Abstract
Background:
Phosphodiesterase type 5 inhibitors (PDE5I) are prescribed for erectile dysfunction and pulmonary hypertension. Despite its widespread use, there are only seven cases of drug-induced liver injury (DILI) associated with PDE5I, none associated with vardenafil or avanafil. We report a patient who had taken vardenafil and tadalafil individually for several years without developing symptoms of liver injury. However, after taking vardenafil and tadalafil together on 2 consecutive days, he developed severe cholestasis.
Methods:
Causality was determined using Roussel Uclaf causality assessment method (RUCAM).
Results:
The patient is a 72-year-old White man in excellent health who drank 2 units of alcohol, three times/week. Previously, he had used vardenafil for more than 2 years and tadalafil for 3 months as single agent for erectile dysfunction without any complications. He took vardenafil and tadalafil for 2 consecutive days and 5 days later, he developed dyspepsia, loss of appetite, jaundice, and intense itching. Liver tests showed mixed cholestatic/hepatocellular pattern of injury. Histology showed marked cholestasis with minimal inflammation. He remained cholestatic for 5 weeks before a full recovery 2 months later. The patient then resumed vardenafil monotherapy with no recurrent liver dysfunction. RUCAM causality score 7 indicates that the combination of PDE5I is probable cause of liver injury. The similarities among the eight cases of PDE5I DILI include a relatively short latency, cholestatic histological features, and complete recovery. Biochemical pattern of liver injury is variable.
Conclusions:
PDE5I DILI is a rare event that can result in severe acute liver injury.
Keywords: drug induced liver injury, hepatitis, phosphodiesterase type 5 inhibitors, tadalafil, vardenafil
Lay Summary: Phosphodiesterase type 5 inhibitors (PDE5I) are widely used in the United States for managing erectile dysfunction and pulmonary hypertension. Previously, there have been seven reports of liver injury associated with PED5I, primarily associated with sildenafil. We report a 72-year-old man who presented severe symptoms of liver injury after taking vardenafil and tadalafil together for 2 consecutive days. Our case adds to the number of reports of liver injury associated with PED5I and is the first to be associated with the combined use of vardenafil and tadalafil. We compare our case and previous cases of PED5I associated liver injury to better understand this rare occurrence. The pattern of blood test abnormalities among the eight cases differs but the findings on liver biopsy are similar. All patients with liver injury due to PED5I made a full recovery within 2 months. Liver injury did not occur in the few instances when patients resumed PED5I.
Introduction
Cyclic guanosine monophosphate (cGMP) is a versatile signaling molecule with one of its functions controlling vasodilation. cGMP is generated by guanylyl cyclase in response to nitric oxide (NO). Levels of cGMP are controlled by breakdown of cGMP by local phosphodiesterase enzymes. Type 5 phosphodiesterase (PDE5) isoenzymes are primarily located in the corpus cavernosum and the pulmonary vasculature. Therefore, phosphodiesterase type 5 inhibitors (PDE5I) are used in the treatment of erectile dysfunction and pulmonary hypertension (1). Approximately 4.5 million prescriptions for sildenafil and tadalafil were written in the United States in 2020 (2). Sildenafil, marketed as Viagra and Revatio, was the first PDE5I approved by the Food and Drug Administration in 1998 (3). To date, three additional PDE5I have been approved: vardenafil, tadalafil, and avanafil.
The most common side effects of PDE5I include headache, flushing, dyspepsia, altered vision, back pain, dizziness, and rhinitis (1). Despite its widespread use, drug-induced liver injury (DILI) associated with PDE5I is uncommon. There are currently four PDE5I approved by the FDA that include sildenafil, tadalafil, vardenafil, and avanafil (1). There are several case reports of cholestasis related to sildenafil (4–9), a single case report associated with tadalafil (10), and no cases related to vardenafil.
