questions remaining, such as what is the trigger and how does it induce the activation of the caspase cascade in cardiomyocytes undergoing heat stress.
The mitochondrion is an important cellular organelle. Under physiological conditions, mitochondria play a key role in bioenergy production and maintenance of intercellular calcium homeostasis. Although it has long been known that injury of mitochondria causes cellular dysfunction and even necrosis, only recently has it been shown that mitochondria are critical in mediating apoptotic signal transduction (Green and Reed 1998; Brenner and Kroemer 2000). Stimuli such as reactive oxygen radicals, intracellular calcium overload, and many cytokines elicit structural and functional changes of mitochondria including disruption of electron transport, intracellular homeostasis, and the opening of mitochondrial membrane permeability transition pore (MPTP). These changes result in the release of proteins located in the mitochondrial inner membrane space and trigger the activation of the caspase family proteases and alteration of cellular reduction-oxidation potential (Mignotte and Vayssiere 1998; Thornberry and Lazebnik 1998). In addition, Bcl-2 also exerts its antiapoptotic effect through the mitochondrial membrane (Kluck et al 1997; Yang et al 1997; Adams and Corry 1998). With the key events regulating apoptotic cell death in mitochondria, mitochondria appear to be a checkpoint in regulating cell life. In a previous study, we have found that cardiomyocyte mitochondrial damage induced by heat stress is related to the cardiac dysfunction (Qian et al 1992, 1999). However, there is no direct evidence about whether the changes in mitochondria induce cardiomyocyte apoptosis. The present study characterizes the effect of heat stress on the mitochondrial structure and function in cardiomyocytes and investigates the role of the mitochondrial changes in the injury process of cardiomyocytes undergoing heat stress. Our findings show that the mitochondria are a crucial trigger for activation of the cell-death pathway in heat-stressed cardiomyocytes.
MATERIALS AND METHODS
Heat-stressed animal model
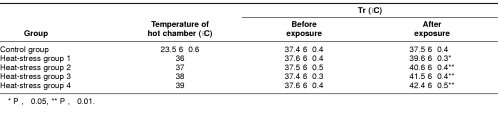
Adult male Wistar rats weighing 200–250 g were randomly divided into 5 groups (4 different degree heat-exposed groups and 1 control group). The heat-exposed rats were put in a hot chamber at various designed temperatures: 36°C, 37°C, 38°C, and 39°C with relative humidity of 75– 85% for about 1.5 hours until their rectal temperature (Tr) reached above 39°C, 40°C, 41°C, and 42°C, respectively (Table 1). The heat exposure was done in the morning from 0700 to 0830 hours. All rats were housed in a pathogen-free environment at room temperature (22–25°C) and maintained on food and water ad libitum before heat exposure.
Table 1.
Changes of the rectal temperature (Tr) of rats before and after heat exposure
Heat-stressed cardiomyocyte model
Cardiomyocytes were isolated from 3-day-old neonatal Wistar rats by a digestion method using trypsin as described previously (Song et al 2000). After animals were killed, the hearts were aseptically removed and minced in the trypsin digestion solution (0.1% trypsin, 0.002% deoxyribonuclease, 0.02% ethylenediaminetetraacetic acid [EDTA] and 0.1%) at 37°C for 10 minutes. The digestion solution of heart tissue was gently tapped through a sterile nylon cell strainer. Debris was allowed to sediment for 3 to 5 minutes at room temperature before transferring the cell suspension and collecting cells by centrifugation at 200 × g for 10 minutes. The cells were cultured in modified Eagle medium (MEM) supplemented with 20% fetal bovine serum, 0.01 mmol/L BrdU, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% CO2-95% air in a humidified incubator. After being cultured for 5 days, the culture bottle of ∼95% confluent cardiomyocytes, 90% of which beat about 140 times/min were selected for experiments. The cells in culture bottles were incubated in a water bath at 37°C, 39°C, 41°C, or 43°C for 40 minutes, to establish the heat-stressed cell model.
Parameters and methods for assay
Rectal temperature
Tr was measured using the Digit Thermometer (TH-1, Institute of Health and Environmental Medicine, Tianjin, China). The Tr values were recorded immediately before and after heat exposure.
Mitochondria function
For isolation and preparation of mitochondria, after heat exposure, the animals were decapitated immediately. Control animals were similarly killed. The hearts were excised, and the left ventricles were separated on an ice dish. A half of ventricular walls was selected for isolating mitochondria, another half of which was used for analyzing adenosine triphosphate (ATP) content in myocardium (shown in the next paragraph beginning “Mitochondrial oxidative phosphorylation”). For isolation of mitochondria, the myocardium was minced into 2 mm3 and washed with 37°C physiological saline (0.9% NaCl) 3 times. The homogenate of myocardium was prepared in ice-cold medium (1:8 wt/vol) containing 250 mM sucrose, 50 mM Tris, and 1 mM EDTA, pH 7.4. The mitochondria were purified by gradient centrifugation (3000 × g for 10 minutes, 9000 × g for 30 minutes). The mitochondria were suspended in sucrose buffer (250 mM sucrose, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid [HEPES], 1 mM EDTA, 1 mM dithiothreitol [DTT], pH 7.4) for up to 2 hours at 4°C.
Mitochondrial oxidative phosphorylation was analyzed with a Clark-type oxygen-electrode (polarography) in a stirred reaction vessel controlled thermostatically at 37°C (Estabrook 1967). An aliquot of the isolated mitochondria was added to medium consisting of 130 mM KCl, 20 mM K2HPO4, pH 7.2, and the following substrates in combination with malate (1 mM): glutamate, pyruvate, acetate (all at 10 mM), or palmitoyl-l-carnitine (5 mM plus bovine serum albumin [BSA], 5:1). A slow rate of oxygen uptake was measured in the presence of substrate and absence of adenosine 5′-diphosphate (ADP). The addition of ADP causes an immediate increase in the rate of oxygen use. The duration of the increased rate of oxygen uptake is dependent on the concentration. There is a proportional relationship between the concentration of oxygen used and the amount of ADP phosphorylated to ATP. The P:O ratio as index of oxidative phosphorylation efficiency was calculated from oxygen-electrode tracings of the microatoms of oxygen used when 250 nM ADP was added. The P:O ratio was calculated as micromoles ADP added/microatoms oxygen used. The respiratory control rate (RCR) was calculated as the ratio of the respiratory rate in the presence of added ADP to the rate obtained following ADP expenditure. At the same time, ATP content in myocardium was measured by fluorescence bioluminescence according to Wattanasirichaigoon et al (1999).
