Abstract
Noise exposure may result in production of auto-antibodies against heat shock proteins (Hsps), which might be of significance in the pathogenesis or prognosis (or both) of auto-immune ear diseases. However, it is not known whether these antibodies are associated with noise-induced hearing loss (NIHL) in workers exposed to noise in occupational settings. Using immunoblotting with human recombinant Hsps, audiological assessment, and multivariate logistic regression models, we investigated the presence of antibodies against Hsp60 and Hsp70 and hearing levels, and analyzed their associations with NIHL in 399 workers exposed to noise between 75 and 115 dB. Our findings showed that the prevalence of positive anti-Hsp70 was significantly higher in the workers with slight and moderate high-frequency hearing loss than in normal workers (P < 0.05). Furthermore, the prevalence of positive anti-Hsp60 in workers with moderate low-frequency NIHL was significantly higher than in the normal (P < 0.01). The levels of anti-Hsp70 and anti-Hsp60 seemed correlated, and the level of anti-Hsp70 better predicted the level of anti-Hsp60. An elevated plasma level of anti-Hsp70 was associated with a nonsignificantly increased risk of high-frequency NIHL (adjusted OR = 1.45; 95% CI = 0.89–2.36) and an elevated plasma level of anti-Hsp60 was associated with a nonsignificantly increased risk of the low-frequency NIHL (adjusted OR = 2.25; 95% CI = 0.85–5.96). These results suggest that the production of anti-Hsp60 and anti-Hsp70 may play a role in the pathogenesis of NIHL, and that anti-Hsps may be a risk factor. The precise mechanisms for the elevation of antibodies against Hsps caused by noise exposure and their possible role in the development of NIHL warrant further investigations.
INTRODUCTION
Noise-induced hearing loss (NIHL) is one of the most prevalent occupational hazards in modern industries. It is a pathological phenomenon that usually progresses over 10–15 years of intensive noise exposure, and then tends to progress more slowly thereafter. It produces a sensor-neural defect that evolves over these years (John 2000). Previous observations have shown that noise can induce changes in immune function both in terms of immunosuppression or immuno-enhancement. In a mouse model, an 8-month exposure to low-frequency noise resulted in a decrease in CD4+ and CD8+ T cells and in IgM and B lymphocytes (Aguas et al 1999a). Interestingly, in lupus-prone mice, exposure to similar low-frequency noise lead to an accelerated expression of the auto-immune disease accompanied by a rise in numbers of IgM + B lymphocytes and CD8+ T cells (Aguas et al 1999b). In male rats exposed to white noise for 150 min/d, serum IgM levels and splenic natural killer (NK) cell activity increased after 24 hours, whereas peripheral phagocytic activity decreased (Van Raaij et al 1996). In the same model, splenic NK activity increased on days 1 and 7 but was lower than controls on day 21. Thus, noise is a stressor, which can affect behavior and neuro-endocrine and immune functions.
Heat shock proteins (Hsps), the phylogenetically conserved proteins induced by numerous physical and physiological stresses, can be induced by noise and toxic drugs (Lim et al 1993). Moreover, Hsps, when induced in response to moderate nontraumatic sound levels, can condition the ear to withstand effects of loud noise and protect from hearing loss, although there is individual variability (Yoshida et al 1999; Altschuler et al 2002). In addition, several lines of evidence suggest that antibody to Hsp70 may be closely associated with the pathogenesis or prognosis (or both) of sensorineural deafness (Billings et al 1995; Veldman 1997).
We have previously observed that antibodies against the inducible member of the Hsp70 family were often associated with exposure to severe noise in steel industry workers (Wu et al 2001). Many observations have shown links between aberrant expression of stress proteins and various disease states (Welch 1992; Minowada and Welch 1995; Wu et al 2001; Xiao et al 2002, 2003). Some Hsps can also present as self-antigens to the immune system, resulting in the production of auto-antibodies to Hsps in patients with inflammatory diseases, auto-immune disorders, or after various infections caused by viruses, bacteria, mycobacteria, and parasites, and with hypertension and atherosclerosis (Kauffmann and Schooel 1994; Schett et al 1995; Frosttegard et al 1997; Pockley et al 2002; Bason et al 2003 and reviewed by Xu [2002] and Pockley [2003]). It has been suggested that antibodies against Hsps might be of significance in the pathogenesis or prognosis (or both) of some diseases (Jarjour et al 1991; Schett et al 1995; Shingai et al 1995; Wu et al 1998, 2001). However, little is known about association between the presence of auto-antibodies against Hsp60 and inducible Hsp70 with NIHL and the role these antibodies may play in development of NIHL. We therefore measured anti-Hsp60 and anti-Hsp70 antibody levels in 399 noise-exposed workers and determined whether the presence of antibodies to Hsp60 or Hsp70 was a risk factor in the development of NIHL.
