Abstract

In the present paper, a facile and efficient synthetic procedure has been applied to obtain dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylates (5a–s), which have subsequently gone through the cyclization in the presence of hydrazine hydrate to afford 12-aryl-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyridazine-8(9H)-ones (7a–q). The molecular structures of these novel compounds were extensively examined through the analysis of spectroscopic data in combination with X-ray crystallography techniques. Following that, the in vitro cytotoxic activities of all derivatives against three human cancer cell lines (Panc-1, PC3, and MDA-MB-231) were comprehensively evaluated alongside the assessment on normal human dermal fibroblast (HDF) cells using the MTT assay. Among the compounds, the 3-nitrophenyl derivative (7m) from the second series showed the best antiproliferative activity against all tested cell lines, particularly against Panc-1 cell line, (IC50 = 12.54 μM), being nearly twice as potent as the standard drug etoposide. The induction of apoptosis and sub-G1 cell cycle arrest in Panc-1 cancer cells by compound 7m was confirmed through further assessment. Moreover, the inhibition of kinases and the induction of cellular apoptosis by compound 7m in Panc-1 cancer cells were validated using the Western blotting assay.
Introduction
Cancer is known as one of the most serious fatal diseases, which happens when the group of cells in the body ignore the physiological cell division rules and grow uncontrollably.1,2 Chemotherapy is one of the most common cancer treatment approaches aiming to halt tumor progression by stopping cancerous cell division and inducing apoptosis.3,4 However, in many cases, there are two major concerns, including nonspecificity of the drug to the cancer cells, which leads to the death of normal body cells, and the drug resistance of cancer cells, which is associated with a decrease in the effectiveness of the treatment.5,6 Based on the reports in the literature, it is evidenced that the most common FDA-approved medicines contain N-heterocycle structure in their skeleton, and the use of heterocycle compounds in drug discovery, especially in most FDA-approved oncological medicines between 2010 and 2015, was further emphasized.7,8
Among the N-heterocycle structures, pyrrole-based compounds, as well as their heterofused derivatives, have drawn considerable attention because of their valuable pharmacological properties such as being anticancer,9−11 antimicrobial,12 antiviral,13 anti-inflammatory,14 and antidiabetic.15 Specifically, the amalgamation of pyrrole with other cyclic structures, notably the fused bicyclic arrangements involving pyrazine or pyridazine, has been extensively explored. This core scaffold has garnered significant attention for the development of novel therapeutics encompassing a diverse spectrum of pharmacological and biological activities.16−20 Particularly, the antiproliferative properties of 1,3-disubstituted pyrrolo[1,2-a]pyrazine derivatives on glioblastoma cell line have been reported by Uygun et al.21 Moreover, Seo et al. utilized pyrrolo[1,2-a]pyrazine core to produce 3,4-dihydropyrrolo[1,2-a]pyrazine derivatives and evaluated the anticancer activity of those compounds against prostate cancer (PC3) and breast cancer (MCF-7) cell lines.22 On the other side, pyrrolopyridazine derivatives have been introduced by their well-known antiviral,19 anti-inflammatory,23 and antimicrobial activities.24 Besides, anticancer and antiproliferative properties of pyrrolopyridazine scaffold derivatives such as pyrrolo[1,2-b]pyridazines and pyrrolo[2,3-d]pyridazine–7-one have been reported.20,25 Xiang and colleagues investigated the role of 2-substituted pyrrolo[1,2-b]pyridazine derivatives to inhibit the poly(ADP-ribose)polymerase (PARP) functions within cancer cells, which results in diminishing cell proliferations.26 Moreover, a new class of Janus kinase (JAK) family inhibitors has been discovered using pyrrolo[1,2-b]pyridazine-3-carboxamide derivatives, which would be promising for a wide variety of human disease treatments.23,27
Considering the importance of pyrrolopyrazine and pyrrolopyridazine derivatives and following our successful previous research on the design of anticancer compounds containing multiple pharmacophores,28−32 a novel series of dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine core structures 5a–s and 7a–q were designed and synthesized (Figure 1) based on the reported similar compound with the same core scaffold displaying a wide range of biological and pharmaceutical functions.22,25,33−35 The synthesis procedure was provided through an efficient one-pot three-step reaction. The cytotoxic effects of all of the mentioned compounds (5a–s and 7a–q) were assessed against three different human cancer cell lines. Further assessments were conducted to identify the potential mechanisms for their anticancer effects.
Figure 1.
Design strategy of final compounds 5a–s and 7a–q.
Results and Discussion
Chemistry
Initially, various substituted arylglyoxal derivatives 1a–s were synthesized according to the literature from the corresponding substituted acetophenones.36 Subsequently, they were reacted with N-aminoethylpyrrole 2 in the presence of acetic acid (HOAc) in dry dichloromethane (DCM) at ambient temperature, completing the reaction within 2 h and yielding intermediates 3a–s. Following nearly complete conversion to the corresponding adduct, triphenylphosphine (PPh3) and dimethylacetylenedicarboxylate (DMAD) 4 were added to the reaction mixture, and stirring was continued for an additional 10 min at room temperature. This resulted in the formation of the desired dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate derivatives 5a–s. Subsequently, upon heating a mixture of these compounds with hydrazine hydrate 6 in ethanol (EtOH) under reflux conditions for 10 h, 12-aryl-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyridazine-8(9H)-one derivatives 7a–q were obtained (Scheme 1).
Scheme 1. Synthetic Approach toward Desirable Compounds 5a–s and 7a–q: (a) HOAc (3 Drops), Dry DCM (0.5 mL), r.t., 2 h; (b) PPh39 (0.5 mmol), DMAD 4 (0.5 mmol); and (c) Hydrazine Hydrate 6 (1 mmol), EtOH (2 mL), Reflux, 10 h.
The structures of the isolated products were determined by analyzing their infrared (IR), 1H NMR, 13C NMR, mass spectrometry, and X-ray data. Partial assignments of the observed resonances can be found in the Experimental Section. The X-ray analysis images of the compounds (from the first series, 5s, and the second series, 7l) are presented in the Supporting Information.
A proposed mechanism for the synthesis of 5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine derivatives 5 is presented in Scheme 2. Initially, the reaction between arylglyoxal 1 and N-ethylaminopyrrole 2 under acidic conditions results in the formation of intermediate 3 through the acid-catalyzed Pictet–Spengler reaction.37 Phosphorus ylide 10 is obtained from the condensation of acetylenic ester 4 and triphenylphosphine 9, and further protonation of 1:1 adduct by NH-acid 3. Then, this intermediate goes through the nucleophilic attack of conjugated acid of NH-acid 11 to make phosphorane 12, which is subjected to an intramolecular Wittig reaction to generate adduct 14.38 Finally, an oxidative aromatization occurs under reaction conditions to produce the desired product 5. The formation of phosphine oxide as a byproduct confirms this step of the proposed mechanism (Scheme 2).
Scheme 2. Plausible Mechanism for the Formation of Compounds 5a–s.
The obvious mechanism to obtain the second series is the domino two nucleophilic additions of hydrazine to ester functionality groups present in compound 5.
Antiproliferative Activity
The in vitro antiproliferative effects of two series of compounds with dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine core structure, including 1-substituted-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c] pyrazine-2,3-dicarboxylates (5a–s) and their pyridazine-fused counterparts 12-aryl-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyridazine-8(9H)-ones (7a–q), were evaluated against three different human cancer cell lines with epidermal growth factor receptor (EGFR) overexpression. The structures of synthesized compounds were varied by attachment of different electron-withdrawing or -donating groups on the phenyl ring. The cytotoxicity of the synthesized compounds was assessed against pancreatic cancer (Panc-1), prostate cancer (PC3), and breast cancer (MDA-MB-231) cell lines, in addition to being evaluated against normal human dermal fibroblasts (HDF) cells. The results were presented as IC50 values (Tables 1 and 2) and compared with etoposide as the reference drug.
Table 1. In Vitro Antiproliferative Effects (IC50, μM) of Compounds 5a–s against Panc-1, PC3, MDA-MB-231, and HDF Cell Linesa.

| compounds | R | Panc-1 | PC3 | MBA-MB-231 | HDF |
|---|---|---|---|---|---|
| 5a | H | 70.13 ± 0.038 | 78.94 ± 0.051 | 71.1 ± 0.05 | >100 |
| 5b | 2-OH | 20.28 ± 0.01 | 22.51 ± 0.018 | 28.2 ± 0.02 | >100 |
| 5c | 3-OH | 72.63 + 0.06 | 90.79 + 0.065 | 87.39 + 0.058 | >100 |
| 5d | 2-Me | 67.88 ± 0.037 | 64.85 ± 0.046 | 78.54 ± 0.061 | >100 |
| 5e | 3-Me | 74.25 ± 0.046 | 92.81 ± 0.071 | 88.1 ± 0.067 | >100 |
| 5f | 4-Me | 70.4 ± 0.05 | 89.26 ± 0.048 | 73.15 ± 0.057 | >100 |
| 5g | 3-OMe | 67.22 ± 0.058 | 85.49 ± 0.065 | 70.75 ± 0.051 | >100 |
| 5h | 4-OMe | 65.3 ± 0.037 | 74.36 + 0.042 | 82.51 + 0.072 | >100 |
| 5i | 2-F | 72.02 ± 0.046 | 89.12 ± 0.065 | 76.14 ± 0.058 | >100 |
| 5j | 4-F | 75.27 ± 0.051 | 89.67 ± 0.072 | 84.21 ± 0.067 | >100 |
| 5k | 3-Cl | 56.98 ± 0.052 | 67.22 ± 0.047 | 75.26 ± 0.044 | >100 |
| 5l | 4-Cl | 73.87 ± 0.048 | 83.33 ± 0.065 | 75.73 ± 0.06 | >100 |
| 5m | 3-Br | 65.74 ± 0.038 | 80.7 ± 0.072 | 71.74 ± 0.064 | >100 |
| 5n | 4-Br | 79.66 ± 0.053 | 91.25 ± 0.072 | 82.74 ± 0.064 | >100 |
| 5o | 3-NO2 | 34.14 ± 0.021 | 40.55 ± 0.026 | 43.67 ± 0.031 | >100 |
| 5p | 4-NO2 | 68.28 ± 0.044 | 82.28 ± 0.057 | 78.92 ± 0.06 | >100 |
| 5q | 3-OMe-4-OH | 43.97 ± 0.025 | 47.68 ± 0.031 | 41.97 ± 0.016 | >100 |
| 5r | 2,4-Cl2 | 69.59 ± 0.048 | 81.07 ± 0.058 | 85.69 ± 0.072 | >100 |
| 5s | 3,4,5-(OMe)3 | 66.01 ± 0.045 | 72.73 ± 0.051 | 75.73 ± 0.06 | >100 |
| etoposide | 24.35 ± 0.001 | 32.15 ± 0.021 | 30.63 ± 0.014 | >100 |
Values are the means of three replicates ± standard deviation (SD).
