Abstract
Heat shock protein 27 (HSP27) modulates actin-dependent cell functions in several systems. We hypothesized that HSP27 modulates wound contraction. Stably transfected fibroblast cell lines that overexpress HSP27 (SS12) or underexpress HSP27 (AS10) were established, and cell behaviors related to wound contraction were examined. First, fibroblast-populated collagen lattice (FPCL) contraction was examined because it has been studied as a wound-healing model. In floating FPCL contraction assays, SS12 cells caused increased contraction, whereas AS10 cells caused reduced contraction. Because floating matrix contraction is thought to be mediated by the tractional force of the cells, cell behaviors related to tractional force were examined. In collagen matrix, SS12 cells elongated faster and to a greater extent and contained longer stress fibers than control cells, whereas AS10 cells were slower to elongate than control cells. SS12 cells attached to the dishes more efficiently than the control, whereas AS10 cells attached less efficiently. Migration of SS12 cells on collagen-coated dishes was also enhanced, although AS10 cells did not differ from the control cells. In summary, HSP27 regulates fibroblast adhesion, elongation, and migration and the contraction of the floating matrix in a manner dependent on the level of its expression.
INTRODUCTION
Wound contraction is the primary process involved in healing large, full-thickness, open wounds (Lindquist 1946). The contraction force in wound healing resides within the granulation tissue, and the wound edge must be attached to the granulation tissue for contraction to occur (Lindquist 1946; Watts et al 1958).
The fibroblast-populated collagen lattice (FPCL) contraction assay has been studied extensively as a tissue culture model of wound contraction. Cells behave differently in floating matrix (released shortly after gelation) from cells in attached matrix (Grinnell 2000). When the freshly polymerized collagen matrix containing fibroblasts is released, cells can cause contraction of the matrix, which is called the floating matrix contraction model. This model has been used to compare the abilities of different cell populations to contract the matrix (reviewed in Grinnell 2000). We used lisophosphatidic acid (LPA) and platelet-derived growth factor BB (PDGF-BB) to stimulate matrix contraction in this study because they have been shown to regulate FPCL contraction by distinct pathways (Grinnell et al 1999).
Heat shock protein 27 (HSP27) modulates actin filament dynamics in a manner dependent on its expression and phosphorylation. In vitro, HSP27 behaves as an actin-capping protein (Miron et al 1991) and has an inhibitory effect on actin polymerization, which is dependent on its phosphorylation status (Benndorf et al 1994). In vivo, overexpression of HSP27 results in stabilization of microfilaments after heat shock (Lavoie et al 1993a), increased pinocytosis (Lavoie et al 1993b), and promotion of cell migration (Rousseau et al 1997; Piotrowicz et al 1998). Anti-HSP27 antibody inhibits the bombesin-induced sustained contraction of permeabilized smooth muscle cells (Bitar et al 1991).
We hypothesized that HSP27 modulates wound contraction in cutaneous wound healing because intact microfilaments are essential for fibroblast-mediated contraction of the matrix (Bell et al 1979; Tomasek and Hay 1984) and HSP27 modulates actin structures (Lavoie et al 1993b; Piotrowicz et al 1998). We have shown that a specific p38 mitogen-activated protein kinase (MAPK) inhibitor and a specific MAPK–extracellular signal-regulated kinase kinase inhibitor inhibit FPCL contraction and wound contraction in rats, as well as HSP27 phosphorylation (Hirano et al 2002). To elucidate the role of HSP27 in wound contraction, we cloned cell lines expressing different amounts of HSP27 and examined cellular behaviors related to fibroblast-mediated contraction.
MATERIALS AND METHODS
Cell culture
The embryonic mouse fibroblast cell line STO was obtained from American Type Culture Collection (ATCC: Manassas, VA, USA). Cells were cultured in Dulbecco modified Eagle medium (DMEM) (GIBCOBRL, Gaithersburg, MD, USA) supplemented with 5% fetal calf serum (FCS) (GIBCOBRL), penicillin (50 U/mL), and streptomycin (50 μg/mL) (GIBCOBRL). Transfectants were cultured with the same media containing 0.1% G418 (GIBCOBRL). The cultures were maintained in a humidified incubator containing an atmosphere of 5% CO2 and 95% air.
Transfections
STO cells were stably transfected by liposome-mediated transfectin using DOTAP (Roche, Indianapolis, IN, USA) according to the protocol provided. The expression vector pcDNA3.0 (Invitrogen, San Diego, CA, USA), with the CMV promoter, was used for transfection with sense-strand wild-type rat HSP27 complementary deoxyribonucleic acid (cDNA) (accession No. M86389) or anti-sense rat cDNA as well as vector only as a control. After 1 week of selection with 0.1% G418, stable transfectants were cloned and grown in selection medium.