MATERIALS AND METHODS
Subjects and evaluation of environmental conditions
This study included 399 workers at the Dongfeng Motor Co in Shiyan, Hubei, China, who worked in different work sites and were exposed to noise between 75 and 115 dB. They were not exposed on a regular basis to other stressful environmental factors, such as heat and dust, and did not have other ear diseases or abnormalities. Noise levels at the workplaces were determined with a sound pressure audiometer (BK-2231, Brüel & Kjaer Company, Naerum, Denmark), and the measurement of noise was performed 9 times at 10 AM, 3 PM, and 5 PM for 3 consecutive days each time, twice per year. Cumulative noise exposures (CNE) were used to represent workers' exposure levels and were calculated according to sound pressure level A and employee time as follows:
| Expc = Leq + 16.61 × log10(T/T0), dBA, |
where T0 = 1 y (Talbott et al 1999).
Evaluation of health status
Health status was evaluated in all workers using a questionnaire, as well as by clinical and laboratory examination. The questionnaire was used to obtain personal and family history including risk factors for hearing loss, such as age, employee years, lifestyle (smoking and drinking), history of explosive noise exposure, and diseases. An industrial hygienist filled out the questionnaire for each worker before the medical examination. The clinical laboratory examination included signs, weight, height, pulse, electrocardiogram, B-echography, blood pressure, blood routine, and hepatic function test. Venous blood was also collected in heparinized tubes to separate plasma for the detection of anti-Hsp60 and anti-Hsp70.
Audiological assessment and definitions of hearing loss
Pure-tone audiometry was performed for both ears at 0.5, 1, 2, 3, 4, 6, and 8 kHz. All auditory testings were performed in a sound-attenuating booth by a trained technician. Audiometry was done using Swiss Midimate RT-150 Audiometer (Brüel and Kjaer) calibrated to ISO 389 (1985-E) for measurement of air conduction. Threshold value was defined as the lowest signal intensity that the subject detected at least 50% of the time, with a minimum of 3 tries. Masking was performed if the subject had a threshold value that differed by 40 dB or more between both ears. Otoscopic examination of the external acoustic meatus and tympanic membrane was done to exclude any ear diseases. Hearing threshold worse than 25 dB in either ear was defined as hearing loss. Hearing loss can either be in the low-frequency range (0.5–2 kHz) or high-frequency range (4–8 kHz). We took the mean threshold of 0.5, 1, and 2 kHz (PTA512) as low-frequency hearing loss and the mean threshold of 4, 6, and 8 kHz (PTA468) as high-frequency hearing loss. Severity of hearing loss was further categorized by the extent of the hearing threshold using the World Health Organization grading: normal (<25 dB); slight (26–40 dB); moderate (41–60 dB); severe (61–80 dB); and profound (worse than 81 dB). In the normal hearing range, we further defined 0–15 dB as normal and 15–25 dB as borderline normal (Stelmachowicz et al 1993).
Determination of anti-Hsp60 and anti-Hsp70
Recombinant human Hsp60 and Hsp70 were obtained through the expression of the human complementary deoxyribonucleic acid coding for the Hsp60 and inducible Hsp70 in NaCl-induced Escherichia coli GJ1168 cells using pET30 as expression vector (Tanguay et al 1993). The immunoblot assay was performed as previously described using the recombinant human Hsps (Wu et al 1998). Briefly, recombinant Hsp60 or Hsp70 were separated on sodium dodecyl sulfate gels and transferred to nitrocellulose membranes, and small membrane pieces (2 × 2 mm) containing the Hsps were incubated with the plasma diluted 1:10, and the presence of anti-Hsp60 or anti-Hsp70 was revealed using 3,3-diaminobenzidine tetrahydrochloride. A brown band on the nitrocellulose strips is regarded as positive and no color on nitrocellulose as negative (see Wu et al 1998, 2001, for further details).
