Key Points
-
•
Axi-cel showed clinically meaningful improvements in QoL in 2L LBCL over SOC at days 100 and 150.
-
•
There was a trend toward faster recovery to baseline QoL in patients who received axi-cel.
Visual Abstract

Abstract
Here, we report the first comparative analysis of patient-reported outcomes (PROs) with chimeric antigen receptor T-cell therapy vs standard-of-care (SOC) therapy in second-line relapsed/refractory large B-cell lymphoma (R/R LBCL) from the pivotal randomized phase 3 ZUMA-7 study of axicabtagene ciloleucel (axi-cel) vs SOC. PRO instruments were administered at baseline, day 50, day 100, day 150, month 9, and every 3 months from randomization until 24 months or an event-free survival event. The quality of life (QoL) analysis set comprised patients with a baseline and ≥1 follow-up PRO completion. Prespecified hypotheses for Quality of Life Questionnaire-Core 30 (QLQ-C30) physical functioning, global health status/QoL, and EQ-5D-5L visual analog scale (VAS) were tested using mixed-effects models with repeated measures. Clinically meaningful changes were defined as 10 points for QLQ-C30 and 7 for EQ-5D-5L VAS. Among 359 patients, 296 (165 axi-cel, 131 SOC) met inclusion criteria for QoL analysis. At day 100, statistically significant and clinically meaningful differences in mean change of scores from baseline were observed favoring axi-cel over SOC for QLQ-C30 global health status/QoL (estimated difference 18.1 [95% confidence interval (CI), 12.3-23.9]), physical functioning (13.1 [95% CI, 8.0-18.2]), and EQ-5D-5L VAS (13.7 [95% CI, 8.5-18.8]; P < .0001 for all). At day 150, scores significantly favored axi-cel vs SOC for global health status/QoL (9.8 [95% CI, 2.6-17.0]; P = .0124) and EQ-5D-5L VAS (11.3 [95% CI, 5.4-17.1]; P = .0004). Axi-cel showed clinically meaningful improvements in QoL over SOC. Superior clinical outcomes and favorable patient experience with axi-cel should help inform treatment choices in second-line R/R LBCL. This trial was registered at www.clinicaltrials.gov as #NCT03391466.
Elsawy et al present the patient-reported outcomes after axicabtagene ciloleucel (axi-cel) administered as second-line therapy in large B-cell lymphoma compared to salvage chemotherapy followed by autologous stem cell transplantation (ASCT) in the ZUMA-7 trial. In clinical responders, treatment with axi-cel delivers better quality-of-life and an earlier return to baseline functioning compared to salvage chemotherapy followed by ASCT. The results imply that, in addition to improving progression-free survival, axi-cel is better tolerated than the previous standard.
Introduction
Outcomes are poor for patients with large B-cell lymphoma (LBCL) who relapse after or are refractory to first-line chemoimmunotherapy.1 The standard-of-care (SOC) therapy with potential for cure for this patient population is high-dose chemotherapy with autologous stem cell transplantation (HDCT-ASCT) if the lymphoma is responsive to salvage chemoimmunotherapy.1, 2, 3 However, many patients may not respond to or may be unable to tolerate second-line chemoimmunotherapy, may not be candidates for HDCT-ASCT, or may relapse early after HDCT-ASCT.4, 5, 6
Axicabtagene ciloleucel (axi-cel) is an engineered, autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in 35 countries for relapsed/refractory (R/R) LBCL after 2 or more prior systemic therapies.7,8 In the pivotal ZUMA-1 (#NCT02348216; clinicaltrials.gov) trial evaluating axi-cel in patients with refractory LBCL, 83% of patients had an objective response and 58% had a complete response,9 and after a median follow-up of 63.1 months, the 5-year overall survival rate was 42.6%.10 Given the high unmet need for better therapies for patients with early relapsed or refractory LBCL and the proven efficacy of CAR T-cell therapy in this disease, we conducted ZUMA-7, a global, randomized, phase 3 study evaluating axi-cel vs SOC in patients with R/R LBCL within 12 months of first-line therapy.11 At the primary analysis, axi-cel was superior to SOC (hazard ratio [HR], 0.398; P < .0001), with a significantly longer median event-free survival (EFS) with axi-cel vs SOC (8.3 vs 2 months) and higher 24-month EFS rates by Kaplan-Meier estimate (40.5% vs 16.3%).11 The safety profile of axi-cel in this study was consistent with previous studies in refractory LBCL, with no newly identified safety concerns.9 Based on the ZUMA-7 trial, axi-cel is now also approved in the United States for adult patients with LBCL that is refractory to or that relapses within 12 months of first-line chemoimmunotherapy.
In addition to safety and efficacy, quality of life (QoL) is a major concern for cancer patients and an important component of the patient experience. Studies have reported that patients with LBCL have worse QoL compared with the general population and a worsening of QoL following SOC chemotherapy.12,13 Patient-reported outcomes (PROs) are also of increasing importance to international regulatory agencies, including the Food and Drug Administration and European Medicines Agency.14, 15, 16 Therefore, ZUMA-7 prospectively evaluated PROs to compare the impact of treatment with axi-cel vs SOC on the overall patient journey. There are multiple oncology-specific PRO assessments that are used in diffuse LBCL17, 18, 19, 20, 21, 22, 23; however, none have been formally validated in patients with diffuse LBCL who have received CAR T-cell therapy.18,19 The ZUMA-7 study used several PRO instruments to assess patient experience, including European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30), EuroQol EQ-5D-5L, and Work Productivity and Activity Impairment Questionnaire: General Health (WPAI:GH). The EORTC QLQ-C30 is a cancer-specific 30-item questionnaire that evaluates the impact of the disease on patient functions, symptoms, overall QoL, and finances over the previous week.17,24 The EQ-5D-5L is a generic measure for capturing health-related QoL on the assessment day.25 Finally, the WPAI:GH measures work productivity and activity impairment during the past 7 days. Results from a recent qualitative patient focus group study support the validity of these instruments in the ZUMA-7 patient population.26 Here, we report the first comparative analysis of PROs with CAR T-cell therapy vs SOC as second-line therapy for R/R LBCL.
