Abstract
Heat shock proteins (Hsps) are constitutively expressed in cells and involved in protein folding, assembly, degradation, intracellular localization, etc, acting as molecular chaperones. However, their overexpression represents a ubiquitous molecular mechanism to cope with stress. Hsps are classified into families, and among them the Hsp70 family appears to be the most evolutionary preserved and distributed in animals. In this study, the expression of Hsp70 and the related messenger ribonucleic acid (mRNA) has been studied in Ostrea edulis after exposure to heat and heavy metals; moreover, levels of metallothioneins (MTs), another class of stress-induced proteins, have contemporaneously been assessed in the same animals. Thermal stress caused the expression of a 69-kDa inducible isoform in gills of O edulis but not in the digestive gland. Northern dot blot analysis confirmed that the transcription of Hsp69-mRNA occurs within 3 hours of stress recovery after oyster exposure at 32 and 35°C. Hsp69-mRNA transcripts were not present in the gills of animals exposed to 38°C after 3 hours of poststress recovery, but they were detected after 24 hours. The expression of the 69-kDa protein in O edulis exposed to 38°C was rather low or totally absent, suggesting that the biochemical machinery at the base of the heat shock response is compromised. Together with the expected increase in MT content, the oysters exposed to Cd showed a significant enhancement of Hsp70, although there was no clear appearance of Hsp69. Interestingly, the levels of MT were significantly increased in the tissues of individuals exposed to thermal stress. Unlike oysters, heat did not provoke the expression of inducible Hsp isoforms in Mytilus galloprovincialis, Tapes philippinarum, and Scapharca inaequivalvis, although it significantly enhanced the expression of constitutive proteins of the 70-kDa family. The expression of newly synthesized Hsp70 isoforms does not seem therefore a common feature in bivalves exposed to thermal stress.
INTRODUCTION
Cell response to unfavorable conditions includes transient enhancement of the expression of polypeptides known as heat shock proteins (Hsps). Hsps are classified into families and the best known have a molecular weight of 60, 70, and 90 kDa; amongst them, the Hsp70 family appears to be the most evolutionary preserved and distributed in animals (De Maio 1999; Feder and Hofmann 1999). Within the same family, some Hsp isoforms exhibit detectable levels in unstressed cells and, therefore, are addressed as constitutive proteins; others are induced in response to stress exposure and, therefore, are addressed as inducible proteins (Lindquist 1986).
The discovery of Hsps was consequent to heat exposure (Lindquist and Craig 1988), and heat does remain the prototypical stimulus eliciting their expression (Scharf et al 1998); however, many other stimuli are known to alter Hsp biosynthesis, such as osmotic (Tirard et al 1997) and oxidative stress (Mager et al 2000), hypoxia (Chang et al 2000), magnetic field (Lin et al 1997), and a number of other physical or chemical stressors (Snyder et al 2001). All these disturbances are known to affect the tertiary structure of proteins, and, therefore, are detrimental to cell homeostasis. The protective role of Hsps is widely documented (Morimoto et al 1997; Vayssier and Polla 1998; Santoro 2000), and all animals studied so far seem to share Hsp overexpression as a universal mechanism for cell survival, with the exception of Hydra oligactis (Bosch et al 1988) and the Antarctic fish Trematomus bernacchii (Hofmann et al 2000). An important role has emerged regarding cross-protective effects of Hsps because their overexpression induced by a mild stress seems to protect cells against otherwise lethal exposure to other stress stimuli. This observation is considered of primary importance and is at the base of new therapeutic strategies against human diseases (Morimoto and Santoro 1998; Santoro 2000).
In recent years, interest has grown regarding the cytoprotective and cross-protective role of Hsps in bivalves, often exposed and highly resistant to a variety of environmental stress stimuli. For example, the possibility that a mild thermal stress could enhance the survival of the oyster population subject to mass mortality has been explored (Clegg et al 1998). Some bivalves, mainly oysters and mussels, are used as sentinel organisms for environmental biomonitoring, and the usefulness of Hsp70 biosynthesis as a biomarker of stress has been suggested, although still under debate (Pyza et al 1997).
Our previous work focused on the heat shock response in the European oyster, Ostrea edulis (Piano et al 2002). A characterization of the Hsp70 expression after thermal stress was performed, showing in particular the expression of an inducible 69-kDa isoform sharply appearing in gills and mantle after exposure at 32°C or more. This study has been carried out to elucidate the Hsp69 expression and related messenger ribonucleic acid (mRNA) transcription after heat exposure in O edulis and to establish whether the appearance of the 69-kDa isoform is a common feature of bivalves. Moreover, the Hsp70 expression has been evaluated in the gills and digestive glands of oysters exposed to heavy metals. Such evaluation has been carried out in parallel with the assessment of metallothioneins (MTs), low–molecular weight soluble polypeptides rich in SH groups, with high affinity for IB and IIB metal ions, known to be involved in heavy-metal homeostasis and overexpressed at increasing metal concentrations in the environment (Viarengo et al 1999a). MTs are widely regarded as indices of metal contamination and, therefore, are used as tools for biomonitoring programs (Viarengo et al 1999b). These experiments were addressed to evaluate the pattern of expression of both groups of cytoprotective proteins in different tissues of oysters exposed to the same stress stimuli.