Statistical analyses
All data were entered into a computerized database. Further data analysis was carried out by using the statistical analysis software SPSS11.0 package. Multivariate logistic regression models were built for a forward stepwise selection procedure. Other analyses were carried out based on the chi-square test and Student's t-test. Statistical inferences are based on the different levels of significance (P < 0.05 or P < 0.01).
RESULTS
Hearing status and characteristics of risk factors of NIHL
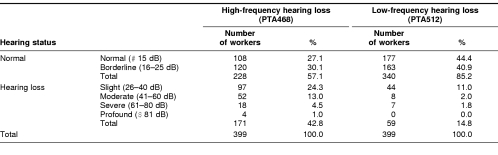
Table 1 summarizes the profiles of hearing levels for different frequencies in the workers who had been exposed to occupational noise. Among the 399 workers tested, 108 (27.1%) and 177 (44.4%) had a normal hearing (≤15 dB) for both high- and low-frequency, respectively; 120 (30.1%) and 163 (40.9%) had a borderline hearing loss (16–25 dB) for both high- and low frequency, respectively. Overall, 171 (42.8%) and 59 (14.8%) workers suffered various hearing loss for both high- and low-frequency, respectively (Table 1). It is apparent that high-frequency hearing loss was more common than low-frequency hearing loss in these workers.
Table 1.
Profiles of hearing levels in the workers exposed to noise
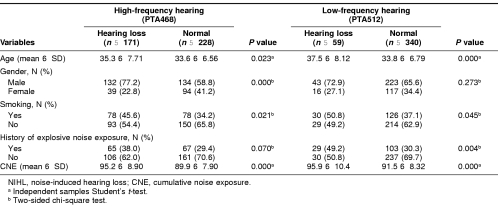
Table 2 lists potential risk factors for NIHL at different frequencies in the workers who had been exposed to occupational noise. The 171 subjects with hearing loss at high frequency and 59 subjects with hearing loss at low frequency were compared with those without loss (considered having a normal hearing) at high frequency (n = 228) or low frequency (n = 340), respectively. Compared with subjects with a normal hearing at high frequency, those who had a hearing loss at high frequency were more likely to be older (P = 0.023), men (P < 0.000), smokers (P = 0.021), and had a higher value of CNE (P = 0.000). However, there was no difference in history of exposure to explosive noise (P = 0.070) (Table 2). In the case of low-frequency hearing, workers with hearing loss were also more likely to be older (P = 0.000), smokers (P = 0.045), and had a higher value of CNE (P = 0.000) than those with normal hearing. No difference in gender was noted (P = 0.273); however, in low frequency, there was difference in history of exposure to explosive noise (P = 0.004) (Table 2).
Table 2.
Differences of factors in workers with NIHL and normal workers
Presence of anti-Hsp60 and anti-Hsp70
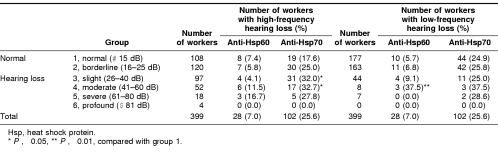
Table 3 details the presence of antibodies against Hsp60 and Hsp70 in workers with normal hearing (group 1, normal; and group 2, borderline) and in those with various NIHL (groups 3–6 or slight to profound hearing loss). Overall, among the 399 workers, 102 (25.6%) subjects had detectable anti-Hsp70, whereas only 28 (7.0%) had detectable anti-Hsp60. Because of the small number of workers who had detectable anti-Hsp60, most of the comparisons for this Hsp were not very meaningful. Compared with group 1 with normal hearing, subjects with slight and moderate hearing loss at high frequency were more likely to have detectable anti-Hsp70 (P < 0.05 for both), whereas no significant differences were observed for the severe and profound groups, perhaps because of the small number of observations in these groups (Table 3). This was also true for the prevalence of detectable anti-Hsp60 in those with the low-frequency hearing loss, in which a difference was observed only for the group of moderate hearing loss (Table 3). No difference in the prevalence of detectable anti-Hsp70 and anti-Hsp60 was observed between the normal and other groups with hearing loss at low frequency and between the normal and other groups with hearing loss at high frequency, respectively (Table 3).
Table 3.