Collagen gel preparation
Type I collagen was extracted from the tails of rats by the method of Bell et al (1979), and collagen lattices were prepared by a modification of that method. Briefly, acid-extracted collagen I, fibroblast cells, 5× DMEM, distilled water, and 0.1 N NaOH were mixed on ice so that the final mixture resulted in 0.9 mg/mL collagen, 2 × 106 cells/mL, a physiological ionic strength, and 1× DMEM. This mixture (1.2 mL) was poured into each 35-mm petri dish (Becton Dickinson, Lincoln Park, NJ, USA). After gelation, which occurred within 5 minutes at 37°C, 2 mL of DMEM medium was added to each dish.
FPCL contraction assay
One hour after gelation, the medium was aspirated, lattices were released, and 2 mL fresh DMEM containing 20 μM LPA (Sigma, St Louis, MO, USA) or 100 ng/mL PDGF-BB (Sigma) was added to each dish. Lattice contraction was quantified by measuring the diameter of 2 axes of each lattice 24 hours after treatment. In studies using the Rho kinase (ROCK) inhibitor Y-27632 (a gift from Welfide Corporation, Osaka, Japan), cultures were treated with a 15 μM solution of the inhibitor for 30 minutes before gel release. The decrease in the average diameter of the 2 axes was used for statistical analysis and was termed contraction. Each group had at least 3 gels assayed. The DNA content of each lattice used was measured as an indirect measurement of cell numbers to directly examine the difference in cell numbers between lines at the end of the experiments. All lattices used were homogenized in a solution containing 4 M guanidine isothiocynate, 25 mM sodium citrate, 0.5% sarcosyl, and 0.1% mercaptoethanol using a polytron 3000 homogenizer (Brinkman Instruments Inc., Westbury, NY, USA). The concentration of DNA was measured by H33258 fluorescence enhancement using a DNA Quant 200 fluorometer (Hoefer Pharmacia Biotech, San Francisco, CA, USA) according to the protocol provided, and total DNA in each gel was calculated.
Western blot analysis
Cloned lines cultured on tissue culture dishes were lysed in RIPA buffer (10 mM Tris–HCl, 1% Triton, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, and 1 mM ethylenediamine-tetraacetic acid). Protein concentrations were determined using a modified Bradford according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA, USA). Protein samples were separated by SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes using a semidry blotting apparatus (Pharmacia Biotech, Piscataway, NJ, USA). Membranes were blocked with 4% dry milk in phosphate-buffered saline (PBS) with 0.1% Tween 20 and incubated with a 1:4000 dilution of polyclonal antibody against hsp25 (Stressgen, Victorica, BC, Canada) for 1 hour. After washing, membranes were incubated with peroxidase-conjugated donkey anti-rabbit IgG (Jackson Immuno Research Laboratories Inc., West Grove, PA, USA) for 1 hour and washed before detection using chemiluminescence (Renaissance, NEN™, Life Science Products, Boston, MA, USA). The same blot was used for Western blotting using monoclonal antibody against β-actin (clone AC-15 from Sigma) to show the relative loading of each lane.
Cell extension assay
The cloned cell lines, SS12, VO, and AS10, were plated in a 0.9% collagen mix at 1 × 106 cells/mL. After 24 hours incubation in DMEM medium supplemented with 5% FCS in a 37°C incubator under an atmosphere of 95% air and 5% CO2, images of 50 cells from each line were acquired on a Zeiss Axiovert microscope equipped with a 10× 0.25NA phase contrast objective lens and captured using a monochrome charge-coupled device (CCD) camera (Panasonic WV-BP310). Cell lengths and ratios of log to short axis were quantified using NIH Image.
To analyze cell extension at earlier times, time-lapse experiments were performed. Cells were plated as described and allowed to equilibrate in a humidified incubator for 30 minutes at 5% CO2. The medium was exchanged with DMEM containing 5% FCS and buffered with 25 mM N-2-hydroxyethyl piperazine-N′-2-ethanesulfonic acid (HEPES), and the dishes were sealed with parafilm and placed on a heated microscope stage. Images were captured every 3 minutes using NIH Image program as described previously (Shelden 1999), and images taken 8 hours and 16 hours after plating are reported.