Table 2. In Vitro Antiproliferative Effects (IC50, μM) of Compounds 7a–q against Panc-1, PC3, MDA-MB-231, and HDF Cell Linesa.
| compounds | R | Panc-1 | PC3 | MBA-MB-231 | HDF |
|---|---|---|---|---|---|
| 7a | H | 92.48 ± 0.05 | 91.25 ± 0.077 | 87.31 ± 0.063 | >100 |
| 7b | 3-OH | 73.48 + 0.055 | 84.44 + 0.062 | 84.61 + 0.067 | >100 |
| 7c | 2-Me | 60.63 ± 0.051 | 65.34 ± 0.052 | 66.17 ± 0.043 | >100 |
| 7d | 3-Me | 65 ± 0.047 | 72.35 ± 0.063 | 65.94 ± 0.051 | >100 |
| 7e | 4-Me | 48.10 ± 0.018 | 53.19 ± 0.024 | 65.47 ± 0.041 | >100 |
| 7f | 3-OMe | 69.12 ± 0.039 | 78.98 ± 0.058 | 64.47 ± 0.05 | >100 |
| 7g | 4-OMe | 60 ± 0.047 | 68.28 ± 0.039 | 61.01 ± 0.029 | >100 |
| 7h | 4-F | 66.4 ± 0.046 | 74.6 ± 0.051 | 70.62 ± 0.054 | >100 |
| 7i | 3-Cl | 21.4 + 0.016 | 30.01 ± 0.02 | 17.9 ± 0.014 | >100 |
| 7j | 4-Cl | 46.95 ± 0.021 | 57.91 ± 0.04 | 53.34 ± 0.037 | >100 |
| 7k | 3-Br | 58.19 ± 0.022 | 66.29 ± 0.037 | 62.01 ± 0.041 | >100 |
| 7l | 4-Br | 73.92 ± 0.043 | 60.04 ± 0.031 | 58.03 ± 0.04 | >100 |
| 7m | 3-NO2 | 12.54 ± 0.001 | 17.66 ± 0.012 | 13.14 ± 0.005 | >100 |
| 7n | 4-NO2 | 46.35 + 0.028 | 60.17 + 0.045 | 61.66 + 0.052 | >100 |
| 7o | 3-OMe-4-OH | 14.95 + 0.001 | 17.76 + 0.014 | 15.09 + 0.001 | >100 |
| 7p | 2,4-Cl2 | 72.48 ± 0.058 | 76.3 ± 0.046 | 81.84 ± 0.063 | >100 |
| 7q | 3,4,5-(OMe)3 | 72.81 ± 0.052 | 81.28 ± 0.06 | 67.4 ± 0.048 | >100 |
| etoposide | 24.35 ± 0.001 | 32.15 ± 0.021 | 30.63 ± 0.014 | >100 |
Values are the means of three replicates ± standard deviation (SD).
The antiproliferative activity of 1-substituted-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate derivatives (5a–s) is presented in Table 1 and Figure 2. According to the results, most of the compounds showed good cytotoxic effects against all tested cell lines (IC50 less than 92 μM). Attachments of different electron-withdrawing groups such as bromo, fluoro, and chloro on the phenyl ring did not cause any significant change in comparison with 5a. As shown in Table 1, compound 5o with 3-NO2 substituent had a comparable antiproliferative activity with etoposide. The best results were observed for compound 5b with 2-OH substituent. This compound showed more potent activity than etoposide, especially against Panc-1 cancer cells (IC50 = 20.3, 22.5, and 28.2 μM against Panc-1, PC3, and MDA-MB-231 cell lines, respectively).
Figure 2.
In vitro cell cytotoxicity: MTT assay results of compounds 5a–s against three cancer lines.
As depicted in Table 2 and Figure 3, all of the 12-aryl-11-hydroxy-5,6-dihydropyrrolo [2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyridazine-8(9H)-ones (7a–q) showed moderate to good antiproliferative activity. Compound 7o with 3-OCH3-4-OH substitutions showed potent results against all tested cancer cells and was about two times more potent than etoposide. However, other compounds with electron-donating groups such as methyl, methoxy, and hydroxy on the C-2, C-3, and C-4 positions exhibited moderate inhibitor activities. Additionally, compounds containing electron-withdrawing groups, such as fluoro and bromo substitutions, had moderate anticancer effects. In contrast, 3-chloro and 3-nitro-containing derivatives showed better anticancer effects than etoposide against all tested cases. The compound 7m, which exhibited the highest potency against all cancer cells, contained a 3-NO2 substitution. According to the IC50 values, compound 7m demonstrated twice the potency of etoposide in all cases, with IC50 = 12.5, 17.7, and 13.1 μM against Panc-1, PC3, and MDA-MB-231 cell lines, respectively.
Figure 3.
In vitro cell cytotoxicity: MTT assay results of compounds 7a–q against three cancer lines.
1-Substituted-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate derivatives and 2-OH containing compound 5b exhibited minimal cytotoxic effects more potent than etoposide with the best results against Panc-1 cancer cells. On the other hand, among the synthesized tetrahydropyrrolo-pyrazino–pyrrolo-pyridazines (7a–q), attachment of electron-withdrawing groups on the phenyl ring had positive effects on the antiproliferative activity of target compounds. However, the nature and position of substituted groups were really important. The best results were seen in compounds 7m, 7i, and 7o, which contained 3-NO2, 3-Cl, and 3-OMe-4-OH substitutions on their phenyl rings. Compound 7m with the highest cytotoxic activity against Panc-1 (IC50 = 12.5 μM) was selected for further biological evaluation.
Apoptosis-Inducing Activity
The flow cytometry analysis was conducted in Panc-1 cells to investigate the mechanism of cell death induced by the highly active compound 7m; in this study, Panc-1 cancer cells were exposed to the IC50 concentration of compound 7m for 48 h. Then, the treated cells were harvested and stained with FITC annexin V and propidium iodide (PI) solutions, respectively. The percentages of apoptotic cells were determined using the flow cytometry annexin V-FITC/PI dual staining assay. Untreated cells were utilized as the negative control. According to the results shown in Figure 4, the treatment of Panc-1 cancer cells with compound 7m at IC50 concentration resulted in 11.01% of cell apoptosis, while the untreated cells exhibited 8.24% of apoptotic cell death. These findings suggested that the cytotoxicity of compound 7m is linked to the induction of cellular apoptosis in a concentration-dependent manner, which is depicted in Figure 4 and tabulated in Table 3.
Figure 4.
Flow cytometric analysis of Panc-1 cells. (A) Nontreated cells as the negative control group; (B) treated with 7m at IC50 concentration.
Table 3. Apoptosis Assay of Panc-1 Cells (Treated with 7m at IC50 Concentration).
| live cells (%) | early apoptosis (%) | late apoptosis (%) | necrosis (%) | |
|---|---|---|---|---|
| control | 91.2 | 4.12 | 4.12 | 0.586 |
| treated | 88.1 | 5.56 | 5.45 | 0.851 |
Cell Cycle Arrest
A cell cycle analysis was conducted to examine the impact of the highly potent compound 7m on the proliferation of cancer cells at specific checkpoints in Panc-1 cancer cells. In this experiment, Panc-1 cancer cells were exposed to compound 7m at its IC50 concentration for 48 h. Untreated cells were used as the negative control in this experiment. After 48 h of treatment with compound 7m, the cancer cells were collected, stained with PI, and analyzed using flow cytometry. The DNA content of the cells was measured by binding to PI, which acts as a fluorescent agent. The results, shown in Figure 4, revealed that treatment of Panc-1 cells with compound 7m at the IC50 concentration increased the proportion of cells in the sub-G1 phase from 1.82% (in the control group) to 5.47% (in the treated cells). These findings provide evidence that compound 7m significantly induces sub-G1 phase arrest in Panc-1 cancer cells (Figure 5).
Figure 5.
Cell cycle analysis of Panc-1 cells by flow cytometric analysis. (A) Nontreated cells as the control group; (B) treated with 7m at IC50 concentration.
Western Blotting Assay
A Western blot analysis was performed in Panc-1 cells to further investigate the kinase inhibition of compound 7m. Panc-1 cancer cells were treated with the IC50 concentration of compound 7m. Subsequently, cell lysates were collected for Western blot analysis to evaluate the expression of caspase-3, cleaved-caspase-3, pro-caspase-3, and B-actin proteins. The results from Figure 6 indicate a downregulation of cleaved-caspase-3 and caspase-3 protein levels in Panc-1 cancer cells, while the protein levels of pro-caspase-3 and β-actin remained unchanged.
Figure 6.