Methods
Study design and patients
ZUMA-7 is a phase 3 randomized, open-label, global, multicenter study evaluating the efficacy of axi-cel vs SOC in adult patients with R/R LBCL.11 Eligible patients were aged ≥18 years with histologically confirmed LBCL per World Health Organization 2016 classification criteria27 who were R/R within 12 months of first-line chemoimmunotherapy, including an anti-CD20 monoclonal antibody and anthracycline-containing regimen, and who were intended to proceed to HDCT-ASCT in case of responsive disease to chemoimmunotherapy. The primary endpoint of ZUMA-7, EFS, and clinical secondary and exploratory endpoints have been reported.11 QoL assessment, measured with EORTC QLQ-C30 and EQ-5D-5L, was a prespecified secondary endpoint, and additional exploratory endpoints evaluated impact of disease and treatment on work productivity and activity. The QoL analysis set was defined as patients who had a baseline PRO and at least 1 measure completed at day 50, 100, or 150 from randomization. Measures at baseline and at least 1 follow-up time point were required to compute changes from baseline.
All patients signed and dated the institutional review board/independent ethics committee–approved consent form before initiating any study-specific procedures or activities that were not part of routine care.
Procedures
Detailed ZUMA-7 procedures have been described.11 Briefly, following screening, patients were randomized in a 1:1 ratio to receive axi-cel or SOC, stratified by response to first-line therapy and second-line age-adjusted International Prognostic Index (IPI; 0-1 vs 2-3) at screening. Patients in the axi-cel arm received a 3-day conditioning chemotherapy regimen (fludarabine 30 mg/m2 per day and cyclophosphamide 500 mg/m2 per day) 5, 4, and 3 days prior to a single IV axi-cel infusion on day 0 at a dose of 2 × 106 anti-CD19 CAR T cells per kg. All patients received axi-cel infusion at a health care facility, followed by daily monitoring for a minimum 7-day observation period. Patients in the SOC arm received 2 to 3 cycles of investigator-selected, protocol-defined, platinum-based salvage combination chemotherapy administered every 2 to 3 weeks, and patients who achieved a complete or partial response proceeded to HDCT and ASCT (supplemental Figure 1, available on the Blood Web site).
PRO assessments
PRO instruments, including the EORTC QLQ-C30, the EuroQol EQ-5D-5L, and the WPAI:GH version 2.0, were administered at baseline (prior to treatment with either conditioning or salvage chemotherapy), day 50, day 100, day 150, month 9, and subsequently every 3 months from randomization up to 24 months or time of EFS event (Table 1). Sites were not required to administer PRO assessments after an EFS event, defined as time from randomization to the earliest date of disease progression per Lugano Classification,28 commencement of new lymphoma therapy, or death from any cause (supplemental Methods).
Table 1.
Study details and completion rates for PRO assessments
| Baseline | Treatment period |
Disease assessment |
Long-term follow-up period |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomization | D50 | D100 | D150 | M9 | M12 | M15 | M18 | M21 | M24 | |||
| Axi-cel | n | 165 | Patients randomly assigned to treatment with axi-cel received a 3-d conditioning chemotherapy regimen consisting of fludarabine 30 mg/m2 per d and cyclophosphamide 500 mg/m2 per d followed by 2 rest days. Patients received a single infusion of axi-cel administered IV at a target dose of 2 × 106 anti-CD19 CAR T cells per kg on treatment day 0. Patients will be hospitalized prior to treatment with axi-cel followed by a minimum 7-d observation period. |
163 | 146 | 110 | 88 | 79 | 67 | 71 | 45 | 32 |
| % | 100 | 98.8 | 88.5 | 66.7 | 53.3 | 47.9 | 40.6 | 43.0 | 27.3 | 19.4 | ||
| Standard-of- care therapy | n | 130 | Patients randomly assigned to SOC will receive a second-line combination chemotherapy regimen (R-ICE, R-DHAP, R-ESHAP, or R-GDP) as selected by the treating investigator. Patients will receive 2 cycles of chemotherapy administered every 2 to 3 weeks. Patients responding to second-line combination chemotherapy after 2 cycles should proceed with 1 additional chemotherapy cycle or directly to HDCT and ASCT. Patients who do not respond to second-line chemotherapy can receive additional treatment off protocol. |
125 | 62 | 56 | 40 | 33 | 26 | 23 | 20 | 12 |
| % | 99.2 | 95.4 | 47.3 | 42.7 | 30.5 | 25.2 | 19.8 | 17.6 | 15.3 | 9.2 | ||
Completion rates within the table reflect completion of the EORTC QLQ-C30 global health status/QoL domain. Differences in sample size at specified timepoints were as follows for the hypothesis-driven scales: physical functioning had 164 patients in the axi-cel arm at baseline and 109 at day 150, and within the SOC therapy arm, 131 patients completed at baseline, 126 at day 50, and 64 at day 100; EQ-5D VAS had 145 patients in the axi-cel arm complete at day 100, 80 at month 12, 70 at month 18, and 32 at month 24, and for the SOC therapy arm, 130 completed at baseline, 126 at day 50, and 65 at day 100. PRO instruments were administered until 24 mo or an EFS event (disease progression, all-cause death, or new lymphoma therapy).