Cell migration assay
Time-lapse experiments were performed to determine cellular migration rates. SS12, AS10, and VO cells were trypsinized, washed with PBS, and plated in collagen lattices at approximately 5 × 104 cells/mL. Cells were incubated for 24 hours in a humidified incubator at 5% CO2 in DMEM supplemented with 5% FCS before exchange of medium with DMEM containing 5% FCS and buffered with 25 mM HEPES. Lattice-containing dishes were sealed with parafilm and placed on a heated microscope stage. Six movies were made for each cell line, with images acquired every 3 minutes, as before, using NIH Image. Migration rates for each cell line were calculated by measuring changes in the location of 60 cells every hour for 8 hours.
Analysis of cellular F-actin
For actin staining, portions of gels were excised 6 hours after plating and fixed in a 4.0% formaldehyde solution in PBS for 30 minutes. The samples were then washed 3 times with PBS before being treated with 0.5% Triton in PBS for 15 minutes. Then, the samples were stained with BODIPY-phalloidin (Molecular Probes, Eugene, OR, USA) in PBS for 30 minutes. After washing with PBS the gels were mounted, examined, and photographed with a Zeiss laser-scanning microscope (LSM510).
Adhesion assay
Cells used in adhesion assays were trypsinized and washed with PBS, and 3.5 × 104 cells were plated on 96-well polystyrene assay plates uncoated or coated with 20 μg/mL collagen. One hour after plating, the cells were washed with PBS and fixed with 4% formaldehyde for 30 minutes before being stained with 0.25% crystal violet in 40% methanol for 3 hours. After removing the crystal violet solution, the plates were washed extensively, dried, and the stain released using 2% SDS in PBS. Stain intensity was quantified by spectrophotometry (570 nm) using a plate reader (Multiscan Plus, Labststem, Franklin, MA, USA). Each line had 10 wells assayed, and experiments were repeated 3 times (n = 30). To confirm that these data represented not only the amount of protein but also the number of cells, 8.7 × 105 cells were also plated on collagen-coated 35-mm petri dishes, and the number of attached cells was determined. One hour after plating, the cells were washed with PBS, fixed, and stained with crystal violet solution, and photographs were taken using a monochrome CCD camera. The number of cells in each of the 6 images was determined.
Growth rate determination
Cells (1 × 103) were plated on 96-well plates and grown for 1 to 4 days before being fixed with 4% formaldehyde for 30 minutes. To indicate the cell number, cells were stained with crystal violet as described. Stain was released and quantified as described previously (n = 24).
Statistical analysis
Data from all experimental groups were expressed as standard error of the mean. An unpaired Student's t-test was used to compare differences between control and experimental groups. Statistical significance was defined as P < 0.05.
RESULTS
Floating matrix contraction is dependent on HSP27 expression
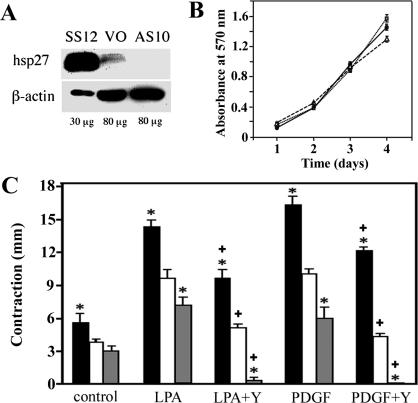
Sense and anti-sense constructs of a full–coding length cDNA for HSP27 (Welsh et al 1996) were cloned into the expression vector pcDNA 3.0 using standard techniques. These constructs were transfected into a mouse embryonic fibroblast cell line that produced a minimal amount of HSP27 (STO [ATCC No. 56-X]). Stably transfected cell lines containing sense or anti-sense constructs were obtained. Western blot analysis of 30 μg protein from the lysate of a cell line transfected with the sense construct (SS12) contained substantially more HSP27 than did 80 μg protein from lysates from the control line (VO), and the anti-sense line AS10 contained less HSP27 than VO did (Fig 1A).
Fig 1.