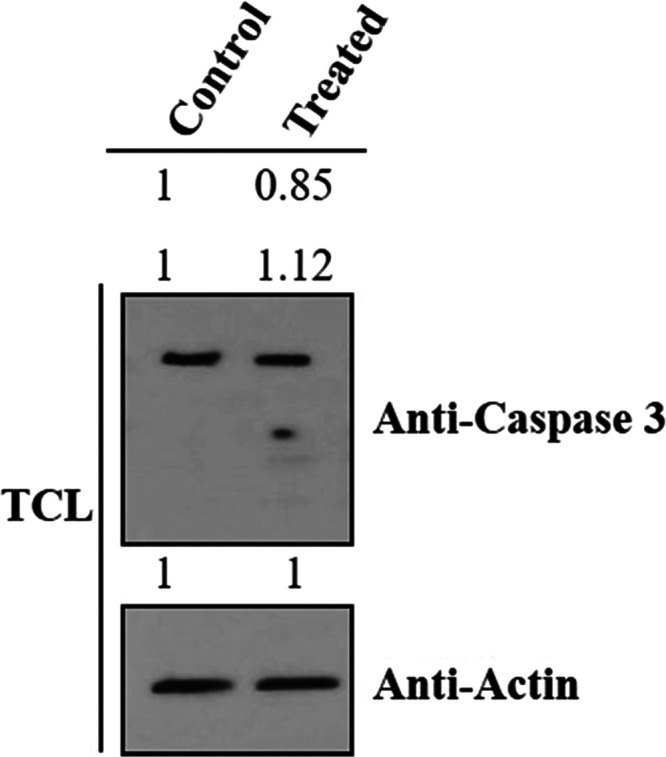
Western blot analysis of compound 7m (sample) in Panc-1 cancer cells. Caspase-3 proteins expression (pro-caspase-3: upper band; cleaved-caspase-3: two lower bands) is shown. Actin used as an internal control (TCL: total cell lysate).
Conclusions
In this study, a novel series of pyrrolopyrazine derivatives has been designed and synthesized. Some of the synthesized compounds exhibit promising anticancer activities. The synthesis pathway for the first series (5a–s) involved an efficient one-pot three-step reaction, resulting in the creation of 19 derivatives. Based on the biological results, most of the synthesized compounds exhibited moderate to good antiproliferative activity. Compound 5b emerged as the most promising candidate, displaying the best results against Panc-1 cell lines (IC50 = 20.3 compared to etoposide IC50 = 24.3 μM). As for the second series (7a–q), compound 7m containing 3-NO2 substitution showed the highest potency. Moreover, this compound demonstrated nearly twice the potency of etoposide against all tested cell lines. Additional evaluation through annexin V-FITC/PI dual staining assay and cell cycle analysis confirmed that compound 7m induced significant apoptotic cell death and caused sub-G1 phase cell cycle arrest in Panc-1 cancer cells. Western blotting analysis further validated the kinase inhibition activity of compound 7m in Panc-1 cancer cells, as evidenced by the downregulation of cleaved-caspase-3 and caspase-3 protein levels. Consequently, this research underscores the promising potential of these pyrropyrazine derivatives as viable candidates for subsequent modifications in the pursuit of innovative anticancer agents.
Experimental Section
General Chemistry
Melting points were determined using a Thermo Scientific 9100 instrument. IR spectra were recorded on Bruker Tensor 27 Equinox 55 infrared spectrophotometer (ν in cm–1). Mass spectra were obtained using an Agilent 5975C VL MSD (ion source: EI+, 70 eV, 230 °C). The 1H and 13C NMR spectra were recorded in dimethyl sulfoxide (DMSO-d6) and deuterated chloroform CDCl3 on a Bruker FT-500 MHz spectrometer, with tetramethylsilane (TMS) as the internal reference. Coupling constants were reported in Hertz (Hz), and chemical shifts were given as δ value (ppm). The detailed characterization data and atom numbering are presented in Figure 7.
Figure 7.

Atom numbering of target compounds 5a–s and 7a–q.
General Procedure for the Synthesis of Dimethyl 1-Substituted-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5a–s)
A solution of arylglyoxal (1 mmol), N-ethyl amino pyrrole (1 mmol), and three drops of acetic acid in DCM (5 mL) was stirred at room temperature for 2 h. Upon completion of the reaction, as indicated by thin layer chromatography (TLC), triphenylphosphine (0.5 mmol) and dimethyl acetylene dicarboxylate (0.5 mmol) in DCM (0.5 mL) were added to the mixture. Then, the reaction mixture was stirred at room temperature for 10 min (monitored by TLC). Finally, the crude product was purified by column chromatography on silicon dioxide (SiO2) using progressively more nonpolar eluent (70:30 petroleum ether/ethyl acetate, v/v), to afford the desired compounds 5a–s in good yield (60–80%).
Dimethyl-1-phenyl-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5a)
Green crystal; yield: 70%; mp 140–142 °C; 1H NMR (500 MHz, CDCl3) δ: 3.71 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.25 (t, 2H, J = 6 Hz, CH2), 4.79 (t, 2H, J = 6.0 Hz, CH2), 5.94 (dd, 1H, J = 3.7, 1.4 Hz, Hb), 6.04 (dd, 1H, J = 3.7, 2.7 Hz, Ha), 6.67 (dd, 1H, J = 2.6, 1.4 Hz, Hc), 7.32–7.36 (m, 1H, HAr), 7.38 (t, 2H, J = 7.9 Hz, HAr), 7.43 (d, 2H, J = 7.9 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.4, 44.0, 51.9, 52.4, 107.2, 109.2, 117.8, 119.3, 120.8, 122.4, 125.1, 127.7, 127.9, 128.5, 130.0, 133.3, 161.1, 166.8; calculated mass: 350.13; electrospray ionization mass spectrometry (ESI-MS) m/z: 350 [M]+. Anal. calcd for C20H18N2O4: C, 68.56; H, 5.18; N, 8.; found: C, 68.33; H, 5.37; N, 7.81%.
Dimethyl-1-(2-hydroxyphenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5b)
Green powder; yield: 60%; mp 200–203 °C; 1H NMR (500 MHz, CDCl3) δ: 3.74 (s, 3H, CH3), 3.85 (s, 3H, CH3), 4.27 (q, 2H, J = 5.2 Hz, CH2), 4.65 (dt, 1H, J = 13.2, 6.4 Hz, CH2), 4.94 (dt, 1H, J = 11.0, 5.0 Hz,CH2), 5.72 (dd, 1H, J = 3.8, 1.6 Hz, Hb), 5.93 (s, 1H, OH), 6.04 (dd, 1H, J = 4.0, 2.4 Hz, Ha), 6.65–6.68 (m, 1H, Hc), 6.95 (t, 1H, J = 7.4 Hz, HAr), 7.06 (d, 1H, J = 8.2 Hz, HAr), 7.21 (d, 1H, J = 7.6 Hz, HAr), 7.31 (t, 1H, J = 7.7 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.5, 43.9, 52.0, 52.7, 107.7, 109.8, 113.1, 117.2, 118.7, 119.0, 120.1, 120.9, 121.1, 121.8, 129.1, 130.2, 132.2, 154.4, 161.0, 167.2; calculated mass: 366.12; ESI-MS m/z: 366.1 [M]+. Anal. calcd for C20H18N2O5: C, 65.57; H, 4.95; N, 7.65.; found: C,65.39; H, 5.12; 7.76%.
Dimethyl-1-(3-hydroxyphenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5c)
White powder; yield: 61%; mp 138–140 °C; 1H NMR (500 MHz, CDCl3) δ: 3.72 (s, 3H, CH3), 3.83 (s, 3H, CH3), 4.21 (t, 2H, J = 6.0 Hz, CH2), 4.75 (t, 2H, J = 5.9 Hz, CH2), 5.61 (s, 1H, OH), 6.02 (d, 1H, J = 3.9 Hz, Hb), 6.05 (s, 1H, Ha), 6.66 (s, 1H, Hc), 6.81 (d, 1H, J = 7.8 Hz, HAr), 6.87 (s, 1H, HAr), 6.96 (d, 1H, J = 7.3 Hz, HAr), 7.23 (t, 1H, J = 7.7 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.3, 43.9, 51.9, 52.5, 107.4, 109.3, 114.8, 116.8, 117.7, 118.9, 120.9, 122.3, 125.0, 127.9, 129.7, 134.6, 155.9, 161.0, 167.1; calculated mass: 366.12; ESI-MS m/z: 366.3 [M]+. Anal. calcd for C20H18N2O5: C, 65.57; H, 4.95; N, 7.65.; found: C, 65.53; H, 5.05; N, 7.56%.
Dimethyl-1-(2-tolyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5d)
Green powder; yield: (70%); mp 158–160 °C; 1H NMR (500 MHz, CDCl3) δ: 2.17 (s, 3H, CH3), 3.65 (s, 3H, CH3), 3.85 (s, 3H, CH3), 4.25 (t, 2H, J = 6.0 Hz, CH2), 4.80–4.82 (m, 2H, CH2), 5.49 (dd, 1H, J = 3.8, 1.5 Hz, Hb), 6.01 (dd, 1H, J = 3.7, 2.7 Hz, Ha), 6.65 (dd, 1H, J = 2.6, 1.4 Hz, Hc), 7.19–7.23 (m, 2H, HAr), 7.27 (d, 2H, J = 2.4 Hz, H–Ar); 13C NMR (125 MHz, CDCl3) δ: 20.1, 43.3, 44.0, 51.8, 52.1, 106.5, 109.5, 118.0, 118.6, 120.7, 122.6, 124.8, 125.8, 127.9, 128.1, 129.9, 130.9, 132.7, 138.1, 161.1, 166.3; calculated mass: 364.14; ESI-MS m/z: 364.14 [M]+. Anal. calcd for C21H20N2O4: C, 69.22; H, 5.53; N, 7.69.; found: C, 69.01; H, 5.81; 7.89%.
Dimethyl-1-(3-tolyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5e)
Cream powder; mp 128–130 °C; yield (66%); 1H NMR (500 MHz, CDCl3) δ: 2.37 (s, 3H, CH3), 3.72 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.25 (t, 2H, J = 6.0 Hz, CH2), 4.79 (t, 2H, J = 5.9, Hz, CH2), 5.97 (d, 1H, J = 3.8 Hz, Hb), 6.06 (d, 1H, J = 3.3 Hz, Ha), 6.67 (d, 1H, J = 2.5 HZ, Hc), 7.15 (d, 1H, J = 7.3 Hz, HAr), 7.22 (d, 1H, J = 7.4 Hz, HAr), 7.27 (d, 2H, J = 10.6 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 21.5, 43.3, 44.0, 51.8, 52.3, 107.1, 109.2, 117.7, 119.4, 120.8, 122.5, 125.1, 127.0, 127.9, 128.3, 128.4, 130.6, 133.1, 137.9, 161.0, 166.8; calculated mass: 364.14; ESI-MS m/z: 364.2 [M]+. Anal. calcd for C21H20N2O4: C, 69.22; H, 5.53; N, 7.69.; found: C, 69.01; H, 5.78; N: 7.91%.