Synthesis activity of mitochondrial H+-adenosine triphosphatase (ATPase) was measured by fluorescence bioluminescence (Zhu et al 1995). Mitochondria were isolated from cardiomyocyte as described above and adjusted to a protein concentration of 1 mg/mL. Ten-microliter samples of mitochondria were mixed with 100 μL luciferin-luciferase solution (containing 200 μg luciferin-luciferase). Adding ADP triggered the ATP synthesis reaction of the mitochondria, and the intensity of emitted light was detected with a luminosity spectrophotometer (FT 632, Beijing medicine instrument factory, Beijing, China). Synthesis activity of mitochondrial H+-ATPase was calculated according to the intensity of emitted light of determined content of ATP in the parallel standard test and described as micromole per minute milligram protein.
Mitochondrial calcium metabolism
Intracellular Ca2+ concentration ([Ca2+]I) was measured by the method of MacCormack et al (1989) with slight modifications. Briefly, the cardiomyocytes were isolated from heat-stressed adult rat heart with collagenase according to Isenberg and Klockner (1982). In the isolated cells, there was approximately 93% cardiomyocytes that were about 100 μm long and 20 μm wide with visible cross-striations and clear cellular edge. The cardiomyocytes were selected and washed twice with phosphate-buffered saline (PBS) and loaded with fura-2 AM (5 μM), 0.04% pluronic F127 (Molecular Probes Inc.) in the buffer (NaCl 118 mM, KCl 4.8 mM, MgSO4 1 mM, NaHCO3 2.4 mM, glucose 11 mM, CaCl2 1.8 mM, pH 7.4) and left at room temperature for 20 minutes. A total of 2 × 105 cells were transferred into cuvette suitable for fluorescence. The cuvette was maintained at 37°C and stirred continuously. The fluorescence of the emitted light was detected in response to excitation at 340 and 380 nm with a spectrophotometer (HITACHI, Japan, F-4500).
Ca2+-ATPase activity was measured with a bioluminescent method, according to a modified Steiner method (Steiner et al 1988). Briefly, 10 μg mitochondrial protein was added in 50 μL reaction medium (20 mM imidazole-HCl [pH 7.0], 100 mM KCl, 5 mM CaCl2, 4 mM ATP) to trigger the enzyme reaction. After incubation in 37°C waterbath for 30 minutes, the reaction was terminated at 100°C for 90 seconds. Two milliliters of luminescence buffer (6 mg luciferin-luciferase in 0.1 M Tris-HCl, pH 7.8) was added to each sample solution. Ten microliters of test sample solution was mixed with 0.2 mL monitoring buffer (0.15 M glycylglycine, 1 mM EDTA, 10 mM MgSO2, pH 7.8). Bioluminescence was recorded with a Luminometer (FT-632, Beijing Bio-instrument Plant, Beijing, China) using ATP (Sigma Chemical Co., St. Louis, MO) as standard.
Calcium content in mitochondria was measured by inductively coupled plasma atomic emission spectrometry (Polarized Zeeman Atomic Absorption Spectrophotometer, Perkin Elmer, Plasma II). Mitochondrial samples containing 5 mg of protein in HNO3 were analyzed for calcium content by the method of Zydowo et al (1985).
Mitochondrial MPT
The changes of mitochondrial MPT were measured by following methods of Petit et al (1998) and Sarah et al (2000).
For detection of mitochondrial swelling, mitochondria were resuspended in the buffer (400 mM mannitol, 10 mM KH2PO4, 5 mg/mL BSA, and 50 mM Tris-HCl, pH 7.2, 4°C) at a concentration of 100 μg protein/10 μL for 30 minutes. For determination of mitochondrial MPT, 1 mg mitochondrial protein was suspended in test buffer (200 mM sucrose, 10 mM Tris-3-(N-morpholino) propane sulphonic acid MOPS (pH 7.4), 5 mM succinate-Tris, 1 mM Tris-phosphate (Pi), 10 μM ethyleneglycol-bis[aminoethylether]-tetraacetic acid [EGTA]–Tris, 2 μM rotenone, 1 μg/mL oligomycin), and the changes of absorbance at 540 nm were monitored before and after addition of 150 μM CaCl2 using spectrophotometer (HITACHI, U-2001 UV/Vis spectrophotometer).
For measuring the change of mitochondrial membrane potential, mitochondria were isolated in a medium composed of 250 mM sucrose, 10 mM Tris-HCl, 5 mM EGTA, pH 7.4. The test solution contained 0.2 M sucrose, 10 mM Tris-MOPS (pH 7.4), 5 mM succinate-Tris, 1 mM Pi-Tris, 10 μM EGTA-Tris, 2 μM rotenone, and 1 μg/mL oligomycin, with a final volume of 2 mL. The analysis was started by the addition of 1 mg mitochondria with 0.2 μM Rhodamine 123 (Rh123). Rh123 is a membrane-permeable cationic fluorophore that accumulates electrophoretically into mitochondria in response to their negative potential. After incubation at 25°C for 5 minutes, the fluorescence intensity of the reaction medium was determined using a F-4500 fluorescence spectrophotometer. To assess the MPTP opening state, 150 μM CaCl2 was added after 1 minute, and then the change of fluorescence intensity in 15 minutes was recorded. The change of membrane potential after Ca2+ addition reflected the original state of the mitochondrial MPT.
Cardiomyocyte injury
Gel electrophoresis of fragmented deoxyribonucleic acid (DNA) was performed. Briefly, cardiomyocytes of heat-stressed rats were lysed in 500 μL of 10 mM Tris, 1 mM EDTA, 0.2% Triton X-100. After centrifugation at 13 000 × g, the precipitates were collected, air dried, resuspended in 10 mM Tris, 1 mM EDTA (pH 7.4), and treated with ribonuclease (RNAse) at 5 μg/mL. Loading buffer containing 15 mM EDTA, 2% sodium dodecyl sulfate (SDS), 50% glycerol, and 0.5% bromophenol blue was added to samples at 1:5 (vol:vol). The samples were heated to 65°C for 10 minutes. Electrophoresis was performed in 0.75% agarose for 2 hours at 90 V. Oligonucleosomal fragments of DNA were observed by staining with ethidium bromide.
Ultrastructural analysis of mitochondria and cardiomyocyte were performed by electron microscopy. The myocardium from heat-stressed rats was fixed in 2.5% glutaraldehyde, 4% osmic acid, and 1% tannic acid. Then, the samples were dehydrated in a series of alcohol solutions and embedded in Epon. Sections were stained with uranyl acetate and lead citrate before examination with the JEM 1200 EXIT electron microscope.
Apoptosis rate was detected with flow cytometry. Apoptotic nuclei appeared as a broad hypodiploid DNA peak that was easily distinguished from the narrow peak of nuclei with normal (diploid) DNA content in the red fluorescence channel. Quantification of apoptotic nuclei was assessed by staining apoptotic nuclei according to Holash et al (1999). Briefly, the plates of ∼90% confluent cardiomyocytes from neonatal rats were washed twice with PBS. The harvested cells were centrifuged at 95 × g for 5 minutes and resuspended in 0.3 mL PBS containing 5% fetal bovine serum. After fixation with 70% ethanol at −20°C overnight, the cells were washed with PBS and incubated with 20 μL RNase A (Sigma) and propidium iodide (PI) for 30 minutes. The PI fluorescence was determined by flow cytometry (America BD Co. FACS Caliber).
