Abstract
The molecular chaperone machinery contains multiple protein components that have 1 or more structural domains composed of tetratricopeptide repeat (TPR) motifs. Many other proteins of separate or unknown function also have TPR domains, so this motif is not exclusive to molecular chaperones. A general function of TPR domains is to bind other polypeptides, but this otherwise prosaic function has been exploited in an assortment of ways that link chaperones and other protein systems into cooperative networks. Among the best-characterized TPR proteins are several cochaperones that participate in assembly and regulation of steroid receptor complexes. Steroid receptors, members of the nuclear receptor subfamily, are hormone-dependent transcription factors that regulate many vertebrate pathways of homeostasis, growth, differentiation, reproduction, and pathology and, as such, have been of great interest to biologists and clinicians. Moreover, the steroid receptors are among the first recognized native clients for chaperones and have been widely studied models for complex chaperone interactions. To provide a coherent, representative minireview of TPR protein function, the scope of this article has been narrowed down primarily to functions of steroid receptor–associated TPR cochaperones.
INTRODUCTION
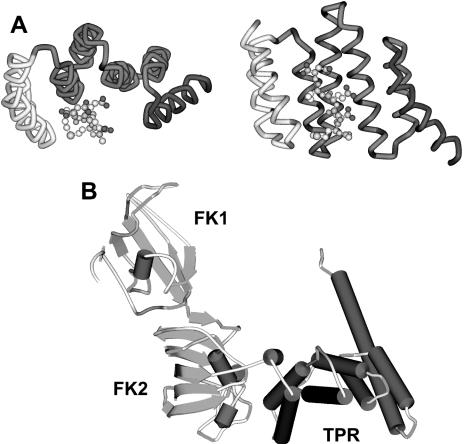
The tetratricopeptide repeat (TPR) is an off-the-shelf protein sequence or structural motif that nature has sampled liberally in eukaryotic lineages. As the name implies, the TPR is characterized by a loose, 34–amino acid consensus motif that is found in tandem repeats of varying number (previously reviewed by Lamb et al 1995; Blatch and Lassle 1999). Numerous crystallographic structures have been solved for TPR-containing proteins and protein fragments. These reveal that a common structural attribute of the TPR motif is the ability to form an antiparallel alpha-helical hairpin. Clustering several of these hairpins in tandem generates a domain with a grooved surface— not unlike a hand with curled fingers—and dimension that can conveniently grasp another polypeptide. Moreover, TPR sidechains projecting into the groove solvent space can readily specify interactions with sidechains from the held polypeptide. A typical TPR fold with interacting peptide ligand is illustrated in Figure 1A. As discussed below, this is a cocrystal between the TPR2a domain of Hop and a peptide representing the C-terminus of Hsp90 (Scheufler et al 2000). Generically, the TPR is an elegant evolutionary solution for generating a flexible, mutable domain that can facilitate specific protein-protein interactions, thus contributing to the fundamental biological importance of coordinating interactions among gene products.
Fig 1.
Tetratricopeptide repeat (TPR) crystal structures. (A) The structure shown was derived from a cocrystal of the Hop TPR2a domain in complex with the MEEVD pentapeptide that terminates Hsp90 (Scheufler et al 2000). Regions of the TPR domain approximately corresponding to the 3 TPR motifs are individually colored (yellow, orange, or pink) along with the final, non-TPR alpha helix (blue). Negatively charged side chains in the MEEVD peptide (ball and stick structure) are colored red. (B) The structure shown is for squirrel monkey FKBP51 (Sinars et al 2003), which contributes to cortisol resistance in New World primates. The 2 FKBP12-like domains are indicated (FK1 and FK2), as is the Hsp90-binding TPR domain. Secondary structures are alpha helix (red cylinders), beta sheet (blue ribbons), and beta turns (green strands). Please refer to cover image for color version of this figure
TPR proteins typically contain, besides the TPR domain itself, 1 or more additional functional domains. In some cases the TPR domain mediates intramolecular interactions among domains, contributing to overall protein conformation and potentially regulating the activity of other domains in a reversible manner. Many TPR proteins contain a TPR domain designed for intermolecular interactions tethered to an enzymatic domain. This arrangement can serve to locally concentrate or better orient an enzymatic activity that can act on a second protein bound directly or indirectly by the TPR domain. As an example, the TPR domain and the attendant peptidylprolyl isomerase (PPIase) domain for a receptor-associated TPR protein, FKBP51, are illustrated in Figure 1B. In yet another arrangement, some TPR proteins contain multiple TPR domains with distinct specificities for protein partners and thereby function as adaptors that coordinate the assembly of multiple interacting proteins into higher-order complexes.
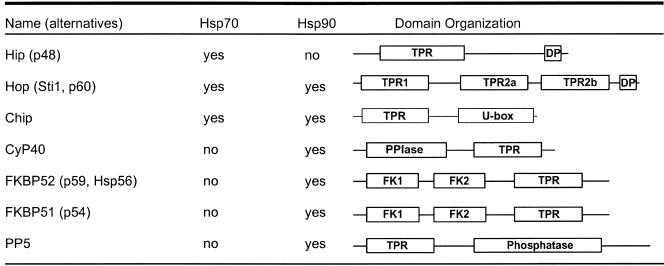
Rather than attempting a comprehensive review of all TPR proteins, this minireview will focus on various TPR proteins that interact with steroid receptor complexes. Because at least 7 TPR proteins—with others yet to be characterized—are known to participate in the assembly of steroid receptor complexes, regulation of receptor activity, and coordination of receptor degradation, steroid receptor pathways provide a convenient focus to narrow the review of TPR proteins while still illustrating the range of functions subserved by TPR proteins. Features of some key receptor-associated TPR cochaperones are summarized in Table 1.
Table 1.
TPR cochaperones in steroid receptor complexes
THE STEROID RECEPTOR–MOLECULAR CHAPERONE CONNECTION
Steroid receptors are hormone-regulated transcription factors that belong to the large nuclear receptor superfamily (Escriva et al 2000; McKenna and O'Malley 2002). A common feature of 5 steroid receptors—those for glucocorticoids (GR), mineralocorticoids, progesterone (PR), androgens, and the estrogen receptor alpha—is an obligate interaction with Hsp90 and other molecular chaperones before hormone-dependent activation (recently reviewed in Pratt and Toft 2003). Around 1985 the laboratories of Toft (Schuh et al 1985), Baulieu (Catelli et al 1985), and Pratt (Sanchez et al 1985) independently discovered Hsp90 in complex with steroid receptors. Soon thereafter, Faber and colleagues (Tai et al 1986) discovered an additional Hsp90-associated protein in several steroid receptor complexes. Although this protein appears in the literature by various names, we now know it as FKBP52, a member of the FK506-binding family of immunophilins and a TPR protein (Lebeau et al 1992; Peattie et al 1992). With the advent of specific antireceptor monoclonal antibodies, rapid and efficient purification of steroid receptor complexes became feasible, and novel receptor-associated proteins were discovered (Smith et al 1990a). The use of cell-free systems, primarily rabbit reticulocyte lysate, to study the assembly of receptor complexes in vitro led to discovery of other receptor components (Smith et al 1990b, 1992; Smith 1993), and genetic approaches with Saccharomyces cerevisiae first identified Hsp40 as required for receptor maturation (Caplan et al 1995; Kimura et al 1995). All the proteins associating with hormone-free receptor, many of which are TPR proteins, are components of the molecular chaperone machinery, and steroid receptor complexes have become a mainstay in the study of complex interactions among molecular chaperones.
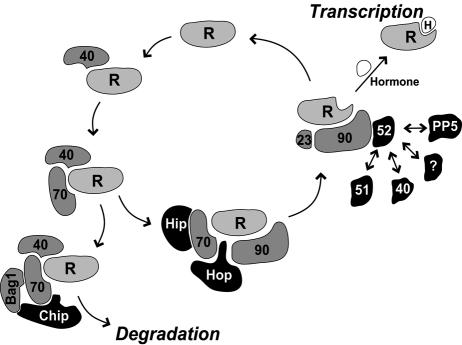
Receptor complexes are assembled with chaperones in an ordered, dynamic manner that promotes functional maturation of the receptor. This pathway is outlined in Figure 2, but the reader is referred to excellent reviews coauthored by Pratt and Toft that cover the details of this process (Pratt and Toft 1997, 2003). Several TPR proteins, as highlighted in Figure 2, participate in receptor-chaperone complexes. A common feature of these TPR proteins is the ability of each to bind Hsp70, Hsp90, or both major chaperones. Although several of the Hsp-binding TPR proteins have been shown to have independent chaperone activity in minimal protein-refolding assays (Bose et al 1996; Freeman et al 1996), the physiological relevance of this independent activity, especially as it relates to steroid receptors, has not been resolved. In their capacities to facilitate functional maturation, regulation, or degradation of steroid receptors, interactions with Hsp70 and Hsp90 appear to be critical.