Effect of heat shock protein 27 (HSP27) on floating fibroblast-populated collagen lattice (FPCL) contraction. (A) Expression of HSP27. Western blot analysis of whole-cell lysates from STO cells transfected with a rat HSP27 sense-strand construct (SS12), with vector only (VO) and with an anti-sense strand HSP27 construct (AS10). (B) Growth curves of 3 lines. Increased cell numbers of SS12 cells (black circles), VO cells (open triangles), and AS10 cells (gray squares) were monitored over 4 days in culture. (C) Floating FPCL contraction. Contraction of floating FPCL populated with SS12 cells (black columns), VO cells (white columns), or AS10 cells (gray columns) was measured at 24 hours with or without treatment with lisophospatidic acid (20 μM) or platelet-derived growth factor BB (100 ng/mL) in the presence and absence of 15 μM Y-27632. *, P < 0.05 vs VO by Student's t-test. +, P < 0.05 vs value of the same cell without Y-27632
Because LPA and PDGF-BB stimulate FPCL contraction by 2 distinct pathways (Grinnell et al 1999), the ability of the transfected cell lines to cause FPCL contraction after treatment with each of these agents was examined. In the floating matrix contraction assay, SS12 cells contracted collagen gels significantly more than did AS10 or VO cells with or without LPA or PDGF treatment. Treatment with LPA or PDGF-BB caused more contraction of FPCLs with all cell lines. As the Rho pathway has been reported to mediate both floating and stressed matrix contraction (Grinnell et al 1999), FPCLs were treated with a ROCK inhibitor, Y-27632 (15 μM). Y-27632 significantly inhibited contraction after LPA and PDGF-BB treatment, with the most dramatic effect being evident in AS10 cultures (Fig 1C). Although equivalent numbers of cells from all lines were plated and cells in floating matrix do not proliferate (Mochitate et al 1991), we performed DNA assay after measuring contraction to confirm that there was an equivalent number of cells in each lattice. There were no differences in DNA content among all lattices (data not shown). We also examined cell growth to confirm that each line grew without impairment. Although there were slight differences among lines, all proliferated in a similar manner, and there was no correlation between HSP27 expression and growth curves (Fig 1B). These data suggest that HSP27 enhances floating matrix contraction without affecting growth rate, regardless of the pathways activated by LPA or PDGF. Floating matrix contraction was partially regulated by ROCK, but the inhibition of ROCK had a greater effect in AS10 cells and was less effective in SS12 cell–mediated contraction. These data suggest that HSP27 regulates contraction by a mechanism independent of ROCK.
Cellular elongation was dependent on HSP27 expression
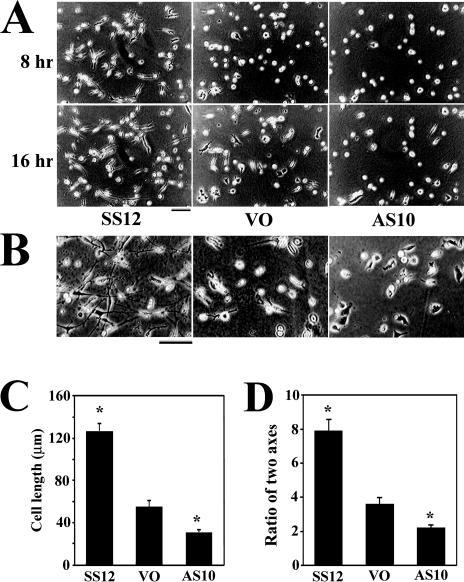
Contraction of the floating matrix is considered to be dependent on tractional forces (Harris et al 1981). Tractional forces appear to be exerted by the contraction of cells, which transfers force to the substrate through cell-matrix adhesive contacts. More elongated cells generate stronger force (Roy et al 1999) than do less-elongated cells. Time-lapse experiments were performed to examine cell elongation in the collagen matrix. SS12, AS10, and VO cells were plated in collagen lattices, and images were captured every 3 minutes for 24 hours. SS12 cells elongated more rapidly in the matrix than did AS10 or VO cells and were significantly longer at 8 hours or 16 hours after plating (Fig 2A). More SS12 cells were elongated in a bipolar manner with a high ratio of the long and short axes by 24 hours than were the other cell lines, whereas AS10 cells remained less elongated with a smaller ratio of the long and short axes (Fig 2 B,C).
Fig 2.
Effect of heat shock protein 27 (HSP27) on cellular elongation in a collagen matrix. (A) Images from time-lapse movies of cells plated in collagen matrix. Images were taken 8 hours and 16 hours after plating. Bar = 100 μm. (B) Photomicrographs of cells in collagen lattices cultured for 24 hours after plating in collagen in a humidified incubator with a 5% CO2–95% air atmosphere. Bar = 100 μm. (C) The average length of the longer axis of 60 SS12, VO, and AS10 cells after 24 hours of culture in collagen. Error bars represent standard error of the mean (SEM). *, P < 0.05 vs VO by Student's t-test. (D) The average ratios of the longer axis vs shorter axis of 60 SS12, VO, and AS10 cells after 24 hours of culture in collagen. Error bars represent SEM. *, P < 0.05 vs VO by Student's t-test
Cellular F-actin structure formation was dependent on HSP27 expression
Collagen lattices containing cells were fixed at 6 hours after plating, and cellular F-actin was stained with BODIPY-phalloidin. Fluorescence micrographs show that SS12 cells were more elongated in a bipolar manner than AS10 or STO cells. Long microfilaments are seen in SS12 cells unlike in VO or AS10. Control cells (VO) showed strong staining at the pseudopodia and appeared to be further in the process of elongation than AS10 cells were at this time. AS10 cells had the least F-actin staining of all 3 lines, and the majority of these cells were round with poorly developed pseudopodia (Fig 3). These data demonstrate that HSP27 expression promotes cell elongation.