R-DHAP, rituximab, dexamethasone, high-dose cytarabine; R-ESHAP, rituximab, etoposide, methylprednisolone, cytarabine, cisplatin; R-GDP, rituximab, gemcitabine, dexamethasone, cisplatin/carboplatin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
The EORTC QLQ-C30 is a self-administered, cancer-specific, 30-item questionnaire with a 1-week recall period.17 It incorporates 5 2- to 5-item functional scales (physical, role, cognitive, emotional, and social), 3 2- to 3-item symptom scales (fatigue, pain, and nausea and vomiting), a 2-item global health status/QoL scale, and numerous single items that assess additional symptoms commonly reported by patients with cancer (eg, dyspnea, loss of appetite, insomnia, constipation, and diarrhea) and the perceived financial impact of the disease. No items are shared between scales. All scales/items are linearly transformed to a 0 to 100 metric, with high functional scale scores representing high/healthy levels of functioning and high scores for symptom scales/items representing high levels of symptoms/problems.24
The EQ-5D-5L is a generic and preference-weighted measure for capturing health-related QoL on the assessment day.25 It is a 2-part self-reported instrument that yields a health utility index score and visual analog scale (VAS) rating. In the first part, 5 domains are evaluated: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each domain is divided into 5 severity levels: “no problem,” “slight problems,” “moderate problems,” “severe problems,” and “extreme problems.” The second part is a VAS where patients are asked to make a global assessment of their current state of health on the date of the assessment, with 0 indicating the worst health they can imagine and 100 indicating the best health they can imagine. The EQ-5D-5L items relate to the respondent’s situation at the time of completion, so the recall period is worded as “your own health state today.” The EQ-5D-5L health utility index score summarizes each health status using a single global score (ie, utility), anchored at 0 (signifying death) and 1 (signifying perfect health), which reflects preferences from the general population. This analysis used the United States time trade-off value set.29
WPAI:GH V2.0 measures work productivity and activity impairment during the past 7 days. It yields 4 scores: absenteeism (proportion of work hours missed), presenteeism (degree that health affects productivity while working), overall work impairment (linear combination of absenteeism and presenteeism), and activity impairment (degree to which health affects regular activities). The 3 employment-related questions of WPAI:GH were only administered to patients who were employed at baseline, and scores are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity.30
Statistical analysis
The null hypothesis for each of the 3 prespecified PRO domains (EORTC QLQ-C30 physical functioning, EORTC global health status/QoL, and EQ-5D-5L VAS) stated that there would be no positive difference in mean change from baseline for axi-cel vs SOC at day 100 from randomization. These 3 PRO domains were tested using a mixed-effects model with repeated measures at day 100, the first assessment period when definitive therapy (axi-cel infusion or ASCT) should have been completed in both cohorts, and at subsequent time points if a statistically significant difference in the unadjusted P value was found at the previous time point. The mixed-effects model with repeated measures was used to examine both individual change in scores over time and differences in changes between groups. Variables for treatment, visit, and treatment by visit interaction, as well as trial stratification variables, were included with the model, which also applied an unstructured covariance matrix for measurements collected across visits. Least-squares means contrasts were used to compare values between treatment arms at each time point. The false discovery rate approach was used to adjust P values across these prespecified endpoints.31 Sensitivity analyses (models 2 and 3) were conducted to control for patterns of missingness and covariates (supplemental Methods). Prespecified exploratory analyses on additional domains of the EORTC QLQ-C30 (role functioning, emotional functioning, cognitive functioning, social functioning, fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties), the EQ-5D-5L (index) and the WPAI:GH (absenteeism, presenteeism, overall work impairment, and activities impairment) scores were performed using similar models. A clinically meaningful change was conservatively defined as 10 points for EORTC, 7 points for EQ-5D-5L VAS score, and 0.06 points for the EQ-5D-5L index (supplemental Methods).32, 33, 34, 35 These thresholds were used to assess change over time and change over time compared between groups.
An additional prespecified exploratory analysis was undertaken for all EORTC QLQ-C30 and EQ-5D scores to understand the time until definitive improvement (TUDI) within patients, which is a variation on the time-to-definitive-deterioration method that has been widely used within oncology trials.36,37 TUDI was analyzed as the percentage of patients reaching a definitive improvement (ie, clinically meaningful improvement, defined as above for each domain and using the same thresholds) over time using Kaplan-Meier–based competing risk models.38 An event was only counted as a definitive improvement if a patient reached the threshold for improvement and at no later time point declined below that threshold. If a patient never reached the threshold for improvement, this patient was censored at the last date of score measurement. Death was treated as a competing risk. TUDI was compared between treatment groups using 2-sided stratified log-rank tests (stratified on randomization factors: response to first-line therapy and second-line age-adjusted IPI at the time of screening).
Descriptive statistics were performed on the QoL analysis set, including baseline characteristics and PRO outcomes by arm over time. All analyses were conducted with SAS version 9.4. An independent data monitory committee supervised the clinical trial.
Role of the funding source
The funder of the study was involved in study design, data collection, data analysis, data interpretation, and writing of the manuscript. All authors had full access to all study data, and the corresponding author had final responsibility for the decision to submit for publication.
Results
QoL population and PRO completion rates
Among 359 patients enrolled between 25 January 2018 and 4 October 2019, 180 patients were randomly assigned to axi-cel and 179 to SOC in the full analysis set.11 Of these, 296 patients (165 axi-cel, 131 SOC; Table 1) met criteria for the QoL analysis set, which required a baseline PRO plus at least 1 follow-up measure. Individuals who experienced an early EFS event and lacked a follow-up PRO measure were ineligible for the QoL analysis set, which, due to the higher number of EFS events, disproportionately affected the SOC arm. Completion rates for PRO assessments are listed in Table 1. After month 9, attrition (eg, due to disease progression, new lymphoma therapy, or death) in the analysis set was substantial, particularly in the SOC arm. This decline in completion rates over time for PRO measures was mostly due to patients experiencing an EFS event (supplemental Table 1) rather than lack of compliance; for all assessments, compliance rates (percentage of patients who completed the PRO questionnaires out of patients expected to complete the questionnaires [those in the QoL analysis set who completed the questionnaire or had not yet had an EFS event]) were above 85% through month 9 and above 83% through month 15 (supplemental Table 2).
Baseline demographic and clinical characteristics
Of the 296 patients in the QoL analysis set, 29.7% were 65 years or older and 66.2% were male (Table 2). The axi-cel cohort had >5% differences compared with SOC in the following baseline characteristics: fewer patients from Europe (axi-cel 18.8% vs SOC 26.0%), more female patients (38.8% vs 27.5%), more patients with Eastern Cooperative Oncology Group performance status 1 (46.1% vs 38.2%), more patients with disease type as HGBL with or without MYC and BCL2 and/or BCL6 rearrangement (22.4% vs 13.0%), more patients with germinal center B-cell–like cell of origin (52.7% vs 42.7%) and fewer not tested (20.6% vs 26.7%), and more patients with status as HGBL double hit (17.6% vs 8.4%), with fewer not tested (17.0% vs 25.2%). No formal statistical testing for differences across treatment arms was undertaken.
Table 2.