Prevalence of antibodies against Hsp60 and Hsp70 in workers with hearing loss
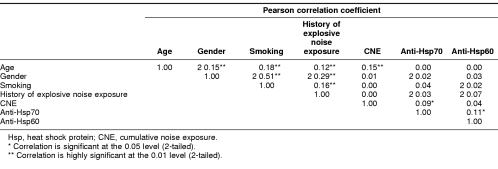
We next analyzed correlation among risk factors related to NIHL and anti-Hsps in these workers. As shown in Table 4, age was correlated with gender, smoking status, history of explosive noise exposure, and CNE score, whereas history of explosive noise exposure was correlated with age, gender, and smoking status as well, but none of these risk factors were correlated with the levels of anti-Hsp70 and anti-Hsp60, except for CNE score, which was weakly correlated with anti-Hsp70 (r = 0.09 and P < 0.05) (Table 4). However, it appeared that the levels of anti-Hsp70 and anti-Hsp60 were correlated (r = 0.11 and P < 0.05).
Table 4.
Correlations among age, gender, smoking, history of explosive noise exposure, CNE, and anti-Hsps in the workers
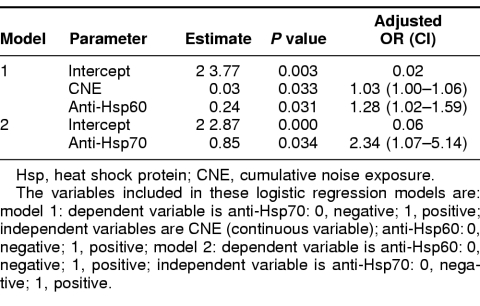
Because CNE values were correlated with anti-Hsp70 but not with anti-Hsp60, we fitted 2 logistic regression models to describe their relationship. As shown in Table 5, anti-Hsp70 levels were significantly predicted by both CNE and anti-Hsp60 levels, and the estimated equation was: anti-Hsp70 = 0.0283 × CNE + 0.2430 × anti-Hsp60 − 3.7661. However, anti-Hsp60 levels were only significantly predicted by anti-Hsp70 levels but not with CNE (therefore, it was omitted in the final model), and the estimated equation was: anti-Hsp60 = 0.8509 × anti-Hsp70 − 2.8658. The data suggested that those who were positive for anti-Hsp60 were 1.28 (95% CI = 1.02–1.59) times likely to be positive for anti-Hsp70, whereas those who were positive for anti-Hsp70 were 2.34 (95% CI = 1.07–5.14) times likely to be positive for anti-Hsp60.
Table 5.
Multiple logistic regression models of risk factors among positive and negative anti-Hsps groups
Association of anti-Hsp60 and anti-Hsp70 with hearing impairment
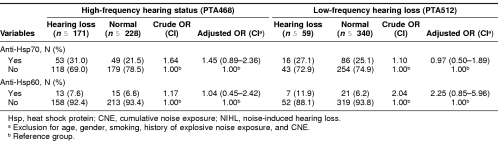
Finally, we calculated the crude, adjusted ORs and 95% CI for the associations of anti-Hsp60 and anti-Hsp70 with NIHL. Compared with those who were negative for anti-Hsp70, workers with positive anti-Hsp70 had a nonsignificantly increased risk (adjusted OR = 1.45; 95% CI = 0.89–2.36) of high-frequency hearing loss but not low-frequency hearing loss (adjusted OR = 0.97; 95% CI = 0.50– 1.89). On the contrary, compared with those who were negative for anti-Hsp60, there was no increased risk (adjusted OR = 1.04; 95% CI = 0.45–2.42) of high-frequency hearing loss in workers with positive anti-Hsp60, but there was a nonsignificantly increased risk (adjusted OR = 2.25; 95% CI = 0.85–5.96) of low-frequency hearing loss in these workers (Table 6).
Table 6.
Logistic regression analysis of anti-Hsp70 and anti-Hsp60 in workers with NIHL and normal workers
DISCUSSION
Excessive exposure to noise results in temporary or permanent changes in hearing ability in both humans and animals (Davis et al 1950; McFadden and Plattsmier 1982; Henry 1984). NIHL was previously known as industrial deafness in exposed workers, and it is still an important problem in occupational health. Similar to many chronic diseases, NIHL has a multifactorial etiology. Major risk factors for hearing loss include increasing age and long-term exposure to continuous noise in occupational settings. However, its precise mechanism and other potent risk factors remain to be explored.