Fig 3.
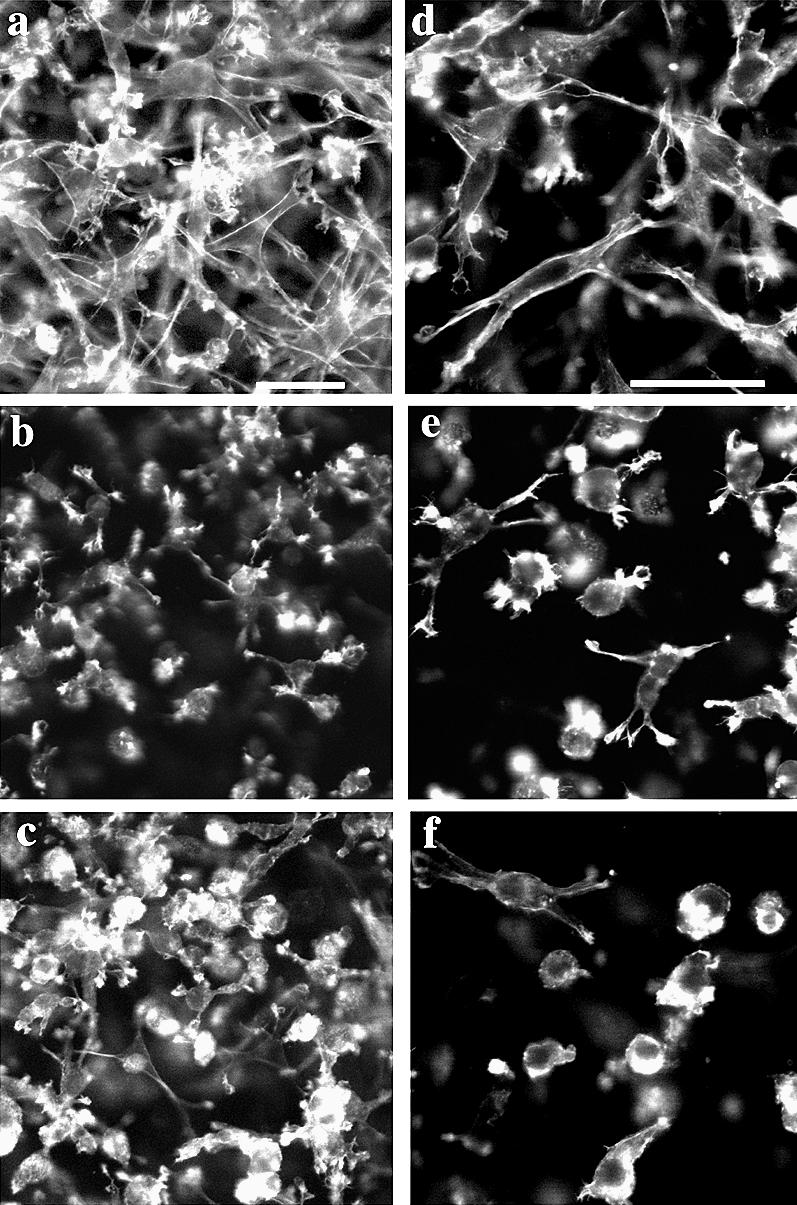
F-actin staining of cells plated in attached collagen lattice. Six hours after plating, cells were fixed and stained with BODIPY-phalloidin to visualize the actin cytoskeleton. Note that SS12 cells (a, d) were more elongated in a bipolar manner than VO cells (b, e), whereas AS10 cells (c, f) were more rounded in appearance. Bar = 50 μm
Cellular adhesion was dependent on HSP27 expression
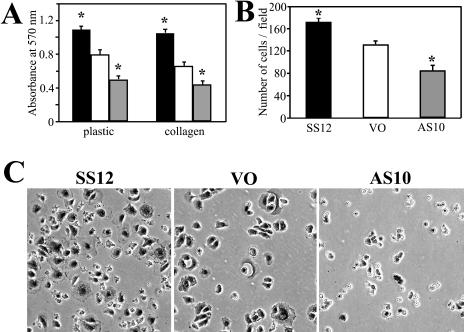
Because adhesion is an important component of tractional force and elongation, the adhesive ability of the 3 lines was examined. Cells were plated on dishes for 1 hour, fixed, and stained. By quantification using crystal violet, SS12 attached more and AS10 attached less than did VO cells to coated or uncoated dishes (n = 30) (Fig 4A). The number of cells attached to coated dishes was also determined by counting to confirm the data using crystal violet absorbance (n = 8) (Fig 4B). From the images, AS10 cells appeared only marginally attached by 1 hour, whereas SS12 and VO cells had already started spreading by this time (Fig 4C).
