Abstract
The high-affinity ligand-binding form of unactivated steroid receptors exists as a multicomponent complex that includes heat shock protein (Hsp)90; one of the immunophilins cyclophilin 40 (CyP40), FKBP51, or FKBP52; and an additional p23 protein component. Assembly of this heterocomplex is mediated by Hsp70 in association with accessory chaperones Hsp40, Hip, and Hop. A conserved structural element incorporating a tetratricopeptide repeat (TPR) domain mediates the interaction of the immunophilins with Hsp90 by accommodating the C-terminal EEVD peptide of the chaperone through a network of electrostatic and hydrophobic interactions. TPR cochaperones recognize the EEVD structural motif common to both Hsp90 and Hsp70 through a highly conserved clamp domain. In the present study, we investigated in vitro the molecular interactions between CyP40 and FKBP52 and other stress-related components involved in steroid receptor assembly, namely Hsp70 and Hop. Using a binding protein-retention assay with CyP40 fused to glutathione S-transferase immobilized on glutathione-agarose, we have identified the constitutively expressed form of Hsp70, heat shock cognate (Hsc)70, as an additional target for CyP40. Deletion mapping studies showed the binding determinants to be similar to those for CyP40-Hsp90 interaction. Furthermore, a mutational analysis of CyP40 clamp domain residues confirmed the importance of this motif in CyP40-Hsc70 interaction. Additional residues thought to mediate binding specificity through hydrophobic interactions were also important for Hsc70 recognition. CyP40 was shown to have a preference for Hsp90 over Hsc70. Surprisingly, FKBP52 was unable to compete with CyP40 for Hsc70 binding, suggesting that FKBP52 discriminates between the TPR cochaperone-binding sites in Hsp90 and Hsp70. Hop, which contains multiple units of the TPR motif, was shown to be a direct competitor with CyP40 for Hsc70 binding. Similar to Hop, CyP40 was shown not to influence the adenosine triphosphatase activity of Hsc70. Our results suggest that CyP40 may have a modulating role in Hsc70 as well as Hsp90 cellular function.
INTRODUCTION
Cyclophilin 40 (CyP40), a 40-kDa cyclosporin A–binding immunophilin first identified in association with unactivated estrogen receptors (Ratajczak et al 1990, 1993), appears to have an accessory role with heat shock protein (Hsp)90 in chaperone protein-folding machinery essential for the activity of diverse intracellular signaling molecules, ranging from steroid receptors to regulatory tyrosine kinases, involved in cell cycle control (Duina et al 1996; Nair et al 1996; Weisman et al 1996). CyP40 shares structural and sequence homology with FKBP51 and FKBP52, proteins of the FK506-binding class identified as common components of apo steroid receptor complexes (Lebeau et al 1992; Peattie et al 1992; Ratajczak et al 1993; Nair et al 1997). These 3 large immunophilins are characterized by an N-terminal immunophilin-like domain together with a conserved C-terminal tetratricopeptide repeat (TPR) domain that provides an interface for protein interaction (Goebl and Yanagida 1991; Ratajczak et al 1993; Nair et al 1997). All target an identical site within Hsp90 through this conserved C-terminal region to form separate steroid receptor complexes containing Hsp90 associated with only 1 of the immunophilins (Radanyi et al 1994; Owens-Grillo et al 1995; Ratajczak and Carrello 1996).
In studies using rabbit reticulocyte lysate, the Toft, Smith, and Pratt laboratories have developed a dynamic model of steroid receptor assembly in which a newly synthesized receptor is conformationally regulated to a high-affinity hormone-binding form through cooperative interaction between the heat shock cognate (Hsc)70 and Hsp90 chaperone systems (Smith and Toft 1993; Hutchinson et al 1994; Johnson and Toft 1995; Smith et al 1995; Chen et al 1996a; Prapapanich et al 1996; Dittmar and Pratt 1997, reviewed by Pratt and Toft 1997). Hsc70 chaperone function is critically dependent on adenosine triphosphatase (ATPase) activity, and cycles of adenosine triphosphate (ATP) binding and hydrolysis modulate substrate interaction and release (Cyr et al 1994; Höhfeld et al 1995; Hartl 1996; Minami et al 1996). In the early stages of receptor assembly, accessory proteins Hsp40, Hip, and Hop sequentially regulate the Hsc70 reaction cycle to enhance the formation of receptor-Hsc70-Hsp90 complexes (Bohen et al 1995; Prapapanich et al 1996; Frydman and Höhfeld 1997). It has been suggested that Hop, which can associate simultaneously with Hsc70 and Hsp90, may promote the efficient transfer of receptor from Hsc70 to Hsp90 (Frydman and Höhfeld 1997). Dissociation of Hop and other Hsc70 chaperone components then allows recruitment of p23 and one or other of the immunophilins to furnish mature receptor complexes (Prapapanich et al 1996; Dittmar and Pratt 1997; Frydman and Höhfeld 1997; Pratt and Toft 1997). These additional, late-stage components are capable of independent chaperone activity (Bose et al 1996; Freeman et al 1996), suggesting that they might, either directly or indirectly via Hsp90, fine-tune receptor conformation for optimal hormone binding and activation.
Interestingly, the 3 immunophilins CyP40, FKBP51, and FKBP52 and the chaperone cofactors Hip and Hop all possess structurally related TPR motifs that mediate their association with Hsp90 or Hsc70 (or both) (Honoré et al 1992; Ratajczak et al 1993; Radanyi et al 1994; Höhfeld et al 1995; Chen et al 1996a; Ratajczak and Carrello 1996; Frydman and Höhfeld 1997; Lässle et al 1997). Hop, which displays a bipartite recognition of both Hsp90 and Hsc70 through separate TPR domains (Honoré et al 1992; Chen et al 1996a; Lässle et al 1997), competes effectively with the immunophilins for Hsp90 binding (Owens-Grillo et al 1996). Similar observations have been noted with a TPR domain fragment of PP5, a serine protein phosphatase identified as an additional component of the unactivated glucocorticoid receptor (Chen et al 1996b; Silverstein et al 1997). Together, these results have led to suggestions of a common TPR interaction site within Hsp90 (Owens-Grillo et al 1996). In the Hsc70 chaperone system, Hip targets the Hsc70 ATPase domain (Höhfeld et al 1995). The presence of Hop, together with Hsp90 and Hsc70, in Hip-targeted immunoprecipitates suggests distinct interaction sites for Hip and Hop in Hsc70 chaperone machinery (Prapapanich et al 1996). Indeed, the interaction domain for Hop has been determined to reside within the C-terminal segment of Hsc70 (Gebauer et al 1997; Demand et al 1998). The C-terminal domain of Hsp90 has also been identified as the interaction site for Hop and TPR-containing immunophilins (Chen et al 1998; Demand et al 1998; Young et al 1998; Carrello et al 1999), and this has been further delineated to the EEVD sequence at the extreme C-terminus of Hsp90 (Chen et al 1998; Carrello et al 1999; Liu et al 1999; Scheufler et al 2000), an interaction motif that is conserved in Hsp70-Hsc70 chaperones (Freeman et al 1995).
Crystal structure determinations for the C-terminal peptides of Hsp70 (GSGSGPTIEEVD) and Hsp90 (MEEVD) with distinct TPR domains in Hop have shown the EEVD motif to be anchored through a similar network of electrostatic interactions with specific residues located in the so-called binding groove (Scheufler et al 2000). Hydrophobic contacts, especially those implicating the isoleucine and methionine residues upstream of the EEVD sequence in Hsp70 and Hsp90, respectively, play a critical role in targeting the chaperones to specific TPR domains (Scheufler et al 2000; Brinker et al 2002). Selective chaperone recognition is achieved by the accommodation of these 2 residues within different hydrophobic pockets of the TPR domains (Scheufler et al 2000; Brinker et al 2002).
Previous studies have demonstrated that Hsp70 selectively targets the TPR domains in small glutamine-rich TPR protein (SGT) (Liu et al 1999; Wu et al 2001; Tobaben et al 2001) and within the N-terminal region of Hop (Chen et al 1996a; Lässle et al 1997; Liu et al 1999; Scheufler et al 2000). On the other hand, the single TPR domain of the cochaperone CHIP is less discriminatory and can bind either Hsp90 or Hsp70 (Prihar et al 1999; Connell et al 2001; Höhfeld et al 2001). Here, we present evidence that the Hsp90 cochaperone, CyP40, although displaying a preference for Hsp90, also interacts with Hsc70. Deletion mapping experiments demonstrated remarkably similar CyP40 structural requirements for interaction with either of the chaperones. CyP40-Hsp90 binding was more sensitive to mutation of core TPR residues involved in forming the primary anchor for the EEVD motif, consistent with the higher binding affinity evident for this interaction. Hop was shown to be an efficient competitor with CyP40 for Hsc70 binding, whereas FKBP52 was unable to compete effectively, suggesting that this immunophilin is selective for Hsp90 interaction. Finally, our study shows that similar to Hop, CyP40 does not alter the ATPase function of Hsp70. Although the biological relevance of CyP40-Hsc70 interaction remains to be established, it is possible that CyP40 has an accessory role in Hsc70 as well as Hsp90 cellular function.
MATERIALS AND METHODS
Generation of CyP40-derived expression constructs
The preparation of expression plasmids for wild-type and mutant hCyP40 185–370 proteins, fused at the N-terminal end to glutathione S-transferase (GST), has been described previously (Ward et al 2002). Plasmids for the protein GST-bCyP40 WT and several deletion mutants (GST-bCyP40 91–370, GST-bCyP40 185–370, GST-bCyP40 1–352, GST-bCyP40 Δ17–213, and GST-bCyP40 1–213) were generated by polymerase chain reaction (PCR) from a bCyP40 complementary deoxyribonucleic acid (cDNA) template and cloned into the pGEX-2T expression vector (Amersham Biosciences, Little Chalfont, UK) as described previously (Ratajczak and Carrello 1996).