MATERIALS AND METHODS
Animals
Oysters and other bivalves were collected by fishermen of “La Bussola” (Rimini, Italy) along the coast of the northwestern Adriatic Sea. Bivalves were maintained in aquaria containing 60 L of aerated artificial 34 ppt sea-water at 18°C, under natural photoperiod. They were fed once a day with appropriate algal slurry (Liquifry Marine; Interpet Ltd, Dorking, UK).
Thermal stress
Bivalves were transferred to a water bath set at the selected temperature for the appropriate time according to the experimental protocol and then returned to 18°C for 1 to 24 hours.
About 50% of the O edulis exposed to 38°C for 1 hour died within 2–3 hours of poststress recovery. All other bivalves survived to 1-hour heat shock at all temperatures tested. Tissues (gills and digestive gland) were rapidly dissected, frozen in liquid nitrogen, and stored at −80°C until analyzed.
Heavy-metal exposure
Ten oysters per treatment were exposed to increasing concentrations (100–500 μg/L) of CdCl2, ZnCl2, or their combinations. Water was renewed every day. All individuals survived the 1-week exposure to heavy metals. At the end of the treatment, tissues were rapidly excised, frozen in liquid nitrogen, and stored at −80°C until use.
Hsp70 determination
Tissues were separately homogenized in an ice-cold 10 mM sodium phosphate buffer, pH 7.4, containing 1% Nonidet-P40; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate (SDS); 1 μg/mL of pepstatin A, E-64, bestatin, leupeptin, and aprotinin; and 25 μg/mL of phenylmethane sulfonyl fluoride. After centrifugation at 500 × g for 5 minutes, supernatants (SNs) were diluted 1:1 with a Laemmli buffer (Laemmli 1970) and boiled for 5 minutes. Fifteen micrograms of homogenate proteins was loaded onto 10% polyacrylamide gels. Electrophoresis was carried out with a Mini Protean III apparatus (28 mA, 2 hours at 4°C), and the resolved proteins were transferred onto a nitrocellulose membrane (300 mA, 1 hour at 4°C). Blots were probed with anti-Hsp70 rat monoclonal antibody; after washings, they were probed with rabbit anti-rat immunoglobulin G (IgG) polyclonal antibody conjugated with horseradish peroxidase. Immunoblots were developed by an enhanced chemiluminescence reagent, and a densitometric analysis of the films was performed by Image Master (Amersham-Pharmacia, Milan, Italy) equipped with TotalLab software.
Hsp70-mRNA detection
The total RNA was extracted from oyster gills by application of 1 mL of TRIzol® reagent to approximately 20 mg of fresh tissue, using the protocol described by Chomezynski and Sacchi (1987). The RNA was quantified by ultraviolet absorbance at 260 nm. Integrity of the total RNA was confirmed by 1% formaldehyde agarose gel electrophoresis. For the Northern dot blot analysis, 20 μg aliquot of total RNA for each sample was spotted onto a nitrocellulose membrane. The nitrocellulose was allowed to air dry and then baked at 80°C for 2 hours. Blots were prehybridized for 30 minutes at 65°C and then hybridized overnight to the fluorescein-labeled molecular probes in a water bath. Immunodetection of the probe hybridization was accomplished by antifluorescein antibodies conjugated to alkaline phosphatase in the presence of a detection reagent. The chemiluminescent visualization was carried out by exposing blots to Hyperfilm™ECL™ for 4–24 hours. Changes in the level of the expression were determined by densitometric analysis. The linearity of the signal was assessed in the range 10–25 μg of the total RNA; 20 μg was chosen to ensure a good signal and to avoid loading more than 20 μL/well.
Development of molecular probes
Probes were developed by cloning the complementary DNA (cDNA) obtained by reverse transcriptase-polymerase chain reaction (RT-PCR) of the total RNA extracted from heat shocked (1 hour at 35°C) oysters. The cDNA fragment of O edulis Hsp69 clone D2 (GenBank accession number: AF416609) was amplified by PCR using Hsp INT-F: 5′CAACCGCAGGGGACACTCATC3′ as forward primer and Hsp INT-R: 5′GTCATTGCTCGTTCCCCCTCA3′ as reverse primer and extracted from agarose gel using Ultrafree®-DA vials. The fluorescein-labeled probe was developed using the Gene Images random prime labeling module. To confirm its specificity, the probe was tested by Northern blotting (data not shown).