We report a patient who had taken vardenafil and tadalafil individually for several years without developing symptoms of liver injury. However, after taking vardenafil and tadalafil together on 2 consecutive days, he developed severe cholestasis. Causality was determined using the updated RUCAM (11).
Case Report
The patient is a 72-year-old White man in excellent health who drank 2 units of alcohol, three times/week. Previously, he had used vardenafil for more than 2 years and tadalafil for 3 months as a single agent for erectile dysfunction without any complications. He took vardenafil and tadalafil for 2 consecutive days and 5 days later, he developed dyspepsia, loss of appetite, jaundice, and intense itching. He did not take any other medication or supplement. The physical exam revealed normal vital signs. He was deeply jaundiced, and the liver edge was palpable two finger breadth below the costal margin. Laboratory studies; alkaline phosphatase 288 U/L, total protein 6.3 g/dL, albumin 4.3 g/dL, total bilirubin 17.3 mg/dL, aspartate aminotransferase (AST) 109 U/L, alanine aminotransferase (ALT) 253 U/L, lactate dehydrogenase 390 U/L, international normalizing ratio (INR) 1.1, white blood count 4,400/μL, 5.9% eosinophil, hemoglobin 13.4 g/dL, platelet 297,000 /μL. Viral (anti-hepatitis A virus IgM antibody, hepatitis B surface antigen, anti-hepatitis B core IgM antibody, and anti-hepatitis C antibody) and autoimmune (anti-nuclear antibody, anti-smooth muscle antibody, anti-mitochondrial antibody) serologies were negative, IgA 227 mg/dL, IgM 59 mg/dL, IgG 974 mg/dL. Magnetic resonance imaging and cholangiography showed liver measuring 14 cm, contracted gallbladder with no intra- or extra-hepatic biliary ductal dilatation.
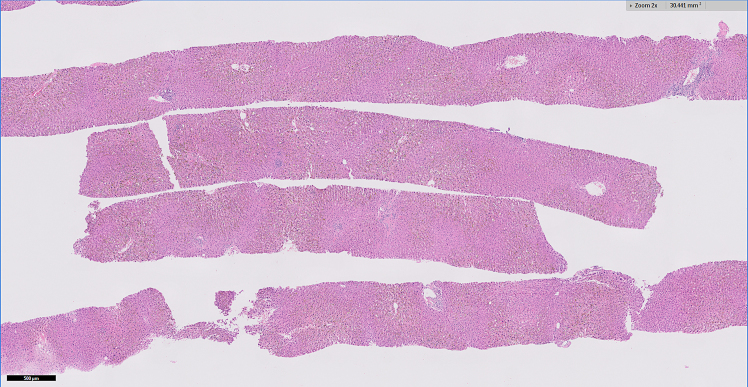
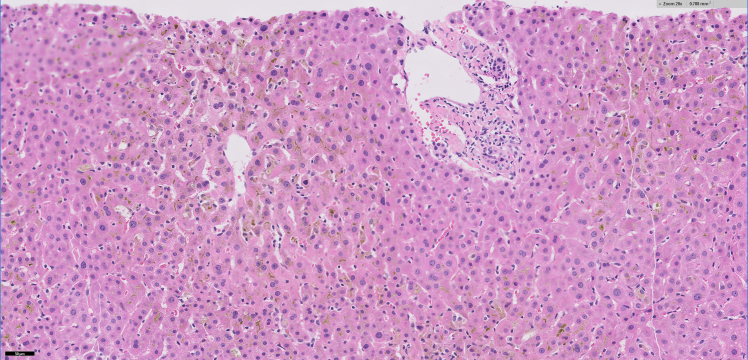
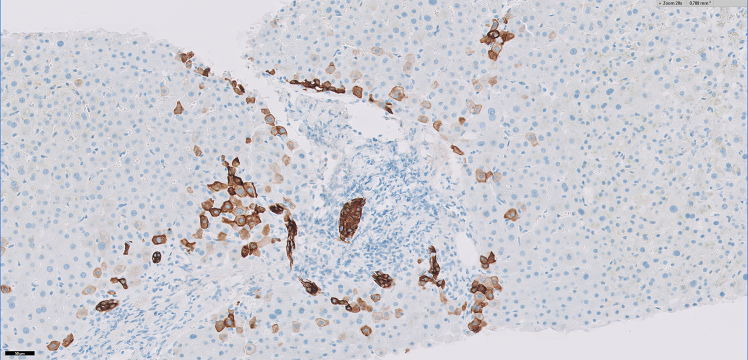
A liver biopsy was performed which showed portal tracts with mild chronic inflammatory infiltrates composed of predominantly lymphocytes mixed with occasional eosinophils without significant interface activity (Figures 1a and 1b). There was absent to minimal bile ductular proliferation as well as absent granulomatous inflammation within the portal tracts and lobules (Figure 1c). The lobules showed moderate to marked canalicular cholestasis with zone 2 and zone 3 distribution pattern accompanied by mild focal lymphocytic infiltrate, occasional acidophilic bodies, and minimal (less than 1%) macrovesicular steatosis. No significant hepatocellular ballooning or Mallory bodies were identified. Trichrome stain demonstrated absent to focal portal fibrosis (Figure 1d). There was minimal (1+) hemosiderosis in sinusoidal Kupfer cells identified by iron stain and no cytoplasmic globules are present on the PAS-Diastase stain. The histological findings on liver biopsy were compatible with cholestatic hepatitis.