Fig 2.
Pathway for assembly of steroid receptor–chaperone complexes. Assembly of steroid receptor complexes is initiated by Hsp40 and proceeds through complexes with Hsp70, Hsp90, and a variety of cochaperones. Receptor complexes containing Hsp90 and p23 have a fully competent hormone-binding domain, and hormone binding releases the receptor from the chaperone assembly cycle and promotes receptor conversion to an active transcription factor. Receptor-lacking hormone recycles through chaperone assembly, eventually being diverted to the proteasome for degradation. Tetratricopeptide repeat cochaperones (highlighted as black objects with white labels) and other components illustrated are steroid receptor monomer (R), Hsp40 (gray object marked 40), Hsp70 (70), Bag1, Chip, Hip, Hop, Hsp90 (90), p23 (23), FKBP52 (52), FKBP51 (51), CyP40 (black object marked 40), PP5, any additional immunophilin-like component (?), and steroid hormone (H)
BINDING OF TPR COCHAPERONES TO HSP70 AND HSP90
The TPR proteins participating in functional maturation of steroid receptors are adapted to bind the major molecular chaperones Hsp70 or Hsp90. There are some common themes in how TPR and Hsp interface, but there also are distinct mechanisms.
Hsp-binding sites in TPR proteins
The TPR domain in all TPR cochaperones is a key mediator of Hsp binding, although regions adjacent to or widely separated from the TPR domain can greatly influence binding. Hip was originally identified as a transient participant during the cell-free assembly of PR complexes (Smith 1993; Prapapanich et al 1996a). Hip was independently identified as an Hsp70-binding protein and was shown to contain TPR motifs (Hohfeld et al 1995). Mutagenic analyses revealed that the TPR motifs are required for Hip binding to Hsp70 (Prapapanich et al 1996b; Irmer and Hohfeld 1997); in addition, an adjacent highly charged region of Hip is necessary for Hsp70 binding, and the C-terminal DP-repeat domain of Hip influences the stability of Hip-Hsp70 complexes (Prapapanich et al 1998).
The protein phosphatase PP5 (Chinkers 1994) binds Hsp90 and contains a TPR domain that is responsible for this interaction (Chen et al 1996a). The 3-dimensional X-ray crystallographic structure was solved for the isolated PP5 TPR domain (Das et al 1998) and revealed architectural similarities between this domain and domains from some non–Hsp-binding TPR proteins. In an insightful mutagenic analysis taking advantage of the PP5 domain structure, Chinkers and colleagues (Russell et al 1999) generated point mutants within the PP5 TPR that efficiently blocked Hsp90 binding and provided early evidence for how this domain interfaces with Hsp90. Some members of the cyclosporin A–binding family of immunophilins (cyclophilins or CyP) and the FK506-binding family of immunophilins (FKBP) bind Hsp90 and contain TPR domains quite similar to the TPR domain of PP5. Deletion mutagenesis showed that the immunophilin TPR domains mediate Hsp90 binding (Radanyi et al 1994; Ratajczak and Carrello 1996; Barent et al 1998) and point mutations in conserved amino acids that align with critical PP5 residues similarly block Hsp90 binding (Chen et al 1996a; Ward et al 2002). Crystal structures for CyP40 (Taylor et al 2001) and FKBP51 (Sinars et al 2003) confirmed the structural similarity shared by each of these Hsp90-binding TPR domains. However, the TPR domain alone does not fully dictate the Hsp90-binding properties of these proteins. For example, FKBP51 and FKBP52 bind Hsp90 with different affinities (Pirkl and Buchner 2001) and assemble differentially into various steroid receptor complexes (Barent et al 1998). These differences were analyzed by mutagenesis and were found to map in part to sequences downstream from the TPR domain (Cheung-Flynn et al 2003).
Hop is another of the major players in the assembly of steroid receptor complexes. A yeast ortholog termed Sti1 was first identified in a screen for genes involved in heat tolerance (Nicolet and Craig 1989). Later, a mammalian ortholog was identified and shown to be induced in cells by viral infection (Honore et al 1992). Hop was identified in a ternary complex with Hsp90 and Hsp70 and shown to be a transient participant in the cell-free assembly of steroid receptor complexes (Smith et al 1993b). Hop contains 3 TPR domains. The first of these, TPR1, mediates binding to Hsp70, and the second domain, TPR2a, mediates Hsp90 binding (Chen et al 1996b; Lassle et al 1997); a binding partner for the third domain, TPR2b, has not been identified, although mutations in TPR2b can reduce binding to either Hsp90 or Hsp70 (Chen and Smith 1998). Similar to Hip, Hop additionally contains a C-terminal DP domain, and mutations within this domain inhibit Hop binding to Hsp70 (Chen and Smith 1998; Nelson et al 2003).
TPR binding sites of Hsp70 and Hsp90
Hsp70 and Hsp90, which otherwise have dissimilar sequences, share a unique structural feature that is recognized by some TPR proteins. Both Hsps terminate with the amino acids EEVD, and several lines of evidence implicate this as a recognition sequence for TPR proteins. Binding of several TPR cochaperones to Hsp90 was reduced in an Hsp90 truncation mutant lacking EEVD (Chen et al 1998); however, other deletion and point mutations within the C-terminal half of Hsp90 selectively affected binding of TPR cochaperones. Additional mutational analyses confirmed the importance of C-terminal sequences in the binding of TPR cochaperones to Hsp90 (Carrello et al 1999; Ramsey et al 2000).
The most definitive evidence for direct interactions between the EEVD sequences of Hsp90 and Hsp70 with TPR domains comes from cocrystallization studies of Hop TPR domains with EEVD-containing peptides (Scheufler et al 2000). Structures were solved for a complex between Hop TPR2a and the pentapeptide MEEVD, which corresponds to the C-terminus of Hsp90 alpha and beta gene products, and for a complex between Hop TPR1 and the octapeptide GPTIEEVD, which terminates both constitutively expressed and stress-inducible forms of Hsp70. Several side chains in the TPR groove, which Scheufler and colleagues termed carboxylate clamp residues, form interactions with negatively charged side chains from EEVD. Unique amino acids adjacent to the EEVD and distinctive side chains within the 2 TPR domain grooves lend specificity to the interactions between TPR1 and GPTIEEVD as compared with TPR2a and MEEVD. Blatch and colleagues have shown that mutations can be introduced into TPR1 such that this domain will bind Hsp90 (Odunuga et al 2003). Also, the carboxylate clamp positions correspond to some of the sites previously identified by Chinkers, where mutations disrupted Hsp90 binding. Despite the importance of EEVD-TPR groove interactions, other sequences in Hsps and TPR proteins can greatly influence protein-protein interactions.
Some experimental evidence for sequences outside the TPR that are important for Hsp binding has been discussed above. Hsp sequences far removed from the EEVD terminus can likewise affect binding to TPR cochaperones. The nucleotide-bound state of either Hsp70 or Hsp90 can profoundly alter TPR binding. Hop appears to recognize only the adenosine 5′-diphosphate (ADP)– bound conformation of Hsp70, as highlighted by Hsp70 K71E, a point mutant that binds adenosine triphosphate (ATP) but is unable to catalyze its hydrolysis (O'Brien et al 1996), which cannot bind Hop. Thus, the N-terminal ATPase domain of Hsp70 determines whether Hop TPR1 can access C-terminal sequences of Hsp70. Drawing into question whether the EEVD of Hsp70 plays a major structural role in binding to Hop, it was observed recently in my laboratory that truncation of up to 50 amino acids from the C-terminus of Hsp70 has little effect on binding of the truncated protein to Hop (Carrigan et al 2004). However, consistent with studies that map Hop binding to the C-terminal region of Hsp70 (Demand et al 1998; Gebauer et al 1998), any Hsp70 truncation that extends more than 50 amino acids from the C-terminus completely loses Hop binding. We speculate that an EEVD-TPR1 interaction is functionally significant but may reflect a conditional interaction that serves to regulate Hsp70 and Hop function. As noted above, mutations of Hsp90 well upstream from the C-terminus had dramatically different effects on TPR cochaperone binding. Collectively, these findings point to critical features of Hsp and TPR cochaperone structure outside the EEVD and TPR domain that influence protein-protein interactions in ways that are functionally relevant. The understanding of the nature and potential role of non–EEVD-TPR interactions would be enhanced greatly by cocrystal structures for Hsp–TPR complexes assembled from full-length proteins.