We found that the prevalence of positive anti-Hsp70 was significantly higher in the workers with slight and moderate high-frequency hearing loss than in normal workers (P < 0.05), and that the prevalence of positive anti-Hsp60 in workers with moderate low-frequency NIHL was also significantly higher than in normal workers (P < 0.01). Some possible reasons for the production of anti-Hsps are (1) genetic factors, (2) viral and bacterial infection, (3) the denaturation and release of Hsps as a result of cell damage, and (4) the presence of antigen-specific lymphocytes (Wu et al 1996). In the present study, we did not find evidence for the involvement of genetic factors or infections that could be responsible for the presence of anti-Hsps antibodies. As an abnormal stress within working conditions, occupational noise can trigger heat shock response to induce the synthesis of Hsps (Lim et al 1993). The elevated and denatured or degraded Hsps or peptides derived from them may become the target of humoral and T cell–mediated immune response and may provide a link between the immune response to infection and auto-immunity caused by T lymphocyte cross-reactivity (Xu et at 1993, 1999; Schett et al 1995). Noise can induce several significant changes in immune functions (Van Raaij et al 1996; Aguas et al 1999a, 1999b).
Our results also show that the elevated plasma level of anti-Hsp70 was associated with a higher risk of high-frequency hearing loss, whereas the elevated plasma level of anti-Hsp60 was associated with a higher risk of low-frequency hearing loss. The presence of anti-Hsps may be involved in the pathogenesis of some diseases or may be prognostic (or both). Several immunoblot studies have demonstrated that many patients with idiopathic sensorineural hearing loss and Meniere's disease have anti-Hsp70 within the inner ear (Moscicki et al 1994; Gottschlich et al 1995), and that the presence of this anti-Hsp70 is highly correlated with the active phase of hearing loss and steroid responsiveness (Moscicki et al 1994). Interestingly, a possible dominant epitope within the Hsp70 molecule has been reported (Bloch et al 1999). Previous studies also have shown that severe noise can induce the production of anti-Hsp70 (Lim et al 1993; Wu et al 2001). Cytoprotection by Hsps was reported in many studies involving the heart and brain of animals (Currie et al 1993; Rajdev et al 2000), and induction of Hsp70 also seems to protect ear from high-level noise and acoustic injury (Yoshida et al 1999; Altschuler et al 2002). It remains to be confirmed whether there is a relation between the increased production of autoantibodies against Hsps and induced Hsps caused by exposure to noise and other stresses during development of NIHL.
Interestingly, our findings also show that the presence of different anti-Hsps is associated with different frequencies of hearing impairment. As the features of NIHL development showed, noise damages the ear first at a frequency of about 4 kHz, and one of the reasons for it is the acoustic resonance characteristic of the external ear. It progresses steadily over the initial decade of exposure to noise and then tends to plateau. Typically, the next affected area is in the 6000-Hz region, followed by the 8000- and the 2000-Hz regions where hearing losses are more slowly progressive (Taylor et al 1965). This process of NIHL and the induction and distribution of Hsp70 during and after stresses including noise may explain why anti-Hsp70 might be one of the risk factors for high-frequency NIHL. With the progression of hearing loss, the damage spreads to the lower frequencies including 2000, 1000, and 500 Hz, and this damage occurs in inner ear. Hsp60 is mainly found in the mitochondria of cells during stress (Morimoto et al 1994), but it (or peptide derived from Hsp60 or mimicking antigens) can also be present at the cell surface where its role is still unclear (Soltys and Gupta 1997; Bocharov et al 2000; Bason et al 2003). However, the detailed mechanism of production of anti-Hsp60 and anti-Hsp70 and their possible significance as a risk factor in the development of NIHL clearly need further investigation.
In summary, our present findings indicate that the presence of different anti-Hsps is associated with different frequency hearing impairment. It remains to be determined whether there is a relationship between the 2 anti-Hsps antibodies and whether these 2 antibodies play any role in the pathogenesis of NIHL.
Acknowledgments
We are particularly grateful to all individuals who voluntarily participated in the study and to many members of the medical personnel of Dongfeng Motor Co, Shiyan, Hubei, for their generous help in the examination and sampling of subjects. This work was supported by research funds from the National Key Basic Research and Development Program (2002CB512905), the National Natural Science Foundation of China (NNSFC) to T.W. T.W. and R.M.T. also acknowledge financial support from the NNSFC of China and the Canadian Institute of Health Research of Canada for a research exchange program and an operating CIHR grant (to R.M.T.).