Figure 1a:
Low power magnification of the core needle biposy showing intact liver architecture with centrovenular cholestasis evident as vague green pigment
Figure 1b:
20x magnification showing portal tract with no significant inflammatory infiltrate. Also, the centrovenular lobules show moderate canalicular cholestasis with increased Kupffer cells within the sinusoids
Figure 1c:
Immunohistochemical stain for Cytokeratin 7 (CK7) highlights the intact bile ducts and demonstrates a lack of biliary proliferative response. Note the CK7 positive “intermediate hepatocytes” at the interface and periportal lobules, indicating a response to cholestatic injury within the hepatocytes
Figure 1d:
Trichrome stain indicates a lack of significant fibrosis.
The patient was started on cholestyramine and ursodeoxycholic acid. His pruritus began abating 5 weeks after and symptoms resolved 2 months after his initial presentation. He resumed taking vardenafil monotherapy for erectile dysfunction with no recurrent liver dysfunction.
The course of the patient's liver tests is shown in Table 1 and the compilation of RUCAM to determine causality is shown in Table 2. The total score is 7 which indicates that the combination of vardenafil and tadalafil is the probable cause of liver injury. Compilation of all eight cases of PDEI acute liver injury is shown in Table 3.
Table 1:
Liver tests of patient
| Alkaline phosphatase (U/L) | Total bilirubin (mg/dL) | Alanine aminotransferase (U/L) | Aspartate aminotransferase (U/L) | Comments | |
|---|---|---|---|---|---|
| Baseline liver tests | 105 | 0.7 | 31 | 25 | |
| Onset | 288 | 17.6 | 25 | 109 | |
| Week 1 | 290 | 17.6 | 214 | 111 | Peak aminotransferase activities |
| Week 2 | 391 | 31.2 | 81 | 64 | Peak bilirubin and alkaline phosphatase levels |
| Week 3 | 340 | 24.9 | 61 | 52 | |
| Week 5 | 233 | 7.5 | 80 | 67 | |
| Week 6 | 219 | 4.5 | 69 | 57 | |
| Week 8 | 137 | 2.3 | 52 | 45 | |
| Week 12 | 97 | 0.9 | 31 | 35 | Complete resolution |
| Week 13 | Resumed vardenafil 3 consecutive days monthly | ||||
| Week 22 | 74 | 0.7 | 35 | 26 | |
| Week 37 | 91 | 0.7 | 30 | 29 |
Table 2:
RUCAM for liver injury
| Possible score | Study patient | |
|---|---|---|
| Time to onset | +2 | +2 |
| • 5–90 days | ||
| • <5 or >90 days | +1 | |
| Course of alkaline phosphatase after cessation of drug | +2 | |
| • Decrease >50% within 8 days | +3 | |
| • Decrease >50% within 30 days | +2 | |
| Risk factors | ||
| • Alcohol + | -1 | -1 |
| • Alcohol - | 0 | |
| • Age ≥55 years | +1 | +1 |
| • Age <55 years | 0 | |
| No concomitant drugs/herbs | 0 | |
| All causes-group I and II-ruled out | +2 | |
| Previous hepatotoxicity | +1 | |
| • Reaction labeled in the product characteristics | +2 | |
| • Reaction published but not labeled | +1 | |
| Response to unintentional re-exposure | 0 | |