Dimethyl-1-(4-tolyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5f)
Green crystal; mp 164–166 °C; yield (68%); 1H NMR (500 MHz, CDCl3) δ: 2.39 (s, 3H, CH3), 3.72 (s, 3H, CH3), 3.83 (s, 3H, CH3), 4.24 (t, 2H, J = 5.8 Hz, CH2), 4.78 (d, 2H, J = 5.8 Hz, CH2), 5.98 (dd, 1H, J = 3.6, 1.4 Hz, Hb), 6.02–6.09 (m, 1H, Ha), 6.66 (dd, 1H, J = 2.8, 1.6 Hz, Hb), 7.19 (d, 2H, J = 7.8 Hz, HAr), 7.32 (d, 2H, J = 8 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 21.4, 43.3, 43.9, 51.8, 52.3, 107.1, 109.1, 117.6, 119.3, 120.7, 122.5, 125.1, 127.9, 129.2, 129.8, 130.1, 137.3, 161.0, 166.9; calculated mass: 364.14; ESI-MS m/z: 364 [M]+. Anal. calcd for C21H20N2O4: C, 69.22; H, 5.53; N, 7.69.; found: C, 68.97; H, 5.72; N, 7.93%.
Dimethyl-1-(3-methoxyphenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5g)
Green powder; mp 138–140 °C; yield (65%); 1H NMR (500 MHz, DMSO-d6) δ: 3.64 (s, 3H, CH3), 3.74 (s, 3H, CH3), 3.77 (s, 3H, CH3), 4.32 (t, 2H, J = 5.6 Hz, CH2), 4.66 (t, 2H, J = 5.7 Hz, CH2), 5.86 (dd,1H, J = 3.7, 1.5 Hz, Hb), 5.98–6.01 (m, 1H, Ha), 6.88 (d,1H, J = 2.2 Hz, Hc), 6.90–6.95 (m, 3H, HAr), 7.33 (t, 1H, J = 7.9 Hz, HAr); 13C NMR (125 MHz, DMSO-d6): δ= 43.0, 43.2, 51.7, 52.0, 55.0, 106.1, 108.4, 113.0, 115.0, 117.4, 117.8, 121.2, 121.6, 121.7, 123.9, 126.9, 129.4, 134.3, 159.0, 160.0, 165.8; calculated mass: 380.14; ESI-MS m/z: 380 [M]+. Anal. calcd for C21H20N2O5: C, 66.31; H, 5.30; N, 7.36.; found: C, 66.08; H, 5.42; C, 7.61%.
Dimethyl-1-(4-methoxyphenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5h)
Green powder; mp 198–200 °C; yield (66%); 1H NMR (500 MHz, CDCl3) δ: 3.72 (s, 3H, CH3), 3.84 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.24 (t, 2H, J = 5.8 Hz, CH2), 4.77 (t, 2H, J = 5.8, CH2), 5.96 (d, 2H, J = 3.8 Hz, Hb), 6.03–6.06 (m, 1H, Ha), 6.66 (s, 1H, Hc), 6.92 (d, 2H, J = 8.4 Hz, HAr), 7.35 (d, 2H, J = 8.4 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.3, 43.9, 51.8, 52.3, 55.3, 107.0, 109.2, 113.9, 117.6, 118.9, 120.7, 122.5, 125.1, 125.3, 128.0, 131.1, 159.1, 161.0, 166.9; calculated mass: 380.14; ESI-MS m/z: 380.2 [M]+. Anal. calcd for C21H20N2O5: C, 66.31; H, 5.30; N, 7.36.; found: C, 66.02; H, 5.47; 7.58%.
Dimethyl-1-(2-fluorophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5i)
Green crystal; mp 138–140 °C; yield (64%); 1H NMR (500 MHz, DMSO-d6) δ: 3.61 (s, 3H, CH3), 3.79 (s, 3H, CH3), 4.32 (t, 2H, J = 5.8 Hz, CH2), 4.64 (t, 2H, J = 5.8 Hz, CH2), 5.60 (d, 1H, J = 3.3 Hz, Hb), 5.96–6.03 (m, 1H, Ha), 6.92 (dd, 1H, J = 2.6, 1.4 Hz, Hc) 7.27 (dt, 2H, J = 14.9, 8.4 Hz, HAr), 7.34 (t, 1H, J = 7.2 Hz, HAr), 7.45 (d, 1H, J = 7.0 Hz, HAr); 13C NMR (125 MHz, DMSO-d6): δ= 43.1, 43.3, 51.8, 51.9, 105.5, 108.6, 110.9, 115.54 (d, J = 22 Hz), 119.4, 120.66 (d, J = 16.5 Hz), 121.0, 121.6, 122.9, 124.3, 127.4, 130.6 (d, J = 8.2 Hz), 132.4, 158.9, 160.3, 160.8, 164.9; calculated mass: 368.12; ESI-MS m/z: 368 [M]+. Anal. calcd for C20H17FN2O4: C, 65.21; H, 4.65; N, 7.60.; found: C, 64.98; H, 4.42; 7.84%.
Dimethyl-1-(4-fluorophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5j)
White powder; mp 170–172 °C; yield (71%); 1H NMR (500 MHz, CDCl3) δ: 3.71 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.25 (t, 2H, J = 5.9 Hz, CH2), 4.78 (t, 2H, J = 5.9 Hz, CH2), 5.89 (dd, 1H, J = 3.7, 0.7 Hz, Hb), 6.06 (dd, 1H, J = 3.7, 2.6 Hz, Ha), 6.66–6.70 (m, 1H, Hc) 7.08 (t, 2H, J = 8.8, HAr), 7.39 (dd, 2H, J = 8.7, 5.5 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.4, 44.0, 51.9, 52.4, 107.0, 109.3, 115.48 (d, J = 21.4 Hz), 118.05 (d, J = 20.7 Hz), 120.9, 122.2, 125.0, 128.0, 129.2, 131.8 (d, J = 8.1 Hz), 161.0, 161.5, 163.5, 166.6; calculated mass: 368.12; ESI-MS m/z: 368.2 [M]+. Anal. calcd for C20H17FN2O4: C, 65.21; H, 4.65; N, 7.60.; found: C, 65.01; H, 4.48; N, 7.89%.
Dimethyl-1-(3-chlorophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5k)
Light green powder; mp 140–142 °C; yield (79%); 1H NMR (500 MHz, CDCl3) δ: 3.73 (s, 3H, CH3), 3.85 (s, 3H, CH3), 4.26 (t, 2H, J = 5.9 Hz, CH2), 4.78 (t, 2H, J = 5.9 Hz, CH2), 5.95 (dd, 1H, J = 3.8, 1.4 Hz, Hb), 6.07 (t, 1H, J = 3.2 Hz, Ha), 6.67–6.71 (1H, m, Hc), 7.30–7.34 (m, 3H, HAr), 7.44 (s, 1H, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.4, 44.0, 52.0, 52.4, 107.2, 109.4, 117.7, 118.2, 121.1, 122.0, 124.8, 127.9, 127.9, 128.3, 129.7, 130.0, 134.2, 135.2, 161.0 166.4; calculated mass: 384.09; ESI-MS m/z: 384.2 [M]+. Anal. calcd for C20H17ClN2O4: C, 62.42; H, 4.45; N, 7.28.; found: C, 62.15; H, 4.74; 7.49%.
Dimethyl-1-(4-chlorophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5l)
Green powder; mp 195–198 °C; yield (62%); 1H NMR (500 MHz, CDCl3) δ: 3.72 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.25 (t, 2H, J = 5.9 Hz, CH2), 4.77 (t, 2H, J = 5.9 Hz, CH2), 5.94 (dd, 1H, J = 3.8, 1.4 Hz, Hb), 6.06 (dd, 1H, J = 3.7, 2.6 Hz, Ha), 6.68 (dd, 1H, J = 2.5, 1.4 Hz, Hc), 7.33–7.39 (4H, m, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.4, 43.9, 51.9, 52.4, 107.1, 109.3, 117.8, 118.1, 121.0, 122.1, 124.8, 127.9, 128.7, 131.4, 131.8, 135.6, 160.9, 166.5; calculated mass: 384.09; ESI-MS m/z: 384 [M]+. Anal. calcd for C20H17ClN2O4: C, 62.42; H, 4.45; N, 7.28.; found: C, 62.21; H, 4.67; N, 7.37%.
Dimethyl-1-(3-bromophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5m)
Green crystal; mp 145–146 °C; yield (73%); 1H NMR (500 MHz, DMSO-d6) δ: 3.65 (s, 3H, CH3), 3.78 (s, 3H, CH3), 4.32 (t, 2H, J = 5.9 Hz, CH2), 4.64 (t, 2H, J = 5.9 Hz, CH2), 5.77 (dd, 1H, J = 3.7, 1.5 Hz, Hb), 6.01 (dd, 1H, J = 3.8, 2.6 Hz, Ha), 6.94 (dd, 1H, J = 2.6, 1.4 Hz, Hc), 7.34 (d, 1H, J = 7.7 Hz, HAr), 7.39 (t, 1H, J = 7.8, HAr), 7.50 (s, 1H, HAr), 7.57 (d, 1H, J = 7.9 Hz, HAr),:13C NMR (125 MHz, DMSO-d6) δ: 43.0, 43.3, 51.8, 52.0, 105.8, 108.5, 115.9, 118.5, 120.9, 121.4, 121.8, 123.3, 127.0, 128.6, 130.3, 130.6, 132.0, 135.4, 160.0, 165.5; calculated mass: 428.04; ESI-MS m/z: 430 [M]+. Anal. calcd for C20H17BrN2O4: C, 55.96; H, 3.99; N, 6.53.; found: C, 56.04; H, 4.11; N, 6.45%.