Metallothionein quantification
MTs were evaluated in different tissues of O edulis according to Viarengo et al (1997) using the Ellman reagent DTNB (5,5′-dilthiobis (2-nitrobenzoic acid)) (Ellman 1959). Tissues were homogenized in a 20 mM Tris-HCl buffer, pH 8.6, supplemented with 0.5 M sucrose, 0.01% β-mercaptoethanol, and a protease inhibitor cocktail. After centrifugation at 30 000 × g for 20 minutes, the SN was resuspended in 49% ethanol plus 3.7% chloroform and recentrifuged at 6000 × g for 10 minutes. Acidified cold 87% ethanol was then added to the SN, and the mixture was stored at −20°C for 1 hour. After centrifugation at 6000 × g for 10 minutes, the pellet was resuspended in 20 mM Tris-HCl buffer, pH 8.6, containing 87% ethanol and 1% chloroform and centrifuged at 6000 × g for 10 minutes. The pellet was dried under a nitrogen gas stream and resuspended in a solution containing 0.16 M NaCl, 0.5 N HCl, and 2 mM ethylenediamine-tetraacetic acid; 0.3 mL aliquots were then added with 4.2 mL of 0.43 M DTNB dissolved in 0.2 M phosphate buffer, pH 8.0, containing 2 M NaCl. Samples were centrifuged at 3000 × g for 5 minutes at room temperature and absorbance of the clear SN determined at 412 nm using reduced glutathione as the reference standard.
Data analysis
Densitometric scanning of the films was performed by ImageMaster equipped with TotalLab software (Amersham-Pharmacia). Data have been subjected to 1-way analysis of variance (SigmaStat, SPSS) to test for significance, and a statistical difference was accepted when P < 0.05. Given the inherent variation between film development, data were normalized to standard samples that had been included in each experimental protocol.
Chemicals
Anti-Hsp70 rat monoclonal antibody was purchased from Affinity BioReagents (Vinci Biochem, Vinci, Italy) and TRIzol® reagent from Invitrogen (San Giuliano Milanese, Milan, Italy). Gene Images CDP-Star detection module, Gene Images random prime labeling module, Hybond N3; nitrocellulose, Hyperfilm™ECL™, and other products for Northern dot blot analysis were from Amersham-Pharmacia Biotech (Milan, Italy). Ultrafree®-DA vials were from Millipore (Milan, Italy). Anti-rat IgG conjugated with horseradish peroxidase, acrylamide, N-N′-bisphenylacrylamide, TEMED N,N,N′N′-tetramethyl-ethylene-diamine, APS ammonium persulfate, protease inhibitor cocktail, Nonidet P-40, Tween 20, SDS, 2-mercaptoethanol, glycine, Kodak emulsion film, and all other reagents were from Sigma-Aldrich (Milan, Italy).
RESULTS
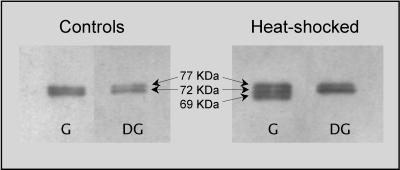
The expression of Hsp70 in the gills and digestive glands of O edulis is illustrated in Figure 1. Two bands of molecular weight estimated at about 77 and 72 kDa are shown in the gills and in the digestive glands of animals in physiological conditions; a 69-kDa protein is induced in gills after exposure to thermal stress (35°C for 1 hour, plus 24 hours of recovery at 18°C). Heat provokes overexpression of constitutive Hsp70 isoforms in the digestive gland, but no appearance of the 69-kDa protein is observed.
Fig 1.
Western blotting to detect proteins of the Hsp70 family in different tissues of Ostrea edulis (G = gills; DG = digestive gland). Heat shocked animals were exposed to 35°C for 1 hour and allowed to recover in aquaria at 18°C for 24 hours before collecting samples. A representative immunoblot out of 5 separate experiments is reported. Hsp, heat shock protein
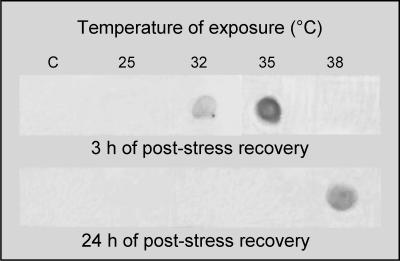
Sequencing of inducible hsp70 genes (GenBank accession number AF416609 and AF416608) allowed us to obtain specific probes to assess the pattern of the Hsp69-mRNA expression in the different tissues after thermal stress. A specific fluorescein-labeled molecular probe was developed by cloning the cDNA obtained by RT-PCR of the total RNA extracted from heat-shocked oysters. To provide information on the Hsp69-mRNA expression after thermal stress at different temperatures, a Northern dot blot analysis was carried out. In Figure 2, the upper panel shows results from a representative experiment during which oysters were exposed to increasing temperatures and allowed to recover for 3 hours at 18°C. Transcripts appear after heat shock at 32°C and further increase after exposure at 35°C. Transcripts are not detected in tissues from unstressed oysters or from animals exposed to 25°C. Interestingly, the Hsp69-mRNA expression is not shown by gills of individuals exposed to 38°C and allowed to recover for 3 hours. However, transcripts clearly appear after 24 hours of recovery period, as shown in Figure 2, lower panel. On the contrary, the transcription of Hsp69-mRNA was not carried out in oysters exposed at a temperature lower than 38°C, when examined 24 hours after the heat shock. The probe never detected the Hsp69-mRNA expression in the digestive glands of oysters exposed to thermal stress (data not shown).
Fig 2.