Necrosis rate was assessed according to the loss of trypan blue exclusion. Just-confluent cardiomyocytes from neonatal rats in 100-mm tissue culture plates were washed with 5 mL Hanks balanced salts (HBS) and 0.5 mL of 2.5% trypan blue solution was added. The cells were incubated for 5 minutes at 37°C and then were washed twice with 10 mL PBS. If necrosis happened, cells would be stained by trypan blue. Using microscopy, the necrosis rate of cells was examined. The percentages of dead cells were estimated after counting 100 cells for 50 times per sample.
bcl-2 Gene transfection to cardiomyocyte
Plasmid PCI-neo and full sequence bcl-2 complementary DNA (cDNA) were cleaved with EcoRI. Expression vector of PCI-neo-bcl-2 was constructed by ligation of bcl-2 into PCI-neo. Cardiomyocytes were seeded into 12-well tissue culture plates and grown in MEM containing 10% fetal calf serum at 37°C. A total of 2.5 μL plasmid were mixed gently with 1 μL lipofectamine (Sigma) and incubated for 30 minutes at room temperature. Cardiomyocytes were washed 3 times with serum-free MEM, then incubated with plasmid-lipofectamine mixture diluted with 400 μL serum-free MEM for 12 hours at 37°C, and 5% CO2 culture medium was replaced with 1 mL MEM containing 20% fetal calf serum and further incubated for 48 minutes.
Transfection efficiency was analyzed with an immunocytochemical technique. Briefly, the transfected cells were washed twice with PBS and fixed by acetone for 2 minutes. The fixed cells were incubated with rabbit bcl-2 antibody (1:200) at 4°C overnight. After washing, the cells were successively incubated with biotin-labeled goat anti-rabbit immunoglobulin G for 1 hour at room temperature and with Avidin-horseradish peroxidase (HRP) for 45 minutes at room temperature. The staining reaction was achieved by incubation with a solution containing 500 mg diaminobenzoate (DAB), 0.3% H2O2 for 5 minutes. Transfection efficiency of cardiomyocytes was evaluated by microscopy.
Release of cytochrome c from mitochondria
The analysis was performed mainly according to Kluck et al (1997). Cardiomyocytes were harvested by centrifugation at 1000 × g for 10 minutes at 4°C. The cell pellets were washed with ice-cold PBS and resuspended with 5 volumes of lysis buffer (0.25 mol/L sucrose, 20 mmol/L HEPES-KOH [pH 7.5], 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 0.1 mmol/L phenylmethylsulfonyl fluoride). The cells were homogenized and then centrifuged at 750 × g for 10 minutes at 4°C. The supernatants were centrifuged at 10 000 × g for 15 minutes at 4°C, and the resulting mitochondria pellets were resuspended in 1× SDS. The supernatants of the 1000 × g spin were further centrifuged at 100 000 × g for 1 hour at 4°C, and the resulting supernatants were the S-100 fraction. Proteins in the supernatant (15 μL) of mitochondria preparation and from the S-100 fraction were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) (10–15%). Immunoblots for the determination of cytochrome c (cyto c) release were performed using a specific cyto c monoclonal antibody (Pharmingen, San Diego, CA, USA).
Caspase-3 protease activity was measured using fluorometry. Cells were collected and washed twice with ice-cold PBS and then resuspended in 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, and 10 mM EGTA containing 50 mM digitonin. After the cells were incubated at 37°C for 10 minutes, the lysates were centrifuged at 15 000 rpm for 3 minutes, and the supernatants were collected. Lysate protein (10 mg) was incubated with 5 mM acetyl-Asp-Glu-Val-Asp-4-methylcoumary1-7-amide (Ac-DEVD-MCA) at 37°C for 5 minutes. The release of 7-amino-4-metrylcouumarin (AMC) was measured by a spectrofluorometer (Hitachi F-2000) using an excitation wavelength of 380 nm and an emission wavelength of 460 nm. One unit was defined as the amount of enzyme required to release 5.2 pmol AMC/min/mg lysate protein at 37°C.
Protein content
Protein content in myocardium and mitochondria were determined by Folin-phenol method with BSA as a standard substance.
Statistical analysis
The double-tail Student's t-test (Nycomed, As, Oslo, Norway) was used to analyze the significance of the difference between control and experimental groups. Differences were considered significant when P ≤ 0.05.
RESULTS
Injury of cardiomyocytes exposed to the heat stress
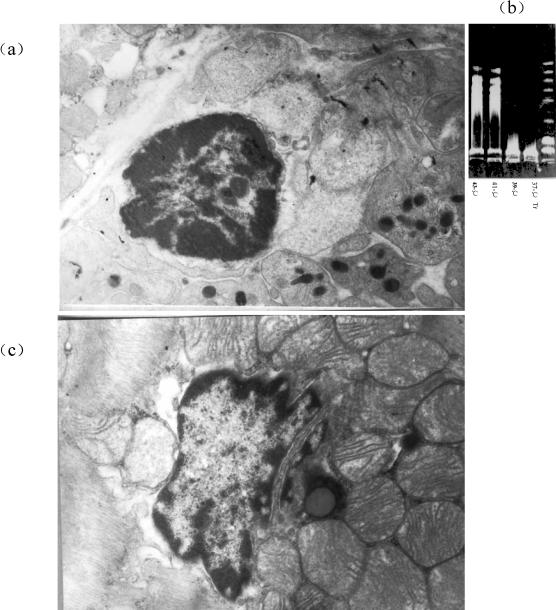
The ultrastructural analysis of myocardium in heat-stressed rats is shown in Figure 1. When the rats were exposed to heat above 39°C, the characteristic condensation of nuclear chromatin could be found in the cardiomyocytes. It is worth noting that alterations of the mitochondria, including reduction of the number of cristae membranes, disruption of the surrounding membranes, and swelling of the organelles, appeared in these cardiomyocytes. The electrophoretic analysis of cardiomyocyte DNA showed the typical DNA ladder of apoptosis. Different from the observation by electron microscopy, the DNA fragmentation did not occur in the rats of 39°C Tr, but only in the heat stress groups of Tr 41°C and 42°C.
Fig 1.