REFERENCES
- Aguas AP, Esaguy N, Grande N, Castro AP, Castelo Branco NA. Effect of low frequency noise exposure on BALB/c mice splenic lymphocytes. Aviat Space Environ Med. 1999a;70(3 Pt 2):A128–A131. [PubMed] [Google Scholar]
- Aguas AP, Esaguy N, Grande NR, Castro AP, Castelo Branco NA. Acceleration of lupus erythematosus-like processes by low frequency noise in the hybrid NZB/W mouse model. Aviat Space Environ Med. 1999b;70(3 Pt 2):A132–A136. [PubMed] [Google Scholar]
- Altschuler RA, Fairfield D, Cho Y, Leonova E, Benjamin IJ, Miller JM, Lomax MI. Stress pathways in the rat cochlea and potential for protection from acquired deafness. Audiol Neurootol. 2002;7:152–156. doi: 10.1159/000058301.1420-3030(2002)007<0152:SPITRC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bason C, Corrocher R, and Lunardi C. et al. 2003 Interaction of antibodies against cytomegalovirus with heat-shock protein 60 in pathogenesis of atherosclerosis. Lancet. 362:1961–1977. [DOI] [PubMed] [Google Scholar]
- Billings PB, Keithley EM, Harris JP. Evidence linking the 68 kilodalton antigen identified in progressive sensorineural hearing loss patient sera with heat shock protein 70. Ann Otol Rhinol Laryngol. 1995;104:181–188. doi: 10.1177/000348949510400302.0003-4894(1995)104<0181:ELTKAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bloch DB, Gutierrez JA, Guerriero V Jr, Rauch SD, Bloch KJ. Recognition of a dominant epitope in bovine heat-shock protein 70 in inner ear disease. Laryngoscope. 1999;109:621–625. doi: 10.1097/00005537-199904000-00019.0023-852X(1999)109<0621:ROADEI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bocharov AV, Vishnyakova TG, Baranova IN, Remaley AT, Patterson AP, Eggerman TL. Heat shock protein 60 is a high-affinity high-density lipoprotein binding protein. Biochem Biophys Res Commun. 2000;277:228–235. doi: 10.1006/bbrc.2000.3663.0006-291X(2000)277<0228:HSPIAH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG. Heat-response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963.0009-7322(1993)087<0963:HALOTN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Davis H, Morgan CT, Hawkins JE Jr, Galambos R, Smith FW. Temporary deafness following exposure to loud tones and noise. Acta Otolaryngol. 1950;88:1–56.0001-6489(1950)088<0001:TDFETL>2.0.CO;2 [PubMed] [Google Scholar]
- Frosttegard J, Lemne C, Andersson B, Zee RVD, Liessling R, Faire U. Association of serum antibodies to heat shock protein 65 with borderline hypertension. Hypertension. 1997;29:40–44. doi: 10.1161/01.hyp.29.1.40.0194-911X(1997)029<0040:AOSATH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gottschlich S, Billings PB, Keithley EM, Weisman MH, Harris JP. Assessment of serum antibodies in patients with rapidly progressive sensorineural hearing loss and Meniere's disease. Laryngoscope. 1995;105:1347–1352. doi: 10.1288/00005537-199512000-00016.0023-852X(1995)105<1347:AOSAIP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Henry KR. Cochlear damage resulting from exposure to four different octave bands of noise at three ages. Behav Neurosci. 1984;98:107–117. doi: 10.1037//0735-7044.98.1.107.0735-7044(1984)098<0107:CDRFET>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jarjour WN, Jeffries BD, Davis JS, Welch WJ, Mimura T, Winfield JD. Autoantibodies to human stress proteins. Arthritis Rheum. 1991;34:1133–1138. doi: 10.1002/art.1780340909.0004-3591(1991)034<1133:ATHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- John J. Occupational hearing loss. Am J Ind Med. 2000;37:112–120. doi: 10.1002/(sici)1097-0274(200001)37:1<112::aid-ajim9>3.0.co;2-#.0271-3586(2000)037<0112:OHL>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kauffmann SHE, Schooel B 1994 Heat shock proteins as antigens in immunity against infection and self. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 495–531. [Google Scholar]