Northern dot blot analysis in gills of controls (C) and thermal-stressed Ostrea edulis exposed to different temperatures for 1 hour and allowed to recover at 18°C for 3 hours (upper panel) or 24 hours (lower panel)
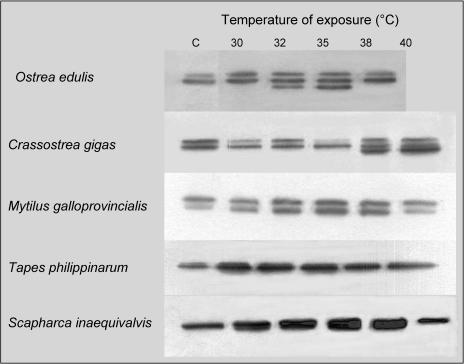
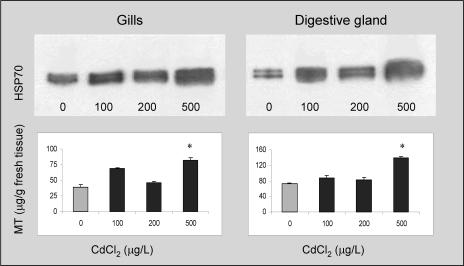
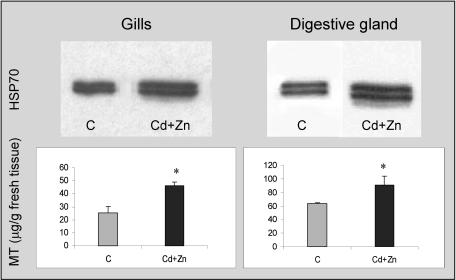
The expression of newly synthesized Hsp70 isoforms does not seem to be a common feature in bivalves exposed to thermal stress. Individuals from different species were exposed to different temperatures for 1 hour and the synthesis of Hsp70 analyzed in their tissues after 24 hours of recovery (Fig 3). Regarding O edulis, we can observe the appearance of the 69-kDa protein at temperatures of 32°C and 35°C; at 38°C, Hsp70 is poorly overexpressed, and Hsp69 is not present or is extremely scarce. Besides O edulis, Crassostrea gigas also displays 2 constitutive isoforms in control conditions and a third lower–molecular weight isoform after thermal stress; this protein is well expressed at 38°C and 40°C, as previously shown by Clegg et al (1998). On the contrary, heat does not provoke the expression of inducible Hsp isoforms in Mytilus galloprovincialis, Tapes philippinarum, and Scapharca inaequivalvis, although it significantly enhances the expression of constitutive proteins of the 70-kDa family. Similar results also were obtained at a lower percentage (8%) of polyacrylamide, but we cannot exclude the possibility that the members of the Hsp70 family in clams can be better resolved by using different experimental approaches such as 2-dimensional electrophoresis. The effects of Cd and Zn on the O edulis Hsp70 expression were evaluated (Fig 4, upper panels). Oysters exposed to different Cd concentrations (within the range 100–500 μg/ L/d) for 7 days show a dose-dependent enhancement of the Hsp70 expression in their gills compared with control animals (322% at the highest Cd concentration). Similarly, Cd induces significant overexpression of Hsp70 in the digestive gland, up to a maximum of 380% compared with control levels. Concentrations lower than 100 μg/L/d caused insignificant effects (data not shown). The image analysis did not indicate clear appearance of the 69-kDa isoform in any tissue after Cd exposure. In the gills and digestive glands of the same animals, levels of MTs were also assessed (Fig 4, lower panels). As expected, oysters treated with Cd show a significant increase in MT content (208% and 160% at 500 μg Cd for gills and digestive glands, respectively). Similar experiments were carried out in the presence of Zn (100–500 μg/L/d), but oysters did not show changes in either the Hsp70 expression or MT levels (data not shown). To investigate the possible interaction between the 2 heavy metals and their integrated effect on oyster tissues, we evaluated the Hsp expression and MT levels in the gills and digestive glands of animals exposed to the combinations Cd + Zn. In both tissues, a significant overexpression of Hsp70 (270% and 297% vs control levels, respectively) is observed in response to Cd + Zn at the highest concentration tested (Fig 5), although the image analysis does not highlight the presence of the lower–molecular weight inducible protein. Significant enhancement of MTs (182% and 145% vs control levels, respectively) is also obtained, but the effect of the combination is always lower than that of Cd alone.
Fig 3.
Western blotting to detect proteins of the Hsp70 family in gills of marine bivalves in control conditions (C) or exposed to different temperatures for 1 hour and allowed to recover at 18°C for 24 hours. For each bivalve species, samples from the same experiment were all blotted together. A representative immunoblot out of at least 3 separate experiments is reported. Hsp, heat shock protein
Fig 4.
Heat shock protein 70 expression (upper panels) and metallothionein content (lower panels) in the gills and digestive gland of Ostrea edulis in control conditions or treated with CdCl2 (100, 200, and 500 μg/L/d) for 1 week. A representative immunoblot out of 3 separate experiments is reported. Samples from the same experiment were all blotted together. Metallothionein values represent the mean ± SE of at least 3 separate experiments. *P < 0.01 vs control levels
Fig 5.