Cardiomyocyte apoptosis in heat-stressed rats. (A) Electron micrographs of cardiomyocyte apoptosis induced by heat stress. The rats were exposed to heat, and the myocardium was selected and examined by electron microscopy (JEM 1200 EXIT). (a) The characteristic apoptotic condensation and surrounding distribution of nuclear chromatin in the cardiomyocyte of heat-stressed rats. (b) The alterations of the mitochondria in cardiomyocytes of heat-stressed rats including reduction of the number of cristae membranes, disruption of the surrounding membranes, and swelling of the organelles. (B). Agarose gel electrophoresis analysis of deoxyribonucleic acid fragmentation from cardiomyocytes of heat-stressed rats
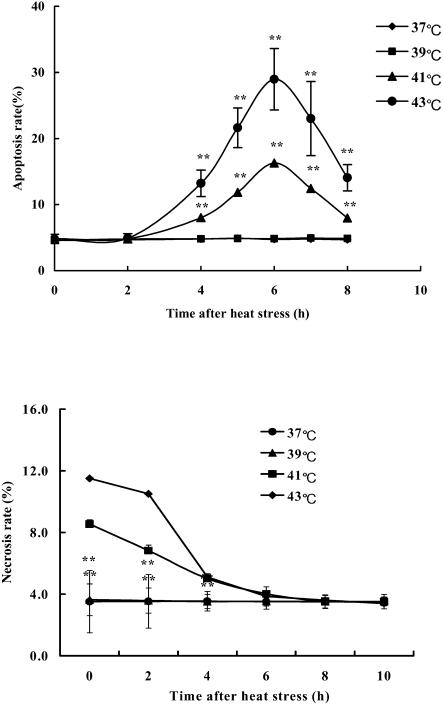
Apoptosis and necrosis often coexist in a pathological process. In vitro, apoptosis rate and necrosis rate in the cardiomyocytes under different loading of heat stress were measured at various times after heat stress by PI staining with flow cytometry and trypan blue staining, respectively. Heat stress resulted in apoptosis and necrosis of cardiomyocytes in a dose- and time-dependent fashion (Fig 2). When cardiomyocytes were exposed to 39°C heat, there was almost no difference of either apoptosis rate or necrosis rate between heat-stressed cells and control cells (37°C group). When the temperature of the heat exposure increased to 41°C, the occurrence of apoptosis and necrosis in heat-stressed cardiomyocytes increased markedly compared with that in the control group. It is also interesting that after heat stress, the necrosis rate reached its peak value immediately, but the apoptosis rate reached its highest level at 6 hours after heat stress. At 6 hours, the apoptosis rate in 43°C heat-stressed cardiomyocytes came up to 30%, an increase of about 3.5 times when compared with control cells.
Fig 2.
Time course of apoptosis and necrosis of cardiomyocytes under heat stress. Cardiomyocytes were isolated from 3-day-old neonatal Wistar rats and exposed to 37°C, 39°C, 41°C, or 43°C heat for 40 minutes. The apoptosis rate was detected with flow cytometry, and necrosis rate was assessed with trypan blue staining method at different times after heat stress. Each point represents the mean ± SEM of 6 separate experiments, *P < 0.05, **P < 0.01 vs 37°C group
Changes of bioenergy metabolism in mitochondria of heat-stressed cardiomyocytes
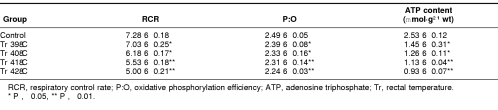
The influence of different heat loading on the mitochondrial oxidative phosphorylation was investigated. The changes of the respiratory control rate (RCR) and P:O in myocardial mitochondria of heat-stressed rats are shown in Table 2. In the Tr 39°C group, the RCR increased significantly. Above 40°C, RCR remarkably decreased by 13.5% as compared with control and continually decreased as Tr increased further. The obvious decrease of P:O started when Tr reached 39°C, and as Tr reached above 42°C, P:O decreased by 11.5% of that in the control group. The results indicated that the oxidative phosphorylation of cardiac mitochondria was impaired. Along with the Tr increase and the decrease of oxidative phosphorylation, ATP content in the myocardium was reduced significantly.
Table 2.
Effect of heat stress on the respiratory function of mitochondria in cardiomyocytes of heat-stressed rats
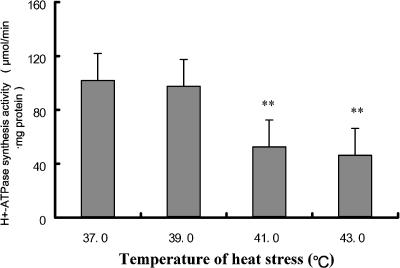
In vitro, the alteration of mitochondrial bioenergy function in heat-exposed cardiomyocytes was determined by analysis of the mitochondrial H+-ATPase synthesis activity. H+-ATPase synthesis activity is a comprehensive evaluation of the mitochondrial structure and function. A comparative test revealed that heat stress resulted in the significant decrease of mitochondrial H+-ATPase synthesis activity. As shown in Figure 3, the H+-ATPase synthesis activity in 42°C heat-stressed cardiomyocytes decreased to 45.2% of that in non–heat stressed cells. The results confirmed that there was heat stress–induced injury of mitochondrial membrane integrity in cardiomyocytes, which in turn caused the dysfunction of H+-ATPase synthesis activity.
Fig 3.
Effect of heat stress H+-ATPase synthesis activity of mitochondria in heat-stressed cardiomyocytes. Cardiomyocytes were isolated from 3-day-old neonatal Wistar rats and exposed to 37°C, 39°C, 41°C, or 43°C heat for 40 minutes. Mitochondria were isolated from the cardiomyocyte, and mitochondrial H+-ATPase synthesis activity was measured by fluorescence bioluminescence. The values are the mean ± SEM, n = 8, *P < 0.05, **P < 0.01 vs 37°C group
Changes of calcium metabolism of mitochondria in heat-stressed cardiomyocytes
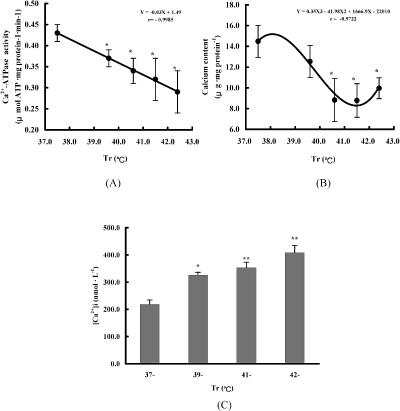
Mitochondria play an important role in sustaining intracellular calcium homeostasis by regulating calcium transport at the mitochondrial membrane. The changes of the Ca2+-ATPase activity and Ca2+ content in mitochondria of cardiomyocytes from heat-stressed rats are shown in Figure 4. Both the Ca2+-ATPase activity and Ca2+ content were reduced as Tr increased and were significantly correlated with each other (Y = 3.21 + 40.35X, r = 0.885, P < 0.05), suggesting that calcium homeostasis in myocardial cells was disrupted. The observation for [Ca2+]I in cardiomyocytes of heat-stressed rats suggests that there was a calcium overload in cardiomyocytes.
Fig 4.