For untagged wild-type bovine CyP40, a bCyP40 cDNA template was amplified by PCR using sequence-specific oligonucleotide 5′ and 3′ primers containing Nde1 and BamH1 restriction enzyme sites, respectively. A TGA stop codon was placed immediately before the BamH1 site. The PCR fragment was ligated into pGEM-T (Promega Corp., Madison, WI, USA), and the sequence integrity of both ends of the insert in an isolated clone was confirmed by automated sequence analysis (Applied Biosystems, Foster City, CA, USA). A Xho1 to Bcl1 fragment excised from a WT bCyP40 cDNA was substituted to eliminate PCR-generated errors within this region. A full-length bCyP40 fragment produced by Nde1 and BamH1 digestion was then cloned into the pET-11 plasmid vector. The pET-11-bCyP40 WT expression plasmid was transformed into the Escherichia coli expression host BL21 (DE3). Overexpression of wild-type bovine CyP40 was induced by 0.4 mM isopropyl b-d-thiogalactoside (IPTG), and lysates of recombinant CyP40 were prepared as described below.
Expression and purification of GST-CyP40 fusion proteins
Expression plasmids for all GST-CyP40–related fusion proteins were transformed in E coli strain BL21 (DE3). The fusion proteins were purified from lysates derived from 0.2 mM IPTG-induced bacterial cultures by affinity chromatography on glutathione-agarose (Amersham Biosciences), as detailed previously (Ratajczak and Carrello 1996; Ward et al 2002). Eluted protein was equilibrated into buffer A (100 mM KCl, 10 mM Tris-HCl, pH 7.3) using Centricon YM-30 centrifugal filter devices (Millipore, Bedford, MA, USA). Protein concentrations were determined by the Bradford assay (Bradford 1976), and the purity of fusion protein preparations was examined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie blue staining.
Recombinant Hsc70, Hsp90β, FKBP52, and Hop
Expression plasmids for rat Hsc70 and human Hop and aminoterminal His-tagged human Hsp90β and FKBP52 have been described previously and were generous gifts provided by JO Thomas (Shi and Thomas 1992), DF Smith (Chen et al 1996a), CT Walsh (Shi et al 1994), and D Peattie (Peattie et al 1992), respectively. The expression plasmid for the ATPase domain of Hsc70, corresponding to the first 402 amino acids of the protein, was derived from the parent full-length rat Hsc70 expression plasmid using PCR. After digestion with Nde1 and BamH1, the amplified deoxyribonucleic acid fragment was cloned into the corresponding sites of a pET-11 expression vector, and sequence integrity was validated by sequence analysis. The expression plasmid for full-length His-tagged rat Hsc70 was prepared by releasing the Hsc70 insert from the parent plasmid with Nde1 and BamH1 digestion, followed by subcloning into pET-28a (+) (Novagen, Madison, WI, USA). The expression plasmid for His-tagged human Hsp90β 527–724 was prepared by PCR using sequence-specific oligonucleotide primers, which allowed the introduction of Nde1 sites at both termini. The amplified fragment was cloned into the pET-28a (+) vector and checked for sequence fidelity. The proteins were overexpressed in bacterial cultures by induction with IPTG (0.1–1 mM). Bacterial lysates were prepared by sonication in lysing buffer (Hsc70, Hop: 10 mM Tris, pH 7.3, containing 100 mM KCl, 1 mM dithiothreitol, 0.2% vol/vol Triton X-100, and 1 mg/mL lysozyme; His-tagged FKBP52, Hsp90β and Hsc70 proteins: 16 mM disodium hydrogen orthophosphate, pH 7.4, containing 0.15 M NaCl, 1% Triton X-100, 5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 5 mM benzamidine) and were cleared of particulate material by ultracentrifugation (100 000 × g for 30 minutes at 4°C). Where indicated, both His-tagged FKBP52, Hsp90β, and Hsc70 were purified from crude lysates by chromatography on nickel-nitrilotriacetic acid (Ni-NTA)-agarose (Qiagen, Gmbh, Germany) according to the manufacturer's instructions. Before use in interaction studies with CyP40, all recombinant FKBP52, Hsp90β, and Hsc70 proteins were equilibrated in binding buffer A. For enzyme-linked immunosorbent assay (ELISA) microtiter plate binding studies (see below), purified His-tagged Hsp90β 527–724 and Hsc70 proteins were equilibrated with NaHCO3, pH 8.5.
Hsc70 binding studies
Hsp70 retention by CyP40 was initially observed in studies with bovine myometrial cytosol under conditions previously described for Hsp90-CyP40 interaction (Ratajczak and Carrello 1996). Briefly, glutathione-agarose charged with GST-bCyP40 185–370 fusion protein was diluted 5-fold with Sepharose 4B, and 40 μL of the diluted gel was then incubated with rotation for 6 hours at 4°C with 200 μL of cytosol prepared in binding buffer (10 mM Tris, pH 7.3, containing 100 mM KCl, 5 mM dithiothreitol, and 10% vol/vol glycerol). After brief microcentrifugation, the pelleted gel was washed eight times with 500 μL of ice-cold binding buffer, the first 4 washes containing 0.2% vol/vol Triton X-100. The washed agarose was resuspended in 50 μL of 2× SDS-PAGE sample buffer, boiled for 5 minutes, and examined for retained proteins by SDS-PAGE with Coomassie blue staining or by Western analysis using the N27 monoclonal antibody to Hsp70 (a generous gift from W. Welch). Interaction studies of Hsc70 with GST-bCyP40 185–370 or with GST as control were performed with similar methodology using 400 μL of induced Hsc70 bacterial lysate.
In experiments designed to examine the binding preference of CyP40 for Hsc70 vs Hsp90, Hsc70 bacterial lysate was supplemented with chelate chromatography-purified, His-tagged Hsp90β to give extracts with Hsp90 to Hsc70 ratios of 1:1, 1:5, and 5:1. Glutathione-agarose containing preabsorbed GST-bCyP40 185–370 fusion protein was diluted with an equal volume of Sepharose 4B, and the diluted gel (50 μL) was then incubated with the mixed Hsp90-Hsc70 preparations (1.35 mL final volume), as described and assessed for Hsp90 and Hsc70 retention by SDS-PAGE.
The ELISA microtiter plate assay for investigating GST-hCyP40 185–370 interactions with His-rHsc70 and His-hHsp90β 527–724 has been described previously (Ward et al 2002). Briefly, using Immulon 4HBX 96-well microtiter plates (Dynex Laboratories Inc, Chantilly, VA, USA), approximately 0.4 μM rHsc70 or 40 nM Hsp90β 527–724 in 100 mM NaHCO3 (pH 8.5) was applied in 50 μL aliquots to triplicate wells for each sample and incubated at 4°C for 3 hours (for each sample, an equivalent amount of bovine serum albumin [BSA] [Promega Corp.] was added to adjacent wells in triplicate as a control for nonspecific binding). All the following steps were as described previously, except that GST-hCyP40 185–370, goat anti-GST, and rabbit anti-goat IgG-HRP were applied in 50 μL volumes. GST-hCyP40 185–370 wild-type and mutant proteins were bound to Hsc70 and Hsp90β 527–724 at concentrations of 2.5 and 0.5 μM, respectively. An assay with GST as control was run in parallel. The resultant color change was quantified at 450 nm using a Titertek Multiskan PLUS spectrophotometer (Flow Laboratories, Australasia Pty Ltd, North Ryde, NSW, Australia). Assays were repeated at least 3 times. Results described in Figure 4B were derived after subtraction of binding levels seen with GST alone. These were 12% and 1.5% of that achieved by wild-type GST-hCyP40 185–370 interaction with Hsc70 and Hsp90β, respectively.
Fig 4.
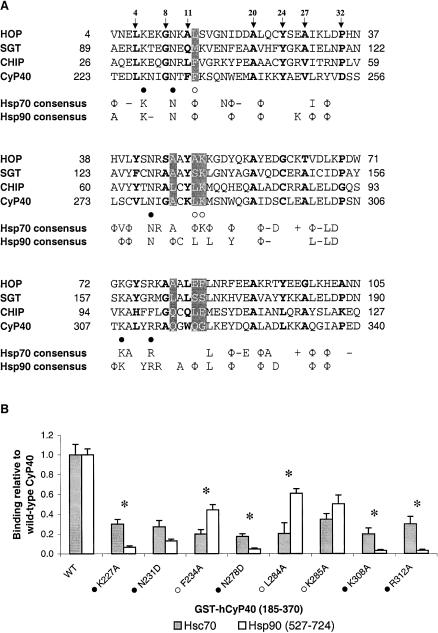
Alignment of tetratricopeptide repeat (TPR) domains of heat shock cognate (Hsc)70–interacting proteins and binding of wild-type and mutant GST-CyP40 185–370 to full-length Hsc70 and heat shock protein (Hsp)90β 527–724. (A) Sequence alignment of the Hsp70-binding TPR1 domain of Hop (residues 4–105), SGT (residues 89–190), and CHIP (residues 26–127) with the TPR units of CyP40 (residues 223–340). All sequences are of human origin. Residues of the 34 amino acid TPR consensus sequence (Sikorski et al 1990) are indicated by motif numbering above the alignment and are shown in bold. Residues that determine chaperone specificity are indicated in white on a gray background. CyP40 residues that were mutated for this study are denoted beneath the alignments and correspond to those associated with the 2-carboxylate clamp (filled circles) and chaperone specificity (open circles). The consensus sequence for Hsc70-interacting proteins was determined on the basis of 3 of 4 residues being identical or conserved in charge (− for Glu or Asp; + for Lys or Arg) or hydrophobic residue (Φ). The consensus for Hsp90-interacting proteins was derived from an alignment of Hsp90-binding TPR domains (TPR2A of Hop, PP5, FKBP51, FKBP52, CyP40, TOM34, TOM70, and CNS1, see Figure 3 Scheufler et al 2000) and was determined on the basis of 5 of 8 conforming residues. (B) Wild-type or mutant GST-hCyP40 was incubated with full-length Hsc70 or hHsp90β 527–724 bound to the wells of a microtiter plate, and the amount of bound CyP40 was detected by incubating with goat anti-GST antibody followed by rabbit anti-goat IgG antibody conjugated to horseradish peroxidase and 3,3′,5,5′-tetramethylbenzidine as substrate. Binding is expressed as a percentage relative to that of wild-type CyP40 185–370 and represents the mean (bars, ±SE) for at least 3 separate assays, each assay being performed in triplicate. Asterisks denote a significant difference (P < 0.05) in percent relative binding of mutants to Hsc70 and hHsp90β 527–724. CyP40, cyclophilin 40; GST, glutathione S-transferase; SGT, small glutamine-rich TPR protein
bCyP40-Hsc70 interaction studies in the presence of FKBP52 and Hop
For experiments with FKBP52, duplicate 100-μL aliquots of Hsc70 bacterial lysate were supplemented with 200 μL of binding buffer or nickel-chelate chromatography–purified recombinant FKBP52. After adjusting volumes to 500 μL with binding buffer, all samples were incubated for 3 hours at 4°C. The mixtures were rotated for an additional 5 hours at 4°C with 50 μL of Sepharose 4B-diluted (1:1) glutathione-agarose charged with GST-bCyP40 185–370 fusion protein, and retained proteins were analyzed by SDS-PAGE as described. For comparison, the binding of CyP40 to Hsp90 in the presence of FKBP52 was investigated in a parallel study, under identical conditions.