Hip is unique among the currently characterized TPR cochaperones in that its binding to Hsp is completely independent of potential EEVD interactions. Hip binds the N-terminal ATPase domain of Hsp70 (Hohfeld et al 1995) in a competitive manner with the non-TPR cochaperone BAG1 (Gebauer et al 1998). Because Hip and Hop bind separate regions of Hsp70, each can coexist in a ternary complex with Hsp70 (Prapapanich et al 1996a; Gebauer et al 1997). The TPR domain of Hip is necessary for Hsp70 binding, but so is a downstream highly charged region. At present, little is precisely known about how Hip interfaces with Hsp70.
REGULATION OF HSP ACTIVITY BY TPR COCHAPERONES
Hsp70 regulation
Hsp70 and Hsp90 each bind and release peptide substrates in an ATPase-dependent manner. Therefore, the Hsp-binding TPR cochaperones can potentially regulate Hsp activity at the level of nucleotide binding, hydrolysis, or release or at the level of substrate binding or release. Hohfeld et al (1995) reported that Hip can stabilize binding of Hsp70 to misfolded substrates. Precisely how Hip does this is unclear, but it may relate to retaining Hsp70 in the ADP-bound conformation recognized by Hip. Interestingly, Hip and Bag1 compete for binding at overlapping sites in the Hsp70 ATPase domain (Gebauer et al 1997; Hohfeld and Jentsch 1997), and Bag1 destabilizes Hsp70 substrate binding (Hohfeld and Jentsch 1997; Takayama et al 1997; Bimston et al 1998). Therefore, the balance of activities between these cochaperones can regulate Hsp70 function and steroid receptor assembly (Kanelakis et al 2000). Hop-Sti1 in some settings has an effect on ATPase. Yeast Sti1 dramatically stimulates activity of the yeast Ssa family of Hsp70 proteins (Wegele et al 2003), although Hop was not observed to stimulate the ATPase activity of vertebrate Hsp70 (Gebauer et al 1998).
Hsp90 regulation
The ATP cycle of Hsp90 is critical for its function (for a recent review see Prodromou and Pearl 2003), but somewhat contrasting mechanisms exist between yeast and mammalian Hsp90. Yeast Hsp90 has a much higher basal ATPase activity, and Sti1 inhibits this activity (Prodromou et al 1999; Richter et al 2003). In contrast, human Hsp90 has very low basal ATPase activity, which can be stimulated by binding to the ligand-binding domain of GR, and Hop is able to inhibit the client-stimulated ATPase (McLaughlin et al 2002). The TPR immunophilins also have distinctive effects on yeast or human Hsp90. Cpr6, a yeast ortholog of CyP40, does not alter the basal ATPase of yeast Hsp90 (Prodromou et al 1999); however, FKBP52 can enhance client-stimulated ATPase of human Hsp90 (McLaughlin et al 2002).
ROLES OF TPR COCHAPERONES IN RECEPTOR ASSEMBLY AND DEGRADATION
Hop-Sti1
Several TPR cochaperones participate transiently at intermediate assembly stages before functional maturation of steroid receptors. There is both in vitro and in vivo evidence that Hop greatly facilitates receptor maturation. Immunodepletion of Hop from rabbit reticulocyte lysate inhibits assembly of PR complexes (Chen et al 1996b), and exclusion of Hop from purified assembly systems inhibits assembly of PR and GR complexes (Dittmar et al 1996; Kosano et al 1998). In the yeast model for steroid receptor function, receptor function is impaired in yeast lacking Sti1 (Chang et al 1997), the yeast ortholog for Hop. The ability of Hop to promote assembly is dependent on simultaneous Hop binding to Hsp70 and Hsp90 (Chen and Smith 1998). When Hop is absent from assembly mixtures, there is no inhibition of Hsp40 and Hsp70 binding to receptor, but Hsp90 is recruited very weakly to the developing complex. Thus, Hop functions in part as an adaptor to promote recruitment of Hsp90 to preexisting Hsp70-receptor complexes. Although the adaptor role may be viewed as passive, this does not diminish the likelihood that Hop serves additional, more active roles in the assembly process. Hop is in a position to coordinate the nucleotide status of Hsp70 and Hsp90 and thereby direct progression of receptor complexes through intermediate assembly stages. Hop may also coordinate recruitment and replacement of other cochaperones in the evolving receptor complex. A better understanding of additional Hop functions is currently limited by the lack of structural or functional analyses that have mapped functions other than simple binding to Hsp70 or Hsp90. There is a great need for discrete Hop mutants that selectively impair putative functions other than simple Hsp binding; such mutants could be readily tested in available cell-free and cellular systems for steroid receptor function.
Hip
Hip participates in steroid receptor assembly, appearing transiently in complexes with approximately the same timing as Hop (Smith 1993). A Hip mutant that binds Hsp70 in an unusually stable manner will inhibit the assembly of PR complexes in rabbit reticulocyte lysate (Prapapanich et al 1998), but this may be a gain-of-function phenomenon rather than a reflection of Hip's requirement for receptor assembly. Addition of Hip to a purified receptor reconstitution system does not enhance or inhibit assembly, although Hip can overcome assembly inhibition mediated by Bag1 (Kanelakis et al 2000). One drawback of the purified assembly system is that receptor complexes are atypically stable and resistant to hormone-dependent dissociation (Pratt and Toft 2003). Hip plus Bag1, and perhaps other cellular factors, may add dynamics that contribute to cycling of receptor through chaperone complexes and facilitating dissociation of the complex subsequent to hormone binding. Because yeast lacks any gene homologous to Hip, genetic approaches with this model have been limited. However, it has been observed in my laboratory that introducing human Hip into yeast will enhance GR-dependent activation of a reporter gene (Nelson et al 2004). Further studies in cellular models should help resolve whether Hip is directly involved in steroid response pathways.
Chip
Chip is a unique Hsp cochaperone that can bind Hsp70 and inhibit Hsp40-stimulated Hsp70 ATPase activity (Ballinger et al 1999). Additionally, Chip contains a U-box ubiquitin ligase domain, and overexpression of Chip decreases steady-state levels of GR in cells by increasing ubiquitinylation and proteolytic degradation of receptor (Connell et al 2001). Therefore, Chip functions as a quality control monitor that can divert chaperone clients and substrates to the proteasome for degradation. General Chip function and mechanisms have been recently reviewed elsewhere (Cyr et al 2002; Wiederkehr et al 2002; Murata et al 2003), and readers are also directed to a recent minireview in this journal (Alberti et al 2003), in which cooperation between Chip and Bag1, a non-TPR nucleotide exchange factor for Hsp70, was discussed in relation to protein quality control.
Other TPR cochaperones
There are several vertebrate FKBP family members in the size range of 35–38 kDa that, unlike FKBP51 and FKBP52, contain a single FK506-binding PPIase domain joined to a TPR domain (Galat 2003). One of these is Xap2, which binds Hsp90 and serves important cellular functions in regulating the arylhydrocarbon receptor (Ma and Whitlock 1997; Meyer et al 1998; Kazlauskas et al 2000). The arylhydrocarbon receptor is an Hsp90 client protein unrelated to steroid receptors, and Xap2 has not been shown to function in steroid receptor complexes. Another 36-kDa family member, FKBP6 (Meng et al 1998), which is essential for male fertility in rodents (Crackower et al 2003), binds Hsp90 and is capable of assembling with steroid receptor complexes in vitro (D. F. Smith, personal communication), but any role this FKBP may play in steroid receptor function is unknown. Cns1p is a unique TPR protein identified in S cerevisiae that binds Hsp90, regulates the activity of steroid receptors plus other Hsp90 clients, and is essential for cell viability (Dolinski et al 1998; Marsh et al 1998; Nathan et al 1999). Cns1p and other Hsp90-binding TPR cochaperones also independently bind Hsp104 (Abbas-Terki et al 2001), an important chaperone for stress tolerance and prion maintenance in yeast. No direct metazoan counterpart for Cns1p has been identified, but there could well be an analogous, currently unrecognized chaperone component in animal cells.