Changes of Ca2+ metabolism in cardiomyocytes of heat-stressed rats. The rats were exposed to heat of different temperatures. The cardiomyocytes and the mitochondria were isolated from myocardium immediately after heat stress. Ca2+-ATPase activity of mitochondria was determined using bioluminescent method, Ca2+ content in mitochondria was assessed using inductively coupled plasma atomic emission spectrometry (ICP) and intracellular Ca2+ concentration of cardiomyocyte was measured by Fura-2 fluorescence method. The values given are the mean ± SEM of 6 separate experiments, *P < 0.05, **P < 0.01 vs 37°C group. (A) Ca2+-ATPase activity of mitochondria in cardiomyocytes of heat-stressed rats. (B) Ca2+ content in mitochondria in cardiomyocytes of heat-stressed rats. (C) Intracellular Ca2+ concentration in cardiomyocyte of heat-stressed rats
Changes of mitochondrial MPT in heat-stressed cardiomyocytes
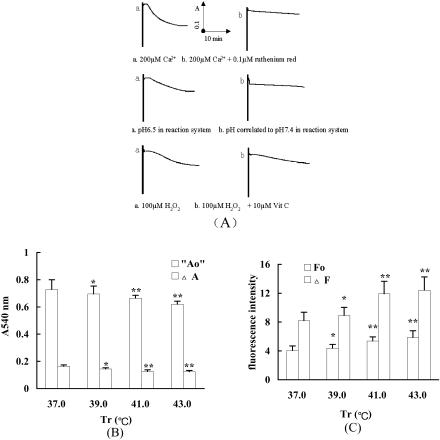
The disorder of oxidative phosphorylation and calcium metabolism would result in disturbance of the intracellular homeostasis such as calcium overload, oxidative stress, and acidosis, which has been identified in our previous and present studies. Isolated myocardial mitochondria were exposed to Ca2+ concentrations up to 200 μmol/L, to 100 μmol/L H2O2, or acidifying mitochondrial surroundings to pH 6.5, mitochondrial MPT alternated evidently. The influence of calcium overload on MPT was the most significant. If ruthenium red, a Ca2+ channel inhibitor, or superoxide dismutase (SOD) was given, or acid-base imbalance in reactive system was corrected, the alteration of mitochondrial MPT was attenuated or disappeared (Fig 5A). The results suggested that heat-induced cell stress may result in injury of mitochondrial MPT. To confirm these findings, we observed the alteration of the light absorption of mitochondria at 540 nm and mitochondrial membrane potential in cardiomyocytes from heat-stressed rats. As shown in Figure 5B, the Ca2+-induced A540-nm increment of mitochondria was reduced significantly as compared with that of non–heat stressed rats. The mitochondrial membrane potential also decreases in a dose-dependent manner with various heat loadings (Fig 5C). Mitochondrial MPT is an important structure for maintaining mitochondrial homeostasis and membrane potential. Although there is now no direct method to detect the opening of the mitochondrial MPTP, the swelling of mitochondria and the decrease of mitochondrial membrane potential undoubtedly suggests the abnormal opening of the mitochondrial MPTP.
Fig 5.
Influence of heat stress on the mitochondrial membrane permeability transition (MPT) in cardiomyocyte. (A) The changes of mitochondrial MPT of cardiomyocyte under the special environmental conditioning induced by heat stress. Isolated mitochondrial MPT was measured with spectrophotometer after exposure to Ca2+ concentration up to 200 μmol/L, 100 μmol/L H2O2, or to acidifying mitochondrial surroundings (pH 6.5) and given ruthenium red, SOD, or correcting acid-base imbalance in reactive system (n = 3). (B) The changes of A540 nm of mitochondria in cardiomyocytes from heat-stressed rats. The swelling of mitochondrial suspension after permeability transition pore opening was assayed by measuring the change of turbidity absorbance at 540 nm before and after 150 nm Ca2+. The values are the mean ± SEM, n = 6, *P < 0.05, **P < 0.01 vs 37°C group. (C) The influence of heat stress on opening of mitochondrial MPT pore in cardiomyocytes of heat-stressed rats. The analysis was started by the addition of 1 mg mitochondria with 0.2 μM Rhodamine 123. After incubation at 25°C for 5 minutes, the fluorescence intensity of the reaction medium was determined immediately using fluorescence spectrophotometer. CaCl2 (150 μM) was added after 1 minute, and the change of fluorescence intensity in 15 minutes was recorded. The change of membrane potential after Ca2+ addition reflected the MPT original state. The values are the mean ± SEM, n = 6, *P < 0.05, **P < 0.01 vs 37°C group
Activation of caspase cascade in heat-stressed cardiomyocytes
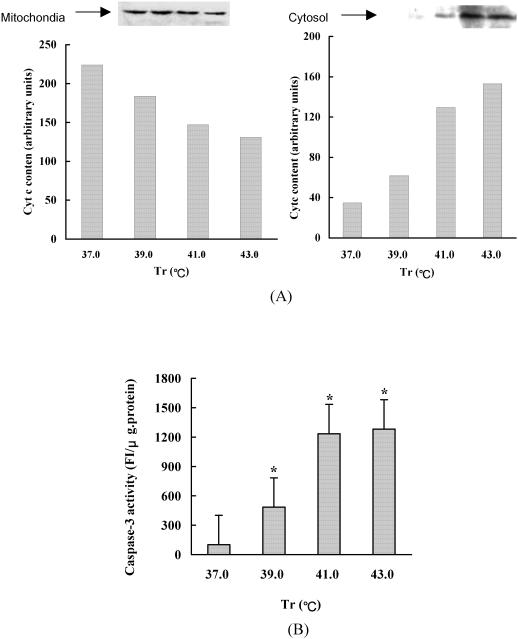
Activation of caspase-3 is mainly dependent on the release of cyto c from the mitochondria and the formation of the “apoptosome.” The changes of intracellular location of cyto c and caspase-3 activity in heat-stressed cardiomyocytes are shown in Figure 6. Heat stress induced the release of cyto c from mitochondria and in turn increased caspase-3 activity up to 13.26 times in 43°C heat-stressed cardiomyocytes compared with that of control cells.
Fig 6.