Fig 4.
Cell adhesion 1 hour after plating. (A) Cells attached to uncoated (plastic) or collagen-coated dishes (collagen) were quantified by staining with crystal violet. Black, white, and gray columns represent SS12, VO, and AS10 cells, respectively. Error bars represent standard error of the mean (SEM). *, P < 0.05 vs VO by Student's t-test. (B) The average number of cells adhered to collagen-coated dishes per 200× field was determined by counting cells attached 1 hour after plating. Error bars represent SEM. *, P < 0.05 vs VO by Student's t-test. (C) Morphology of each line 1 hour after being plated on collagen-coated dishes. Note that attached AS10 cells did not spread as well as VO or SS12 cells
Cell migration was dependent on HSP27 expression
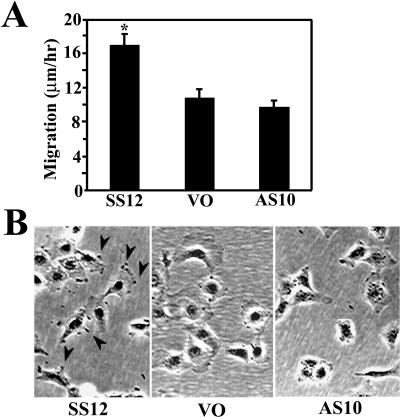
Cellular ability to exert tractional forces is thought to be related to cell motility (Harris et al 1981; Ehrlich and Rajaratnam 1990), although it has been demonstrated that nonmotile cells generate strong forces in a matrix (Roy et al 1999). Cellular migration is a process dependent on cell-matrix adhesion and actin polymerization. To test the effect of altered HSP27 levels on cellular locomotion, we performed 6 time-lapse experiments for each cell line over an 8-hour period, and the migration rates of 60 cells were calculated. SS12 cells migrated a significantly greater distance than VO cells on collagen-coated dishes, whereas there was no significant difference between VO and AS10 cell migration (Fig 5A). During these experiments, we noticed that lamellipodia protrusions and ruffling were more obvious in SS12 cells (data not shown), and they appeared more pronounced in SS12 cells (Fig 5B) than in the other lines.
Fig 5.
Cell migration on collagen-coated dishes. (A) The average migration rate for each cell line was calculated using 6- and 8-hour–time-lapse movies. Error bars represent standard error of the mean. *, P < 0.05 vs VO by Students t-test. (B) Photomicrographs of each cell line 24 hours after plating. SS12 cells have more actively moving and pronounced lamellae (arrow heads) compared with the relatively smooth contours of VO and AS10 cells
DISCUSSION
In this study we examined the role of HSP27 in fibroblast-mediated collagen contraction. Cells overexpressing HSP27 (SS12 cells) demonstrated enhanced contraction in the floating matrix contraction assay, whereas cells underexpressing HSP27 (AS10) demonstrated a reduced ability to contract collagen gels. The difference in contraction among lines was consistent with or without treatment by LPA or PDGF. These data suggest that HSP27 modulates some fundamental cellular activity leading to matrix contraction.
In trying to understand how HSP27 regulates matrix contraction, we undertook studies to determine the effect(s) of HSP27 on cellular functions related to the generation of tractional forces. The generation of tractional force in collagen matrix by elongated cells with strong cell-matrix adhesion has been reported to be the strongest (Roy et al 1999). We observed enhanced elongation in SS12 cells compared with VO cells and reduced elongation in AS10 cells compared with VO cells. Cellular adhesion with the matrix is essential for cellular elongation to occur. We demonstrated that SS12 cells are more adhesive not only to collagen-coated dishes but also to polystyrene dishes, whereas AS10 cells are less adhesive than control (VO) cells. Adhesion is regulated by focal contacts containing integrins, microfilaments, and other proteins involved in forming the focal adhesion complex. HSP27 has been shown to stabilize actin filaments (Lavoie et al 1993a), which may account for enhanced adhesion in SS12 cells. Recently, Hic-5 was identified as a binding protein of HSP27 (Jia et al 2001). Hic-5, like paxillin, has been reported to bind the focal adhesion structural protein vinculin (Thomas et al 1999) and the signal protein focal adhesion kinase (Fujita et al 1998). Hic-5 can reduce integrin-mediated cell spreading, presumably by competing with paxillin for sites on paxillin-binding proteins and by inhibiting paxillin tyrosine phosphorylation (Nishiya et al 2001). HSP27 expression may also affect cellular adhesion by modulating the availability of Hic-5.