Another unique TPR cochaperone, termed Tpr2, contains 2 TPR domains plus a J domain homologous to the Hsp40 family of chaperones (Murthy et al 1996). Like Hop, Tpr2 binds both Hsp70 and Hsp90, but unlike Hop, Tpr2 stimulates the release of substrates from Hsp90, as shown by Brychzy et al (2003). Through the TPR2 J domain, Hsp70 refolding activity is stimulated by Tpr2 as much as by Hsp40. Although Tpr2 has not yet been identified in steroid receptor complexes, Brychzy et al found that either increasing or decreasing Tpr2 protein levels in cells decreases GR-dependent activation of a reporter gene. A major conclusion of this study is that Tpr2 may promote the retrograde transfer of steroid receptors or other protein substrates to Hsp70. However, if Tpr2 concentration lies within a narrow range, it can instead favor efficient maturation of Hsp90-bound receptor complexes. As demonstrated by these novel findings, there may well be additional, undiscovered TPR cochaperones in the chaperone machinery that fine-tune the handling of steroid receptors and other client proteins.
REGULATION OF STEROID RECEPTOR FUNCTION BY TPR COCHAPERONES
Based on in vitro receptor assembly assays with purified proteins, the addition of immunophilins or PP5 to these assays does not enhance or inhibit generation of the hormone-binding capacity of steroid receptors. Therefore, the late-stage TPR proteins are probably not required for folding of the receptor's ligand-binding domain into a competent state for hormone binding. As listed below, however, there are clues that immunophilins regulate receptor activity at the cellular level. First, we must bear in mind that TPR immunophilins and PP5 are typically coexpressed in cells and that they compete for binding Hsp90 and assembling with steroid receptor complexes (Owens-Grillo et al 1995; Ratajczak and Carrello 1996; Nair et al 1997). Under steady-state assembly conditions the immunophilins exchange rapidly on steroid receptor complexes (Barent et al 1998), resulting in a dynamic mixture of receptor complexes distinguished by which Hsp90-binding TPR protein is incorporated into the complex. Because of rapid exchange of TPR proteins, any given receptor will effectively sample the environment for all available TPR proteins, but these proteins do not incorporate into receptor complexes strictly based on the relative abundance of each TPR protein (Nair et al 1997). There are distinct patterns of selectivity for immunophilins by different steroid receptors (Silverstein et al 1997; Barent et al 1998), and hormone binding can stimulate a rapid shift in selectivity (Smith et al 1993a; Davies et al 2002).
Hormone-binding affinity
The first indication that receptor-associated immunophilins can alter hormone-binding affinity came from studies of cortisol insensitivity in New World monkeys. Scammell and colleagues have shown that FKBP51 lowers GR hormone-binding affinity (Reynolds et al 1999; Denny et al 2000) and that FKBP51 is overexpressed in tissues of New World compared with Old World primates (Scammell et al 2001). The Scammell group has further shown that FKBP51 from squirrel monkey, one of the cortisol-resistant New World primates, is more potent in repressing GR hormone-binding affinity than human FKBP51, although the human protein can also lower GR affinity (Reynolds et al 1999; Denny et al 2000). Interestingly, squirrel monkey FKBP51 can partially inhibit reporter gene activation mediated by PR (Hubler et al 2003). Crystal structures have been solved for both human and squirrel monkey FKBP51 (Hubler et al 2003), but there was no structural difference that immediately suggested differences in protein activity. At the amino acid sequence level various primate FKBP51 sequences are greater than 90% identical, although there are several nonconservative amino acid differences scattered over the sequence. The mechanism by which FKBP51 can lower GR hormone-binding affinity has not been determined, but it may relate to a contrasting effect of FKBP52 on GR activity.
My laboratory has recently used the yeast model as a convenient background for testing the function of FKBP51 and FKBP52 because there is no counterpart to these TPR-containing FKBPs in S cerevisiae (Riggs et al 2003). When human FKBP52 is introduced into yeast expressing GR, hormone-dependent GR transcriptional activity is enhanced as much as 10-fold at subsaturating levels of hormone, a potentiation that reflects an increase in GR hormone-binding affinity. Similar potentiation was not observed by expressing human FKBP51, PP5, or CyP40 or by overexpressing the endogenous yeast cyclophilins Cpr6 or Cpr7. Mapping studies revealed that FKBP52-mediated potentiation of GR activity required a functional Hsp90-binding TPR domain plus an active PPIase domain. On the basis of the aforementioned studies in New World primates, we anticipated that FKBP51 would inhibit GR function, but this was not observed. On the other hand, coexpression of FKBP51 and FKBP52 in yeast eliminated potentiation of GR activity, suggesting that FKBP51 may act to reverse or prevent the actions of FKBP52. The repressive effect of FKBP51 did not result solely from competitive displacement of FKBP52 from GR complexes because coexpression of PP5 with FKBP52 did not reduce potentiation. Our working model in current studies is that FKBP52 PPIase activity targets 1 or more prolines in the ligand-binding domain of GR. This proposed mechanism is similar to the ability of FKBP52 to alter the conformation and function of interferon regulatory factor-4, which has also been shown to be PPIase dependent (Mamane et al 2000).
Dynein binding and receptor translocation
The Pratt laboratory has led studies on the potential role of receptor-associated immunophilins and PP5 as facilitators of receptor translocation from the cytoplasmic to nuclear compartments (Pratt et al 1999). Pratt's group and others have published observations (Davies et al 2002; Galigniana et al 2002) that FKBP52, CyP40, and PP5 can bind dynein, one of the major microtubule-associated motor proteins. Dynein binding to FKBP52 localizes to an N-terminal region containing the PPIase and the drug-binding domain (Galigniana et al 2001); however, FK506 does not disrupt dynein binding, and PPIase activity does not appear to be important. Several lines of evidence have been published that suggest that 1 or more of the dynein-binding TPR cochaperones may speed trafficking of GR to the nucleus after hormone binding; however, definitive proof of this mechanism is lacking. In the yeast model where FKBP52 was shown to specifically enhance GR activity, dynein was excluded as a mediator of GR potentiation (Riggs et al 2003). Experimental determinations in vertebrate cells of the relevance of dynein-TPR cochaperone interactions would be greatly facilitated by TPR point mutants that disrupt dynein binding, but such mutants have not been generated so far.
Other potential functions
Because PP5, one of the receptor-associated TPR cochaperones, contains a protein phosphatase domain and steroid receptors are phosphoproteins, one could reasonably speculate that PP5 regulates the phosphorylation status of steroid receptors. However, evidence of such a role for PP5 has not been forthcoming. Receptors have multiple phosphorylation sites, and there is evidence that differential phosphorylation of these sites can alter receptor localization, proteolytic stability, and transcriptional activity (Weigel 1996; Bodwell et al 1998; Wang et al 2002; Qiu and Lange 2003). One group (Zuo et al 1999; Dean et al 2001) reported that knockdown of PP5 expression by antisense deoxyoligonucleotide stimulates translocation of GR from the cytoplasmic to nuclear compartments in the absence of hormone, suggesting that PP5 inhibits hormone-independent transport. This finding contrasts somewhat with the report (Chen et al 1996a) that a dominant negative mutant of PP5 inhibits GR transactivation. Further work is needed to determine what role(s) PP5 plays in GR function at the cellular level.
REGULATION OF TPR COCHAPERONE FUNCTION
As with any protein, there are multiple ways in which the activity of a TPR cochaperone can be regulated. Below are several mechanisms that appear to be used by 1 or more receptor-associated TPR proteins.
Transcriptional control
Expression of several TPR cochaperones can be induced by heat shock or other cellular stressors. Sti1 protein levels increase in yeast in response to heat shock or an amino acid analog (Nicolet and Craig 1989), and Hop levels double in mammalian cells in response to viral infection (Honore et al 1992). FKBP52, which has also been termed Hsp56, is inducible by heat shock (Catelli et al 1985; Sanchez 1990), and the promoter region of the FKBP52 gene contains consensus response elements for the heat shock transcription factor (Massol et al 2003; Scammell et al 2003). CyP40 messenger ribonucleic acid (RNA) was strongly induced by heat shock in human MCF7 breast cancer cells, but there was no change in the steady-state level of CyP40 protein, perhaps because of a concomitant decrease in CyP40 protein half-life (Mark et al 2001).