CyP40 binding to Hsc70 and Hsp90 in the presence of Hop was studied using similar methodology. Briefly, 0, 10, 20, 50, 100, and 200 μL aliquots of induced Hop bacterial lysate was added to separate 200-μL aliquots of Hsc70 lysate, and the final volume for all samples was adjusted to 500 μL with binding buffer. After 3 hours at 4°C, the extracts were rotated for 5 hours at 4°C with 50 μL of diluted glutathione-agarose–containing GST-bCyP40. Proteins retained on the resin were recovered with 2× SDS-PAGE sample buffer and analyzed by SDS-PAGE. A parallel study with 200 μL of uninduced Hop lysate was conducted as control. For the corresponding experiment with Hsp90, we preincubated extracts (150 μL) containing nickel chelate chromatography–purified Hsp90 with 0- to 1.0-mL aliquots of the same induced Hop bacterial lysate in a total volume of 1.2 mL. After incubation with resin containing immobilized GST-bCyP40, retained proteins were recovered with SDS-PAGE sample buffer and analyzed as described. Protein bands were quantitated by densitometric scanning using Image Quant (Molecular Dynamics, Sunnyvale, CA, USA) software.
ATPase assays
Purification of human Hsp70 and yeast Ydj-1 proteins used in ATPase assays was performed as described previously (Johnson et al 1998). The Hsp70-catalyzed hydrolysis of ATP was measured according to previously described methodology (Bochner and Ames 1982). Briefly, Hsp70 (2 μM) was incubated with 1 μM [α32P]ATP (2 mCi/mL, DuPont NEN, Boston, MA, USA) at 30°C for 20 minutes in a buffer containing 40 mM N-2-hydroxythylpiperazine-N′-2-ethane-sulfonic acid–KOH, pH 7.4, 50 mM KCl, 2 mM MgCl2, and 2 mM dithiothreitol. Reactions were stopped by the addition of an equal volume of a “stop” solution containing unlabeled adenosine 5′ monophosphate, adenosine 5′ diphosphate (ADP), ATP, and ethylenediamine-tetraacetic acid (12 mM each). Ydj-1, the yeast homologue of Hsp40 (Caplan and Douglas 1991), was added to some samples at one-tenth the molar concentration of Hsp70 or at 200 nM. CyP40 was added at one, two, or four times the molar concentration of Hsp70, either in the absence or in the presence of Ydj-1.
Thin-layer chromatography (TLC) was used to assess the extent of ATP hydrolysis. Aliquots (2.5 μL) of each ATPase reaction were spotted on cellulose PEI TLC sheets (Selecto Scientific Inc, Suwanee, GA, USA), and ascending chromatography in 0.5 M LiCl and 2 N formic acid was performed for 1 hour at room temperature. After completion of chromatography, the TLC plates were air-dried, and the radioactive spots corresponding to ADP and ATP were quantified by the STORM-840 phosphorimaging system (Molecular Dynamics). The ratio of ADP to ATP was used to calculate percent ATP hydrolysis. A blank consisting of all the reaction components, added to the stop solution at the zero reaction time point, was run at each concentration of CyP40 protein assayed. The data plotted in Figure 7 were derived from the mean results of 3 independently performed experiments.
Fig 7.
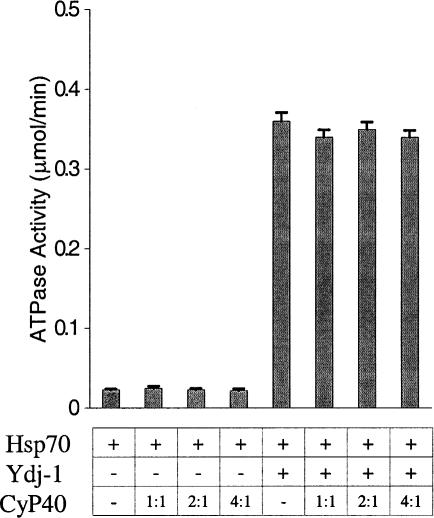
Influence of cyclophilin 40 (CyP40) on heat shock protein (Hsp)70 adenosine triphosphatase (ATPase) function. The hydrolysis of 32P-labeled adenosine triphosphate (ATP) by Hsp70 (2 μM) was determined in the absence (−) or presence (+) of Ydj-1 (200 nM), with or without purified human CyP40 at molar ratios equivalent to one, two, or four times the heat shock protein (Hsp)70 concentration. After thin-layer chromatography of the reaction mixture, the ratio of adenosine 5′ diphosphate and ATP was quantitated by phosphorimaging and used to calculate the level of ATP hydrolysis. ATPase activity is expressed in micromoles per minute. The data represent the means (bars, ±SE) of results from 3 separate experiments
RESULTS
The interaction site for Hsc70 is located in the CyP40 C-terminal domain
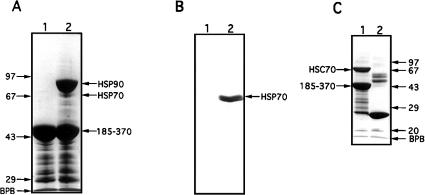
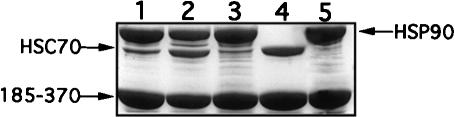
We have previously shown that the critical regions for Hsp90 binding reside in the C-terminal half of CyP40 and include the TPR domain together with acidic and basic elements flanking the N- and C-terminal ends of the TPR domain, respectively (Ratajczak and Carrello 1996). All these structural features are retained in the GST-bCyP40 185–370 fusion protein (Ratajczak and Carrello 1996), which is deleted in the N-terminal, cyclophilin-like domain. Use of this construct, immobilized on glutathione-agarose, in pull-down assays for Hsp90 binding showed the clear retention of Hsp90 from bovine myometrial cytosol and suggested that additional protein(s), approximately 70 kDa in size, might also be retained (Fig 1A, lane 2). Our contention that the observed minor protein bands represented Hsp70 was confirmed by Western analysis of the same extracts using a Hsp70-specific monoclonal antibody (Fig 1B, lane 2). By performing the pull-down assay with bacterial lysates containing the recombinant 70-kDa Hsc protein (Hsc70), we were able to demonstrate the direct interaction of CyP40 with members of the Hsp70 protein family (Fig 1C, lane 1). In addition, our results showed that the C-terminal domain of CyP40 mediates Hsc70 interaction. Under conditions that were identical to those used for interaction studies with full-length Hsc70, we were unable to observe retention of the bacterially expressed Hsc70 ATPase domain on glutathione-agarose gel charged with GST-bCyP40 185–370 fusion protein (data not shown). CyP40 then, does not recognize the ATPase domain of Hsc70.
Fig 1.
Interaction of cytosolic heat shock protein (Hsp)70 and recombinant heat shock cognate (Hsc)70 with the C-terminal domain of cyclophilin 40 (CyP40). (A) Glutathione-agarose (40 μL) charged with glutathione S-transferase (GST)-bCyP40 185–370 (see Fig 2A) was incubated with rotation for 6 hours at 4°C with bovine myometrial cytosol (200 μL) prepared in binding buffer. After centrifugation, the gel was washed eight times with binding buffer, the first 4 washes containing 0.2% vol/vol Triton X-100. Retained proteins (lane 2) were extracted from the gel with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and were analyzed by SDS-PAGE on a 10% wt/vol polyacrylamide gel followed by Coomassie blue staining. Fusion protein-charged gel not exposed to cytosol was used as control (lane 1). (B) A parallel study was performed as in (A) except that proteins separated by SDS-PAGE were electrotransferred to nitrocellulose membrane (Amersham Boisciences) and immunoblotted with N27 (Hsp70) monoclonal antibody. (C) GST and GST-bCyP40 185–370 were immobilized on glutathione-agarose and the protein-charged gels (40 μL) were incubated as described in (A) with bacterial lysate (400 μL) containing recombinant Hsc70. Contaminating proteins were removed by washing, and retained proteins were analyzed by SDS-PAGE on a 12.5% polyacrylamide gel followed by Coomassie blue staining. Proteins recovered with GST (control) are shown in lane 2. Protein molecular weight markers (Pharmacia) are shown on the left (panel A) and on the right (panel C). BPB, bromophenol blue
Similar CyP40 structural requirements for Hsp90 and Hsc70 interaction
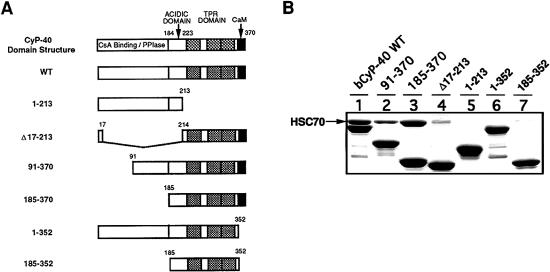
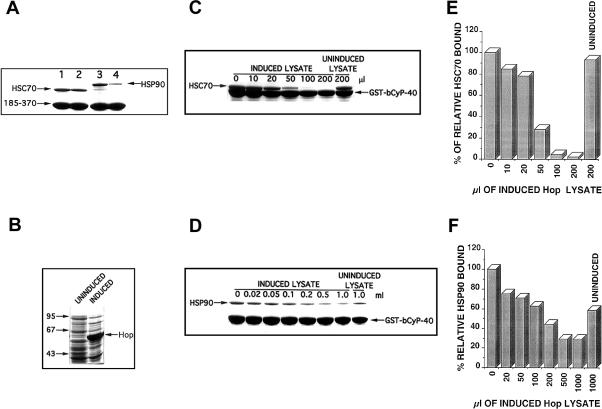
To better define the CyP40-binding domain for Hsc70, we made use of the same GST-based CyP40 deletion mutants previously used in mapping studies for CyP40-Hsp90 interaction (Ratajczak and Carrello 1996) (Fig 2A). In addition to the N-terminal deletions 91–370 and 185–370, these included the Δ17–213 construct, which has a disrupted acidic domain, and the 1–352 fusion protein, which is missing a positively charged α-helical region downstream of the core TPR domain (Ratajczak and Carrello 1996; Taylor et al 2001). Results of pull-down assays with Hsc70 bacterial lysates showed that efficient Hsc70 binding was displayed by all constructs having an intact C-terminal domain (Fig 2B). The N-terminal 1–213 region of CyP40 was unable to mediate Hsc70 interaction (Fig 2B). Deletion of the extreme C-terminal domain (as in constructs 1–352 and 185–352) and partial deletion of the acidic domain (as in Δ17–213) led to a dramatic loss of Hsc70 binding efficiency (Fig 2B). Our results suggested that multiple C-terminal elements of CyP40, incorporating the TPR domain and adjacent acidic and basic regions, are important for determining Hsc70 interaction. Significantly, our results also indicated that the structural requirements for CyP40 binding to Hsp90 and Hsc70 appear to be similar.