Dimethyl-1-(4-bromophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5n)
Green crystal; mp 210–214 °C; yield (60%); 1H NMR (500 MHz, CDCl3) δ: 3.72 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.25 (t, 2H, J = 5.9 Hz, CH2), 4.78 (t, 2H, J = 5.9 Hz, CH2), 5.94 (dd, 1H, J = 3.7, 1.3 Hz, Hb), 6.07 (dd, 1H, J = 3.5, 2.9 Hz, Ha), 6.68 (dd, 1H, J = 2.6, 1.5 Hz, Hc), 7.31 (d, 2H, J = 8.5 Hz, HAr), 7.51 (d, 2H, J = 8.5 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 43.4, 44.0, 52.0, 52.4, 107.2, 109.4, 117.9, 118.2, 121.0, 121.9, 122.1, 124.8, 127.9, 131.7, 132.3, 161.0, 166.6; calculated mass: 428; ESI-MS m/z: 428 [M]+. Anal. calcd for C20H17BrN2O4: C, 55.96; H, 3.99; N, 6.53.; found: C, 56.02; H, 4.02; N, 6.36%.
Dimethyl-1-(3-nitrophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5o)
Green powder; mp 180–182 °C; yield (71%); 1H NMR (500 MHz, CDCl3) δ: 3.74 (s, 3H, CH3), 3.86 (s, 3H, CH3), 4.28 (t, 2H, J = 5.8 Hz, CH2), 4.79 (t, 2H, J = 5.8 Hz, CH2), 5.86 (dd, 1H, J = 3.8, 1.4 Hz, Hb), 6.06 (dd, 1H, J = 3.8, 2.6 Hz, Ha), 6.68–6.72 (m, 1H, Hc), 7.57 (t, 1H, J = 7.9 Hz, HAr), 7.79 (d, 1H, J = 7.6 Hz, HAr), 8.21 (d, 1H, J = 8.2 Hz, HAr), 8.33 (s, 1H, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.5, 44.0, 52.1, 52.4, 107.0, 109.5, 116.5, 118.9, 121.4, 121.6, 122.6, 124.4, 125.1, 128.0, 129.4, 135.3, 136.4, 148.4, 160.9, 166.1; calculated mass: 395.11; ESI-MS m/z: 395.1 [M]+. Anal. calcd for C20H17N3O6: C, 60.76; H, 4.33; N, 10.63.; found: C, 60.98; H, 4.16; N, 10.51%.
Dimethyl-1-(4-nitrophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5p)
Dark orange powder; mp 230–232 °C; yield (68%); 1H NMR (500 MHz, CDCl3) δ: 3.73 (s, 3H, CH3), 3.86 (s, 3H, CH3), 4.29 (t, 2H, J = 5.9 Hz, CH2), 4.79 (t, 2H, J = 5.9 Hz, CH2), 5.94 (dd, 1H, J = 3.8, 1.4 Hz, Hb), 6.08 (dd, 1H, J = 3.8, 2.6 Hz, Ha), 6.69–6.74 (m, 1H, Hc),7.63 (d, 2H, J = 8.7 Hz, HAr), 8.25 (d, 2H, J = 8.6 Hz, HAr); 13C NMR(125 MHz, DMSO-d6) δ: 43.1, 43.4, 52.0, 52.5, 106.3, 108.6, 115.4, 119.2, 120.5, 122.0, 123.0, 123.7, 127.1, 130.6, 140.4, 146.5, 160.0, 165.3; calculated mass: 395.11; ESI-MS m/z: 395.3 [M]+. Anal. calcd for C20H17N3O6: C, 60.76; H, 4.33; N, 10.63.; found: C, 60.92; H, 4.12; N, 10.38%.
Dimethyl-1-(4-hydroxy-3-methoxyphenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5q)
Light green powder; mp 215–216 °C; yield (80%); 1H NMR (500 MHz, CDCl3) δ: 3.73 (s, 3H, CH3), 3.83 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.24 (t, 2H, J = 5.8 Hz, CH2), 4.78 (t, 2H, J = 5.8 Hz, CH2), 5.73 (s, 1H, OH), 6.03 (d, 1H, J = 3.9 HZ, Hb), 6.06 (dd, 1H, J = 3.8, 2.3 Hz, Ha), 6.67 (dd, 1H, J = 2.7, 1.4 Hz, Hc), 6.93 (s, 2H, HAr), 6.96 (s, 2H, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.3, 44.0, 51.8, 52.4, 56.0, 107.2, 109.2, 112.7, 114.5, 117.5, 119.1, 120.8, 122.4, 125.2, 128.0, 145.3, 146.4, 161.0, 167.0; calculated mass: 396.13; ESI-MS m/z: 396.2 [M]+. Anal. calcd for C21H20N2O6: C, 63.63; H, 5.09; N, 7.07.; found: C, 63.42; H, 4.91; N, 7.35%.
Dimethyl-1-(2,4-dichlorophenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5r)
Dark green powder; mp 161–163 °C; yield (72%); 1H NMR (500 MHz, CDCl3) δ: 3.69 (s, 3H, CH3), 3.86 (s, 3H, CH3), 4.22–4.31 (m, 2H, CH2), 4.77 (t, 2H, J = 5.9 Hz, CH2), 5.64 (dd, 1H, J = 3.4, 1.8 Hz, Hb), 6.07 (dd, 1H, J = 3.3 Hz, Ha), 6.66–6.74 (m, 1H, Hc), 7.30 (d, 2H, J = 2.2 HZ, HAr), 7.51 (d, 1H, J = 1.8 Hz, HAr); 13C NMR (125 MHz, CDCl3) δ: 43.3, 43.8, 51.9, 52.1, 106.8, 109.5, 115.1, 119.2, 120.9, 121.9, 123.7, 127.1, 128.0, 129.5, 131.2, 133.4, 134.5, 135.9, 161.1, 165.4; calculated mass: 418.05; ESI-MS m/z: 418.2 [M]+. Anal. calcd for C20H16Cl2N2O4: C, 57.30; H, 3.85; N, 6.68.; found: C, 57.47; H, 3.69; N, 6.73%.
Dimethyl-1-(3,4,5-trimethoxyphenyl)-5,6-dihydrodipyrrolo[1,2-a:2′,1′-c]pyrazine-2,3-dicarboxylate (5s)
Green crystal; mp 164–167 °C; yield (60%);1H NMR (500 MHz, CDCl3) δ: 3.75 (s, 3H, CH3), 3.80 (s, 6H, 2CH3), 3.83 (s, 3H, CH3), 3.89 (s, 3H, CH3), 4.25 (t, 2H, J = 5.8 Hz, CH2), 4.78 (t, 2H, J = 5.9 Hz, CH2), 6.07 (dd, 1H, J = 3.6, 2.4 Hz, Hb), 6.13 (dd, 1H, J = 3.8, 1.4 Hz, Ha), 6.65–6.70 (3H, m, Hc, 2 HAr); 13C NMR (125 MHz, CDCl3) δ: 43.3, 43.9, 51.8, 52.4, 56.2, 61.0, 107.0, 107.4, 109.2, 117.6, 118.9, 120.9, 122.2, 125.1, 127.8, 128.5, 137.5, 153.1, 160.8, 167.0; calculated mass: 440.16; ESI-MS m/z: 440 [M]+. Anal. calcd for C23H24N2O7: C, 62.72; H, 5.49; N, 6.36.; found: C,62.89; H, 5.72; N, 6.64%.
General Procedure for the Synthesis of 12-Aryl-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d]pyridazine-8(9H)-one (7a–q)
To a solution of phthalate 5a–s (1 mmol) in EtOH (2 mL), hydrazine hydrate (1 mmol) 6 was added. Then, the mixture was refluxed for 10 h. After cooling to room temperature and evaporating the solvents under reduced pressure, the crude product was washed with water, followed by filtration and drying under vacuum, resulting in the cyclic hydrazide 7a–q with yields of 70–82%.
11-Hydroxy-12-phenyl-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d] pyridazin-8(9H)-one (7a)
White powder; mp 350–353 °C; yield (75%); 1H NMR (500 MHz, DMSO-d6): 4.34 (t, 2H, J = 5.5 Hz, CH2), 4.89 (t, 2H, J = 5.7 Hz, CH2), 5.77–5.82 (m, 1H, Hb), 5.97 (t, 1H, J = 3.2 Hz, Ha), 6.39 (d, 1H, J = 2.2 Hz, Hc) 7.31–7.36 (m, 1H, HAr), 7.39 (t, 2H, J = 7.1 Hz, HAr), 7.42 (d, 2H, J = 6.8 Hz, HAr). 13C NMR (125 MHz, DMSO-d6) δ: 42.6, 43.3, 106.3, 108.3, 113.3, 117.1, 121.6, 121.7, 124.8, 126.9, 127.5, 128.4, 130.9, 133.5, 152.6, 153.5; calculated mass: 318.11; ESI-MS m/z: 318 [M]+. Anal. calcd for C18H14N4O2: C, 67.92; H, 4.43; N, 17.60.; found: C, 67.78; H, 4.51; N, 17.86%.
11-Hydroxy-12-(3-hydroxyphenyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7b)
White powder; mp 341–343 °C; yield (72%); 1H NMR (500 MHz, DMSO-d6) δ: 4.34 (t, 2H, J = 5.9 Hz, CH2), 4.86 (t, 2H, J = 5.8 Hz, CH2), 5.84 (d, 1H, J = 3.7 Hz, Hb), 5.99 (t, 1H, J = 3.2 Hz, Ha), 6.74 (d, 1H, J = 8 Hz, Hc), 6.81 (d, 2H, J = 7.9 Hz, HAr), 6.93 (s, 1H, HAr), 7.17 (t, 1H, 7.5 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.6, 43.3, 106.6, 108.3, 113.4, 113.9, 116.5, 117.0, 117.9,121.5, 121.6, 124.6, 128.4, 128.7, 131.4, 134.7, 156.6; calculated mass: 334.11; ESI-MS m/z: 336.2 [M]+. Anal. calcd for C18H14N4O3: C, 64.67; H, 4.22; N, 16.76.; found: C, 64.48; H, 4.39; N, 16.91%.