CyP40, FKBP52, and FKBP51 genes are all inducible by hormones. The cardiac cytokine cardiotrophin-1 stimulates FKBP52 gene expression, and overexpression of FKBP52 may be responsible for cardiotrophin-induced cardiac hypertrophy (Railson et al 2001). Cyp40 and FKBP52 levels increase modestly in MCF7 cells treated with estradiol (Mark et al 2001), an increase that was attributed both to increased gene transcription and longer message half-life. FKBP51 gene expression can be greatly increased by corticosteroids and was the basis for initial identification of FKBP51 by 1 group (Baughman et al 1995). Given the ability of FKBP51 to repress GR function, as discussed above, GR-mediated induction of FKBP51 gene expression suggests a feedback mechanism for modulating cellular responsiveness to GR (Cheung and Smith 2000). One group suggested recently that hormone-induced FKBP51 expression could be a useful clinical marker for GR sensitivity and bioavailability in patients (Vermeer et al 2003). Progestins (Kester et al 1997; Hubler et al 2003) or androgens (Amler et al 2000; Zhu et al 2001) can also stimulate FKBP51 gene expression, which suggests that the FKBP51 promoter may contain 1 or more of the consensus response elements that are a common target for GR, PR, and AR. A hormone-responsive region was mapped to the promoter region of the FKBP51 gene, but this region did not contain a prototypical steroid response element (Hubler et al 2003).
Covalent modification
Most of the TPR cochaperones are known phosphoproteins. In Blatch's laboratory it was shown that Hop can be phosphorylated by casein kinase II and by cdc2 at distinct sites, and it was predicted that phosphorylation may regulate Hop activity in a cell cycle–dependent manner. FKBP52 is another phosphoprotein. The Baulieu group has shown that it is a substrate for casein kinase II and fails to bind Hsp90 upon phosphorylation (Miyata et al 1997). In an unusual twist on TPR cochaperone function, FKBP52 has been identified as the cellular factor that directly binds a specific single-stranded region of an adeno-associated virus (AAV) genome and inhibits deoxyribonucleic acid (DNA) synthesis (Qing et al 2001). Binding of FKBP52 to AAV DNA and inhibition of DNA synthesis requires phosphorylation at serine-threonine or, more importantly, at a tyrosine residue of FKBP52; moreover, a specific tyrosine phosphatase has been shown to inactivate FKBP52 and prevent AAV transduction of cells in a transgenic mouse model (Qing et al 2003). Tyrosine phosphorylation of FKBP52 may also regulate its activity in steroid receptor complexes, but this possibility has not been explored.
Alternative conformations
There have been intriguing suggestions that TPR domains are capable of undergoing dramatic conformational rearrangements. Crystallographic studies have uncovered alternative conformers for the peroxisomal multi-TPR protein PEX5 (Gatto et al 2000; Kumar et al 2001) and for CyP40 (Taylor et al 2001). The TPR motif typically conforms as a helix-turn-helix, in which the 2 helices align in an antiparallel manner. Three or more tandem repeats of the core motif continue this pattern to form the characteristic domain (Fig 1A). Alternative PEX5 and CyP40 crystals revealed that turn regions can instead be incorporated with adjacent helices into a single extended helix. Kumar speculated that a PEX5 TPR may alternatively assume folded-back or extended conformations—what they termed the jackknife model (Kumar et al 2001)—as a means of regulating PEX5 interactions. Crystal structures for full-length FKBP51 (Sinars et al 2003) showed a typical fold for the core TPR motifs, but downstream sequences formed an extended helix (Fig 1B), similar to that seen with CyP40 and PEX5. Mutagenic analyses within this region of FKBP51 and the corresponding region of FKBP52 showed that sequences within and contiguous to the TPR greatly influence Hsp90 binding (Barent et al 1998; Cheung-Flynn et al 2003). Recalling the jackknife model, mutant results suggested that the extended helix may fold back in the Hsp90-bound FKBP to become part of the core TPR domain (Cheung-Flynn et al 2003). Interestingly, the Baulieu group has suggested (Massol et al 1992) that calmodulin binds the same TPR-adjacent region of FKBP52 that we have implicated in Hsp90 binding and potential refolding. Further structural studies are needed to confirm whether native TPR proteins are adapted to assume alternative conformations and, if so, what signals stimulate the switch between conformations.
Small-molecule regulators of TPR cochaperones
FKBP52 and FKBP51, like other FK506-binding proteins, bind FK506, rapamycin, and related compounds; CyP40, like other cyclophilins, binds cyclosporin A (for a recent comprehensive review see Galat 2003). These natural product drugs bind to and inhibit the respective PPIase domains of FKBP and cyclophilin family members. Endogenous small-molecule ligands for the immunophilins have not been identified, but the existence of such ligands in vertebrate cells remains an intriguing possibility.
The primary mechanism of action for these immunosuppressive drugs, rather than PPIase inhibition, is an induced tight association of the drug-immunophilin complex with other cellular proteins that signal T-cell activation. Within the normal therapeutic range for these drugs, the most important immunophilin targets are small members of these families, FKBP12 and CyPA, rather than the TPR-containing members. Still, the possible participation of TPR immunophilins in some drug effects has not been ruled out.
Attempts to use immunosuppressive drugs in cellular models to target steroid receptor function through receptor-associated immunophilins have been confounded by the pleiotropic effects of these drugs in cells. The challenge has been to compare drug effects in cells that either lack or contain a specific immunophilin. Taking advantage of the yeast model for steroid receptor function in which we were able to selectively introduce human FKBP51 or FKBP52, we found that FK506 could reverse FKBP52-stimulated GR activity (Riggs et al 2003). We confirmed that this was due to inhibition of FKBP52 PPIase activity by mutation of active site residues in FKBP52. As small interfering RNA and gene-targeting technologies are applied to receptor-associated immunophilins in vertebrate cells and organisms, even more relevant models will become available for teasing out drug actions that are directly mediated by receptor-associated immunophilins. Further structural studies on individual PPIase domains may also suggest rationales for designing drugs that are highly selective for one or other immunophilin.
In several laboratories it has been observed that certain lipid compounds can act allosterically to regulate the protein phosphatase activity of PP5 (Chen and Cohen 1997; Chinkers 2001). The Chinkers laboratory has identified long-chain fatty acid CoA esters that activate PP5 at physiologically relevant concentrations (Ramsey and Chinkers 2002), suggesting that these may be endogenous regulators of PP5 function. Fatty acids and Hsp90 bind noncompetitively to the PP5 TPR domain, and either factor appears to relieve an inhibitory intramolecular interaction involving the C-terminal phosphatase domain. Given the similarity of Hsp90-binding TPR domains from PP5, Hop, FKBP51, FKBP52, CyP40, and others, it will be useful to determine whether in one or other of these proteins there is a similar allosteric regulatory site, perhaps accommodating unique small ligands.
Finally, there is potential for developing novel therapeutic reagents that bind specifically to the TPR pocket of cochaperones. On the basis of the specificity apparent from Hop TPR–EEVD peptide cocrystal structures, Hartl and colleagues have explored peptide features that confer specificity (Brinker et al 2002). A better comparative understanding of TPR ligand-binding pockets and native peptide ligands can contribute to development of peptide mimetics that would target clinically relevant pathways involving TPR cochaperones.
CONCLUDING REMARKS
The TPR cochaperones in steroid receptor complexes are representative of a much broader array of TPR proteins that function in multiple cellular compartments and are expressed in all eukaryotes. The mechanisms by which these proteins function and the mechanisms that regulate TPR activity are diverse. Although this review focused largely on steroid receptor complexes, the reader should bear in mind that the receptor-associated TPR cochaperones have been observed in complexes of many of the more than 100 known clients for Hsp90. It is highly likely that each of the TPR cochaperones will have important responsibilities in other client pathways, and there is also a proven ability of some of the TPR cochaperones to function independently of Hsp70 or Hsp90. Because some of the TPR cochaperones are already known drug targets, there is a reasonable prospect for future drugs and molecular therapies that will target individual TPR cochaperones as a means of enhancing or inhibiting cellular processes. However, much more remains to be learned than what is already known about TPR proteins. As should be apparent from the discussion above, there is a general need in the chaperone field for additional genetic models in mammalian cells and whole animals that demonstrate the physiological importance of TPR cochaperones in steroid receptor and other cellular pathways. With the advent of RNA interference approaches that are efficacious in mammalian cells, we should soon have cellular models for the knockdown of endogenous cochaperone expression and experimental replacement with mutant gene products. Likewise, with the rapid expansion of mouse gene knockout models, we can hope that appropriate mouse genetic models will soon be available.