Fig 2.
Deletion mapping of the cyclophilin 40 (CyP40) binding domain for heat shock cognate (Hsc)70. (A) The domain structure of CyP40 is shown schematically and includes the acidic domain, the tetratricopeptide repeat domain (shaded) and putative calmodulin (CaM) binding site (black). Also shown are glutathione S-transferase (GST)-bCyP40 WT and CyP40 deletion mutants (Ratajczak and Carrello 1996). GST was fused in-frame to the 5′ end of CyP40 complementary deoxyribonucleic acid fragments. Numbers refer only to CyP40 amino acids. (B) Wild-type bCyP40 and its deletion mutants were immobilized as GST fusion proteins on glutathione-agarose and assayed for Hsc70 binding as described in Figure 1. In each lane, the corresponding bCyP40 construct is identified by the major protein band
CyP40 has a binding preference for Hsp90 over Hsc70
Having shown that CyP40 is able to bind both Hsp90 and Hsc70, we next examined whether CyP40 displays a binding preference for one Hsp over the other. Figure 3 shows the results of a binding study in which the C-terminal 185–370 construct was exposed to bacterial lysates containing either recombinant Hsc70 (lane 4) or Hsp90 (lane 5) alone or to lysate mixtures in which the ratios of Hsp90 to Hsc70 during incubation were 1:1 (lane 1), 1:5 (lane 2), and 5:1 (lane 3). When exposed to equal concentrations of the Hsps, the 185–370 construct displayed a preferential interaction with Hsp90 (Fig 3, lane 1). This same binding preference was still evident at Hsc70 levels that were 5-fold greater than those of Hsp90 (Fig 3, lane 2). In parallel experiments, under the same reaction conditions, we were unable to detect Hsc70 interaction with His-tagged Hsp90 immobilized on nickel-chelate agarose (not shown) thus discounting possible interference in our study from this mode of interaction. Taken together, our results show clearly that CyP40 has a preferential affinity for Hsp90.
Fig 3.
Determination of preferential binding of cyclophilin 40 (CyP40) for heat shock protein (Hsp)90 over heat shock cognate (Hsc)70. Bacterial lysate containing recombinant Hsc70 was supplemented with chelate-purified, His-tagged Hsp90β to give extracts with Hsp90 to Hsc70 ratios of 1:1, 1:5, and 5:1. Aliquots (50 μL) of glutathione-agarose containing immobilized GST-bCyP40 185–370 fusion protein were incubated with the mixed Hsp90-Hsc70 preparations and analyzed for Hsp90 and Hsc70 retention by SDS-PAGE as described in Figure 1. Hsp90-Hsc70 ratios were: lane 1, 1:1; lane 2, 1:5; lane 3, 5:1; lane 4, neat Hsc70; lane 5, neat Hsp90. GST, glutathione S-transferase; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Mutational analysis of the interaction site for Hsc70 in CyP40
Investigations of Hsp70 and Hsp90 interaction with Hop have defined an association between the C-terminal regions of these chaperones, incorporating the conserved EEVD motif, with TPR domains located at the N-terminal end of Hop (TPR1) and centrally in the Hop protein (TPR2A), respectively (Chen et al 1996a, 1998; Lässle et al 1997; Demand et al 1998; Young et al 1998; Carrello et al 1999; Liu et al 1999; Scheufler et al 2000). Both Hsc70 and Hsp90 recognize TPR-containing cochaperones through a fingerprint 2-carboxylate clamp motif resulting from electrostatic interactions between the aspartate residue in the common C-terminal EEVD peptide and conserved residues within the TPR domain (Scheufler et al 2000). The binding is stabilized through hydrophobic interactions with the penultimate valine residue serving a general anchoring role for the EEVD sequence and the isoleucine and methionine residues within the extended respective C-terminal peptides for Hsc70 (GAGSGPTIEEVD) and Hsp90 (MEEVD) functioning as the major determinants of binding specificity (Scheufler et al 2000; Wu et al 2001; Brinker et al 2002).
To gain further insights into the structural elements within TPR domains that might favor binding selectivity for C-terminal regions in Hsp70 and Hsp90, we aligned the TPR1 sequence of Hop with the corresponding sequences of other Hsp70-binding TPR proteins, namely SGT (Liu et al 1999; Tobaben et al 2001; Wu et al 2001) and CHIP (Ballinger et al 1999; Liu et al 1999; Prihar et al 1999), in addition to CyP40 (Fig 4A). Similar to CyP40, CHIP is capable of binding Hsp90 as well as Hsc70 (Prihar et al 1999; Connell et al 2001; Höhfeld et al 2001). The aligned sequences were used to derive a consensus sequence for Hsp70 interaction (Fig 4A). A similar approach, based on the alignment of Hsp90-binding TPR domains described previously by Scheufler et al (2000), was used to obtain a consensus interaction sequence for TPR proteins with Hsp90 (Fig 4A). Although the 2 consensus binding sequences for Hsp70 and Hsp90 were remarkably similar, some differences were noted. Specifically, within the first TPR unit, the negative charge conserved at position 3 in Hsp70-binding proteins was retained in only 2 of 8 proteins interacting with Hsp90, including CyP40. Similarly, within the third TPR unit, the negatively charged residues at amino acid positions 19 and 33 were retained only in 2 of 8 and 1 of 8 TPR proteins that bind to Hsp90, respectively. On the other hand, an aspartate residue that is highly conserved at position 23 of the third TPR unit among Hsp90-binding TPR proteins was retained in only 1 interactant (CyP40) with Hsp70.
Of the amino acids N-terminal to the EEVD motif, the isoleucine residue within the Hsp70 C-terminal peptide, GPTIEEVD, contributes most to specific recognition of TPR1, the N-terminal TPR domain of Hop (Scheufler et al 2000; Brinker et al 2002). X-ray crystallographic data have revealed the isoleucine to be accommodated in a hydrophobic cavity formed by the residues Ala46, Ala49, and Lys50, within the second repeat of TPR1 (Scheufler et al 2000). These residues correspond to Ala281, Leu284, and Lys285 in CyP40, suggesting that the binding cavity for isoleucine is partially conserved within the CyP40 TPR domain.
We have previously performed a structure-based mutational analysis of selected residues within the CyP40 TPR domain to identify key residues required for efficient interaction with Hsp90 (Ward et al 2002). Thus, 5 residues—Lys227, Asn231, Asn278, Lys308, and Arg312— corresponding to the 2-carboxylate clamp motif in CyP40 (Fig 4A) were shown to be essential for Hsp90 binding. Three other residues—Phe234, Leu284 and Lys285 (Fig 4A)—contributed significantly to the interaction. As noted above, these residues may also play a role in determining chaperone-binding specificity. Using an ELISA microtiter plate assay with purified His-tagged full-length rat Hsc70 and CyP40 185–370 wild-type and mutated proteins fused to GST, we next examined these same 8 residues for their contribution to CyP40-Hsc70 interaction. The corresponding assay with His-tagged human Hsp90β 527–724 was performed in parallel. This served as a control and also allowed a direct comparison of the relative importance of these residues for CyP40 interaction with both chaperones. Consistent with our previously published data for Hsp90, mutation of the CyP40 residues involved in forming the 2-carboxylate clamp (ie, Lys227Ala, Asn231Asp, Asn278Asp, Lys308Ala, and Arg312Ala) led to low binding levels, whereas intermediate levels of interaction were seen with remaining mutants (Phe234Ala, Leu284Ala, and Lys285Ala) (Fig 4B). All the mutations had a pronounced negative impact on CyP40-Hsc70 interaction (Fig 4B). However, the variability in binding levels was much less than that observed with Hsp90, suggesting a more uniform contribution of the residues to Hsc70 recognition. Interactions via the 2-carboxylate clamp (ie, those involving Lys227, Asn231, Asn278, Lys308, and Arg312) appeared to be more markedly affected by the mutations in CyP40 binding to Hsp90 as against Hsc70, indicating that the primary EEVD anchor for Hsp90 is better accommodated in the CyP40-binding groove. On the other hand, our results suggest that CyP40-Hsc70 recognition might be favored in secondary interactions with residues such as Phe234 and Leu284 (Fig 4B), both predicted to determine binding specificity.