- Lim HH, Jenkins OH, Myers MW, Miller JM, Altschuler RA. Detection of HSP72 synthesis after acoustic overstimulation in rat cochlea. Hear Res. 1993;69:146–150. doi: 10.1016/0378-5955(93)90102-7.0378-5955(1993)069<0146:DOHSAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS 1982 Exposure-induced loudness shifts and threshold shifts. In: New Perspectives on Noise-Induced Hearing Loss, ed Hamernik RP, Henderson D, Salvi R. Raven Press, New York, 363–373. [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Investig. 1995;95:3–12. doi: 10.1172/JCI117655.0021-9738(1995)095<0003:CIOTSR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C 1994 The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Moscicki RA, San Martin JE, Quintero CH, Rauch SD, Nadol JB Jr, Bloch KJ. Serum antibody to inner ear proteins in patients with progressive hearing loss. Correlation with disease activity and response to corticosteroid treatment. JAMA. 1994;272:611–616.0098-7484(1994)272<0611:SATIEP>2.0.CO;2 [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5.0140-6736(2003)362<0469:HSPARO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2002;42:235–238. doi: 10.1161/01.HYP.0000086522.13672.23.0194-911X(2002)042<0235:SHSPLP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791.0364-5134(2000)047<0782:MORHSP>2.0.CO;2 [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van Der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 65 mediate endothelial cytotoxicity. J Clin Investig. 1995;96:2569–2577. doi: 10.1172/JCI118320.0021-9738(1995)096<2569:AAHSPM>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai R, Maeda T, Onishi S, Yamamoto Y. Autoantibody against 70 kD heat-shock protein in patients with autoimmune liver diseases. J Hepatol. 1995;23:382–390. doi: 10.1016/0168-8278(95)80195-2.0168-8278(1995)023<0382:AAKHPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21:315–320. doi: 10.1006/cbir.1997.0144.1065-6995(1997)021<0315:CSLOTK>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stelmachowicz PG, Gorga MP 1993 Auditory function tests. In: Otolaryngology—Head and Neck Surgery, ed Cummings CW, Marker LA. Mosby Year Book, St Louis, MO. [Google Scholar]
- Talbott EO, Gibson LB, Burks A, Engberg R, McHugh KP. Evidence for a dose-response relationship between occupational noise and blood pressure. Arch Environ Health. 1999;54:71–78. doi: 10.1080/00039899909602239.0003-9896(1999)054<0071:EFADRB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock stress proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205.0192-253X(1993)014<0112:TEOHSS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Taylor W, Pearson J, Main A, Burns W. Study on noise and hearing in jute weaving. J Acoust Soc Am. 1965;32:135–137. doi: 10.1121/1.1909580.1520-8524(1965)032<0135:SONAHI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Raaij Van, Oortgiesen M, Timmerman HH, Dobbe CJ, Van Loveren H. Time-dependent differential changes of immune function in rats exposed to chronic intermittent noise. Physiol Behav. 1996;60:1527–1533. doi: 10.1016/s0031-9384(96)00327-7.0031-9384(1996)060<1527:TDCOIF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Veldman JE. Immune-mediated sensorineural hearing loss with or without endolymphatic hydrops: a clinical and experimental approach. Ann N Y Acad Sci. 1997;830:179–186. doi: 10.1111/j.1749-6632.1997.tb51889.x.0077-8923(1997)830<0179:ISHLWO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063.0031-9333(1992)072<1063:MSRCPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wu T, Ma J, and Chen S. et al. 2001 Association of plasma antibodies against the inducible Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones. 6:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Tanguay RM, Wu Y, He H, Xu D, Feng J, Shi W, Zhang G. Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperatures and carbon monoxide. Biomed Environ Sci. 1996;9:370–379.0895-3988(1996)009<0370:POATHS>2.0.CO;2 [PubMed] [Google Scholar]
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones. 1998;3:161–167. doi: 10.1379/1466-1268(1998)003<0161:poaths>2.3.co;2.1466-1268(1998)003<0161:POATHS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Chen S, and Li J. et al. 2002 Association of Hsp70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones. 7:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Wu T, and Ren A. et al. 2003 Basal and inducible levels of Hsp70 in patients with acute heat illness induced during training. Cell Stress Chaperones. 8:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–1559. doi: 10.1161/01.atv.0000029720.59649.50.1079-5642(2002)022<1547:ROHSPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.cir.100.11.1169.0009-7322(1999)100<1169:AOSATH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu Q, Willeit J, and Marosis M. et al. 1993 Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 341:255–259. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J Neurosci. 1999;19:10116–10124. doi: 10.1523/JNEUROSCI.19-22-10116.1999.0270-6474(1999)019<10116:HSAPFP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]