Acknowledgments
The author regrets that many relevant studies on TPR proteins could not be covered in this minireview. Work in the author's laboratory was supported by NIH R01 DK44923, NIH R01 DK48218, and the Mayo Foundation.
REFERENCES
- Abbas-Terki T, Donze O, Briand PA, Picard D. Hsp104 interacts with hsp90 cochaperones in respiring yeast. Mol Cell Biol. 2001;21:7569–7575. doi: 10.1128/MCB.21.22.7569-7575.2001.0270-7306(2001)021<7569:HIWHCI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Esser C, Hohfeld J. BAG-1—a nucleotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones. 2003;8:225–231. doi: 10.1379/1466-1268(2003)008<0225:bnefoh>2.0.co;2.1466-1268(2003)008<0225:BNEFOH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amler LC, Agus DB, and LeDuc C. et al. 2000 Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 60:6134–6141. [PubMed] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535.0270-7306(1999)019<4535:IOCANT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. doi: 10.1210/mend.12.3.0075.0888-8809(1998)012<0342:AOFCAM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Baughman G, Wiederrecht GJ, Campbell NF, Martin MM, Bourgeois S. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15:4395–4402. doi: 10.1128/mcb.15.8.4395.0270-7306(1995)015<4395:FANTIC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimston D, Song J, Winchester D, Takayama S, Reed JC, Morimoto RI. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 1998;17:6871–6878. doi: 10.1093/emboj/17.23.6871.0261-4189(1998)017<6871:BANROH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N.0265-9247(1999)021<0932:TTRASM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bodwell JE, Webster JC, Jewell CM, Cidlowski JA, Hu JM, Munck A. Glucocorticoid receptor phosphorylation: overview, function and cell cycle-dependence. J Steroid Biochem Mol Biol. 1998;65:91–99. doi: 10.1016/s0960-0760(97)00185-4.0960-0760(1998)065<0091:GRPOFA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. doi: 10.1126/science.274.5293.1715.0193-4511(1996)274<1715:CFOHP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brinker A, Scheufler C, Von Der Mulbe F, Fleckenstein B, Herrmann C, Jung G, Moarefi I, Hartl FU. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 × Hop × Hsp90 complexes. J Biol Chem. 2002;277:19265–19275. doi: 10.1074/jbc.M109002200.0021-9258(2002)277<19265:LDBTDR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brychzy A, Rein T, Winklhofer KF, Hartl FU, Young JC, Obermann WM. Cofactor Tpr2 combines two TPR domains and a J domain to regulate the Hsp70/Hsp90 chaperone system. EMBO J. 2003;22:3613–3623. doi: 10.1093/emboj/cdg362.0261-4189(2003)022<3613:CTCTTD>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ, Langley E, Wilson EM, Vidal J. Hormone-dependent transactivation by the human androgen receptor is regulated by a dnaJ protein. J Biol Chem. 1995;270:5251–5257. doi: 10.1074/jbc.270.10.5251.0021-9258(1995)270<5251:HTBTHA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Carrello A, Ingley E, Minchin RF, Tsai S, Ratajczak T. The common tetratricopeptide repeat acceptor site for steroid receptor-associated immunophilins and hop is located in the dimerization domain of Hsp90. J Biol Chem. 1999;274:2682–2689. doi: 10.1074/jbc.274.5.2682.0021-9258(1999)274<2682:TCTRAS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Carrigan PE, Nelson GM, Roberts PJ, Stoffer J, Riggs DL, Smith DF. Multiple domains of the co-chaperone Hop are important for Hsp70 binding. J Biol Chem. 2004;279:16185–16193. doi: 10.1074/jbc.M314130200.0021-9258(2004)279<16185:MDOTCH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Catelli MG, Binart N, Jung-Testas I, Renoir JM, Baulieu EE, Feramisco JR, Welch WJ. The common 90-kd protein component of non-transformed ‘8S’ steroid receptors is a heat-shock protein. EMBO J. 1985;4:3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x.0261-4189(1985)004<3131:TCKPCO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Nathan DF, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318.0270-7306(1997)017<0318:IVAOTH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996a;271:32315–32320. doi: 10.1074/jbc.271.50.32315.0021-9258(1996)271<32315:TTRDOP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen MX, Cohen PT. Activation of protein phosphatase 5 by limited proteolysis or the binding of polyunsaturated fatty acids to the TPR domain. FEBS Lett. 1997;400:136–140. doi: 10.1016/s0014-5793(96)01427-5.0014-5793(1997)400<0136:AOPPBL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen S, Prapapanich V, Rimerman RA, Honore B, Smith DF. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Mol Endocrinol. 1996b;10:682–693. doi: 10.1210/mend.10.6.8776728.0888-8809(1996)010<0682:IOPAMO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen S, Smith DF. Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J Biol Chem. 1998;273:35194–35200. doi: 10.1074/jbc.273.52.35194.0021-9258(1998)273<35194:HAAAIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen S, Sullivan WP, Toft DO, Smith DF. Differential interactions of p23 and the TPR-containing proteins Hop, Cyp40, FKBP52 and FKBP51 with Hsp90 mutants. Cell Stress Chaperones. 1998;3:118–129. doi: 10.1379/1466-1268(1998)003<0118:diopat>2.3.co;2.1466-1268(1998)003<0118:DIOPAT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: an update. Mol Endocrinol. 2000;14:939–946. doi: 10.1210/mend.14.7.0489.0888-8809(2000)014<0939:MCIWSR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Roberts PJ, Riggs DL, Smith DF. C-terminal sequences outside the tetratricopeptide repeat domain of FKBP51 and FKBP52 cause differential binding to Hsp90. J Biol Chem. 2003;278:17388–17394. doi: 10.1074/jbc.M300955200.0021-9258(2003)278<17388:CSOTTR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chinkers M. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc Natl Acad Sci U S A. 1994;91:11075–11079. doi: 10.1073/pnas.91.23.11075.0027-8424(1994)091<11075:TOADPP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M. Protein phosphatase 5 in signal transduction. Trends Endocrinol Metab. 2001;12:28–32. doi: 10.1016/s1043-2760(00)00335-0.1043-2760(2001)012<0028:PPIST>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618.1465-7392(2001)003<0093:TCCRPT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Crackower MA, Kolas NK, and Noguchi J. et al. 2003 Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science. 300:1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DM, Hohfeld J, Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4.0376-5067(2002)027<0368:PQCUEU>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192.0261-4189(1998)017<1192:TSOTTR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200.0021-9258(2002)277<4597:ANFSIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dean DA, Urban G, and Aragon IV. et al. 2001 Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demand J, Luders J, Hohfeld J. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol. 1998;18:2023–2028. doi: 10.1128/mcb.18.4.2023.0270-7306(1998)018<2023:TCDOHP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785.0013-7227(2000)141<4107:SMIFIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Hutchison KA, Owens-Grillo JK, Pratt WB. Reconstitution of the steroid receptor hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271:12833–12839. doi: 10.1074/jbc.271.22.12833.0021-9258(1996)271<12833:ROTSRH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dolinski KJ, Cardenas ME, Heitman J. CNS1 encodes an essential p60/Sti1 homolog in Saccharomyces cerevisiae that suppresses cyclophilin 40 mutations and interacts with Hsp90. Mol Cell Biol. 1998;18:7344–7352. doi: 10.1128/mcb.18.12.7344.0270-7306(1998)018<7344:CEAESH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva H, Delaunay F, Laudet V. Ligand binding and nuclear receptor evolution. Bioessays. 2000;22:717–727. doi: 10.1002/1521-1878(200008)22:8<717::AID-BIES5>3.0.CO;2-I.0265-9247(2000)022<0717:LBANRE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718.0193-4511(1996)274<1718:MCMCAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity–targets–functions. Curr Top Med Chem. 2003;3:1315–1347. doi: 10.2174/1568026033451862.1568-0266(2003)003<1315:PTIIBD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, Murphy PJ, Chinkers M, Radanyi C, Renoir JM, Zhang M, Pratt WB. Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry. 2002;41:13602–13610. doi: 10.1021/bi020399z.0006-2960(2002)041<13602:BOHITC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–14889. doi: 10.1074/jbc.M010809200.0021-9258(2001)276<14884:ETTPID>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gatto GJ Jr, Geisbrecht BV, Gould SJ, Berg JM. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat Struct Biol. 2000;7:1091–1095. doi: 10.1038/81930.1072-8368(2000)007<1091:PTSRBT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gebauer M, Zeiner M, Gehring U. Proteins interacting with the molecular chaperone hsp70/hsc70: physical associations and effects on refolding activity. FEBS Lett. 1997;417:109–113. doi: 10.1016/s0014-5793(97)01267-2.0014-5793(1997)417<0109:PIWTMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gebauer M, Zeiner M, Gehring U. Interference between proteins Hap46 and Hop/p60, which bind to different domains of the molecular chaperone hsp70/hsc70. Mol Cell Biol. 1998;18:6238–6244. doi: 10.1128/mcb.18.11.6238.0270-7306(1998)018<6238:IBPHAP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1 [published erratum appears in EMBO J; 2 February 1998; 17(3):847] EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209.0261-4189(1997)016<6209:GROTHC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3.0092-8674(1995)083<0589:HANCII>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Honore B, Leffers H, Madsen P, Rasmussen HH, Vandekerckhove J, Celis JE. Molecular cloning and expression of a transformation-sensitive human protein containing the TPR motif and sharing identity to the stress-inducible yeast protein STI1. J Biol Chem. 1992;267:8485–8491.0021-9258(1992)267<8485:MCAEOA>2.0.CO;2 [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092.0013-7227(2003)144<2380:TFIFIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Irmer H, Hohfeld J. Characterization of functional domains of the eukaryotic co-chaperone Hip. J Biol Chem. 1997;272:2230–2235. doi: 10.1074/jbc.272.4.2230.0021-9258(1997)272<2230:COFDOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kanelakis KC, Murphy PJ, Galigniana MD, Morishima Y, Takayama S, Reed JC, Toft DO, Pratt WB. Hsp70 interacting protein Hip does not affect glucocorticoid receptor folding by the hsp90-based chaperone machinery except to oppose the effect of BAG-1. Biochemistry. 2000;39:14314–14321. doi: 10.1021/bi001671c.0006-2960(2000)039<14314:HIPHDN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Poellinger L, Pongratz I. The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J Biol Chem. 2000;275:41317–41324. doi: 10.1074/jbc.M007765200.0021-9258(2000)275<41317:TIPXRU>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kester HA, van der Leede BM, van der Saag PT, van der Burg B. Novel progesterone target genes identified by an improved differential display technique suggest that progestin-induced growth inhibition of breast cancer cells coincides with enhancement of differentiation. J Biol Chem. 1997;272:16637–16643. doi: 10.1074/jbc.272.26.16637.0021-9258(1997)272<16637:NPTGIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yahara I, Lindquist S. Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science. 1995;268:1362–1365. doi: 10.1126/science.7761857.0193-4511(1995)268<1362:ROTPCY>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973.0021-9258(1998)273<32973:TAOPRC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kumar A, Roach C, Hirsh IS, Turley S, deWalque S, Michels PA, Hol WG. An unexpected extended conformation for the third TPR motif of the peroxin PEX5 from Trypanosoma brucei. J Mol Biol. 2001;307:271–282. doi: 10.1006/jmbi.2000.4465.0022-2836(2001)307<0271:AUECFT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, Hieter P. Tetratricopeptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4.0376-5067(1995)020<0257:TRITTO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lassle M, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, murine protein mSTI1. Characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876.0021-9258(1997)272<1876:SMPMCO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lebeau MC, Massol N, Herrick J, Faber LE, Renoir JM, Radanyi C, Baulieu EE. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992;267:4281–4284.0021-9258(1992)267<4281:PAHBPC>2.0.CO;2 [PubMed] [Google Scholar]
- Ma Q, Whitlock JP Jr.. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272:8878–8884.0021-9258(1997)272<8878:ANCPTI>2.0.CO;2 [PubMed] [Google Scholar]
- Mamane Y, Sharma S, Petropoulos L, Lin R, Hiscott J. Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity. 2000;12:129–140. doi: 10.1016/s1074-7613(00)80166-1.1074-7613(2000)012<0129:PROIAB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mark PJ, Ward BK, Kumar P, Lahooti H, Minchin RF, Ratajczak T. Human cyclophilin 40 is a heat shock protein that exhibits altered intracellular localization following heat shock. Cell Stress Chaperones. 2001;6:59–70. doi: 10.1379/1466-1268(2001)006<0059:hciahs>2.0.co;2.1466-1268(2001)006<0059:HCIAHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JA, Kalton HM, Gaber RF. Cns1 is an essential protein associated with the hsp90 chaperone complex in Saccharomyces cerevisiae that can restore cyclophilin 40-dependent functions in cpr7Delta cells. Mol Cell Biol. 1998;18:7353–7359. doi: 10.1128/mcb.18.12.7353.0270-7306(1998)018<7353:CIAEPA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol N, Lebeau MC, Renoir JM, Faber LE, Baulieu EE. Rabbit FKBP59-heat shock protein binding immunophillin (HBI) is a calmodulin binding protein. Biochem Biophys Res Commun. 1992;187:1330–1335. doi: 10.1016/0006-291x(92)90448-t.0006-291X(1992)187<1330:RFSPBI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Massol N, Lebeau MC, Schumacher M, Baulieu EE. Promoter activity and gene structure of rabbit FKBP52. DNA Cell Biol. 2003;22:505–511. doi: 10.1089/10445490360708919.1044-5498(2003)022<0505:PAAGSO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4.0092-8674(2002)108<0465:CCOGEB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McLaughlin SH, Smith HW, Jackson SE. Stimulation of the weak ATPase activity of human hsp90 by a client protein. J Mol Biol. 2002;315:787–798. doi: 10.1006/jmbi.2001.5245.0022-2836(2002)315<0787:SOTWAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Meng X, Lu X, Morris CA, Keating MT. A novel human gene FKBP6 is deleted in Williams syndrome. Genomics. 1998;52:130–137. doi: 10.1006/geno.1998.5412.0888-7543(1998)052<0130:ANHGFI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. 1998;18:978–988. doi: 10.1128/mcb.18.2.978.0270-7306(1998)018<0978:HBVXPI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Chambraud B, and Radanyi C. et al. 1997 Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: regulation of HSP90-binding activity of FKBP52. Proc Natl Acad Sci U S A. 94:14500–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata S, Chiba T, Tanaka K. CHIP: a quality-control E3 ligase collaborating with molecular chaperones. Int J Biochem Cell Biol. 2003;35:572–578. doi: 10.1016/s1357-2725(02)00394-1.1357-2725(2003)035<0572:CAQELC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Murthy AE, Bernards A, Church D, Wasmuth J, Gusella JF. Identification and characterization of two novel tetratricopeptide repeat-containing genes. DNA Cell Biol. 1996;15:727–735. doi: 10.1089/dna.1996.15.727.1044-5498(1996)015<0727:IACOTN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nair SC, Rimerman RA, Toran EJ, Chen S, Prapapanich V, Butts RN, Smith DF. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol Cell Biol. 1997;17:594–603. doi: 10.1128/mcb.17.2.594.0270-7306(1997)017<0594:MCOHFA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. Identification of SSF1, CNS1, and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc Natl Acad Sci U S A. 1999;96:1409–1414. doi: 10.1073/pnas.96.4.1409.0027-8424(1999)096<1409:IOSCAH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GM, Huffman H, Smith DF. Comparison of the carboxy-terminal DP-repeat region in the co-chaperones Hop and Hip. Cell Stress Chaperones. 2003;8:125–134. doi: 10.1379/1466-1268(2003)008<0125:cotcdr>2.0.co;2.1466-1268(2003)008<0125:COTCDR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GM, Prapapanich V, Carrigan PE, Roberts PJ, Riggs DL, and Smith DF 2004 The Hsp70 co-chaperone Hip enhances functional maturation of glucocorticoid receptor. Mol Endocrinol. in press. [DOI] [PubMed] [Google Scholar]