Hop and FKBP52 differ in their ability to compete with CyP40 for Hsc70 binding
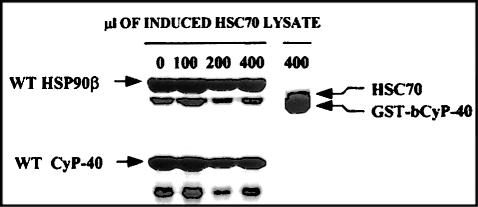
CyP40 and FKBP52 target an identical interaction site in Hsp90 (Radanyi et al 1994; Owens-Grillo et al 1995; Ratajczak and Carrello 1996). The binding is mediated by a conserved C-terminal domain, suggesting that these immunophilins present a unique binding surface that is conducive for stable association with Hsp90. Because identical structural elements within CyP40 appear to be involved in the recognition of both Hsp90 and Hsc70, we next tested the influence of FKBP52 on CyP40-Hsc70 interaction. Figure 5A, lanes 1 and 3, shows that similar levels of Hsc70 and Hsp90, respectively, were retained by GST-bCyP40 185–370 resin after exposure of the gel to bacterial lysates containing the recombinant Hsps. Preincubation of these same lysates with equal quantities of recombinant FKBP52 confirmed the inhibition of Hsp90 binding to CyP40 (Fig 5A, compare lanes 3 and 4), but the amount of Hsc70 retained was relatively unaltered (Fig 5A, compare lanes 1 and 2). When expressed in quantitative terms, the presence of FKBP52 decreased the Hsp90-CyP40 ratio from 0.32 to 0.08 (Fig 5A, lanes 3 to 4), whereas the corresponding Hsc70-CyP40 ratio was only marginally decreased from 0.35 to 0.28 (Fig 5A, lanes 1 to 2). The result confirms previous observations that FKBP52 does not interact directly with Hsp70 family members (Czar et al 1994) and signifies a divergence in the behavior of CyP40 and FKBP52. That is, whereas CyP40 displays a dual recognition of Hsp90 and Hsc70, FKBP52 has specificity for Hsp90.
Fig 5.
Competition of FKBP52 and Hop for heat shock cognate (Hsc)70 and heat shock protein (Hsp)90 binding by cyclophilin 40 (CyP40). (A) Hsc70 bacterial lysate (100 μL) was incubated at 4°C for 3 hours in the presence and absence of purified recombinant FKBP52. Glutathione-agarose charged with glutathione S-transferase (GST)-bCyP40 185–370 fusion protein was added to each mixture and after rotation for additional 5 hours at 4°C retained proteins were determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis as described. Binding to Hsp90 in the presence of FKBP52 was investigated under identical conditions. Lane 1, neat Hsc70; lane 2, Hsc70 plus FKBP52; lane 3, neat Hsp90; lane 4, Hsp90 plus FKBP52. (B) Lysates (2 μL) from uninduced and isopropyl b-d-thiogalactoside–induced bacterial cultures overexpressing recombinant human Hop were submitted to SDS-PAGE and proteins were observed by Coomassie blue staining. (C) Induced Hop lysate was added in 0, 10, 20, 50, 100, and 200 μL aliquots to separate tubes containing Hsc70 bacterial lysate (200 μL), and all volumes were adjusted to 500 μL with binding buffer. After brief mixing, the extracts were allowed to stand at 4°C for 3 hours. Glutathione-agarose (50 μL) containing immobilized GST-bCyP40 fusion protein was added to each tube and the mixtures were incubated with rotation for an additional 5 hours at 4°C. Gel-retained proteins were extracted with SDS-PAGE sample buffer and analyzed by SDS-PAGE with Coomassie blue staining. A parallel study with 200 μL of uninduced Hop lysate in Hsc70 bacterial lysate was conducted as control. (D) Induced Hop bacterial lysate was added in 0, 0.02, 0.05, 0.1, 0.2, 0.5, and 1.0 mL aliquots to tubes containing extracts (150 μL) of purified, recombinant Hsp90. Total volumes were adjusted to 1.2 mL and after preincubation for 3 hours at 4°C the mixtures were rotated with glutathione-agarose (50 μL) charged with GST-bCyP40 fusion protein. Gel-retained proteins were determined by SDS-PAGE as described. Results from a parallel study using 1.0 mL of uninduced Hop bacterial lysate served as control. (E) After quantitation of Hsc70 and GST-bCyP40 protein in (C) by densitometric scanning, the amount of Hsc70 bound to gel-immobilized GST-bCyP40 was expressed relative to that observed before the addition of induced Hop lysate as control (100%). (F) Hsp90 and GST-bCyP40 proteins in (D) were quantitated by densitometry, and retained Hsp90 was expressed relative to that bound in the absence of induced Hop lysate as control (100%)
The structural similarity shared by CyP40 and Hop through their TPR domains (Honoré et al 1992; Ratajczak et al 1993) and their common targeting of Hsp90 and Hsp70 suggested that CyP40 and Hop might compete for Hsp70 binding. Figure 5C,E shows that preincubation of Hsc70 bacterial lysates with increasing volumes of a lysate preparation containing bacterially expressed Hop was able to effectively inhibit CyP40-Hsc70 interaction. Direct binding of Hop to CyP40 was not observed, suggesting that the effect of Hop is mediated via its interaction with Hsc70. Under similar binding conditions, an effect of recombinant Hop on CyP40 interaction with Hsp90 was only observed at much higher Hop concentrations (Fig 5D,F).
To discount the possibility that similar to Hop, CyP40 might be capable of associating with both Hsp90 and Hsc70 together to form a trimeric complex, we carried out an experiment in which His-tagged Hsp90, immobilized on chelate-agarose gel, was exposed to lysate containing bacterially expressed wild-type bovine CyP40. The gel containing Hsp90-bound CyP40 was then incubated with increasing amounts of Hsc70 bacterial lysate. To eliminate nonspecific binding of Hsc70, wash buffers containing 35 mM imidazole and 0.2% vol/vol Triton X-100 were included in our experimental conditions. As a positive control for Hsc70-CyP40 interaction, we conducted a binding study in which glutathione-agarose charged with GST-bCyP40 was incubated with a corresponding maximal amount of Hsc70 bacterial lysate under identical buffer conditions. We were unable to detect retention of Hsc70 together with Hsp90 and CyP40 even at the highest Hsc70 concentrations used (Fig 6). Under similar conditions, however, we observed a strong band attributed to Hsc70 retained by GST-bCyP40 (Fig 6). Increasing levels of Hsc70 had very little influence on the Hsp90-CyP40 binding profile apart from a slight diminution of retained CyP40, consistent with the expected competition of Hsc70 for CyP40 binding. Our results then confirm that the interaction of Hsp90 and Hsc70 with CyP40 is mutually exclusive.
Fig 6.
Determination of a mutually exclusive interaction of heat shock protein (Hsp)90 and heat shock cognate (Hsc)70 with cyclophilin 40 (CyP40). Ni-NTA agarose was charged with His-tagged Hsp90β and mixed 1:1 with Sepharose 4B. Bacterial lysate (150 μL) for wild-type bCyP40 was added to the diluted gel (40 μL), and after dilution to 500 μL total volume with binding buffer containing 35 mM imidazole and 0.2% Triton X-100, the mixture was rotated at 4°C for 3 hours. After removal of unbound proteins by successive washing, the gel containing Hsp90-bound CyP40 was exposed to 0, 100, 200, and 400 μL of induced Hsc70 bacterial lysate. Reaction volumes were brought to 500 μL with binding buffer containing imidazole and Triton X-100, and incubation was continued with rotation at 4°C for 3 hours. Gel-retained proteins were recovered with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and analyzed by SDS-PAGE with Coomassie blue staining. Glutathione-agarose containing immobilized GST-bCyP40 was mixed 1:5 with Sepharose 4B. The diluted gel (40 μL) was incubated as described above with 400 μL of Hsc70 lysate and gel-retained proteins were analyzed by SDS-PAGE. GST, glutathione S-transferase
CyP40 does not alter the ATPase activity of Hsc70
The Hsp70 ATPase cycle is defined by a process of ATP binding, hydrolysis, and nucleotide exchange that facilitates the binding and folding of peptide substrates (Hartl 1996). The intrinsic ATPase activity of Hsc70 is stimulated by Hsp40 family members (Liberek et al 1991; Minami et al 1996), and this is further modulated by Hsp70-associated TPR-containing cofactors (Höhfeld et al 1995; Demand et al 1998; Johnson et al 1998; Ballinger et al 1999; Tobaben et al 2001). Although Ydj-1 stimulated Hsp70 ATPase activity ∼15-fold, coincubation with CyP40 in amounts up to 4-fold molar excess over Hsp70 failed to alter either the basal level or the enhanced level of activity (Fig 7). Our results suggest that CyP40 does not play a role in regulating the Hsp70 ATPase cycle.
DISCUSSION
This report presents the first evidence of an interaction between CyP40 and the major cellular chaperone Hsc70 and demonstrates that the association is mediated by a protein-binding interface located within the CyP40 carboxy terminus. Remarkably, structural elements we have identified to be important for CyP40 recognition of Hsc70 are identical to those previously determined for CyP40-Hsp90 interaction (Ratajczak and Carrello 1996). The essential structural features include the TPR domain in combination with flanking charged subdomains (Ratajczak et al 1993; Ratajczak and Carrello 1996). Although we considered that, through its chaperone function, Hsc70 might bind some of the CyP40 deletion mutants by recognizing the nonnative form of these proteins, the strong similarity between the critical binding parameters for both Hsp90 and Hsc70 makes this unlikely. Taken together, our results suggest the existence of a similar binding surface for CyP40 in both Hsps.