| Total score for the case | +7 |
Table 3:
Summary of cases of liver injury associated with phosphodiesterase type 5 inhibitors
| Patient characteristics | Type of PDI | Age | Gender | Ethnicity | Time to onset (days) | Peak bilirubin (mg/dL) | Peak alkaline phosphatase (U/L) | Peak ALT (U/L) | Peak AST (U/L) | Presentation to peak bilirubin (weeks) | Presentation to peak alkaline phosphatase (weeks) | Presentation to peak ALT (weeks) | Peak INR | R factor Pattern of injury | Time from peak to resolution (weeks) | Rechallenge | Alcohol Intake | How long been taking | Histology | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref [Essaid | Tadalafil | 38 | Male | Not available | 5 | 2.5 × ULN | 4 × ULN | 4 x ULN | 3 × ULN | 1.0 Cholestatic | 8 | No | None | 5 days | No | |||||
| Ref [Nissan] | Sildenafil | 65 | Male | Caucasian | 17 | 2.06 | 326 | 984 | 1342 | 1 | 1 | 1 | 1.37 | >5 Hepatocellular | 30 | No | One ounce/month | 2.5 weeks | No | |
| Ref [Maroy] | Sildenafil | 65 | Male | Not available | 1 | Not reported | 5 × ULN | 113 × ULN | 114 × ULN | 1 | 1 | >5 Hepatocellular | 4 | Yes | None | > 1year | No | |||
| Ref [Balian] | Sildenafil | 56 | Male | Not available | 21 | 6.5 x ULN | 2.5 × ULN | 8 × ULN | 3.5 × ULN | 1 | 1 | 1 | 3.2 Mixed Cholestatic | 4 | No | <10 g/day | Yes Cholestasis | |||
| Ref [Enomoto] | Sildenafil | 58 | Male | Not available | 30 | 8.5 | 476 | 64 | 42 | 0.5 Cholestatic | 16 | No | No excessive alcohol intake | 1 month | Yes Cholestasis Minimal portal inflammation | |||||
| Ref [Wolfhagen] | Sildenafil | 59 | Male | Not available | 7 | 19 | 225 | 1,665 | 1,077 | 10 | 9 | 1 | > 5 Hepatocellular | 13 | No | 1 unit/week | 3 months | Yes Cholestasis Minor infiltrate with predominant eosinophils | ||
| Ref [Daghfous] | Sildenafil | 49 | Male | Not available | 28 | 0.62 | Normal | 1.2 × ULN | 7.4 × ULN | 1 | 4 | > 5 Hepatocellular | 3 | Yes | Information not provided | 1 month | No | |||
| Patient in this case report | Vardenafil Tadalafil | 72 | Male | Caucasian | 5 | 31.2 | 391 | 214 | 111 | 2 | 2 | 1 | 1.2 | 1.9 Cholestatic | 12 | Yes | 2 units, 3 times/week | Vardenafil individually >2 years Tadalafil individually 3 months | Cholestasis |
Discussion
Despite the widespread use of PDE5I, this patient represents only the eighth case of PDE5I associated acute liver injury and the first involving the combination of vardenafil and tadalafil. This scenario raises possible mechanisms for liver injury. The combination of vardenafil and tadalafil results in a higher level of PDE5I but this is unlikely to be a factor since much higher doses of PDE5I are used in patients treated for pulmonary hypertension (12). The second potential mechanism is the combination itself, causing liver injury since the patient did not have recurrent symptoms when he resumed tadalafil alone.