- Nicolet CM, Craig EA. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3638–3646. doi: 10.1128/mcb.9.9.3638.0270-7306(1989)009<3638:IACOSA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MC, Flaherty KM, McKay DB. Lysine 71 of the chaperone protein Hsc70 is essential for ATP hydrolysis. J Biol Chem. 1996;271:15874–15878. doi: 10.1074/jbc.271.27.15874.0021-9258(1996)271<15874:LOTCPH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Odunuga OO, Hornby JA, Bies C, Zimmermann R, Pugh DJ, Blatch GL. Tetratricopeptide repeat motif-mediated Hsc70-mSTI1 interaction. Molecular characterization of the critical contacts for successful binding and specificity. J Biol Chem. 2003;278:6896–6904. doi: 10.1074/jbc.M206867200.0021-9258(2003)278<6896:TRMHIM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Owens-Grillo JK, Hoffmann K, Hutchison KA, Yem AW, Deibel MR Jr, Handschumacher RE, Pratt WB. The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor. J Biol Chem. 1995;270:20479–20484. doi: 10.1074/jbc.270.35.20479.0021-9258(1995)270<20479:TCAICA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ, Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992;89:10974–10978. doi: 10.1073/pnas.89.22.10974.0027-8424(1992)089<10974:EACOHF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol. 2001;308:795–806. doi: 10.1006/jmbi.2001.4595.0022-2836(2001)308<0795:FAOTHH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Nair SC, Rimerman RA, Smith DF. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an Hsp70-binding protein. Mol Endocrinol. 1996a;10:420–431. doi: 10.1210/mend.10.4.8721986.0888-8809(1996)010<0420:MCOHPA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Smith DF. Mutation of Hip's carboxy-terminal region inhibits a transitional stage of progesterone receptor assembly. Mol Cell Biol. 1998;18:944–952. doi: 10.1128/mcb.18.2.944.0270-7306(1998)018<0944:MOHCRI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Toran EJ, Rimerman RA, Smith DF. Mutational analysis of the hsp70-interacting protein Hip. Mol Cell Biol. 1996b;16:6200–6207. doi: 10.1128/mcb.16.11.6200.0270-7306(1996)016<6200:MAOTHP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Silverstein AM, Galigniana MD. A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37. Cell Signal. 1999;11:839–851. doi: 10.1016/s0898-6568(99)00064-9.0898-6568(1999)011<0839:AMFTCT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303.0163-769X(1997)018<0306:SRIWHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201.0071-3384(2003)228<0111:ROSPFA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3:301–323. doi: 10.2174/1568009033481877.1568-0096(2003)003<0301:SAFROH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prodromou C, Siligardi G, and O'Brien R. et al. 1999 Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 18:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001.0022-538X(2001)075<8968:AVTMGT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Li W, and Zhong L. et al. 2003 Adeno-associated virus type 2-mediated gene transfer: role of cellular T-cell protein tyrosine phosphatase in transgene expression in established cell lines in vitro and transgenic mice in vivo. J Virol. 77:2741–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85:147–157. doi: 10.1016/s0960-0760(03)00221-8.0960-0760(2003)085<0147:MKCMFO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Radanyi C, Chambraud B, Baulieu E. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci U S A. 1994;91:11197–11201. doi: 10.1073/pnas.91.23.11197.0027-8424(1994)091<11197:TAOTIF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railson JE, Lawrence K, Buddle JC, Pennica D, Latchman DS. Heat shock protein-56 is induced by cardiotrophin-1 and mediates its hypertrophic effect. J Mol Cell Cardiol. 2001;33:1209–1221. doi: 10.1006/jmcc.2001.1384.0022-2828(2001)033<1209:HSPIIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Chinkers M. Identification of potential physiological activators of protein phosphatase 5. Biochemistry. 2002;41:5625–5632. doi: 10.1021/bi016090h.0006-2960(2002)041<5625:IOPPAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Russell LC, Whitt SR, Chinkers M. Overlapping sites of tetratricopeptide repeat protein binding and chaperone activity in heat shock protein 90. J Biol Chem. 2000;275:17857–17862. doi: 10.1074/jbc.M001625200.0021-9258(2000)275<17857:OSOTRP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Carrello A. Cyclophilin 40 (CyP-40), mapping of its hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for hsp90 binding. J Biol Chem. 1996;271:2961–2965. doi: 10.1074/jbc.271.6.2961.0021-9258(1996)271<2961:CCMOIH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429.0021-972X(1999)084<0663:GRITSM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Richter K, Muschler P, Hainzl O, Reinstein J, Buchner J. Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the ATPase cycle. J Biol Chem. 2003;278:10328–10333. doi: 10.1074/jbc.M213094200.0021-9258(2003)278<10328:SIANIO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, and Chirillo SC. et al. 2003 The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 22:1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LC, Whitt SR, Chen MS, Chinkers M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J Biol Chem. 1999;274:20060–20063. doi: 10.1074/jbc.274.29.20060.0021-9258(1999)274<20060:IOCRRF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sanchez ER. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990;265:22067–22070.0021-9258(1990)265<22067:HANHSP>2.0.CO;2 [PubMed] [Google Scholar]
- Sanchez ER, Toft DO, Schlesinger MJ, Pratt WB. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985;260:12398–12401.0021-9258(1985)260<12398:ETTKPA>2.0.CO;2 [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three new world primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696.1095-6840(2001)124<0152:OOTFIF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scammell JG, Hubler TR, Denny WB, Valentine DL. Organization of the human FK506-binding immunophilin FKBP52 protein gene (FKBP4) Genomics. 2003;81:640–643. doi: 10.1016/s0888-7543(03)00090-9.0888-7543(2003)081<0640:OOTHFI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2.0092-8674(2000)101<0199:SOTDCC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schuh S, Yonemoto W, Brugge J, Bauer VJ, Riehl RM, Sullivan WP, Toft DO. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985;260:14292–14296.0021-9258(1985)260<14292:ADBPCT>2.0.CO;2 [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224.0021-9258(1997)272<16224:PPIAMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 2003;100:868–873. doi: 10.1073/pnas.0231020100.0027-8424(2003)100<0868:SOTLFP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860.0888-8809(1993)007<1418:DOHSPP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith DF, Baggenstoss BA, Marion TN, Rimerman RA. Two FKBP-related proteins are associated with progesterone receptor complexes. J Biol Chem. 1993a;268:18365–18371.0021-9258(1993)268<18365:TFPAAW>2.0.CO;2 [PubMed] [Google Scholar]
- Smith DF, Faber LE, Toft DO. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990a;265:3996–4003.0021-9258(1990)265<3996:POUPRA>2.0.CO;2 [PubMed] [Google Scholar]
- Smith DF, Schowalter DB, Kost SL, Toft DO. Reconstitution of progesterone receptor with heat shock proteins. Mol Endocrinol. 1990b;4:1704–1711. doi: 10.1210/mend-4-11-1704.0888-8809(1990)004<1704:ROPRWH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith DF, Stensgard BA, Welch WJ, Toft DO. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992;267:1350–1356.0021-9258(1992)267<1350:AOPRWH>2.0.CO;2 [PubMed] [Google Scholar]
- Smith DF, Sullivan WP, Marion TN, Zaitsu K, Madden B, McCormick DJ, Toft DO. Identification of a 60-kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol Cell Biol. 1993b;13:869–876. doi: 10.1128/mcb.13.2.869.0270-7306(1993)013<0869:IOAKSP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai PK, Maeda Y, Nakao K, Wakim NG, Duhring JL, Faber LE. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986;25:5269–5275. doi: 10.1021/bi00366a043.0006-2960(1986)025<5269:AKPAWP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887.0261-4189(1997)016<4887:BMTCAO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Dornan J, Carrello A, Minchin RF, Ratajczak T, Walkinshaw MD. Two structures of cyclophilin 40 folding and fidelity in the tpr domains. Structure (Camb) 2001;9:431–438. doi: 10.1016/s0969-2126(01)00603-7.0969-2126(2001)009<0431:TSOCFA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab. 2003;88:277–284. doi: 10.1210/jc.2002-020354.0021-972X(2003)088<0277:GIILFM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200.0021-9258(2002)277<26573:DTPCOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ward BK, Allan RK, and Mok D. et al. 2002 A structure-based mutational analysis of cyclophilin 40 identifies key residues in the core tetratricopeptide repeat domain that mediate binding to Hsp90. J Biol Chem. 277:40799–40809. [DOI] [PubMed] [Google Scholar]
- Wegele H, Haslbeck M, Reinstein J, Buchner J. Sti1 is a novel activator of the Ssa proteins. J Biol Chem. 2003;278:25970–25976. doi: 10.1074/jbc.M301548200.0021-9258(2003)278<25970:SIANAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Weigel NL 1996 Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 319(Pt 3). 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr T, Bukau B, Buchberger A. Protein turnover: a CHIP programmed for proteolysis. Curr Biol. 2002;12:R26–R28. doi: 10.1016/s0960-9822(01)00644-3.0960-9822(2002)012<R26:PTACPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22:1399–1403. doi: 10.1093/carcin/22.9.1399.0143-3334(2001)022<1399:SIFOTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon I, Honkanen RE. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–8857. doi: 10.1021/bi990842e.0006-2960(1999)038<8849:TPPTPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]