Although CyP40 displays a dual recognition of Hsp90 and Hsc70, our study shows that its binding preference is for Hsp90. It is possible, however, that the level of CyP40-Hsc70 interaction achieved in the presence of Hsp90 is still significant in the context of a role for CyP40 in Hsc70 function. Hsp90 is generally more abundant in cells than Hsc70, with cellular concentrations estimated to be in the range of 5–10 and 5 μM, respectively (Nadeau et al 1994). However, localized differences may exist in the relative levels of the 2 Hsps within cellular compartments. Hsp90, for example, is predominantly localized within the cytoplasm of unstressed cells, although some Hsp90 is also present in the nucleus (Gase et al 1984; Akner et al 1992; Perdew et al 1993). Hsc70, on the other hand, has been shown to be diffusely located in both cytoplasmic and nuclear compartments (Gase et al 1984). Application of cellular stress results in a redistribution of both Hsps to the nucleus, but Hsc70 also concentrates in nucleoli (Velazquez and Lindquist 1984; Welch and Feramisco 1984). A cell cycle dependence has been noted for the cellular location of Hsc70, with the protein being localized to the nucleus and nucleolus during S phase (Milarski and Morimoto 1986; Milarski et al 1989). CyP40 displays a varied localization between cytoplasm, nucleus, and nucleoli in a number of breast cancer cell lines we have examined (Mark et al 2001), although an almost exclusive nucleolar location has previously been reported for the protein in rat pulmonary endothelial cells (Owens-Grillo et al 1996).
The present study has shown that individual residues defining the TPR clamp domain of CyP40 each contribute significantly to CyP40-Hsc70 interaction. Single substitution of these key residues reduced Hsc70 binding levels to 20%–30% of those seen with wild-type CyP40. Our current results, together with earlier observations (Ward et al 2002), showed consistently lower binding levels for the mutated clamp residues with Hsp90, perhaps reflecting a steric incompatibility of Hsc70 with CyP40, compared with Hsp90. The mutation of additional TPR domain residues, thought to determine binding specificity for Hsc70 and Hsp90, also significantly inhibited CyP40-Hsc70 interaction. Blatch and colleagues have recently completed a mutational analysis of selected amino acids within TPR1 of murine Hop to establish the significance of these residues in both binding and specificity associated with Hop-Hsc70 interaction (Odunuga et al 2003). They concluded that strictly conserved clamp domain residues within the first (Lys8, Asn12) and second (Asn43) TPR units of Hop (see Fig 4A) form part of a network of interactions, both within and outside of the TPR domain, that contribute to overall Hop-Hsc70 recognition. They also confirmed the critical importance of Ala49 and Lys50, located within the second TPR motif, in determining Hop-Hsc70 interaction specificity (Odunuga et al 2003). These residues are topologically equivalent to residues Leu284 and Lys285 in CyP40 (Fig 4A). Among Hsc70-interacting proteins, there appears to be only a limited conservation of TPR domain residues that mediate interaction specificity (Fig 4A). The TPR domains of CyP40 and CHIP then may display a broader binding specificity, enabling these proteins to recognize both Hsc70 and Hsp90 (Ballinger et al 1999; Connell et al 2001; Brinker et al 2002). There is now accumulating evidence that sequences within an extended α-helical region, downstream of the TPR domain, may also contribute to the differential recognition of the Hsc70 and Hsp90 chaperones (Ratajczak and Carrello 1996; Barent et al 1998; Ballinger et al 1999; Cheung-Flynn et al 2003).
Under carefully controlled identical conditions, FKBP52 was shown to inhibit CyP40-Hsp90 interaction but was unable to compete effectively with CyP40 for Hsc70 binding. The result is consistent with earlier observations (Owens-Grillo et al 1996) that FKBP52 does not interact with Hsc70 and suggests that structural differences between FKBP52 and CyP40 may determine the specificity of FKBP52 for Hsp90 interaction.
Hip and Hop are strongly implicated in steroid receptor assembly and target Hsc70 during the assembly process through their protein-interacting TPR domains (Chen et al 1996a; Prapapanich et al 1996; Frydman and Höhfeld 1997; Lässle et al 1997). The observation that Hip and Hop can exist together in Hsc70 complexes (Prapapanich et al 1996) suggests that Hsc70 harbors distinct binding sites for these proteins. Functional domains previously described for Hsc70 include a 44-kDa ATPase domain located at the amino terminus and a 10-kDa carboxyterminal fragment thought to mediate interaction with DnaJ-like proteins (Chappell et al 1987; Cyr et al 1994; Hartl 1996). These functional regions are separated by an intermediate 18-kDa segment proposed to facilitate chaperone substrate binding (Wang et al 1993; Cyr et al 1994; Hartl 1996). Hip functions both as a regulator of Hsc70 function and as a molecular chaperone and binds to the ATPase domain of Hsc70 in a reaction that is facilitated by DnaJ (Hsp40) (Höhfeld et al 1995; Hartl 1996; Minami et al 1996). In the presence of ATP/Mg2+, a more transient association exists between Hip and Hsc70, eventually resulting in the dissociation of the Hip-Hsc70 complex (Höhfeld et al 1995; Minami et al 1996; Frydman and Höhfeld 1997). The CyP40-Hsc70 interaction is also sensitive to ATP/Mg2+ (results not shown). However, although the CyP40 TPR domain, together with adjacent acidic and basic regions, bears a structural resemblance to that in Hip (Ratajczak et al 1993; Höhfeld et al 1995; Frydman and Höhfeld 1997), we have been unable to demonstrate interaction between CyP40 and the ATPase domain of Hsc70. Our results suggest indirectly that the site for CyP40 interaction is located within the 25-kDa carboxy terminal end of Hsc70.
Hop interacts simultaneously with Hsc70 and Hsp90 through the N-terminal TPR1 domain and the central TPR2A domain, respectively (Chen et al 1996a; Frydman and Höhfeld 1997; Lässle et al 1997). A comparison of our competitive binding results between Hop and CyP40 for Hsc70 and Hsp90 interaction showed that Hop inhibits CyP40 binding to Hsc70 more effectively. Under the same conditions, the CyP40-Hsp90 interaction was much more stable, suggesting that Hsp90 might have a preference for association with CyP40 over Hop. This result contrasts with that of an earlier report (Owens-Grillo et al 1996) that Hop blocks the binding of CyP40 to Hsp90 and that a CyP40 deletion mutant containing the essential elements for efficient Hsp90 interaction (Hoffman and Handschumacher 1995) was unable to inhibit Hop binding to Hsp90, even at high concentrations. These findings led to a proposal that Hop and the immunophilins CyP40 and FKBP52 might share common or overlapping interaction sites within Hsp90 (Owens-Grillo et al 1996). Clearly, our result is more consistent with the second alternative and would be in keeping with a steroid receptor assembly model in which the Hop (and perhaps Hsc70) component of the Hsp90-Hop-Hsc70 complex associated with the receptor is replaced by one or other of the immunophilins and p23 (Chen et al 1996a; Owens-Grillo et al 1996).
Collectively, our results point to a common interaction site for Hop and CyP40 located within the C-terminal domain of Hsc70. This region mediates peptide binding, displays a regulatory function for Hsc70 ATPase activity, and serves as an anchor point from which DnaJ (Hsp40) can modulate Hsc70 chaperone function (Wang et al 1993; Cyr et al 1994; Freeman et al 1995; Höhfeld et al 1995; Hartl 1996; Minami et al 1996). The C-terminal domain is also an essential determinant of Hsc70 self-association, a process thought to be intimately involved in regulating the chaperone function of the protein (Benaroudj et al 1997). As previously observed for Hop (Demand et al 1998; Johnson et al 1998), CyP40 was shown not to influence the ATPase activity of Hsc70. The role of CyP40 in Hsc70 function then differs from those defined for other Hsc70-interacting TPR proteins. CHIP, for example, inhibits the refolding of nonnative proteins by blocking the Hsp70 ATPase cycle (Ballinger et al 1999). SGT, on the other hand, is capable of enhancing the weak intrinsic ATPase activity of Hsc70, and this is dramatically activated in combination with the synaptic vesicle cysteine string protein (CSP) (Tobaben et al 2001). CSP contains a J domain typical of the universally conserved DnaJ-Hsp40 chaperone family (Georgopoulos and Welch 1993; Hendrick and Hartl 1993). Similarly, Sti1, the yeast homologue of Hop, has been shown to potently activate the ATPase cycle of Ssa1, a cytosolic Hsp70 family member in Saccharomyces cerevisiae (Wegele et al 2003). This contrasts with the behavior of Hop in the mammalian Hsp70 system (Demand et al 1998; Johnson et al 1998). However, Hop interaction with Hsp70 is favored by the conversion of Hsp70-ATP to the ADP-bound Hsp70 conformation resulting from Hsp40 stimulation or during the chaperoning of substrate proteins (or both) (Johnson et al 1998; Hernandez et al 2002). In a model for the role of Hop in the assembly of Hsp90-Hop-Hsp70 complexes, it has been proposed that initial association of Hop with Hsp90 imparts conformational changes to Hop that allow high-affinity, selective recognition of Hsp70 in the ADP-bound (Hernandez et al 2002). The model further asserts that protein-protein interactions during Hsp90-Hop-Hsp70 complex assembly may alter both the conformation and function of the individual chaperone components. Recent evidence for the involvement of a second site, upstream of the EEVD motif, as the preferred initial contact in the interaction of Hsp70 with the TPR1 domain of Hop has led to speculation that the EEVD-TPR1 interaction becomes physiologically relevant during ATP hydrolysis and subsequent steroid receptor heterocomplex assembly (Carrigan et al 2004). The EEVD-TPR1 interaction may then be considered to mediate a regulatory function of Hop.