HSP27 modulation of F-actin and enhanced adhesion could be sufficient to account for the difference in elongation among the cell lines used in these studies, although other mechanisms may also be involved. The process of cell elongation in a collagen matrix has been described as occurring in 4 steps: (1) formation of filopodia, (2) movement of filopodia to opposite ends of the cell, (3) extension of pseudopodia, and (4) elongation of the cell (Tomasek and Hay 1984). Intact microfilaments are required in all steps except the first step, whereas microtubules are essential only in the last step of elongation (Tomasek and Hay 1984). Time-lapse experiments were initiated 30 minutes after cell and collagen mixtures were poured, so we were not able to detect any differences in the formation of filopodia, which may have occurred during this time. By 8 hours after plating, extension of pseudopodia (step 3) was observed in the majority of SS12 cells, whereas VO cells exhibited pseudopodia extension between 16 hours and 24 hours after plating. Initial pseudopodia formation was seen in some of the AS10 cells by 16 hours; however, these cells failed to form an elongated morphology by 24 hours after plating (data not shown). The ratio of the longer and shorter axes of SS12 cells was larger than that of VO cells, and that of AS10 cells was smaller than that of VO cells. It appears that steps 3 and 4, both of which are regulated by microfilaments, were affected by expression of HSP27. It is possible that HSP27 positively regulates actin polymerization in this system, as well as enhances cell-matrix adhesion.
We examined cell migration, an adhesion-dependent process, on collagen-coated dishes. We observed that fibroblasts that overexpressed HSP27 (SS12) migrated more rapidly than control cells (VO) or cells that underexpress HSP27 (AS10). This observation is consistent with that of Piotrowicz et al (1998), who demonstrated that overexpression of wild type HSP27 enhanced endothelial cell migration. However, enhanced migration on collagen-coated dishes is not likely to fully account for enhanced collagen matrix contraction. Cells embedded in collagen matrix demonstrated protrusion and retraction of the pseudopodia only at the ends of elongated SS12 cells, and most SS12 cells moved little except for the small movement of the pseudopodia (data not shown). AS10 cells also demonstrated similar movement in pseudopodia, although because these cells were significantly less elongated than SS12 cells, this movement involved a larger proportion of the overall cell length. Interestingly, it has been reported that the active migration of cells in a collagen matrix is associated with the disruption of tension on the matrix (Roy et al 1999). These observations imply that although SS12 cell migration on collagen-coated dishes is enhanced, this may not be the reason these cells demonstrate enhanced collagen matrix contraction.
The observations that overexpression of HSP27 in STO cells causes enhanced adhesion and cellular elongation within the collagen gels may account for the enhanced matrix contraction observed. Both SS12 cell–enhanced migration and elongation may be due to HSP27-mediated increased focal adhesions or actin rearrangement–F-actin stabilization because these processes are essential components of both cellular functions.
The Rho-ROCK pathway regulates stress fiber formation (Ridley and Hall 1992; Hotchin and Hall 1995) and has been reported to modulate FPCL contraction (Grinnell et al 1999). The inhibitor of ROCK (Y-27632) significantly inhibited contraction in all 3 lines. It was most effective in AS10 cells and least effective in SS12 cells. These data indicate that the HSP27-regulated component of FPCL contraction is independent of ROCK.
In summary, we showed that expression of HSP27 regulates floating FPCL contraction, cellular adhesion, elongation in collagen, and motility. The molecular mechanisms of HSP27 modulation of adhesion, elongation, and motility need to be further elucidated.
Acknowledgments
The authors thank Dr Taketo Yamada, Dr Jun-ich Hata, Dr Rainer Benndorf, and Dr Michael J. Welsh for allowing the use of their equipment and space for some of the experiments. This work was supported by grants from the National Institute of Environmental Health Sciences (R01 ES011196 to E.A.S.) and (PO1 ES11188 to M.J. Welsh, Principal Investigator) and funding from the University of Michigan Department of Surgery Research Advisory Committee (R.R.G.).