11-Hydroxy-12-(o-tolyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d] pyridazin-8(9H)-one (7c)
Yellow powder; mp 321–323 °C; yield (77%); 1H NMR (500 MHz, DMSO-d6) δ: 2.06 (s,3H, CH3), 4.34 (t, 2H, J = 5.9 Hz, CH2), 4.88–4.90 (m, 2H, CH2), 5.38 (d, 1H, J = 3.7 Hz, Hb), 5.94 (t, 1H, J = 3.1 Hz, Ha), 6.91 (s, 1H, Hc), 7.19–7.20 (m, 2H, HAr), 7.28–7.29 (m, 2H, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 19.9, 42.6, 43.3, 106.0, 108.6, 117.9, 121.6, 121.8, 124.6, 125.3, 127.4, 128.6, 129.5, 130.5, 131.0, 133.6, 134.2, 136.2, 137.5, 155.4; calculated mass: 332.13. ESI-MS m/z: 332.3 [M]+. Anal. calcd for C19H16N4O2: C, 68.66; H, 4.85; N, 16.86.; found: C, 68.44; H, 5.07; N, 16.98%.
11-Hydroxy-12-(m-tolyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d] pyridazin-8(9H)-one (7d)
Cream powder; mp 331–333 °C; yield (71%); 1H NMR (500 MHz, DMSO-d6) δ: 2.37 (s, 3H, CH3), 4.34 (t, 2H, J = 5.8 Hz, CH2), 4.88 (t, 2H, J = 5.8 Hz, CH2), 5.78 (d,1H, J = 3.8 Hz, Hb), 5.97 (t, 1H, J = 3.2 Hz, Ha), 6.89–6.99 (m, 1H, Hc), 7.15 (d, 1H, J = 7.3 Hz, HAr) 7.20 (d, 1H, J = 7.4 Hz, HAr), 7.23 (s, 1H, HAr), 7.28 (t, 1H, J = 7.5 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 21.0, 42.6, 43.3, 106.3, 108.3, 113.4, 117.2, 121.7, 124.7, 127.5, 127.6, 128.0, 128.4, 128.9, 131.5, 133.4, 136.4, 152.7, 153.6; calculated mass: 332.13; ESI-MS m/z: 332.1 [M]+. Anal. calcd for C19H16N4O2: C, 68.66; H, 4.85; N, 16.86.; found: C, 68.37; H, 4.97; N, 17.03%.
11-Hydroxy-12-(p-tolyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo[2,3-d] pyridazin-8(9H)-one (7e)
White powder; mp 340–343 °C; yield (71%); 1H NMR (500 MHz, DMSO-d6) δ: 2.37 (s, 3H, CH3), 4.35 (t, 2H, J = 5.7 Hz, CH2), 4.86 (t, 2H, J = 5.5 Hz, CH2), 5.81 (dd, 1H, J = 3.8, 1.4 Hz, Hb), 5.98 (t, 1H, J = 3.2 Hz, Ha), 6.93 (t, 1H, J = 2.0 Hz, Hc), 7.20 (d, 2H, J = 7.8 Hz, HAr), 7.29 (d, 2H, J = 7.9 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 20.9, 42.7, 43.3, 106.5, 108.4, 116.0, 116.9, 121.5, 121.8, 124.2, 128.2, 128.8, 130.2, 130.7, 136.1, 151.8, 153.2; calculated mass: 332.13; ESI-MS m/z: 332.3 [M]+. Anal. calcd for C19H16N4O2: C, 68.66; H, 4.85; N, 16.86.; found: C, 68.42; H, 5.11; N, 17.12%.
11-Hydroxy-12-(3-methoxyphenyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7f)
Yellow powder; mp 280–283 °C; yield (75%); 1H NMR (500 MHz, DMSO-d6) δ: 3.75 (s, 3H, OCH3), 4.34 (t, 2H, J = 5.8 Hz, CH2), 4.87 (t, 2H, J = 4.9 Hz, CH2), 5.85 (d, 1H, J = 3.7 Hz, Hb), 5.98–5.99 (m, 1H, Ha), 6.91–6.93 (m, 2H, Hc, HAr), 6.96–7.00 (m, 2H, HAr), 7.30 (t, 1H, J = 7.8 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.7, 43.3, 55.0, 106.6, 108.4, 112.5, 113.1, 116.6, 117.2, 121.5, 121.8, 123.2, 124.6, 128.6, 134.8, 152.5, 153.4, 158.6, 168.7; calculated mass: 348.12; ESI-MS m/z: 348.5 [M]+. Anal. calcd for C19H16N4O3: C, 65.51; H, 4.63; N, 16.08.; found: C, 65.28; H, 4.73; N, 16.27%.
11-Hydroxy-12-(4-methoxyphenyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7g)
White powder; mp 337–338 °C; yield (73%); 1H NMR (500 MHz, DMSO-d6) δ: 3.81 (s, 3H, OCH3), 4.34 (t, 2H, J = 4.9 Hz, CH2), 4.87 (t, 2H, J = 5.4 Hz, CH2), 5.83 (dd, 1H, J = 3.7, 1.6 Hz, Hb), 5.99 (t, 1H, J = 3.1 Hz, Ha), 6.92–6.93 (m, 1H, Hc), 6.96 (d, 2H, J = 8.0 Hz, HAr), 7.33 (d, 2H, J = 8.4 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.6, 43.3, 55.0, 106.4, 108.4, 113.0, 113.1, 117.1, 121.6, 121.7, 124.5, 125.4, 128.6, 131.9, 152.6, 153.2, 158.3; calculated mass: 348.12; ESI-MS m/z: 348.2 [M]+. Anal. calcd for C19H16N4O3: C, 65.51; H, 4.63; N, 16.08.; found: C, 65.29; H, 4.78; N, 16.25%.
12-(4-Fluorophenyl)-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7h)
White powder; mp 340–342 °C; yield 0.241g (72%); 1H NMR (500 MHz, DMSO-d6) δ: 4.35 (t, 2H, J = 5.7 Hz, CH2), 4.87 (t, 2H, J = 5.9 Hz, CH2), 5.80 (d, 1H, J = 3.7 Hz, Hb), 6.00 (t, 1H, J = 3.1 Hz, Ha), 6.94 (d, 1H, J = 2.5 Hz, Hc), 7.22 (t, 2H, J = 8.6 Hz, HAr), 7.45 (dd, 2H, J = 8.3, 5.6 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.7, 43.3, 106.4, 108.4, 112.0, 114.5 (d, J = 21.2 Hz), 117.1, 121.6 (d, J = 74.1 Hz), 124.5, 125.7, 129.6, 132.8 (d, J = 7.5 Hz), 152.4, 155.5, 162.4, 164.4; calculated mass: 336.10; ESI-MS m/z: 336.2 [M]+. Anal. calcd for C18H13FN4O2: C, 64.28; H, 3.90; N, 16.66.; found: C, 64.46; H, 3.71; N, 16.83%.
12-(3-Chlorophenyl)-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo [2,3-d]pyridazin-8(9H)-one (7i)
Yellow powder; mp 268–270 °C; yield (78%); 1H NMR (500 MHz, DMSO-d6) δ: 4.34 (t, 2H, J = 5.0 Hz, CH2), 4.89 (t, 2H, J = 5.1 Hz, CH2), 5.82–5.83 (m, 1H, Hb), 6.01 (d, 1H, J = 3.1 Hz, Ha), 6.94 (d, 1H, J = 2.6 Hz, Hc), 7.38–7.44 (m, 3H, HAr), 7.47 (s, 1H, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.6, 43.3, 106.2, 108.4, 111.8, 117.2, 121.3, 121.9, 125.2, 126.7, 128.2, 129.3, 129.6, 130.6, 132.1, 135.8, 148.3, 152.7; calculated mass: 352.07; ESI-MS m/z: 352.2 [M]+. Anal. calcd for C18H13ClN4O2: C, 61.28; H, 3.71; N, 15.88.; found: C, 61.05; H, 3.94; N, 15.82%.
12-(4-Chlorophenyl)-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7j)
White powder; mp 335–337 °C; yield (70%); 1H NMR (500 MHz, DMSO-d6) δ: 4.36 (dd, 2H, J = 6.9, 4.8 Hz, CH2), 4.86 (t, 2H, J = 5.7 Hz, CH2), 5.81–5.83 (m, 1H, Hb), 6.01 (1H, t, J = 3.2 Hz, Ha), 6.95 (t, 1H, J = 2 Hz, Hc), 7.41–7.45 (4H, m, HAr); 13C NMR (125 MHz, CDCl3) δ: 42.7, 43.3, 106.6, 108.5, 116.0, 117.0, 121.2, 122.1, 124.5, 125.4, 127.7, 131.8, 132.2, 132.7, 152.7, 154.9; calculated mass: 352.07; ESI-MS m/z: 352.2 [M]+. Anal. calcd for C18H13ClN4O2: C, 61.28; H, 3.71; N, 15.88.; found: C, 61.09; H, 3.91; N, 15.98%.
12-(3-Bromophenyl)-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5]pyrrolo [2,3-d]pyridazin-8(9H)-one (7k)
White powder; mp 267–270 °C; yield (80%); 1H NMR (500 MHz, DMSO-d6) δ: 4.35 (t, 2H, J = 5.8 Hz, CH2), 4.88 (t, 2H, J = 5.6 Hz, CH2), 5.82 (dd, 1H, J = 3.8, 1.4 Hz, Hb), 5.95–6.14 (m, 1H, Ha), 6.96 (d, 1H, J = 2.1 Hz, Hc), 7.37 (t, 1H, J = 7.9, HAr), 7.46 (d, 1H, J = 7.3 Hz, HAr), 7.55 (d, 1H, J = 7.9 Hz, HAr), 7.60 (s, 1H, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.7, 43.3, 106.3, 108.5, 111.4, 114.4, 120.7, 121.2, 122.1, 122.8, 128.7, 129.7 (2), 130.0, 133.4, 135.9, 152.4, 153.2; calculated mass: 396.02; ESI-MS (m/z): 398 [M]+. Anal. calcd for C18H13BrN4O2: C, 54.43; H, 3.30; N, 14.10.; found: C, 54.16; H, 3.51; N, 14.27%.