Heat shock protein 70 expression (upper panels) and metallothionein content (lower panels) in the gills and digestive gland of Ostrea edulis in control conditions (C) or treated with CdCl2 + ZnCl2 (500 μg/L/d) for 1 week. A representative immunoblot out of 3 separate experiments is reported. Samples from the same experiment were all blotted together. *P < 0.01 vs control levels
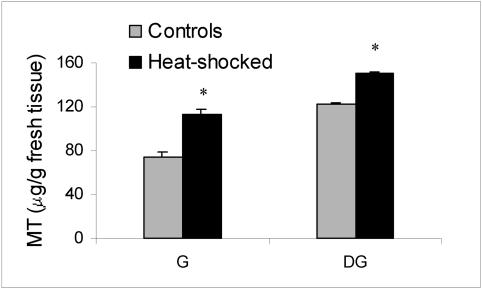
MT levels were also assessed in oysters not treated with heavy metals but exposed to thermal stress for 1 hour at 35°C and allowed to recover at 18°C for 24 hours. Interestingly, the MT levels in both gills and digestive glands are significantly increased by heat (Fig 6).
Fig 6.
Metallothionein levels in gills (G) and digestive gland (DG) of Ostrea edulis exposed to thermal stress (35°C, 1 hour) and allowed to recover at 18°C for 24 hours. *P < 0.01 vs basal levels
DISCUSSION
Hsps are constitutively expressed in cells and involved in protein folding, assembly, degradation, intracellular localization, etc, acting as molecular chaperones (Fink 1999). However, evidence is available that transient acceleration of hsp gene transcription is essential for cell survival after stress exposure. In general, the Hsp overexpression represents a ubiquitous molecular mechanism to cope with stress, but animals show individual responses with different thresholds of sensitivity and tissue specificity (Hofmann 1999). The protein expression analysis indicated the appearance of a 69-kDa Hsp isoform in gills of oysters exposed to thermal stress. The expression of such lower–molecular weight isoforms was first observed in the gills of C gigas (Clegg et al 1998), and we have shown that this is true also for the mantle of oysters exposed to a temperature of 32°C or more (Piano et al 2002). The Hsp69-mRNA expression analysis reported in this study confirmed that thermal stress induces new synthesis of the 69-kDa protein in gills. Accordingly, transcripts were not detected in control animals and in oysters exposed to 25°C, the temperature at which no protein overexpression was detected by Western blotting. Interestingly, the Hsp69-mRNA was not transcribed in the gills of animals exposed to 38°C within 3 hours of recovery. This is well in agreement with the absence or extremely low expression of the related protein observed in the gills of O edulis by Western blotting (Fig 3); the lack of the cytoprotective response could be related to the high bivalve mortality observed after this treatment. To further analyze such phenomena, the Hsp69-mRNA expression caused by 1 hour of heat shock at 38°C was assessed after 24 hours of recovery; Northern dot blot analysis indicated a significant but late mRNA transcription. We can argue that the high temperature partially compromises the biochemical machinery at the base of the heat shock response (both the mRNA transcription or protein translation, or both), which is indeed delayed or reduced, with possible lethal consequence for the animals. In contrast to the gills, Hsp69-mRNA and the corresponding protein were never detected in the digestive glands of oysters exposed to thermal stress. These results might suggest that the oyster digestive glands are scarcely affected by heat and possibly that Hsp70 only acts as a constitutive molecular chaperone in this tissue. Different tissue specificity and temporal patterns of Hsp induction have therefore been observed in O edulis, in agreement with similar observations reported for other organisms in response to stress (Sanders et al 1994; Smith et al 1999). As observed by other authors (Hofmann and Somero 1995), different constitutive members of the Hsp70 family are present in the gills of unstressed Mitilidae; in our experimental conditions, the exposure to temperatures ≥32°C provoked the overexpression of such proteins, without transient enhancement of any inducible isoform. To our knowledge, no information is available as to the heat shock response in the 2 clams T philippinarum and S inaequivalvis, besides this study. Two constitutive Hsp70 isoforms appeared in the gills of clams under our experimental conditions and, similar to mussels, exposure to temperatures ≥30°C provoked their significant overexpression. All mollusks, however, showed a lower expression of Hsp70 at the highest temperature tested (38°C for O edulis and 40°C for the other bivalves).
Hsps of the 70-kDa family have proven useful as a part of a suite of stress indices for environmental biomonitoring (Snyder et al 2001). Their use has also been criticized because of the variability of their basal levels in organisms and the variety of stress stimuli, besides heat, responsible for their production (Pyza et al 1997). In agreement with other authors (Feder and Hofmann 1999), we have indeed observed that the expression of constitutive Hsp isoforms is highly fluctuating (Piano et al 2002) in the presence or absence of stimuli. In the lucky case of the oysters (O edulis and C gigas), however, the sharp appearance of the Hsp69 may represent per se a clear response to stress, without the need for further quantification. The nonspecificity of the Hsp response remains, however, a limit for the use of these proteins as stress indices, although their evaluation may give interesting indications if used within a battery of biomarkers.