Baseline demographic and clinical characteristics in the QoL analysis set
| Category | Overall, n (%) (N = 296) | Axi-cel, n (%) (n = 165) | SOC therapy, n (%) (n = 131) | |
|---|---|---|---|---|
| Geographic region | Australia | 4 (1.4) | 2 (1.2) | 2 (1.5) |
| Europe | 65 (22.0) | 31 (18.8) | 34 (26.0) | |
| Israel | 5 (1.7) | 4 (2.4) | 1 (0.8) | |
| North America | 222 (75.0) | 128 (77.6) | 94 (71.8) | |
| Observed baseline Eastern Cooperative | 0 | 170 (57.4) | 89 (53.9) | 81 (61.8) |
| Oncology Group performance status | 1 | 126 (42.6) | 76 (46.1) | 50 (38.2) |
| Age group | <65 y | 208 (70.3) | 119 (72.1) | 89 (67.9) |
| ≥65 y | 88 (29.7) | 46 (27.9) | 42 (32.1) | |
| Sex | Female | 100 (33.8) | 64 (38.8) | 36 (27.5) |
| Male | 196 (66.2) | 101 (61.2) | 95 (72.5) | |
| Race | Asian | 17 (5.7) | 11 (6.7) | 6 (4.6) |
| Black or African American | 14 (4.7) | 8 (4.8) | 6 (4.6) | |
| White | 247 (83.4) | 134 (81.2) | 113 (86.3) | |
| Other | 18 (6.1) | 12 (7.3) | 6 (4.6) | |
| Response to first-line therapy | Primary refractory | 208 (70.3) | 119 (72.1) | 89 (67.9) |
| Relapse ≤ 6 mo of first-line therapy | 16 (5.4) | 9 (5.5) | 7 (5.3) | |
| Relapse > 6 and ≤ 12 mo of first-line therapy | 72 (24.3) | 37 (22.4) | 35 (26.7) | |
| Second-line age-adjusted IPI total score | 0-1 | 171 (57.8) | 96 (58.2) | 75 (57.3) |
| 2–3 | 125 (42.2) | 69 (41.8) | 56 (42.7) | |
| Disease type per investigator | DLBCL, NOS | 187 (63.2) | 101 (61.2) | 86 (65.6) |
| HGBL with or without MYC and BCL2 and/or BCL6 rearrangement | 54 (18.2) | 37 (22.4) | 17 (13.0) | |
| Large-cell transformation from follicular lymphoma | 40 (13.5) | 19 (11.5) | 21 (16.0) | |
| Other | 15 (5.1) | 8 (4.8) | 7 (5.3) | |
| Cell of origin (molecular subgroup) | GCB | 143 (48.3) | 87 (52.7) | 56 (42.7) |
| Non-GCB | 84 (28.4) | 44 (26.7) | 40 (30.5) | |
| Not tested | 69 (23.3) | 34 (20.6) | 35 (26.7) | |
| Double-/triple-hit status per investigator | HGBL double hit | 40 (13.5) | 29 (17.6) | 11 (8.4) |
| HGBL triple hit | 17 (5.7) | 6 (3.6) | 11 (8.4) | |
| Negative | 178 (60.1) | 102 (61.8) | 76 (58.0) | |
| Not tested | 61 (20.6) | 28 (17.0) | 33 (25.2) |
Percentages are based on total evaluable in the QoL analysis set.
BCL2, B-cell lymphoma 2; BCL6, B-cell lymphoma 6; DLBCL, NOS, DLBCL not otherwise specified; GCB, germinal center B-cell like; HGBL, high-grade B-cell lymphoma; MYC, MYC gene; NOS, not otherwise specified.
EORTC QLQ-C30 physical functioning, EORTC global health status/QoL, and EQ-5D-5L VAS
Baseline PRO scores are listed in supplemental Table 3. For patients in the QoL analysis set, impairment in both arms compared with baseline was observed at day 50 (supplemental Table 4). At day 100, there was a statistically significant and clinically meaningful difference in mean change of scores from baseline in favor of axi-cel for the prespecified hypothesis endpoints of EORTC QLQ-C30 global health status/QoL (estimated difference, 18.1 [95% CI, 12.3-23.9]; adjusted P < .0001), EORTC QLQ-C30 physical functioning (13.1 [95% CI, 8.0-18.2]; adjusted P < .0001), and EQ-5D-5L VAS (13.7 [95% CI, 8.5-18.8]; adjusted P < .0001; Figure 1). Sensitivity analyses showed similar results with retained significance at day 100 (supplemental Table 5). Furthermore, scores significantly favored axi-cel over SOC for global health status/QoL (estimated difference 9.8 [95% CI, 2.6-17.0]; adjusted P = .0124) and EQ-5D-5L VAS (11.3 [95% CI, 5.4-17.1]; adjusted P = .0004) at day 150; this difference was clinically meaningful for EQ-5D-5L VAS. For the prespecified endpoints, the mean estimated scores for the axi-cel arm had numerically returned to or exceeded scores at baseline by day 150 vs month 9 or later for the SOC arm (supplemental Figure 2).
Figure 1.
Mixed model with repeated measures for change from baseline for secondary PRO endpoints (QoL analysis set). Results populated only through month 15 due to lack of model convergence when using time points. Horizontal lines represent the minimally important difference thresholds for clinically meaningful change and are provided for clarity of interpretation. This model included variables for treatment, time, and treatment by time interaction (primary analysis) and controlled for response to first-line therapy (primary refractory, relapse = 6 months of first-line therapy vs relapse > 6 and = 12 months of first-line therapy) and age-adjusted IPI (0-1 vs 2-3) at time of screening. Patients who had PRO assessments after an EFS event (supplemental Results) were not censored to avoid biasing results by excluding patients with worse outcomes. ∗P < .05.
Exploratory PRO analyses
Additional exploratory analyses of PRO endpoints (eg, other EORTC QLQ-C30 domains, EQ-5D-5L index, WPAI:GH) also showed improvements with axi-cel over SOC (supplemental Tables 6-8). The differences in change from baseline were all in favor of axi-cel for nausea and vomiting, diarrhea, insomnia, and appetite loss measures at day 100; role functioning at day 100 and day 150; and social functioning, fatigue, and dyspnea measures at day 100, day 150, and month 9 (Figures 2 and 3). The differences in change from baseline for the EQ-5D-5L index (United States value set) was in favor of axi-cel at day 100 (estimated difference, 0.081 [95% CI, 0.024-0.138]; adjusted P = .0112). Patients treated with axi-cel had significantly lower mean absenteeism and lower mean activities impairment at day 100 than those treated with SOC (supplemental Figure 3). Results were similar when models were adjusted for patterns of missingness and other covariates (supplemental Tables 6-8).