12-(4-Bromophenyl)-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7l)
White powder; mp 330–332 °C; yield (72%); 1H NMR (500 MHz, DMSO-d6) δ: 4.35 (t, 2H, J = 5.6 Hz, CH2), 4.85 (t, 2H, J = 5.5 Hz, CH2), 5.84 (d, 1H, J = 2.5 Hz, Hb), 5.98–6.03 (m, 1H, Ha), 6.95 (s, 1H, Hc) 7.38 (d, 2H, J = 8.3 Hz, HAr), 7.59 (d, 2H, J = 8.3 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.8, 43.3, 106.7, 108.6, 111.5, 116.9, 120.4, 121.1, 122.2, 124.4, 129.0, 130.6, 132.6, 133.1, 148.6, 152.2; calculated mass: 396.02; ESI-MS (m/z): 396.1 [M]+. Anal. calcd for C18H13BrN4O2: C, 54.43; H, 3.30; N, 14.10.; found: C, 54.21; H, 3.57; N, 14.31%.
11-Hydroxy-12-(3-nitrophenyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7m)
Yellow powder; mp 320–322 °C; yield (76%); 1H NMR (500 MHz, DMSO-d6) δ: 4.38 (t, 2H, J = 5.9 Hz, CH2), 4.90 (t, 2H, J = 5.8 Hz, CH2), 5.88 (d, 1H, J = 3.8 Hz, Hb), 6.02 (t, J = 3.2 Hz, 1H, Ha), 6.99 (t, J = 2.0 Hz, 1H, Hc), 7.69–7.72 (m, 1H, HAr), 7.95 (d, 1H, J = 7.6 Hz, HAr), 8.21 (d, 1H, J = 8.3 Hz, HAr), 8.29 (s, 1H, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.7, 43.3, 106.3, 108.6, 117.0, 119.3, 120.9, 121.8, 122.2, 124.9, 125.4, 125.9, 129.1, 135.1, 137.7, 147.2, 151.3, 153.5; calculated mass: 363.10; ESI-MS (m/z): 363.1 [M]+. Anal. calcd for C18H13N5O4: C, 59.50; H, 3.61; N, 19.28.; found: C, 59.21; H, 3.68; N, 19.12%.
11-Hydroxy-12-(4-nitrophenyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino[1′,2′:1,5] pyrrolo[2,3-d]pyridazin-8(9H)-one (7n)
Yellow powder; mp 230–232 °C; yield (70%); 1H NMR (500 MHz, DMSO-d6) δ: 4.38 (t, 2H, J = 5.9 Hz, CH2), 4.89 (t, 2H, J = 5.8 Hz, CH2), 5.92 (d, 1H, J = 3.8 Hz, Hb), 6.02 (t, 1H, J = 3.1 Hz Ha), 7.00 (s, 1H, Hc), 7.75 (2H, d, J = 8.3 Hz, HAr), 8.26 (d, 2H, J = 8.3 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.8, 43.2, 106.8, 108.6, 111.0, 117.0, 120.8, 122.4, 122.7, 125.1, 128.7, 131.4, 132.0, 132.2, 132.3, 133.1, 140.9, 146.2, 152.1, 153.3; calculated mass: 363.10; ESI-MS (m/z): 363.2 [M]+. Anal. calcd for C18H13N5O4: C, 59.50; H, 3.61; N, 19.28.; found: C, 59.38; H, 3.66; N, 19.26%.
11-Hydroxy-12-(4-hydroxy-3-methoxyphenyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino [1′,2′:1,5]pyrrolo[2,3-d]pyridazin-8(9H)-one (7o)
Yellow powder; mp 288–290 °C; yield (82%); 1H NMR (500 MHz, DMSO-d6) δ: 3.72 (s, 3H, OCH3), 4.34 (t, 2H, J = 5.8 Hz, CH2), 4.86 (t, 2H, J = 5.8 Hz, CH2), 5.91 (d, 1H, J = 3.7 Hz, Hb), 6.00 (t, 1H, J = 3.1 Hz, Ha), 6.79–6.83 (m, 2H, HAr, Hc), 6.93 (d, 2H, J = 14.4 Hz, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.7, 43.3, 55.6, 106.6, 108.4, 113.4, 114.8, 115.3, 117.1, 121.7, 123.4, 124.0, 124.2, 129.0, 146.0, 147.0, 152.3, 155.3; calculated mass: 364.12; ESI-MS (m/z): 364.2 [M]+. Anal. calcd for C19H16N4O4: C, 62.63; H, 4.43; N, 15.38.; found: C, 62.81; H, 4.66; N, 15.67%.
12-(2,4-Dichlorophenyl)-11-hydroxy-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino [1′,2′:1,5]pyrrolo[2,3-d]pyridazin-8(9H)-one (7p)
White powder; mp 260–262 °C; yield (70%); 1H NMR (500 MHz, DMSO-d6) δ: 4.34 (t, 2H, J = 5.8 Hz, CH2), 4.89 (t, 2H, J = 5.8 Hz, CH2), 5.82–5.83 (1H, m, Hb), 6.01–6.02 (m 1H, Ha), 6.94 (d, 1H, J = 2.5 Hz, Hc), 7.40–7.42 (2H, m, HAr), 7.47 (s, 1H, HAr); 13C NMR (125 MHz, DMSO-d6) δ: 42.6, 43.3, 106.4, 108.8, 121.0, 122.1, 127.0, 128.6, 128.7, 128.8, 131.4, 131.5, 132.0, 133.0, 133.1, 134.1, 135.4, 156.8; calculated mass: 386.03; ESI-MS (m/z): 386.1 [M]+. Anal. calcd for C18H12Cl2N4O2: C, 55.83; H, 3.12; N, 14.47.; found: C, 55.59; H, 3.31; N, 14.68%.
11-Hydroxy-12-(3,4,5-trimethoxyphenyl)-5,6-dihydropyrrolo[2″,1″:3′,4′]pyrazino [1′,2′:1,5]pyrrolo[2,3-d]pyridazin-8(9H)-one (7q)
White powder; mp 328–330 °C; yield (70%); 1H NMR (500 MHz, DMSO-d6) δ: 3.73 (s, 9H, 3CH3), 4.36 (t, 2H, J = 5.9 Hz, CH2), 4.88 (t, 2H, J = 5.9 Hz, CH2), 5.97 (d, 1H, J = 3.8 Hz, Hb), 6.04 (t, 1H, J = 3.1 Hz, Ha), 6.74 (s, 2H, Hc, HAr), 6.95 (s, 1H, HAr); 13C NMR (125 MHz, CDCl3) δ: 42.7, 43.3, 55.9, 60.1, 106.7, 108.4, 113.2, 117.0, 121.5, 121.8, 124.4, 128.6, 136.6, 152.1; calculated mass: 408.14; ESI-MS (m/z): 408.3 [M]+. Anal. calcd for C21H20N4O5: C, 61.76; H, 4.94; N, 13.72.; found: C, 61.93; H, 4.73; N, 13.87%.
Cytotoxicity Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide MTT was used for the evaluation of the cytotoxicity effects of the target compounds against Panc-1, PC3, and MB-231 cancer cell lines. All cells were obtained from the National Cell Bank of Iran (Pastor Institute, Tehran, Iran) and subcultured in RPMI-1640 media and DMEM (with 10% FBS) (Gibco, Milano, Italy). The cancer cells were maintained at 37 °C, 5% CO2, and 100% relative humidity. The cancer cells were cultured in a 96-well microtiter plate, with 5 × 103 cancer cells in each well and 50 μM of different concentrations of target compounds in 0.1% DMSO (n = 3). Before the addition of MTT to each well, the plates were incubated for 48 h. After the addition of MTT, the plates were incubated for an additional 3 h. The negative control consisted of 0.1% DMSO, and a known cytotoxic agent, etoposide, was used as the positive control. Absorbance was measured with a multiplate reader (Bio-Rad PR4100). The optical density of the purple formazan dye crystals was assessed at 570 nm, providing an indication of the relative number of viable cells. The concentration of a compound yielding 50% inhibition (IC50) of cell growth, relative to control cell growth (100% inhibition), was determined as the mean ± standard error (SE) from the dose–response curves obtained in at least three independent experiments. Analysis was performed using GraphPad Prism software.39
Analysis of Cellular Apoptosis
Panc-1 cancer cells were seeded into 6-well plates and incubated overnight at 37 °C under 5% CO2. Then, the cancer cells were treated with an IC50 concentration of 7m for 48 h. The untreated cancer cells were used as the negative control. After incubation for 48 h, the treated cells were trypsinized, washed with phosphate-buffered saline (PBS) twice, and centrifuged at 1200 rpm to collect the cells. After suspending the cells in 500 μL of binding buffer, 5 μL of annexin V-APC and Propidium iodide (PI) were added successively and mixed well. After 15 min of incubation at room temperature in the dark, cell apoptosis was analyzed by flow cytometry using FACSCalibur Becton-Dickinson instrument.40
Cell Cycle Assessment
The inhibition of cancer cell proliferation at specific checkpoints was investigated through cell cycle analysis in the Panc-1 cell line using fluorescence microscopy and a PI staining assay. PI can bind to DNA and emit fluorescence, the intensity of which is proportional to the DNA content. The cells were treated with an IC50 concentration of test compound (7m) for 48 h. Then, the cells were trypsinized, washed with PBS, and centrifuged at 1000 rpm for 5 min. The collected Panc-1 cells were fixed with 70% cold ethanol. After washing with phosphate-buffered saline (PBS), the fixed cells were treated with RNase A (0.1 mg/mL) and incubated for 30 min, followed by treatment with PI (50 mg/mL) and incubated for an additional 15 min. The cell cycle distribution of each mitotic phase was calculated using a Novocyte flow cytometer (ACEA Biosciences).41 The data were analyzed using NovoExpress 1.1.0 software. All experiments were performed in triplicate.