The effect of heat stress on the cytochrome c (cyto c) release from mitochondria and caspase-3 activity in cardiomyocytes. (A) The changes of cyto c distribution in cardiomyocytes of heat-stressed rats. Proteins in the supernatant (15 μL) of mitochondria preparation and from the S-100 fraction of cardiomyocytes were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10–15%). Immunoblots for the determination of cyto c release were performed using a specific cyto c monoclonal antibody, n = 4. (B) The effect of heat stress on the caspase-3 activity in cardiomyocytes. The 10 mg lysate protein of cardiomyocytes was incubated with 5 mM Ac-DEVD-MCA at 37°C for 5 minutes. The release of 7-amino-4-metrylcouumarin was measured by a spectrofluorometer (Hitachi F-2000) using an excitation wavelength of 380 nm and an emission wavelength of 460 nm. The values are the mean ± SEM, n = 8, *P < 0.05, **P < 0.01 vs 37°C group
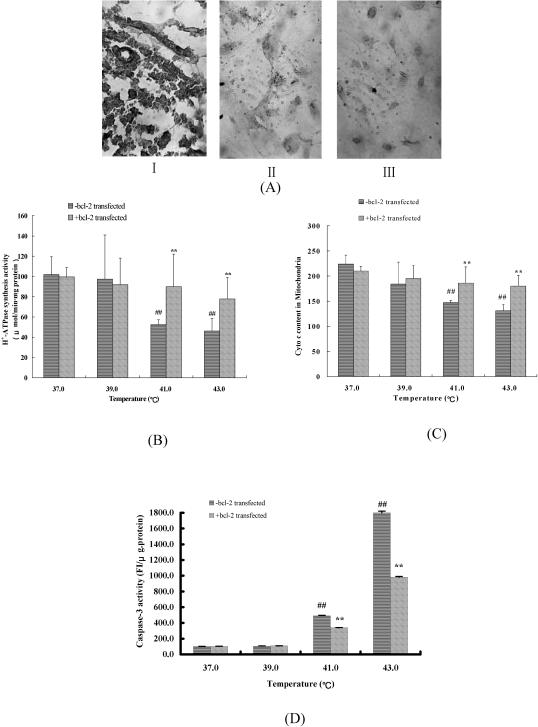
Influence of bcl-2 transfection on the heat-stressed cardiomyocytes
Bcl-2 prevents cells from undergoing apoptosis induced by various stimuli in a wide variety of cell types. Bcl-2 has an inhibitory effect on the mitochondrial MPTP (Kluck et al 1997; Yang et al 1997). To explore the role of mitochondria in initiating the heat stress–induced cell death pathway, Bcl-2 was used as a tool to block opening of the mitochondrial MPTP. The bcl-2 was transfected into cardiomyocytes and overexpressed (Fig 7A). The cells transfected with the plasmid PCI-neo without bcl-2 as control and the cells transfected with plasmid PCI-neo containing bcl-2 cDNA were exposed to heat of 39°C, 41°C, or 43°C. The effect of Bcl-2 overexpression on mitochondria in heat-stressed cardiomyocytes is presented in Figure 7B,C. Bcl-2 overexpression had no effect on the routine cultured cells or on 39°C heat-stressed cardiomyocytes, while 41°C or 43°C heat stress was given, H+-ATPase synthesis activity of mitochondria in Bcl-2 overexpressing–cardiomyocytes was raised 71.42% and 68.52%, respectively, compared with that of non-bcl-2– transfected cells. Similarly, the heat stress–induced release of cyto c from mitochondria to cytosol reduced markedly in bcl-2–transfected cells, compared with non-bcl-2–transfected cells. Moreover, Bcl-2 overexpression suppressed the caspase-3 protease activity in heat-stressed cardiomyocytes (Fig 7D).
Fig 7.
The protective effects of bcl-2 transfection on the mitochondria of cardiomyocytes under heat stress. (A) Immunohistochemical expression of the cardiomyocytes transfected with the plasmid PCI-neo without bcl-2 (-bcl-2 transfected cardiomyocytes) and the cardiomyocytes transfected with plasmid PCI-neo containing bcl-2 complementary deoxyribonucleic acid (cDNA) (bcl-2 transfected cardiomyocytes) (200×). I. Lymphatic tissue as positive control. II. The cardiomyocytes transfected with plasmid PCI-neo containing bcl-2 cDNA. III. The cardiomyocytes transfected with the plasmid PCI-neo without bcl-2. Cells expressing Bcl-2 show a yellow immunostaining. (B) Effect of bcl-2 transfection on H+-adenosine triphosphatase synthesis activity of mitochondria in heat-stressed cardiomyocytes. The values are the mean ± SEM, n = 8. *P < 0.05, **P < 0.01 vs -bcl-2 transfected group, #P < 0.05, ##P < 0.01 vs 37°C group. (C) Effect of bcl-2 transfection on the cytochrome c release from mitochondria. The values are the mean ± SEM, n = 5. *P < 0.05, **P < 0.01 vs -bcl-2 transfected group #P < 0.05, ##P < 0.01 vs 37°C group. (D) The effect of Bcl-2 overexpression on caspase-3 activity of heat-stressed cardiomyocytes. The values are the mean± SEM, n = 8. *P < 0.05, **P < 0.01 vs -bcl-2 transfected group, #P < 0.05, ##P < 0.01 vs 37°C group
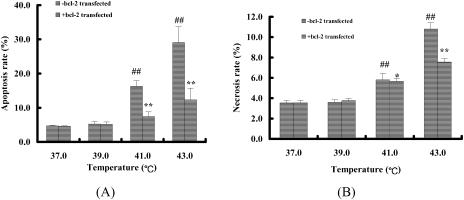
After heat stress, Bcl-2 overexpression significantly inhibited the increase of the apoptosis rate of cardiomyocytes almost in various heat-stress groups, but reduced the necrosis rate only in 43°C heat-stress group (Fig 8). A statistical analysis indicated there was significant correlation between the H+-ATPase synthesis activity of mitochondria and the apoptosis rate in Bcl-2 overexpressing–heat-stressed cardiomyocytes (Y = −0.3806X + 41.84, r = 0.9977).
Fig 8.
The effect of Bcl-2 overexpression on apoptosis and necrosis rate of heat-stressed cardiomyocytes. (A) The effect of Bcl-2 overexpression on apoptosis rate of heat-stressed cardiomyocytes. The values are the mean ± SEM, n = 8. *P < 0.05, **P < 0.01 vs -bcl-2 transfected group, #P < 0.05, ##P < 0.01 vs 37°C group. The effect of Bcl-2 overexpression on necrosis rate of heat-stressed cardiomyocytes. The values are the mean ± SEM, n = 8. *P < 0.05, **P < 0.01 vs -bcl-2 transfected group, #P < 0.05, ##P < 0.01 vs 37°C group
DISCUSSION
Adaptation to heat stress depends on strengthening the capacity of the cardiovascular system to perform greater amounts of work. Through increase of cardiac output, vasodilatation, and the blood flow rate, heat is dissipated more rapidly and body heat load decreased. If the heat exposure is prolonged or the environmental temperature is too high, imbalance between heat load and regulation capacity of body occurs, body temperature rises to a lethal limit, and the cardiovascular system collapses. The cardiovascular dysfunction induced by heat stress results from not only the decrease in cardiac stroke volume caused by the increase of heart rate and decrease of circulating blood volume, but also the decrease of cardiomyocyte contractile capacity under heat. In this present study, we demonstrate that heat stress resulted in apoptosis that coexisted with necrosis. Apoptosis is a genetically determined cell death induced by diverse stimuli and plays an important role in regulating the development and repair of tissue injury. Cardiomyocytes, being postmitotic, were thought to have minimal capacity to undergo apoptosis, but evidence accumulates that apoptosis occurs after and during myocardial infarction or coronary arteriosclerosis (Edward 1997; Zamzami et al 1997; Liu et al 2002). Apoptosis removes the injured cells and limits the myocardium injury. However, excess cell death results in decreased cardiac contraction and heart failure. At 4 hours after heat stress, cardiomyocyte apoptosis was evidently increased compared with control cells. At 6 hours after heat stress, cardiomyocyte apoptosis rate was further increased to a peak level of 30% and was the principle mechanism of myocardium injury. This result suggests that after heat stress, there is only a 3 to 4 hours opportunity for intervention to suppress apoptotic cell death.