Figure 2.
Mixed model with repeated measures for change from baseline for exploratory EORTC QLQ-C30 functional scales (QoL analysis set). Results populated only through month 15 due to lack of model convergence when using time points. For EORTC QLQ-C30 domains, figures are based on model 1. Horizontal lines represent the minimally important difference thresholds for clinically meaningful change and are provided for clarity of interpretation. This model included variables for treatment, time, and treatment by time interaction (primary analysis) and controlled for response to first-line therapy (primary refractory, relapse = 6 months of first-line therapy vs relapse > 6 and = 12 months of first-line therapy) and age-adjusted IPI (0-1 vs 2-3) at time of screening. Patients who had PRO assessments after an EFS event (supplemental Results) were not censored to avoid biasing results by excluding patients with worse outcomes. ∗P < .05.
Figure 3.
Mixed model with repeated measures for change from baseline for exploratory EORTC QLQ-C30 symptom scales (QoL analysis set). Results populated only through month 15 due to lack of model convergence when using time points. For EORTC QLQ-C30 domains, figures based on model 1. Horizontal lines represent the minimally important difference thresholds for clinically meaningful change and are provided for clarity of interpretation. This model included variables for treatment, time, and treatment by time interaction (primary analysis) and controlled for response to first-line therapy (primary refractory, relapse = 6 months of first-line therapy vs relapse > 6 and = 12 months of first-line therapy) and age-adjusted IPI (0-1 vs 2-3) at time of screening. Patients who had PRO assessments after an EFS event (supplemental Results) were not censored to avoid biasing results by excluding patients with worse outcomes. ∗P < .05.
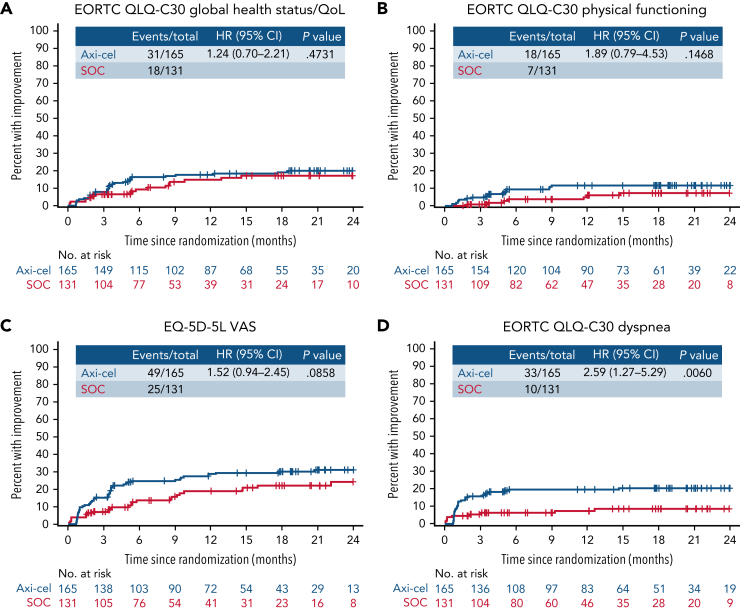
Within exploratory TUDI analyses, for the EORTC QLQ-C30 global health status/QoL, there was no significant difference demonstrated in TUDI (HR, [95% CI] 1.24 [0.7-2.21]), though a numerically greater proportion of patients experienced improvement in the axi-cel arm compared with patients in the SOC arm. This was similar for EORTC QLQ-C30 physical functioning (HR, 1.89 [95% CI, 0.79-4.53]) and the EQ-5D-5L VAS (HR, 1.52 [95% CI, 0.94-2.45]; Figure 4). For EORTC QLQ-C30 dyspnea, the difference in TUDI was statistically significant in favor of axi-cel (HR, 2.59 [95% CI, 1.27-5.29]; P =.0060). Full TUDI analyses and curves are provided in supplemental Table 9 and supplemental Figure 4.
Figure 4.
Time until definitive improvement Kaplan-Meier curves for exploratory EORTC QLQ-C30 global health status/QoL, EORTC QLQ-C30 physical functioning, EQ-5D-5L VAS, and EORTC QLQ-C30 dyspnea. Cumulative incidence of improvement (greater than the minimally important difference) in scores from baseline with no further decline, controlling for death as a competing risk; an event was defined as the first time reaching the threshold for improvement and no deterioration below the threshold at later time points. Patients who had PRO assessments after an EFS event (supplemental Results) were not censored to avoid biasing results by excluding patients with worse outcomes. + on the curve, censor; CIF, cumulative incidence function; NE, not estimable.
Discussion
ZUMA-7, the first randomized, global, multicenter phase 3 study of axi-cel vs SOC as second-line treatment of R/R LBCL, showed that treatment with axi-cel results in statistically significant and clinically meaningful improvements in QoL over SOC at days 100 and 150 for prespecified endpoints, as measured by multiple validated PRO instruments. Additionally, mean return to baseline QoL was demonstrated at day 150 with axi-cel vs month 9 for SOC, suggesting a faster recovery of QoL. Exploratory analyses of PRO endpoints also showed improvements in QoL with axi-cel over SOC. Moreover, patients treated with axi-cel had significantly lower mean WPAI:GH absenteeism and lower mean activities impairment at day 100 than those treated with SOC, suggesting a faster return to baseline functioning at the workplace and at home. This finding is notable, given that only patients employed at screening completed the absenteeism domain. Together with the recently published superior EFS,11 these results highlight that definitive therapies cannot be compared solely based on response and survival rates and that patient experience and QoL must be evaluated alongside of curative potential. ZUMA-7 demonstrated that the benefit to patients of axi-cel over SOC is multifaceted, with a significant improvement in not only efficacy but also QoL that affects patients’ overall sense of well-being.