Among the seven case reports and our patients, all patients were male who were taking PDE5I for erectile dysfunction. All but one case involved sildenafil, with the single non-sildenafil case involving tadalafil (10). Two of these case reports stand out and warrant closer examination. Daghfous et al. reported a 49-year-old male with predominantly AST elevation with normal bilirubin, alkaline phosphatase, gamma glutamyl transferase levels. AST normalized within 20 days (4). Liver biopsy was not taken. The clinical picture was consistent with an ischemic event (13) or acute muscle injury (14). The case report by Nissan et al. (7) was a 65-year-old patient with cirrhosis who remained compensated despite acute hepatocellular injury, and fully recovered. There were no cases of acute liver failure or death, and all patients made a complete recovery.
Our patient had used vardenafil and tadalafil individually for several years without problems but developed severe cholestasis 5 days after taking vardenafil and tadalafil together for the first time. After his liver tests resolved, he resumed tadalafil (without vardenafil) without recurrence of symptoms. One patient had used PDE5I for 12 months without issues, but the other cases had taken PDE5I for 5 days to 3 months which is the usual latency for drug induced liver injury (11).
The latency (time to onset from the beginning of drug exposure) is relatively short for PDE5I associated liver injury; 5 days for our patient and median 12 (range: 1–30) days among all the cases. The recovery time loosely correlated with the severity of jaundice ranging from 3 to 16 (median 10) weeks. Including our case, there were three re-challenges none of which resulted in recurrent liver injury, although our patient did not retake the combination and only took vardenafil without tadalafil. None of the patients drank alcohol excessively.
The biochemical pattern of liver injury among the eight cases of PDE5I DILI was variable (four hepatocellular, three cholestatic, and one hepatocellular–cholestatic mixed). However, the liver biopsy findings of four cases were congruent with the biochemical presentation in showing the various histological types of liver injury. Our case report demonstrated a slightly more severe histological picture than what was typically observed in the PDE5I injury. Specifically, our case displayed a mild cholestatic hepatitis characterized by canicular cholestasis with conspicuous inflammatory infiltrates and a modest degree of single hepatocyte necrosis. These findings were similar with the cases reported by Enomoto et al. and Balian et al. which both showing mixed cholestatic-hepatocellular injury pattern (6,8). The increased necro-inflammatory activity present in our patient may be attributable to combined PDE5 inhibitor use producing a mixed cholestatic-hepatocellular injury albeit with a predominant cholestatic biochemical pattern.
In summary, despite the widespread use of PDE5I, liver injury associated with this class of drug is extremely rare. We report only the eighth case of PDE5I associated liver injury that presented with severe cholestasis; clinically, biochemically, and histologically, which resolved 2 months later. The similarities among the cases include a relatively short latency, cholestatic features seen on liver biopsy and complete recovery. However, the biochemical pattern of liver injury among the cases is variable.
Funding Statement
Funding: No funding was received for this work.
Contributions:
Conceptualization, T-L Fong; Data curation, T-L Fong, B Xu, DR Braxton; Investigation, T-L Fong, B Xu, DR Braxton; Methodology, T-L Fong, B Xu, DR Braxton; Writing – Original Draft, B Xu, DR Braxton and T-L Fong; Writing – Review & Editing, B Xu, DR Braxton, and T-L Fong.
Ethics Approval:
This study was approved by the institutional review board at Hoag Memorial Hospital Presbyterian.
Informed Consent:
The authors confirm that informed patient consent has been secured from the patient.