The most-important biological functions of mitochondria are oxidative metabolism and calcium metabolism, both having a direct bearing on the energy supply for myocardial contraction. Under physiological conditions, mitochondrial function depends on the mitochondrial integrity. On the basis of the dependence of mitochondrial oxygen use on the availability of inorganic phosphate or ADP, the concept of “respiratory control rate” serves as a foundation for understanding of oxidative phosphorylation. RCR and P: O are sensitive indexes reflecting the structure and oxidative metabolism of mitochondria. As heat stress increased, both were decreased as shown in Table 2. Our previous study showed that heat stress resulted in swollen mitochondria with broken cristae and low matrix density, and a decrease of ATP content in the myocardium, indicating that the mitochondria was 1 of the main organelles injured by heat (Qian et al 1992; Song et al 2000). It is now confirmed that the energy production from oxidative metabolism in mitochondria of cardiomyocytes was suppressed under heat stress, whereas heat exposure induced an increase of cardiac function requiring greater energy supply, so that the ATP content in cardiomyocytes decreased significantly. It is because of the uncoupling of oxidative phosphorylation, heat stress induced the dysfunction of myocardial construction as well as the aggravation of body heat load, and finally results in cardiac fatigue. The broken respiratory chain produced a lot of reactive oxygen species (ROS), thus leading to cellular oxidative stress. The oxidative phosphorylation of mitochondria is closely correlated to calcium balance. Ca2+-ATPase on the mitochondrial membrane is the key factor in regulating and maintaining calcium homeostasis of cells and mitochondria. After the fall of Ca2+-ATPase activity, the disruption of intracellular calcium homeostasis occurs, such as the decrease of the Ca2+ uptake of mitochondria from cytoplasm and intracellular Ca2+ overload (Fig 2). Because the oxidative enzymes in the mitochondrial respiratory chain, including α-ketoglutaric dehydrogenase, pyruvate dehydrogenase, and isocitric dehydrogenase are regulated by Ca2+ concentration in the mitochondria matrix, the disturbance of mitochondrial calcium may further change the direction of oxidative phosphorylation and aggravate the uncoupling of oxidative phosphorylation (McCormack and Denton 1989; Chien 2000). The intracellular Ca2+ overload would overactivate a series of Ca2+-dependent protein kinases in the cytoplasm and cause serious impairment of mitochondria, cardiomyocytes, and even mitochondrial DNA.
The opening of mitochondrial MPTP is a key event in triggering the cell-death pathway (Mignotte and Vayssiere 1998; Kroemer and Reed 2000; Ferri and Kroemer 2001). Many stimuli cause the mitochondrial MPTP to open, and subsequently, a series of cytological effects occur, including loss of mitochondrial transmembrane potentials, uncoupling of respiratory chain, leakage of mitochondrial Ca2+, excess generation of ROS, and release of resident mitochondria proteins such as apoptosis initiating factor, cyto c and second mitochondria-derived activator of caspase (sMac) (Chal et al 2000). These entail a biogenetic catastrophe culminating in the disruption of plasma membrane integrity (necrosis) or the activation and action of apoptogenic protease with secondary endonuclease activation (or both) and consequent oligonucleosomal DNA fragmentation (apoptosis). In the present study, we have found that heat stress induced directly the opening of mitochondrial MPTP, which caused the alteration of mitochondrial MPT. Subsequent to the alterations, the release of cyto c and increase of caspase-3 activity occur. These results suggest that the change of MPT mediates activation of the apoptotic process in cardiomyocyte under heat stress.
Bcl-2 is an inhibitor of mitochondria-mediated apoptosis. Although its mechanism remains unclear, Bcl-2 was believed to have a role in regulating the opening of mitochondrial MPTP. Some researchers found that Bcl-2 interacts directly on the component proteins such as voltage-dependent anion channel (VDAC) (also termed porin) forming the mitochondrial MPTP to inhibit the opening of mitochondrial MPT (Yang et al 1997; Adams et al 1998). After transfecting bcl-2 into cardiomyocytes, mitochondria were protected effectively. As a result, the cardiomyocyte apoptosis was suppressed significantly compared with that in non-bcl-2–transfected cardiomyocytes undergoing heat stress (Fig 8). It is interesting that Bcl-2 overexpression decreased necrosis rate only in 43°C heat-stress group, but had no influence on the 39°C, 40°C, or even 41°C heat-stressed cardiomyocytes. This observation consisted with the evidence that only 43°C heat stress induced opening of the great majority of mitochondrial MPTPs and induced the collapsing of mitochondria, which caused necrosis. These results confirm that Bcl-2 overexpression protected mitochondria from heat stress–induced injury effectively and suggests the mitochondria injury, especially alteration of mitochondrial MPT was a key event in triggering the heat stress–induced apoptosis and necrosis of cardiomyocytes. Many factors such as toxin, chemical mediators, and cytokinine, as well as nuclear coding TR3 and p53 are able to trigger the opening of mitochondrial MPTP (Li et al 2000a, b; Marchenko et al 2000). Although the role of Ca2+ overload, ROS, and osmotic pressure in inducing cardiomyocyte apoptosis have been investigated, we have not yet determined the mechanism of mitochondrial MPTP opening induced by heat stress. The Fas pathway is also an important signal pathway, which induced caspase-8 activation. Caspase-8 could activate caspase-3 directly and also resulted in the opening of mitochondrial MPTP (Li et al 1998; Nakamura and Ueda 2000). Fas pathway may be another mechanism for triggering the opening of mitochondria MPTP, which remains to be explored.
In conclusion, the damage of mitochondrial structure and function and its secondary biological effects is an important mechanism of myocardium injury induced by heat stress, in which the alteration of mitochondrial MPT is a key point to trigger the apoptosis pathway. The mitochondria-mediated apoptosis as main cellular basis happened 4–6 hours after heat stress. Protecting mitochondria may be a new medical measure for prevention and therapy of cardiomyocyte injury in heat stress. In considering the drug design, the finding is of great importance for understanding the target and best time of therapeutic intervention.