Through its chaperone function Hsc70 regulates the signal transduction properties of steroid receptors (Pratt and Toft 1997) and heat shock transcription factor (Morimoto 1993). There is now accumulating evidence (Cyr et al 1994; Bohen et al 1995; Höhfeld et al 1995; Chen et al 1996a; Hartl 1996; Minami et al 1996; Prapapanich et al 1996; Frydman and Höhfeld 1997) that several chaperone accessory proteins including DnaJ (Hsp40), Hip, Hop, BAG-1/Hap-46 (Takayama et al 1997; Zeiner et al 1997), and the Nm23-related protein, p16 (Leung and Hightower 1997), might help diversify the chaperone activities of Hsc70. BAG-1/Hap-46 binds to the ATPase domain of Hsc70 and functions as a potent inhibitor of Hsc70 protein-folding activity (Takayama et al 1997; Zeiner et al 1997). It is interesting that Hop, through its interaction with the C-terminal domain of Hsc70, has the ability to counter these inhibitory effects (Gebauer et al 1997). Our observation of CyP40 interaction with Hsc70 lends support for a modulating role for CyP40 in Hsc70 function. An attractive possibility is that CyP40 may mimic some of the actions of Hop. Alternatively, Hsc70-CyP40 association might provide a means of regulating the still undefined cellular function(s) of CyP40.
Acknowledgments
The authors are grateful to Drs John Thomas for providing the Hsc70 expression plasmid, Christopher Walsh for the Hsp90β expression plasmid, Bill Welch for the N27 Hsc70 monoclonal antibody, Debra Peattie for the FKBP52 expression plasmid, and David Smith for the Hop expression plasmid. This work was supported by the National Health and Medical Research Council of Australia and the National Breast Cancer Foundation.
REFERENCES
- Akner G, Mossberg K, Sundqvist KG, Gustafsson JA, Wikstrom AC. Evidence for reversible, non-microtubule and non-microfilament-dependent nuclear translocation of Hsp90 after heat shock in human fibroblasts. Eur J Cell Biol. 1992;58:356–364.0171-9335(1992)058<0356:EFRNAN>2.0.CO;2 [PubMed] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin L-Y, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535.0270-7306(1999)019<4535:IOCANT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. doi: 10.1210/mend.12.3.0075.0888-8809(1998)012<0342:AOFCAM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Benaroudj N, Fouchaq B, Ladjimi MM. The COOH-terminal peptide binding domain is essential for self-association of the molecular chaperone HSC70. J Biol Chem. 1997;272:8744–8751. doi: 10.1074/jbc.272.13.8744.0021-9258(1997)272<8744:TCPBDI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bochner BR, Ames BN. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982;257:9759–9769.0021-9258(1982)257<9759:CAOCNB>2.0.CO;2 [PubMed] [Google Scholar]
- Bohen SP, Kralli A, Yamamoto KR. Hold'em and fold'em: chaperones and signal transduction. Science. 1995;268:1303–1304. doi: 10.1126/science.7761850.0193-4511(1995)268<1303:HAFCAS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bose S, Weikl T, Bügl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. doi: 10.1126/science.274.5293.1715.0193-4511(1996)274<1715:CFOHP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3.0003-2697(1976)072<0248:ARASMF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brinker A, Scheufler C, von der Mulbe F, Fleckenstein B, Herrmann C, Jung G, Moarefi I, Hartl F-U. Ligand discrimination by TPR domains: relevance and selectivity of EEVD-recognition in Hsp70-Hop-Hsp90 complexes. J Biol Chem. 2002;277:19265–19275. doi: 10.1074/jbc.M109002200.0021-9258(2002)277<19265:LDBTDR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Caplan AJ, Douglas MG. Characterization of YDJ1: a yeast homologue of the bacterial DnaJ protein. J Cell Biol. 1991;114:609–621. doi: 10.1083/jcb.114.4.609.0021-9525(1991)114<0609:COYAYH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrello A, Ingley E, Minchin RF, Tsai S, Ratajczak T. The common tetratricopeptide repeat acceptor site for steroid receptor-associated immunophilins and Hop is located in the dimerization domain of Hsp90. J Biol Chem. 1999;274:2682–2689. doi: 10.1074/jbc.274.5.2682.0021-9258(1999)274<2682:TCTRAS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Carrigan PE, Nelson GM, Roberts PJ, Stoffer J, Riggs DL, Smith DF. Multiple domains of the co-chaperone Hop are important for Hsp70 binding. J Biol Chem. 2004;279:16185–16193. doi: 10.1074/jbc.M314130200.0021-9258(2004)279<16185:MDOTCH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chappell TG, Konforti BB, Schmid SL, Rothman JE. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987;262:746–751.0021-9258(1987)262<0746:TACOAC>2.0.CO;2 [PubMed] [Google Scholar]
- Chen S, Prapapanich V, Rimerman RA, Honoré B, Smith DF. Interactions of p60, a mediator of progesterone receptor assembly with heat shock proteins Hsp90 and Hsp70. Mol Endocrinol. 1996a;9:670–678. doi: 10.1210/mend.10.6.8776728.0888-8809(1996)009<0670:IOPAMO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996b;271:32315–32320. doi: 10.1074/jbc.271.50.32315.0021-9258(1996)271<32315:TTRDOP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen S, Sullivan WP, Toft DO, Smith DF. Differential interactions of p23 and the TPR-containing proteins Hop, CyP40, FKBP52 and FKBP51 with Hsp90 mutants. Cell Stress Chaperones. 1998;3:118–129. doi: 10.1379/1466-1268(1998)003<0118:diopat>2.3.co;2.1466-1268(1998)003<0118:DIOPAT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung-Flynn J, Roberts PJ, Riggs DL, Smith DF. C-terminal sequences outside the tetratricopeptide repeat domain of FKBP51 and FKBP52 cause differential binding to Hsp90. J Biol Chem. 2003;278:17388–17394. doi: 10.1074/jbc.M300955200.0021-9258(2003)278<17388:CSOTTR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618.1465-7392(2001)003<0093:TCCRPT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cyr DM, Langer T, Douglas MG. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x.0376-5067(1994)019<0176:DPMCAS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Czar MJ, Owens-Grillo JK, Dittmar KD, Hutchinson KA, Zacharek AM, Leach KL, Deibel MR Jr., Pratt WB. Characterization of the protein-protein interactions determining the heat shock protein (Hsp90.Hsp70.Hsp56) heterocomplex. J Biol Chem. 1994;269:11155–11161.0021-9258(1994)269<11155:COTPID>2.0.CO;2 [PubMed] [Google Scholar]
- Demand J, Höhfeld J. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol. 1998;18:2023–2028. doi: 10.1128/mcb.18.4.2023.0270-7306(1998)018<2023:TCDOHP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KD, Pratt WB. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. J Biol Chem. 1997;272:13047–13054. doi: 10.1074/jbc.272.20.13047.0021-9258(1997)272<13047:FOTGRB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Duina AA, H-CJ Chang, Marsh JA, Lindquist S, Gaber RF. A cyclophilin function in Hsp90-dependent signal transduction. Science. 1996;274:1713–1715. doi: 10.1126/science.274.5293.1713.0193-4511(1996)274<1713:ACFIHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulator motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x.0261-4189(1995)014<2281:IOARMI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin CyP40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718.0193-4511(1996)274<1718:MCMCAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Frydman J, Höhfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0.0376-5067(1997)022<0087:CGITTH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gase J-M, Renoir J-M, Radanyi C, Joab I, Tuohimaa P, Baulieu E. Progesterone receptor in chick oviduct: an immunohistochemical study with antibodies to distinct receptor components. J Cell Biol. 1984;99:1193–1201. doi: 10.1083/jcb.99.4.1193.0021-9525(1984)099<1193:PRICOA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer M, Zeiner M, Gehring U. Proteins interacting with the molecular chaperone Hsp70/Hsc70: physical associations and effects on refolding activity. FEBS Lett. 1997;417:109–113. doi: 10.1016/s0014-5793(97)01267-2.0014-5793(1997)417<0109:PIWTMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125.0743-4634(1993)009<0601:ROTMHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Goebl M, Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991;16:173–177. doi: 10.1016/0968-0004(91)90070-c.0376-5067(1991)016<0173:TTSHAN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl F-U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0.0028-0836(1996)381<0571:MCICPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hendrick JP, Hartl F-U. Molecular chaperone functions of heat shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025.0066-4154(1993)062<0349:MCFOHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hernandez MP, Sullivan WP, Toft DO. The assembly and intermolecular properties of the Hsp70-Hop-Hsp90 molecular chaperone complex. J Biol Chem. 2002;277:38294–38304. doi: 10.1074/jbc.M206566200.0021-9258(2002)277<38294:TAAIPO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hoffman K, Handschumacher RE. Cyclophilin 40: evidence for a dimeric complex with Hsp90. Biochem J. 1995;307:5–8. doi: 10.1042/bj3070005.0264-6021(1995)307<0005:CEFADC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;21:885–890. doi: 10.1093/embo-reports/kve206.1469-221X(2001)021<0885:FTCTTG>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J, Minami Y, Hartl F-U. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3.0092-8674(1995)083<0589:HANCII>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Honoré B, Leffers H, Madsen P, Rasmussen HH, Vandekerckhove J, Celis JE. Molecular cloning and expression of a transformation-sensitive human protein containing the TPR motif and sharing identity to the stress-inducible Yeast Protein STI1. J Biol Chem. 1992;267:8485–8491.0021-9258(1992)267<8485:MCAEOA>2.0.CO;2 [PubMed] [Google Scholar]
- Hutchinson KA, Dittmar KD, Czar MJ, Pratt WB. Proof that Hsp70 is required for assembly of the glucocorticoid receptor into a heterocomplex with Hsp90. J Biol Chem. 1994;269:5043–5049.0021-9258(1994)269<5043:PTHIRF>2.0.CO;2 [PubMed] [Google Scholar]
- Johnson BD, Schumacher RJ, Ross ED, Toft DO. Hop modulates Hsp70/Hsp90 interactions in protein folding. J Biol Chem. 1998;273:3679–3686. doi: 10.1074/jbc.273.6.3679.0021-9258(1998)273<3679:HMHIIP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnson JL, Toft DO. Binding of p23 and Hsp90 during assembly with the progesterone receptor. Mol Endocrinol. 1995;9:670–678. doi: 10.1210/mend.9.6.8592513.0888-8809(1995)009<0670:BOPAHD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lässle M, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, mouse protein mSTI1. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876.0021-9258(1997)272<1876:SMPM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lebeau M-C, Massal N, Herrick J, Faber LE, Renoir J-M, Radanyi C, Baulieu E-E. p59, an Hsp90-binding protein. J Biol Chem. 1992;267:4281–4284.0021-9258(1992)267<4281:PAHP>2.0.CO;2 [PubMed] [Google Scholar]