It is a matter of fact that other stimuli besides heat evoke the “heat shock response” in animal tissues, from changes in salinity and hypoxia to hydrocarbon and heavy-metal exposure (Snyder et al 2001). Cd has been shown to induce the Hsp expression in mammalian (Beyersmann and Hechtenberg 1997) and nonmammalian cells (Carginale et al 2002). Moreover, the accumulation of Cd has been documented for a long time in gills of oysters and other bivalves, and Cd has been reported to stimulate a net increase in MT in several tissues (Viarengo et al 1980, 1985; Roesijadi and Klerks 1989; Bebianno et al 1993; Roméo and Gnassia-Barelli 1995; Lemoine et al 2000). In our study, the digestive gland was used as the tissue mostly involved in the detoxification phenomena, and gills were chosen on the basis of previous data indicating that in bivalve species dissolved metals are mostly accumulated by these organs, where the greatest MT response is also observed (Viarengo et al 1985; Roesijadi 1986). Exposure to increasing Cd concentrations elicited significant increment of both Hsp70 as well as MTs in the 2 tissues. These data indicate that the same stress stimulus, namely Cd, is able to simultaneously enhance the expression of 2 different classes of cytoprotective proteins and that the biochemical machinery at the base of the “heat shock response” is activated at the same Cd concentrations eliciting a significant increase in MTs.
Tissues of oysters exposed to Zn did not show changes in the Hsp70 or MT content at any concentration tested. Unclear observations about the effect of Zn on MT accumulation are reported, but in general, this metal is considered a weaker inducer of MTs in comparison to Cd (Roesijadi 2000). Zn has also been specifically reported as a weak inducer of MT synthesis in oysters (Alonso and Martin-Mateo 1996).
The combination Cd + Zn was tested at the highest metal concentration (500 μg/L) used in previous experiments, and it caused significant increment of the Hsp70 expression and the MT content in both the gills and the digestive gland. Remarkably, in all cases, the effect of the combination Cd + Zn was lower than the effect of Cd alone. This could be ascribed to a lower water-filtering rate by the animal exposed to such high amounts of contaminants; however, an interaction between the metals or a competition during their cellular uptake could be another possible explanation for the reduced effect of Cd. In Perna viridis, for example, the preexposure to Zn has a negative effect on Cd assimilation and potentially reduces Cd uptake (Blackmore and Wang 2002); Meshitsuka et al (1987) reported that cadmium uptake is inhibited by Cu(II) and affected by Zn(II) in cultured cells.
A last but interesting observation has been produced by some experiments carried out to evaluate the Hsp70 expression after heat exposure, in which the gill and digestive gland MT content has also been measured. As shown in Figure 6, in both tissues, the MT level had significantly increased after 1 hour exposure at 35°C. Previous data (Serafim et al 2002) showed that MT synthesis induced by heavy metals is greater at higher temperature, a phenomenon easily correlated with the increased metabolism caused by the higher water temperature. They also noted, however, that MT levels in noncontaminated mussels kept at 25°C were significantly higher than in those kept at 5°C. Both reports, therefore, suggest that a direct effect of temperature on the MT gene expression must be taken into account that would imply the presence of a heat-inducible element on the MT-gene promoters. This hypothesis is indeed supported by a recent study in which a heat shock factor–binding site has been found along the distal part of a M galloprovincialis MT promoter sequence subjected to functional analysis using specific algorithms (Dondero and Viarengo 2001). More work is needed in this direction.
Taken together, the present observations give further insights into the tissue- and species-specificity of the heat shock response; moreover, the simultaneous activation of 2 different cytoprotective mechanisms in oysters exposed to Cd or to heat was shown. The physiological meaning of such effects will challenge future research, leading to a more complete knowledge of bivalve response to stress and in general to a better understanding of cross-protection phenomena.
Acknowledgments
The research has been granted by Ministero dell'Università e della Ricerca Scientifica (MURST, ex 40% 2000). A special thanks to Dr Fausto Tinti for his valuable suggestions and to Mrs Leda Dewaure for improving the language of the manuscript.