Measures of general health status (EORTC QLQ-C30 global health status/QoL and EQ-5D-5L VAS) and physical functioning (EORTC QLQ-C30) were chosen for the prespecified hypotheses due to the importance of general health status as a summary measure and because previous research has shown that physical functioning is negatively impacted by ASCT.39 We hypothesized that physical functioning may be less impacted and for a shorter duration of time following CAR T-cell therapy. Although both scores provide complementary information, EQ-5D-5L VAS scores, compared with EQ-5D-5L index, have been shown to be more sensitive to reported change (improvement or deterioration) in previous studies, and the EQ-5D-5L VAS scores may represent a more holistic measure of patient well-being than can be captured by the EQ-5D-5L index and its 5 components.40
We chose day 100 from randomization as the time point to begin hypothesis testing as this was the first time point when definitive therapy (either axi-cel infusion or ASCT) should have been completed in both cohorts. Whereas the first disease assessment was completed at day 50, PRO assessment at this timepoint would evaluate QoL of axi-cel vs salvage chemotherapy and not definitive therapy (HDCT-ASCT). At day 50, patients in both arms reported impairment compared with baseline, which highlights the extended negative impact of salvage chemotherapy followed by HDCT-ASCT on the overall patient journey. However, recovery of QoL to baseline is faster in the axi-cel arm, reflected in the significant difference in QoL at day 100; therefore, SOC is a longer journey that impacts QoL, even for patients who respond to salvage chemotherapy and are candidates for definitive therapy with HDCT-ASCT.
Despite the limitation of imbalances in sample sizes and patient characteristics between the 2 groups in the QoL analysis set, similar results were found in the sensitivity analysis after adjusting for the pattern of missingness through day 150. Because at least 1 follow-up PRO measure was required at day 50, 100, or 150, individuals who already had an EFS event, and therefore could stop completing PRO questionnaires, were not included, which happened disproportionately more frequently in the SOC arm. This is reflected in fewer patients in the QoL analysis set in the SOC arm at the start and increasing imbalance at later time points and is consistent with the clinical outcomes seen in ZUMA-7, with more than a fourfold difference in median EFS (8.3 months vs 2 months) and a 2.5-fold greater 2-year EFS (40.5% vs 16.3%) in favor of axi-cel over SOC after a median follow-up of 24.9 months.11 Notably, at late time points, no statistically significant or clinically meaningful difference in QoL was observed, in accordance with previous observations that among both patients treated with CAR T-cell therapy or transplant, QoL measures are initially impaired but improve over time.39 However, score comparisons at later time points, particularly after month 9, warrant cautious interpretation as attrition due to disease progression, new lymphoma therapy, or death was disproportionately higher on the SOC arm and could contribute to the selection of patients with the best outcomes.
Furthermore, patients in the ZUMA-7 QoL analysis set had numerically higher mean scores at baseline for EORTC QLQ-C30 global health status/QoL and physical functioning scores than the general population (based on norm data for the EORTC QLQ-C30 measures).41 The scores remained higher than these benchmarks at later time points except day 50 for both groups, coinciding with expected early treatment toxicity, and day 100 for the SOC group. Similar patterns are also observed with EQ-5D-5L index and VAS scores. Therefore, the data suggest not only a faster recovery of QoL with axi-cel compared with SOC but a return to a level of health-related QoL that could be expected in the general population. Moreover, the exploratory TUDI analyses suggest a trend toward faster improvement of QoL above baseline, which was already comparable to or better than the general population at baseline, for the axi-cel arm compared with the SOC arm on multiple PRO measures.
Although PRO measurements are currently more commonly used in the oncology space, there is a paucity of peer-reviewed literature on health-related QoL in second-line LBCL.20 PRO data have been assessed in single-arm studies with CAR T-cell therapy, including in third-line LBCL,42, 43, 44, 45 and in a recent presentation, improved QoL in second-line LBCL was suggested with lisocabtagene maraleucel, another anti-CD19 CAR T-cell therapy, vs SOC.46 One of the few studies analyzing QoL among CAR T-cell therapy and transplant recipients is a recent congress presentation reporting superior short-term QoL in patients with hematologic malignancies after CAR T-cell therapy vs autologous or allogeneic stem cell transplantation, although QoL was initially impaired in both groups and improved over time.39 More studies with this comparator are expected as second-line studies, including PRO data, are completed.47 PRO data add a useful layer of information to aid in the decision-making process when choosing a treatment option. These data reflect patient views of a treatment and its value beyond efficacy. Therefore, the ZUMA-7 study helps fill an important gap in the literature with implications for patient care.
There are also several intrinsic methodological limitations to consider. One limitation is that WPAI:GH, except for the activity impairment domain, captures productivity within the sphere of the workplace only, even though productivity in other settings can also be affected. Another limitation of the PRO instruments is that some patients with higher baseline scores may have demonstrated smaller improvements, a common issue when improvement was defined based on the change of scores in any outcome measures with a maximum value (eg, 100 for EORTC QLQ-C30 and EQ-5D-5L VAS). Finally, although severe neurologic events are rare, reversible, and resolve in patients with R/R LBCL treated with axi-cel,11,48 long-term cognitive impairments in patients treated with CAR T-cell therapy are a potential serious complication. There is a lack of PRO scales that assess either events specific to CAR T-cell therapy or general, long-term neurocognitive dysfunction. Although for our assessment period no significant impairment was observed in cognitive, emotional, or social functioning among axi-cel–treated patients compared with SOC (with improvement relative to SOC observed for the latter), improved PRO tools may be needed to capture the nuanced experience of this patient population.
In conclusion, the ZUMA-7 study shows that treatment with axi-cel results in clinically meaningful improvement in QoL over SOC at day 100 as measured by multiple validated PRO instruments. These findings support the idea that sustained clinical benefit confers high QoL and that patients can reach these goals with greater likelihood and faster recovery to baseline QoL with axi-cel compared with SOC. The superior clinical outcomes and favorable patient experience with axi-cel over SOC should help inform treatment choices in second-line R/R LBCL.