- Leung S-M, Hightower LE. A 16-kDa protein functions as a new regulatory protein for Hsc70 molecular chaperone and is identified as a member of the Nm23/nucleoside diphosphate kinase family. J Biol Chem. 1997;272:2607–2614. doi: 10.1074/jbc.272.5.2607.0021-9258(1997)272<2607:AKPFAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874.0027-8424(1991)088<2874:ECDAGH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F-H, Wu S-J, Hu S-M, Hsiao C-D, Wang C. Specific interaction of the 70-kDa heat shock cognate protein with tetratricopeptide repeats. J Biol Chem. 1999;274:34425–34432. doi: 10.1074/jbc.274.48.34425.0021-9258(1999)274<34425:SIOTKH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mark PJ, Ward BK, Kumar P, Lahooti H, Minchin RF, Ratajczak T. Human cyclophilin 40 is a heat shock protein that exhibits altered intracellular localization following heat shock. Cell Stress Chaperones. 2001;6:59–70. doi: 10.1379/1466-1268(2001)006<0059:hciahs>2.0.co;2.1466-1268(2001)006<0059:HCIAHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski KL, Morimoto RI. Expression of human Hsp70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986;83:9517–9521. doi: 10.1073/pnas.83.24.9517.0027-8424(1986)083<9517:EOHHDT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski KL, Welch WJ, Morimoto RI. Cell cycle-dependent association of Hsp70 with specific cellular proteins. J Cell Biol. 1989;108:413–423. doi: 10.1083/jcb.108.2.413.0021-9525(1989)108<0413:CCAOHW>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Höhfeld J, Ohtsuka K, Hartl F-U. Regulation of the heat shock protein 70 reaction cycle by the mammalian DnaJ homolog Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617.0021-9258(1996)271<19617:ROTHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637.0193-4511(1993)259<1409:CISTAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nadeau K, Nadler SG, Saulnier M, Tepper MA, Walsh CT. Quantitation of the interaction of the immunosuppressant deoxyspergualin and analogs with Hsc70 and Hsp90. Biochemistry. 1994;33:2561–2567. doi: 10.1021/bi00175a027.0006-2960(1994)033<2561:QOTIOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nair SC, Rimerman RA, Toran EJ, Chen S, Prapapanich V, Butts RN, Smith DF. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol Cell Biol. 1997;17:594–603. doi: 10.1128/mcb.17.2.594.0270-7306(1997)017<0594:MCOHFA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgall TE, Smith DF. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription for Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2.1466-1268(1996)001<0237:APOMIC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odunuga OO, Hornby JA, Bies C, Zimmermann R, Pugh DJ, Blatch GL. Tetratricopeptide repeat motif-mediated Hsc70-mSTI1 interaction: molecular characterization of the critical contacts for successful binding and specificity. J Biol Chem. 2003;278:6896–6904. doi: 10.1074/jbc.M206867200.0021-9258(2003)278<6896:TRMHIM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Owens-Grillo JK, Czar MJ, Hutchinson KA, Hoffmann K, Perdew GH, Pratt WB. A model of protein targeting by immunophilins and other proteins that bind Hsp90 via tetratricopeptide repeat domains. J Biol Chem. 1996;271:13468–13475. doi: 10.1074/jbc.271.23.13468.0021-9258(1996)271<13468:AMOPTB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Owens-Grillo JK, Hoffmann K, Hutchinson KA, Yem AW, Deibel MR Jr, Handschumacher RE, Pratt WB. The cyclosporin A-binding immunophilin CyP40 and the FK506-binding immunophilin Hsp56 bind to a common site on Hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor. J Biol Chem. 1995;270:20479–20484. doi: 10.1074/jbc.270.35.20479.0021-9258(1995)270<20479:TCAICA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Peattie DA, Harding MW, Fleming MA, De Cenzo MT, Lippke JA, Livingston DJ, Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992;89:10974–10978. doi: 10.1073/pnas.89.22.10974.0027-8424(1992)089<10974:EACOHF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH, Hord N, Hollenback CF, Welsh MJ. Localization and characterization of the 86- and 84-kDa heat shock proteins in Hepa 1c1c7 cells. Exp Cell Res. 1993;209:350–356. doi: 10.1006/excr.1993.1320.0014-4827(1993)209<0350:LACOTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Nair SC, Rimerman RA, Smith DF. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an Hsp70-binding protein. Mol Endocrinol. 1996;10:420–431. doi: 10.1210/mend.10.4.8721986.0888-8809(1996)010<0420:MCOHPA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrinol Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Prihar G, Gonzalez de Chavez F, Baker M, Crook R, McGowan E, Grover A, Hardy J, Hutton M. A novel presenilin-1 interacting protein containing tetratricopeptide repeats. NeuroReport. 1999;10:1409–1415. doi: 10.1097/00001756-199905140-00005.0959-4965(1999)010<1409:ANPIPC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Radanyi C, Chambraud B, Baulieu E-E. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci U S A. 1994;91:11197–11201. doi: 10.1073/pnas.91.23.11197.0027-8424(1994)091<11197:TAOTIF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak T, Carrello A. Cyclophilin 40 (CyP40), mapping of its Hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for Hsp90 binding. J Biol Chem. 1996;271:2961–2965. doi: 10.1074/jbc.271.6.2961.0021-9258(1996)271<2961:CCMOIH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Carrello A, Mark PJ, Warner BJ, Simpson RJ, Moritz RL, House AK. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59) J Biol Chem. 1993;268:13187–13192.0021-9258(1993)268<13187:TCCOTU>2.0.CO;2 [PubMed] [Google Scholar]
- Ratajczak T, Hlaing J, Brockway MJ, Hähnel R. Isolation of untransformed bovine estrogen receptor without molybdate stabilization. J Steroid Biochem. 1990;35:543–553. doi: 10.1016/0022-4731(90)90197-z.0022-4731(1990)035<0543:IOUBER>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl F-U, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2.0092-8674(2000)101<0199:SOTDCC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shi Y, Brown ED, Walsh CT. Expression of recombinant human casein kinase II and recombinant heat shock protein 90 in Escherichia coli and characterization of their interactions. Proc Natl Acad Sci U S A. 1994;91:2767–2771. doi: 10.1073/pnas.91.7.2767.0027-8424(1994)091<2767:EORHCK>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Thomas JO. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992;12:2186–2192. doi: 10.1128/mcb.12.5.2186.0270-7306(1992)012<2186:TTOPIT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990;60:307–317. doi: 10.1016/0092-8674(90)90745-z.0092-8674(1990)060<0307:ARAAMI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Chen M-S, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor-Hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224.0021-9258(1997)272<16224:PPIAMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith DF, Toft DO. Steroid receptors and their associated proteins. Mol Endocrinol. 1993;7:4–11. doi: 10.1210/mend.7.1.8446107.0888-8809(1993)007<0004:SRATAP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimerman RA. Progesterone receptor structure and function altered by geldanamycin, an Hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804.0270-7306(1995)015<6804:PRSAFA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;18:4887–4896. doi: 10.1093/emboj/16.16.4887.0261-4189(1997)018<4887:BMTCAO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Dornan J, Carrello A, Minchin RF, Ratajczak T, Walkinshaw MD. Two structures of cyclophilion 40: folding and fidelity in the TPR domains. Structure. 2001;9:431–438. doi: 10.1016/s0969-2126(01)00603-7.0969-2126(2001)009<0431:TSOCFA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tobaben S, Thakur P, Fernandez-Chacon R, Sudhof TC, Rettig J, Stahl B. A trimeric protein complex functions as a synaptic chaperone machine. Neuron. 2001;31:987–999. doi: 10.1016/s0896-6273(01)00427-5.0896-6273(2001)031<0987:ATPCFA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Velazquez JM, Lindquist S. Hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984;36:655–662. doi: 10.1016/0092-8674(84)90345-3.0092-8674(1984)036<0655:HNCDES>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang T-F, Chang J, Wang C. Identification of the peptide binding domain of Hsc70. J Biol Chem. 1993;268:26049–26051.0021-9258(1993)268<26049:IOTPBD>2.0.CO;2 [PubMed] [Google Scholar]
- Ward BK, Allan RK, and Mok D. et al. 2002 A structure-based mutational analysis of cyclophilin 40 identifies key residues in the core tetratricopeptide repeat domain that mediate binding to Hsp90. J Biol Chem. 277:40799–40809. [DOI] [PubMed] [Google Scholar]
- Wegele H, Haslbeck M, Reinstein J, Buchner J. Sti1 is a novel activator of the Ssa proteins. J Biol Chem. 2003;278:25970–25976. doi: 10.1074/jbc.M301548200.0021-9258(2003)278<25970:SIANAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Weisman R, Creanor J, Fantes P. A multicopy suppressor of a cell cycle defect in S. pombe encodes a heat shock inducible 40 kDa cyclophilin-like protein. EMBO J. 1996;15:447–456.0261-4189(1996)015<0447:AMSOAC>2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Feramisco JR. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984;259:4501–4513.0021-9258(1984)259<4501:NANLOT>2.0.CO;2 [PubMed] [Google Scholar]
- Wu S-J, Liu F-H, Hu S-M, Wang C. Different combinations of the heat shock cognate protein 70 (Hsc70) C-terminal functional groups are utilized to interact with distinct tetratricopeptide repeat-containing proteins. Biochem J. 2001;359:419–426. doi: 10.1042/0264-6021:3590419.0264-6021(2001)359<0419:DCOTHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Obermann WM, Hartl FU. Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of Hsp90. J Biol Chem. 1998;273:18007–18010. doi: 10.1074/jbc.273.29.18007.0021-9258(1998)273<18007:SBOTRP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zeiner M, Gebauer M, Gehring U. Mammalian protein RAP46: an interaction partner and modulator of 70 kDa heat shock proteins. EMBO J. 1997;16:5483–5490. doi: 10.1093/emboj/16.18.5483.0261-4189(1997)016<5483:MPRAIP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]