REFERENCES
- Alonso JI, Martin-Mateo MC. Induction and characterization of metallothionein in different organs of Ostrea edulisL. Biol Trace Elem Res. 1996;53:85–94. doi: 10.1007/BF02784547.0163-4984(1996)053<0085:IACOMI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bebianno MJ, Nott J, Langston WJ. Cadmium metabolism in the clam Ruditapes decussata: the role of metallothioneins. Aquat Toxicol. 1993;27:315–334.0166-445X(1993)027<0315:CMITCR>2.0.CO;2 [Google Scholar]
- Beyersmann D, Hechtenberg S. Cadmium, gene regulation, and cellular signalling in mammalian cells. Toxicol Appl Pharmacol. 1997;144:247–261. doi: 10.1006/taap.1997.8125.0041-008X(1997)144<0247:CGRACS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Blackmore G, Wang WX. Uptake and efflux of Cd and Zn by the green mussel Perna viridis after metal preexposure. Environ Sci Technol. 2002;36:989–995. doi: 10.1021/es0155534.0013-936X(2002)036<0989:UAEOCA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bosch TC, Krylow SM, Bode H, Steele RE. Thermotolerance and synthesis of heat shock proteins: these responses are present in Hydra attenuata but absent in Hydra oligactis. Proc Natl Acad Sci U S A. 1988;85:7927–7931. doi: 10.1073/pnas.85.21.7927.0027-8424(1988)085<7927:TASOHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carginale V, Capasso C, Scudiero R, Parisi E. Identification of cadmium-sensitive genes in the Antarctic fish Chionodraco hamatus by messenger RNA differential display. Gene. 2002;299:117–124. doi: 10.1016/s0378-1119(02)01020-x.0378-1119(2002)299<0117:IOCGIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chang J, Knowlton AA, Wasser JS. Expression of heat shock proteins in turtle and mammal hearts: relationship to anoxia tolerance. Am J Physiol Regul Integr Comp Physiol. 2000;278:209–214. doi: 10.1152/ajpregu.2000.278.1.R209.0363-6119(2000)278<0209:EOHSPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chomezynski P, Sacchi N. Single step method of RNA isolation by acid guanidinum thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999.0003-2697(1987)162<0156:SSMORI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clegg JS, Uhlingher KR, Jackson SA, Cherr GN, Rifkin E, Friedman CS. Induced thermotolerance and heat shock protein-70 family in Pacific oyster Crassostrea gigas. Mol Mar Biol Biotech. 1998;7:21–30.1053-6426(1998)007<0021:ITAHSP>2.0.CO;2 [Google Scholar]
- De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001.1073-2322(1999)011<0001:HSPFTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dondero F, Viarengo A 2001 Identification of a putative metallothionein promoter from the mediterranean mussel Mytilus galloprovincialis. 11 Int. Symp. on pollutant responses in marine organisms, PRIMO 11, Plymouth UK, 10–13 July 2001. Abs 1102. [Google Scholar]
- Ellman GL. Tissue sulphyhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6.0003-9861(1959)082<0070:TSG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243.0066-4278(1999)061<0243:HPMCAT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425.0031-9333(1999)079<0425:CPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hofmann G, Somero G. Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus. J Exp Biol. 1995;198:1509–1518. doi: 10.1242/jeb.198.7.1509.0022-0949(1995)198<1509:EFPDAE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hofmann GE. Ecologically relevant variant in induction and function of heat shock proteins in marine organism. Am Zool. 1999;39:889–900.0003-1569(1999)039<0889:ERVIIA>2.0.CO;2 [Google Scholar]
- Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN. Heat-shock protein expression is absent in the antarctic fish Trematomus bernacchii (family Nototheniidae) J Exp Biol. 2000;203:2331–2339. doi: 10.1242/jeb.203.15.2331.0022-0949(2000)203<2331:HPEIAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the heat of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0.0028-0836(1970)227<0680:COSPDT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lemoine S, Bigot Y, Sellos D, Cosson RP, Laulier M. Metallothionein isoform in Mytilus edulis (Mollusca, Bivalvia): complementary DNA characterization and quantification of expression in different organs after exposure to cadmium, zinc and copper. Mar Biotechnol. 2000;2:195–203. doi: 10.1007/s101269900026.1436-2228(2000)002<0195:MIIMEM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lin H, Opler M, Head M, Blank M, Goodman R. Electromagnetic field exposure induces rapid, transitory heat shock factor activation in human cells. J Cell Biochem. 1997;66:482–488. doi: 10.1002/(sici)1097-4644(19970915)66:4<482::aid-jcb7>3.0.co;2-h.0730-2312(1997)066<0482:EFEIRT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443.0066-4154(1986)055<1151:THSR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Ann Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022<0631:THP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mager WH, de Boer AH, Siderius MH, Voss HP. Cellular responses to oxidative and osmotic stress. Cell Stress Chaperones. 2000;5:73–75. doi: 10.1379/1466-1268(2000)005<0073:crtoao>2.0.co;2.1466-1268(2000)005<0073:CRTOAO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshitsuka S, Ishizawa M, Nose T. Uptake and toxic effects of heavy metal ions: interactions among cadmium, copper and zinc in cultured cells. Experientia. 