REFERENCES
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274.0027-8424(1979)076<1274:POATSB>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784.0021-9258(1994)269<20780:PASOOM>2.0.CO;2 [PubMed] [Google Scholar]
- Bitar KN, Kaminski MS, Hailat N, Cease KB, Strahler JR. Hsp27 is a mediator of sustained smooth muscle contraction in response to bombesin. Biochem Biophys Res Commun. 1991;181:1192–1200. doi: 10.1016/0006-291x(91)92065-r.0006-291X(1991)181<1192:HIAMOS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ehrlich H, Rajaratnam J. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell. 1990;22:407–417. doi: 10.1016/0040-8166(90)90070-p.0040-8166(1990)022<0407:CLFVCC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fujita H, Kamiguchi K, Cho D, Shibanuma M, Morimoto C, Tachibana K. Interaction of Hic-5, a senescence-related protein, with focal adhesion kinase. J Biol Chem. 1998;273:26516–21. doi: 10.1074/jbc.273.41.26516.0021-9258(1998)273<26516:IOHASP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x.0962-8924(2000)010<0362:FCGSAM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Grinnell F, Ho CH, Lin YC, Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999;274:918–923. doi: 10.1074/jbc.274.2.918.0021-9258(1999)274<0918:DITROF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0.0028-0836(1981)290<0249:FTAAMF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hirano S, Rees RS, Gilmont RR. MAP kinase pathways involving hsp27 regulate fibroblast-mediated wound contraction. J Surg Res. 2002;102:77–84. doi: 10.1006/jsre.2001.6315.0022-4804(2002)102<0077:MKPIHR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hotchin N, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857.0021-9525(1995)131<1857:TAOIAC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE. Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem. 2001;276:39911–39918. doi: 10.1074/jbc.M103510200.0021-9258(2001)276<39911:IACOAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. J Biol Chem. 1993a;268:3420–3429.0021-9258(1993)268<3420:IOCHHG>2.0.CO;2 [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993b;268:24210–24214.0021-9258(1993)268<24210:MOAMDA>2.0.CO;2 [PubMed] [Google Scholar]
- Lindquist G 1946 The healing of skin defects. Acta Chir Scand. 94:Suppl 94. 1–163. [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255.0021-9525(1991)114<0255:AKIOAP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochitate K, Pawelek P, Grinnel F. Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp Cell Res. 1991;193:198–207. doi: 10.1016/0014-4827(91)90556-a.0014-4827(1991)193<0198:SROCCG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nishiya N, Tachibana K, Shibanuma M, Mashimo JI, Nose K. Hic-5-reduced cell spreading on fibronectin: competitive effects between paxillin and Hic-5 through interaction with focal adhesion kinase. Mol Cell Biol. 2001;21:5332–5345. doi: 10.1128/MCB.21.16.5332-5345.2001.0270-7306(2001)021<5332:HCSOFC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481.0892-6638(1998)012<1481:HSPKEA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7.0092-8674(1992)070<0389:TSGPRR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rousseau S, Houle F, Landry J, Hout J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380.0950-9232(1997)015<2169:PMKABV>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roy P, Petroll WM, Chuong CJ, Cavanagh HD, Jester JV. Effect of cell migration on the maintenance of tension on a collagen matrix. Ann Biomed Eng. 1999;27:721–730. doi: 10.1114/1.227.0090-6964(1999)027<0721:EOCMOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shelden E. Major role for active extension in the formation of processes by ras-transformed fibroblasts. Cell Motil Cytoskelet. 1999;42:12–26. doi: 10.1002/(SICI)1097-0169(1999)42:1<12::AID-CM2>3.0.CO;2-W.0886-1544(1999)042<0012:MRFAEI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thomas SM, Hagel M, Turner CE. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J Cell Sci. 1999;112:181–190. doi: 10.1242/jcs.112.2.181.0021-9533(1999)112<0181:COAFAP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J Cell Biol. 1984;99:536–549. doi: 10.1083/jcb.99.2.536.0021-9525(1984)099<0536:AOTROM>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts GT, Grillo HC, Gross J. The role of granulation tissue in contraction. Ann Surg. 1958;148:153–160. doi: 10.1097/00000658-195808000-00002.0003-4932(1958)148<0153:TROGTI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ, Wu W, Parvinen M, Gilmont RR. Variation in expression of hsp27 messenger ribonucleic acid during the cycle of the seminiferous epithelium and co-localization of hsp27 and microfilaments in Sertoli cells of the rat. Biol Reprod. 1996;55:141–151. doi: 10.1095/biolreprod55.1.141.0006-3363(1996)055<0141:VIEOHM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]