Conflict-of-interest disclosure: M.E. reports honoraria from Kite, Bristol Myers Squibb, Novartis, Pfizer, and Janssen and consulting or advisory role with Kite, Bristol Myers Squibb, Novartis, Pfizer, and Janssen. J.C.C. reports consultancy fees for Kite, AbbVie, Janssen, ADC Therapeutics, Karyopharm, Kymera, Genmab, and Novartis; speakers' bureau participation for Morphosys, Epizyme, Bristol Myers Squibb, BeiGene, and AstraZeneca; and research funding from AstraZeneca, Merck, ADC Therapeutics, and Adaptive. I.A. reports speakers’ bureau participation for Kite and Novartis. L.W. reports consultancy or advisory role for Novartis, MSD, Bristol Myers Squibb, AstraZeneca, and Roche; research funding from Roche; and expert testimony from AstraZeneca and Novartis. K.C. reports consultancy or advisory role for Kite, Roche, Takeda, Celgene, Atara, Gilead, Janssen, and Incyte; speakers' bureau for Roche, Takeda, Kite, Gilead, and Incyte; and travel support from Roche, Takeda, Kite, Janssen, and Bristol Myers Squibb/Celgene. K.O. reports consultancy or advisory role for Kite. K. Davison reports consulting or advisory role for Merck, AstraZeneca, Janssen, Novartis, Gilead, AbbVie, and Celgene. J.D.R. reports consultancy or advisory role with Kite, Novartis, Amgen, MSD, Roche, and Bristol Myers Squibb/Celgene; speakers' bureau for Kite, Novartis, and Roche; and research funding from Kite and Novartis. S.D. reports consultancy or advisory role for Kite, Atara Biotherapeutics, and Bristol Myers Squibb and research funding from Jazz Pharmaceuticals and Miltenyi Biotech. K. Dorritie reports research funding from Kite, Juno/Bristol Myers Squibb, Hoffman-LaRoche, Janssen, and Genmab. S.J. reports consultancy or advisory role for Kite, Juno, Novartis, Takeda, and CRISPR Therapeutics and research funding from Kite and Novartis. J.R. reports stock or other ownership in ADC Therapeutics and AstraZeneca; honoraria from Takeda, ADCT, and Bristol Myers Squibb; consultancy or advisory role for Takeda, ADCT, Bristol Myers Squibb, and Novartis; speakers' bureau from Takeda and ADCT; research funding from Takeda; and expert testimony from Takeda and ADCT. F.M. reports consultancy or advisory role for Roche, Bristol Myers Squibb, Gilead, Epizyme, Genmab, Novartis, and AbbVie; speakers’ bureau from Roche; and expert testimony from Roche and Genentech. D.C. reports research funding from MedImmune, AstraZeneca, Clovis, Eli Lilly, 4SC, Bayer, Celgene, and Roche. A.M.G.-S. reports honoraria from Roche, Celgene, Janssen, Servier, and Gilead; consulting or advisory role with Roche, Bristol Myers Squibb/Celgene, Morphosys, Kyowa Kirin, Clinigen, Eusa Pharma, Novartis, Gilead, Servier, and Incyte; research funding from Janssen; expert testimony from Gilead; and travel support Roche, Celgene, Servier, and Kern Pharma. D.T. reports consultancy or advisory role for Partner, Takeda, EUSA, Kite, Kyowa Kirin, and Magenta; speakers' bureau participation for Takeda and Kite; and research funding from Bristol Myers Squibb, Kite, Genentech, Incyte, and Fate Therapeutics. R.K. reports consulting or advisory role with BeiGene, Kite, Morphosys, Karyopharm, Bristol Myers Squibb/Celgene, Genentech, Roche, Janssen, and Pharmacyclics; speakers’ bureau from AstraZeneca, BeiGene, Kite, and Morphosys; and research funding from Kite, Bristol Myers Squibb/Celgene, and Takeda. N.K. reports honoraria from Gilead, Novartis, and Celgene and consultancy or advisory role for Gilead, Novartis, and Celgene. C. Thieblemont reports honoraria from Bristol Myers Squibb/Celgene, AbbVie, Takeda, Novartis, Roche, Kite and Incyte; consultancy or advisory role for Bristol Myers Squibb/Celgene, AbbVie, Takeda, Roche, Novartis, Kite, and Incyte; and travel support from Bristol Myers Squibb/Celgene, Takeda, Roche, Novartis, and Kite. G.E. reports consultancy or advisory role with Kite and Gilead Sciences. P.D. reports consulting or advisory role for AbbVie, AstraZeneca, bluebird bio, Bristol Myers Squibb, Gilead Sciences, Janssen, and Novartis and research funding from Riemser. R.M. reports honoraria from Gilead; consultancy or advisory role Gilead Science; and travel support from Gilead. N.J., W.-J.W., and C.T.S. report employment with OPEN Health; consultancy or advisory role for Kite through employment at OPEN Health; and research funding from multiple clients through employment at OPEN Health. J.T.S. and C. To report employment with Kite and stock or other ownership in Gilead. P.C. reports employment with Kite; stock or other ownership in Gilead; and travel support from Kite. M.J.K. reports honoraria from Kite, Novartis, Miltenyi Biotech, Roche, and Bristol Myers Squibb/Celgene; consultancy or advisory role for Kite, Roche, Bristol Myers Squibb/Celgene, Novartis, and Miltenyi Biotech; research funding from Kite, Roche, Takeda, and Celgene; and travel support from Kite, Roche, Novartis, and Miltenyi Biotech. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank and acknowledge all ZUMA-7 investigators and contributing Kite members as well as ZUMA-7 patients for their contributions to this research; Gab Castaigne, Anik Patel, and Sachin Vadgama for their critical review of study materials; Marco Schupp and Yin Yang for their input on the study design; Anna Purdum for her contributions as head of Heath Economics and Outcomes Research; and Grace Lewis and Skye Geherin of Nexus Global Group Science for medical writing assistance.
This study was supported by Kite, a Gilead Company.
Authorship
Contribution: C.T.S. and N.J. drafted the PRO-specific statistical analysis plan, with input on study design from J.T.S. and P.C.; C.T.S., N.J., and W.-J.W. did the statistical analysis, including access and verification of the data; all authors contributed to the conduct, analysis, verification, and interpretation of data and writing of the manuscript, with medical writing support funded by the sponsor; and all authors participated in writing the article, provided feedback throughout the development process, and approved the final submitted version.