1987;43:151–156. doi: 10.1007/BF01942833.0014-4754(1987)043<0151:UATEOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat shock response: regulation and function of heat shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29.0071-1365(1997)032<0017:THSRRA>2.0.CO;2 [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotech. 1998;16:833–838. doi: 10.1038/nbt0998-833.1087-0156(1998)016<0833:SRAHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Piano A, Asirelli C, Caselli F, Fabbri E. Hsp70 expression in thermally stressed Ostrea edulis, a commercially important oyster in Europe. Cell Stress Chaperones. 2002;7:250–257. doi: 10.1379/1466-1268(2002)007<0250:heitso>2.0.co;2.1466-1268(2002)007<0250:HEITSO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyza E, Mak P, Kramarz P, Laskowski R. Heat shock proteins (HSP70) as biomarkers in ecotoxicological studies. Ecotoxicol Environ Saf. 1997;38:244–251. doi: 10.1006/eesa.1997.1595.0147-6513(1997)038<0244:HSPHAB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roesijadi G. Mercury-binding proteins from the marine mussel, Mytilus edulis. Environ Health Perspect. 1986;65:45–48. doi: 10.1289/ehp.866545.0091-6765(1986)065<0045:MPFTMM>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesijadi G. Metal transfer as a mechanism for metallothionein-mediated metal detoxification. Cell Mol Biol (Noisy-le-grand) 2000;46:393–405.0145-5680(2000)046<0393:MTAAMF>2.0.CO;2 [PubMed] [Google Scholar]
- Roesijadi G, Klerks PL. Kinetic analysis of cadmium binding to metallothionein and other intracellular ligands in oyster gills. J Exp Zool. 1989;251:1–12.0022-104X(1989)251<0001:KAOCBT>2.0.CO;2 [Google Scholar]
- Roméo M, Gnassia-Barelli M. Metal distribution in different tissues and in subcellular fractions of the Mediterranean clam Ruditapes decussatus treated with cadmium, copper, or zinc. Comp Biochem Physiol. 1995;111C:457–463.0010-406X(1995)111C<0457:MDIDTA>2.0.CO;2 [Google Scholar]
- Sanders BM, Martin LS, Howe SR, Nelson WG, Hegre ES, Phelps D. Tissue-specific differences in accumulation of stress proteins in Mytilus edulis exposed to a range of copper concentrations. Toxicol Appl Pharmacol. 1994;125:206–213. doi: 10.1006/taap.1994.1066.0041-008X(1994)125<0206:TDIAOS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59:55–63. doi: 10.1016/s0006-2952(99)00299-3.0006-2952(2000)059<0055:HSFATC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scharf K-D, Hohfeld I, Nover L. Heat stress response and heat stress transcription factors. J Biosci. 1998;23:313–329.0250-5991(1998)023<0313:HSRAHS>2.0.CO;2 [Google Scholar]
- Serafim MA, Company RM, Bebianno MJ, Langston WJ. Effect of temperature and size on metallothionein synthesis in the gills of Mytilus galloprovincialis exposed to cadmium. Mar Environ. 2002;54:361–365. doi: 10.1016/s0141-1136(02)00121-6. [DOI] [PubMed] [Google Scholar]
- Smith TR, Tremblay GC, Bradley TM. Hsp70 and a 54 kDa protein (Osp54) are induced in salmon (Salmo salar) in response to hyperosmotic stress. J Exp Zool. 1999;284:286–298. doi: 10.1002/(sici)1097-010x(19990801)284:3<286::aid-jez6>3.0.co;2-j.0022-104X(1999)284<0286:HAAKPO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Snyder MJ, Girvetz E, Mulder EP. Induction of marine mollusc stress proteins by chemical or physical stress. Arch Environ Contam Toxicol. 2001;41:22–29. doi: 10.1007/s002440010217.0090-4341(2001)041<0022:IOMMSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tirard CT, Grossfeld RM, Levine JF, Kennedy-Stoskopf S. Effect of osmotic shock on protein synthesis of oyster hemocytes in vitro. Comp Biochem Physiol. 1997;116A:43–49.0010-406X(1997)116A<0043:EOOSOP>2.0.CO;2 [Google Scholar]
- Vayssier M, Polla BS. Heat shock proteins chaperoning life and death. Cell Stress Chaperones. 1998;3:221–227. doi: 10.1379/1466-1268(1998)003<0221:hspcla>2.3.co;2.1466-1268(1998)003<0221:HSPCLA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viarengo A, Burlando B, Cavaletto M, Marchi B, Ponzano E, and Blasco J 1999a Role of metallothionein against oxidative stress in mussel Mytilus galloprovincialis. Am J Physiol 277:1612–1619. [DOI] [PubMed] [Google Scholar]
- Viarengo A, Burlando B, Dondero F, Marrò A, Fabbri R. Metallothionein as a tool in biomonitoring programmes. Biomarkers. 1999b;4:455–466. doi: 10.1080/135475099230615.1354-750X(1999)004<0455:MAATIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Viarengo A, Palmero S, Zanicchi G, Capelli R, Vaissiere R, Orunesu M. Role of metallothionein in Cu and Cd accumulation and elimination in the gill and digestive gland cells of Mytilus galloprovincialis Lam. Mar Environ Res. 1985;16:23–36.0141-1136(1985)016<0023:ROMICA>2.0.CO;2 [Google Scholar]
- Viarengo A, Pertica M, Mancinelli G, Zanicchi G, Orunesu M. Rapid induction of copper-binding proteins in the gills of metal exposed mussels. Comp Biochem Physiol. 1980;67C:215–218. doi: 10.1016/0306-4492(80)90021-0.0010-406X(1980)67C<0215:RIOCPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Viarengo A, Ponzano E, Dondero F, Fabbri R. A simple spectrophotometric method for metallothionein evaluation in marine organism: an application to mediterranean and antarctic molluscs. Mar Environ Res. 1997;44:68–84.0141-1136(1997)044<0068:ASSMFM>2.0.CO;2 [Google Scholar]