Registry and the Registration No. of the Study/Trial:
N/A
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
References
- 1.Dhaliwal A, Gupta M. PDE5 inhibitors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK549843/ [Google Scholar]
- 2.ClinCalc DrugStats Database. Sildenafil - Drug Usage Statistics. https://clincalc.com/DrugStats/Drugs/Sildenafil
- 3.U.S. Food and Drug Administration. Drug Approval Package: Viagra (sildenafil citrate). https://www.accessdata.fda.gov/drugsatfda_docs/NDA/98/viagra/viagra_toc.cfm
- 4.Daghfous R, El Aidli S, Zaiem A, Loueslati MH, Belkahia C. Sildenafil-associated hepatotoxicity. Am J Gastroenterol. 2005;100(8):1895–6. PMID: [DOI] [PubMed] [Google Scholar]
- 5.Wolfhagen FH, Vermeulen HG, de Man RA, Lesterhuis W. Initially obscure hepatotoxicity attributed to sildenafil. Eur J Gastroenterol Hepatol. 2008;20(7):710–2. 10.1097/MEG.0b013e3282f2bbb5. PMID: [DOI] [PubMed] [Google Scholar]
- 6.Enomoto M, Sakaguchi H, Ominami M, et al. Sildenafil-induced severe cholestatic hepatotoxicity. Am J Gastroenterol. 2009;104(1):254–5. 10.1038/ajg.2008.18. PMID: [DOI] [PubMed] [Google Scholar]
- 7.Nissan R, Poperno A, Stein GY, et al. A case of hepatotoxicity induced by adulterated “Tiger King”, a Chinese herbal medicine containing sildenafil. Curr Drug Saf. 2016;11(2):184–8. 10.2174/1574886311207040257. PMID: [DOI] [PubMed] [Google Scholar]
- 8.Balian A, Touati F, Huguenin B, et al. Hépatite aiguë mixte probablement induite par le sildénafil (Viagra) chez un malade sans autre facteur de risque [Probable sildenafil (Viagra) induced acute hepatitis in a patient with no other risk factors]. Gastroenterol Clin Biol. 2005;29(1):89. 10.1016/s0399-8320(05)80705-5. PMID: [DOI] [PubMed] [Google Scholar]
- 9.Maroy B. Hépatite aiguë cytolytique probablement due à la prise de sildénafil (Viagra) [Cytolytic acute hepatitis probably due to sildenafil (Viagra)]. Gastroenterol Clin Biol. 2003;27(5):564–5. PMID: [PubMed] [Google Scholar]
- 10.Essaid A, Timraz A. Hépatite aiguë cholestatique probablement induite par le tadalafil (Cialis) [Cholestatic acute hepatitis induced by tadalafil (Cialis)]. Gastroenterol Clin Biol. 2010;34(4–5):e1–e2. 10.1016/j.gcb.2010.01.001. PMID: [DOI] [PubMed] [Google Scholar]
- 11.Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17(1):14. 10.3390/ijms17010014. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karedath J, Dar H, Ganipineni VDP, et al. Effect of Phosphodiesterase-5 (PDE-5) inhibitors on clinical outcomes in patients with pulmonary hypertension: a meta-analysis of randomized control trials. Cureus. 2023;15(1):e33363. 10.7759/cureus.33363. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrion J. Hypoxic hepatitis. Liver Int. 2012;32(7):1039–52. 10.1111/j.1478-3231.2011.02655.x. PMID: [DOI] [PubMed] [Google Scholar]
- 14.Han JH, Kwak JY, Lee SS, et al. Markedly elevated aspartate aminotransferase from non-hepatic causes. J Clin Med. 2022;12(1):310. 10.3390/jcm12010310. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- ClinCalc DrugStats Database. Sildenafil - Drug Usage Statistics. https://clincalc.com/DrugStats/Drugs/Sildenafil
- U.S. Food and Drug Administration. Drug Approval Package: Viagra (sildenafil citrate). https://www.accessdata.fda.gov/drugsatfda_docs/NDA/98/viagra/viagra_toc.cfm