Footnotes
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620. [published correction appears in Blood. 2018;131(5):587–588] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahid U, Akbar F, Amaraneni A, et al. A review of autologous stem cell transplantation in lymphoma. Curr Hematol Malig Rep. 2017;12(3):217–226. doi: 10.1007/s11899-017-0382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 4.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57. doi: 10.1038/bmt.2015.213. [DOI] [PubMed] [Google Scholar]
- 6.van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35(5):544–551. doi: 10.1200/JCO.2016.69.0198. [DOI] [PubMed] [Google Scholar]
- 7.YESCARTA® (axicabtagene ciloleucel) Prescribing information . Kite Pharma, Inc; 2021. https://www.fda.gov/media/108377/download [Google Scholar]
- 8.YESCARTA® (axicabtagene ciloleucel) [summary of product characteristics] Kite Pharma EU B.V.; Amsterdam, the Netherlands: 2021. https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf [Google Scholar]
- 9.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson C, Locke FL, Ghobadi A, et al. Long-term (≥4 year and ≥5 year) overall survival (OS) by 12- and 24-month event-free survival (EFS): an updated analysis of ZUMA-1, the pivotal study of axicabtagene ciloleucel (axi-cel) in patients (Pts) with refractory large B-cell lymphoma (LBCL) Blood. 2021;138(suppl 1):1764. [Google Scholar]
- 11.Locke FL, Miklos DB, Jacobson CA, et al. All ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 12.Oerlemans S, Issa DE, van den Broek EC, et al. Health-related quality of life and persistent symptoms in relation to (R-) CHOP14, (R-)CHOP21, and other therapies among patients with diffuse large B-cell lymphoma: results of the population-based PHAROS-registry. Ann Hematol. 2014;93(10):1705–1715. doi: 10.1007/s00277-014-2099-8. [DOI] [PubMed] [Google Scholar]
- 13.Hafez R, Hussein S, Ismail M. Definitive salvage chemotherapy for the treatment of refractory/relapsed non-Hodgkin lymphoma, a single center experience. Alex J Med. 2018;54(4):679–683. [Google Scholar]
- 14.Food and Drug Administration Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. https://www.fda.gov/media/77832/download [DOI] [PMC free article] [PubMed]
- 15.Food and Drug Administration Core Patient-Reported Outcomes in Cancer Clinical Trials. https://fda.report/media/149994/Core-Patient-Reported-Outcomes-in-Cancer-Clinical-Trials_508ed.pdf
- 16.European Medicines Agency Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man: the use of patient-reported outcome (PRO) measures in oncology studies. https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf
- 17.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 19.Hlubocky FJ, Webster K, Beaumont J, et al. A preliminary study of a health related quality of life assessment of priority symptoms in advanced lymphoma: the National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy - Lymphoma Symptom Index. Leuk Lymphoma. 2013;54(9):1942–1946. doi: 10.3109/10428194.2012.762977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin V, Oak B, Snider J, Epstein J. Health-related quality of life (HRQOL) burden in patients with relapsed/refractory diffuse large B-cell lymphoma (RR-DLBCL) and non-Hodgkin’s lymphoma (RR-NHL) J Clin Oncol. 2020;38(15 suppl) [Google Scholar]
- 21.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 22.Shah J, Shacham S, Kauffman M, et al. Health-related quality of life and utility outcomes with selinexor in relapsed/refractory diffuse large B-cell lymphoma. Future Oncol. 2021;17(11):1295–1310. doi: 10.2217/fon-2020-0946. [DOI] [PubMed] [Google Scholar]
- 23.Thieblemont C, Howlett S, Casasnovas R.-O., et al. Lenalidomide maintenance for diffuse large B-cell lymphoma patients responding to R-CHOP: quality of life, dosing, and safety results from the randomised controlled REMARC study. Br J Haematol. 2020;189(1):84–96. doi: 10.1111/bjh.16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A. 3rd Edition. European Organisation for Research and Treatment of Cancer; Brussels, Belgium: 2001. The EORTC QLQ-C30 Scoring Manual. [Google Scholar]
- 25.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng R, Scippa K, Locke FL, Snider JT, Jim H. Patient perspectives on health-related quality of life in diffuse large B-cell lymphoma treated with car T-cell therapy: a qualitative study. Oncol Ther. 2022;10(1):123–141. doi: 10.1007/s40487-021-00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheson BD, Fisher RI, Barrington SF, et al. United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–941. doi: 10.1016/j.jval.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 32.Maringwa JT, Quinten C, King M, et al. EORTC PROBE project and the Lung Cancer Group. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer. 2011;19(11):1753–1760. doi: 10.1007/s00520-010-1016-5. [DOI] [PubMed] [Google Scholar]
- 33.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5(1):70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 35.Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713–1721. doi: 10.1016/j.ejca.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 36.Bonnetain F, Dahan L, Maillard E, et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma. Eur J Cancer. 2010;46(15):2753–2762. doi: 10.1016/j.ejca.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Anota A, Hamidou Z, Paget-Bailly S, et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24(1):5–18. doi: 10.1007/s11136-013-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 39.Sidana S, Thanarajasingam G, Griffin J, et al. Patient experience of chimeric antigen receptor (CAR)-T cell therapy vs. stem cell transplant: Longitudinal patient reported adverse events, cognition and quality of life. Blood. 2019;134(suppl_1):794. [Google Scholar]
- 40.Stark RG, Reitmeir P, Leidl R, König HH. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis. 2010;16(1):42–51. doi: 10.1002/ibd.20989. [DOI] [PubMed] [Google Scholar]
- 41.Nolte S, Liegl G, Petersen MA, et al. EORTC Quality of Life Group. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153–163. doi: 10.1016/j.ejca.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Ruark J, Mullane E, Cleary N, et al. Patient-reported neuropsychiatric outcomes of long-term survivors after chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2020;26(1):34–43. doi: 10.1016/j.bbmt.2019.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XS, Srour SA, Whisenant M, et al. Patient-reported symptom and functioning status during the first 12 months after chimeric antigen receptor T cell therapy for hematologic malignancies. Transplant Cell Ther. 2021;27(11):930.e1–930.e10. doi: 10.1016/j.jtct.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maziarz RT, Waller EK, Jaeger U, et al. Patient-reported long-term quality of life after tisagenlecleucel in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(4):629–637. doi: 10.1182/bloodadvances.2019001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patrick DL, Powers A, Jun MP, et al. Effect of lisocabtagene maraleucel on HRQoL and symptom severity in relapsed/refractory large B-cell lymphoma. Blood Adv. 2021;5(8):2245–2255. doi: 10.1182/bloodadvances.2020003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abramson JS, Solomon SR, Arnason JE, et al. Improved quality of life (QOL) with lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, compared with standard of care (SOC) as second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): results from the phase 3 Transform study. Blood. 2021;138(suppl 1):3845. [Google Scholar]
- 47.Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–639. doi: 10.1056/NEJMoa2116596. [DOI] [PubMed] [Google Scholar]
- 48.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.