REFERENCES
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1328. doi: 10.1126/science.281.5381.1322.0193-4511(1998)281<1322:TBPFAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Adams JW, Sakata Y, and Davis MG. et al. 1998 Enhanced Gq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. 95:10140–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SB, Watkins SC, Hastings TG. Quantitative biochemical and ultrastructural comparison of mitochondrial permeability transition in isolated brain and liver mitochondria: evidence for reduced sensitivity of brain mitochondria. Exp Neurol. 2000;164:415–425. doi: 10.1006/exnr.2000.7438.0014-4886(2000)164<0415:QBAUCO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brenner C, Kroemer G. Mitochondria: the death signal integrators. Science. 2000;289:1150–1151. doi: 10.1126/science.289.5482.1150.0193-4511(2000)289<1150:MTDSI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chal J, Du C, Wu JW, Kyin S, Wang XD, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514.0028-0836(2000)406<0855:SABBOA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chien KR. Genomic circuits and the integrative biology of cardiac diseases. Nature. 2000;407:227–231. doi: 10.1038/35025196.0028-0836(2000)407<0227:GCATIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Edward THY. Life and death in the cardiovascular system. Circulation. 1997;95:782–786. doi: 10.1161/01.cir.95.4.782.0009-7322(1997)095<0782:LADITC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Estabrook RW. Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol. 1967;10:41–56.0076-6879(1967)010<0041:MRCATP>2.0.CO;2 [Google Scholar]
- Ferri KF, Kroemer G. Mitochondria—the suicide organelles. BioEssays. 2001;23(2):111–115. doi: 10.1002/1521-1878(200102)23:2<111::AID-BIES1016>3.0.CO;2-Y.0265-9247(2001)023<0111:MSO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309.0193-4511(1998)281<1309:MAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994.0193-4511(1999)284<1994:VCRAGI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Isenberg G, Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB Medium.”. Pflueg Arch. 1982;395:6–18. doi: 10.1007/BF00584963.0031-6768(1982)395<0006:CTVMPB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5.0092-8674(1997)088<0347:PCDIAD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bal-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132.0193-4511(1997)275<1132:TROCCF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994.1078-8956(2000)006<0513:MCOCD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Li H, Kolluri SK, and Gu J. et al. 2000a Cytochrome c release and apoptosis induced by mitochondrial targeting of orphan receptor TR3-nur77-NGFI-B. Science. 289:1159–1164. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1.0092-8674(1998)094<0491:COBBCM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Willams RS. Cytochrome c deficiency causes embryonic letality and attenuates stress induced apoptosis. Cell. 2000b;12:389–399. doi: 10.1016/s0092-8674(00)80849-1.0092-8674(2000)012<0389:CCDCEL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu J, Tian Z, Gao B, Kunos G. Dose-dependent activation of antiapoptotic and proapoptotic pathways by ethanol treatment in human vascular endothelial cells: differential involvement of adenosine. J Biol Chem. 2002;277:20927–20933. doi: 10.1074/jbc.M110712200.0021-9258(2002)277<20927:DAOAAP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- MacCormack JG, Browne HW, Dawes NJ. Studies on mitochondrial Ca2+ transport and matrix Ca2+ using fura-2–loaded rat heart mitochondria. Biochim Biophys Acta. 1989;973:420–427. doi: 10.1016/s0005-2728(89)80384-6.0006-3002(1989)973<0420:SOMCTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria: a potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202.0021-9258(2000)275<16202:DSLOPP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McCormack JG, Denton RM. The role of Ca2+ ion in the regulation of intramitochondrial metabolism and energy production in rat heart. Mol Cell Biochem. 1989;89:121–125.0300-8177(1989)089<0121:TROCII>2.0.CO;2 [PubMed] [Google Scholar]
- Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x.0014-2956(1998)252<0001:MAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7.0092-8674(1997)088<0355:ABDF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ueda T. Fas-mediated apoptosis in adrimyclin induced cardiomyopathy in rat in vivo study. Circulation. 2000;102:572–578. doi: 10.1161/01.cir.102.5.572.0009-7322(2000)102<0572:FAIAIC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Petit PX, Goubern M, Diolez P, Susin SA, Zamzami N, Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998;426:111–116. doi: 10.1016/s0014-5793(98)00318-4.0014-5793(1998)426<0111:DOTOMM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Qian LJ, Cheng SQ, Wu MP. Changes of mitochondrial function in heat stressed rats. Chin J Pathol Physiol. 1999;15:333–335. [Google Scholar]
- Qian LJ, Wu MP, Chen XJ, Cheng SQ. The changes of ATP content in ventricle myocytes of heat shocked rats and its mechanism. Chin J Ind Hyg Occup Dis. 1992a;10:233–236.1001-9391(1992)010<0233:TCOACI>2.0.CO;2 [Google Scholar]
- Song XL, Qian LJ, Li FZ. Injury of heat stress on rat cardiomyocytes. Chin J Appl Physiol. 2000;16:227–230.1000-6834(2000)016<0227:IOHSOR>2.0.CO;2 [Google Scholar]
- Steenland K. Epidemiology of occupation and coronary heart disease: research agenda. Am J Ind Med. 1996;30:495–499. doi: 10.1002/(SICI)1097-0274(199610)30:4<495::AID-AJIM16>3.0.CO;2-#.0271-3586(1996)030<0495:EOOACH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Steiner M, Bauer H, Krassnigg F, Schill WB, Adam H. Micromethod for determination of ATPase activities using bioluminescence technique. Clin Chim Acta. 1988;77:107–114. doi: 10.1016/0009-8981(88)90313-0.0009-8981(1988)077<0107:MFDOAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463.0193-4511(1995)267<1445:MAGOCS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464.0193-4511(1995)267<1456:AITPAT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1309–1316. doi: 10.1126/science.281.5381.1312.0193-4511(1998)281<1309:CEW>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wattanasirichaigoon S, Menconi MJ, Delude RL, Fink MP. Effect of mesenteric ischemia and reperfusion or hemorrhagic shock on intestinal mucosal permeability and ATP content in rats. Shock. 1999;12(2):127–133. doi: 10.1097/00024382-199908000-00006.1073-2322(1999)012<0127:EOMIAR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, and Bhalla K. et al. 1997 Prevention of apoptosis by Bcl-2: release of cytochrome C from mitochondria blocked. Science. 275:1129–1132. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Hirsch T, Dallaporte B. Mitochondrial implication in accidental and programmed cell death: apoptosis and necrosis. J Bioenerg Biomembr. 1997;29:185–193. doi: 10.1023/a:1022694131572.0145-479X(1997)029<0185:MIIAAP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang GG, He HZ, and Zhang W 1989 Thermal Physiology and Hygiene. Shanghai Science and Technology Press, Shanghai, 82–96. [Google Scholar]
- Zhu SJ, Wang XM, Jiao XM, Liu SS. The influence of oxygen free radicals on synthetic and hydrolytic activities of H+-ATPase in mitochondria of rat myocardium and the protective effect of DS-182. Chin J Pathol Physiol. 1995;11:42–45. [Google Scholar]
- Zydowo MM, Swiercynski J, Nagel G, Wrzolkowa T. The respiration and calcium content of heart mitochondria from rats with vitamin D-induced cardionecrosis. Biochem J. 1985;226:155–161. doi: 10.1042/bj2260155.0264-6021(1985)226<0155:TRACCO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]