Western Blot Analysis
Western blot analysis was carried out in Panc-1 cancer cells to assess the inhibition of EGFR by compound 7m. In this experiment, primary antibodies were purchased from Santa Cruz Biotechnology. Protein concentration was determined using the Bradford reagent, and lysates were resolved on 15% sodium dodecyl sulfate- polyacrylamide (SDS-PAGE) gels. Panc-1 cancer cells were treated with an IC50 concentration of 7m, and the results were evaluated after 48 h. Furthermore, the cells were lysed in a lysis buffer, and the proteins were electrophoresed on SDS-PAGE, transferred to a poly(vinylidene difluoride) (PVDF) membrane, and soaked with primary and secondary antibodies solutions. β-actin was used as a Western blot loading control. The resulting bands were visualized using a Western blot chemiluminescence reagent (PerkinElmer Life Sciences) and analyzed using an immunoblotted filter (LAS-4000 Fuji Film).
Acknowledgments
This research is supported by the Department of Chemistry, Semnan University, as a fellowship for the Ph.D. dissertation of Azam Barghi Lish (grant No: 2514). The authors would like to express their gratitude to Dr. Fariba Peytam from Tehran University of Medical Sciences for her guidance and cooperation. The authors also appreciate the scientific advice and comments of Dr. Sina Farzaneh from the University of Massachusetts Amherst. The authors are deeply thankful to Dr. Setareh Moghimi from Tehran University of Medical Sciences for the critical feedback that greatly improved the quality of this manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c04167.
5s (CIF)
7l (CIF)
1H NMR for derivatives 5a–s and 7a–q; 13C NMR for derivatives 5a–s and 7a–q; mass spectrometry for derivatives 5a–s and 7a–q (Figures S3–S110); X-ray crystallographic data of 5s (Tables S1–S7); X-ray crystallographic data of 7l (Tables S8–S13), the crystal structure of 5s (CCDC 2256715) (Figure S1), and the crystal structure of 7l (CCDC 2256716) (Figure S2) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chhikara B. S.; Parang K. Global Cancer Statistics 2022: the trends projection analysis. Chem. Biol. Lett. 2023, 10 (1), 451. [Google Scholar]
- Matthews H. K.; Bertoli C.; de Bruin R. A. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23 (1), 74–88. 10.1038/s41580-021-00404-3. [DOI] [PubMed] [Google Scholar]
- Kuderer N. M.; Desai A.; Lustberg M. B.; Lyman G. H. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 2022, 19 (11), 681–697. 10.1038/s41571-022-00685-3. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Liu Y.; Ma X.; Hu H. The influence of cell cycle regulation on chemotherapy. Int. J. Mol. Sci. 2021, 22 (13), 6923 10.3390/ijms22136923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A.; Farooq U.; Singh R.; Dubey V. P.; Kumar S.; Kumari R.; Kumar K.; Naik B. D.; Dhar K. L. Chemotherapy treatment and strategy schemes: A review. Open Acc. J. Toxicol. 2018, 2, 555–600. 10.19080/OAJT.2018.02.555600. [DOI] [Google Scholar]
- Bukowski K.; Kciuk M.; Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21 (9), 3233 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J. Med. Chem. 2014, 57 (24), 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Martins P.; Jesus J.; Santos S.; Raposo L. R.; Roma-Rodrigues C.; Baptista P. V.; Fernandes A. R. Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules 2015, 20 (9), 16852–16891. 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Petri G.; Spanò V.; Spatola R.; Holl R.; Raimondi M. V.; Barraja P.; Montalbano A. Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem. 2020, 208, 112783 10.1016/j.ejmech.2020.112783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic-Kurt Z.; Bakar-Ates F.; Aka Y.; Kutuk O. Design, synthesis and in vitro apoptotic mechanism of novel pyrrolopyrimidine derivatives. Bioorg. Chem. 2019, 83, 511–519. 10.1016/j.bioorg.2018.10.060. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; El-Damasy A. K.; Seo S. H.; Gadhe C. G.; Pae A. N.; Jeong N.; Hong S. S.; Keum G. Novel 5, 6-disubstituted pyrrolo [2, 3-d ] pyrimidine derivatives as broad spectrum antiproliferative agents: Synthesis, cell based assays, kinase profile and molecular docking study. Bioorg. Med. Chem. 2018, 26 (21), 5596–5611. 10.1016/j.bmc.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Raimondi M. V.; Listro R.; Cusimano M. G.; La Franca M.; Faddetta T.; Gallo G.; Schillaci D.; Collina S.; Leonchiks A.; Barone G. Pyrrolomycins as antimicrobial agents. Microwave-assisted organic synthesis and insights into their antimicrobial mechanism of action. Bioorg. Med. Chem. 2019, 27 (5), 721–8.13. 10.1016/j.bmc.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Curreli F.; Belov D. S.; Ahmed S.; Ramesh R. R.; Kurkin A. V.; Altieri A.; Debnath A. K. Synthesis, Antiviral Activity, and Structure–Activity Relationship of 1, 3-Benzodioxolyl Pyrrole-Based Entry Inhibitors Targeting the Phe43 Cavity in HIV-1 gp120. ChemMedChem 2018, 13 (21), 2332–2348. 10.1002/cmdc.201800534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczukowski Ł.; Redzicka A.; Wiatrak B.; Krzyżak E.; Marciniak A.; Gębczak K.; Gębarowski T.; Świątek P. Design, synthesis, biological evaluation and in silico studies of novel pyrrolo [3, 4-d] pyridazinone derivatives with promising anti-inflammatory and antioxidant activity. Bioorg. Chem. 2020, 102, 104035 10.1016/j.bioorg.2020.104035. [DOI] [PubMed] [Google Scholar]
- Li Z.; Pan M.; Su X.; Dai Y.; Fu M.; Cai X.; Shi W.; Huang W.; Qian H. Discovery of novel pyrrole-based scaffold as potent and orally bioavailable free fatty acid receptor 1 agonists for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2016, 24 (9), 1981–1987. 10.1016/j.bmc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Garzan A.; Willby M. J.; Ngo H. X.; Gajadeera C. S.; Green K. D.; Holbrook S. Y.; Hou C.; Posey J. E.; Tsodikov O. V.; Garneau-Tsodikova S. Combating enhanced intracellular survival (Eis)-mediated kanamycin resistance of Mycobacterium tuberculosis by novel pyrrolo [1, 5-a] pyrazine-based Eis inhibitors. ACS Infect. Dis. 2017, 3 (4), 302–309. 10.1021/acsinfecdis.6b00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkova M. A.; Mokrov G. V.; Gudasheva T. A.; Seredenin S. B. Novel pyrrolo [1, 2-a] pyrazines (TSPO Ligands) with anxiolytic activity dependent on neurosteroid biosynthesis. Pharm. Chem. J. 2016, 50, 501–504. 10.1007/s11094-016-1476-0. [DOI] [Google Scholar]
- Micheli F.; Bertani B.; Bozzoli A.; Crippa L.; Cavanni P.; Di Fabio R.; Donati D.; Marzorati P.; Merlo G.; Paio A.; Perugini L. Phenylethynyl-pyrrolo [1, 2-a] pyrazine: A new potent and selective tool in the mGluR5 antagonists arena. Bioorg. Med. Chem. Lett. 2008, 18 (6), 1804–1809. [DOI] [PubMed] [Google Scholar]
- Wiscount C. M.; Williams P. D.; Tran L. O.; Embrey M. W.; Fisher T. E.; Sherman V.; Homnick C. F.; Staas D. D.; Lyle T. A.; Wai J. S.; Vacca J. P. 10-Hydroxy-7, 8-dihydropyrazino [1′, 2′: 1, 5] pyrrolo [2, 3-d] pyridazine-1, 9 (2H, 6H)-diones: potent, orally bioavailable HIV-1 integrase strand-transfer inhibitors with activity against integrase mutants. Bioorg. Med. Chem. Lett. 2008, 18 (16), 4581–4583. [DOI] [PubMed] [Google Scholar]
- Popovici L.; Amarandi R. M.; Mangalagiu I. I.; Mangalagiu V.; Danac R. Synthesis, molecular modelling and anticancer evaluation of new pyrrolo [1, 2-b] pyridazine and pyrrolo [2, 1-a] phthalazine derivatives. J. Enzyme Inhib. Med. Chem. 2019, 34 (1), 230–243. 10.1080/14756366.2018.1550085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun M. T. Synthesis of novel 1, 3-disubstituted pyrrolo [1, 2-a] pyrazine derivatives and antiproliferative effects on glioblastoma cell line. Monatsh. Chem. 2021, 152, 513–522. 10.1007/s00706-021-02761-3. [DOI] [Google Scholar]
- Seo Y.; Lee J. H.; Park S. H.; Namkung W.; Kim I. Expansion of chemical space based on a pyrrolo [1, 2-a] pyrazine core: Synthesis and its anticancer activity in prostate cancer and breast cancer cells. Eur. J. Med. Chem. 2020, 188, 111988 10.1016/j.ejmech.2019.111988. [DOI] [PubMed] [Google Scholar]
- Hynes J. Jr.; Wu H.; Kempson J.; Duan J. J.; Lu Z.; Jiang B.; Stachura S.; Tokarski J. S.; Sack J. S.; Khan J. A.; Lippy J. S.; et al. Discovery of potent and efficacious pyrrolopyridazines as dual JAK1/3 inhibitors. Bioorg. Med. Chem. Lett. 2017, 27 (14), 3101–3106. 10.1016/j.bmcl.2017.05.043. [DOI] [PubMed] [Google Scholar]
- Butnariu R. M. Mangalagiu II. New pyridazine derivatives: Synthesis, chemistry and biological activity. Bioorg. Med. Chem. 2009, 17 (7), 2823–2829. [DOI] [PubMed] [Google Scholar]
- Meade E. A.; Wotring L. L.; Drach J. C.; Townsend L. B. Synthesis and antiproliferative and antiviral activity of carbohydrate-modified pyrrolo [2, 3-d] pyridazin-7-one nucleosides. J. Med. Chem. 1997, 40 (5), 794–801. 10.1021/jm960631x. [DOI] [PubMed] [Google Scholar]
- Xiang H. Y.; Chen J. Y.; Huan X. J.; Chen Y.; Gao Z. B.; Ding J.; Miao Z. H.; Yang C. H. Identification of 2-substituted pyrrolo [1, 2-b] pyridazine derivatives as new PARP-1 inhibitors. Bioorg. Med. Chem. Lett. 2021, 31, 127710 10.1016/j.bmcl.2020.127710. [DOI] [PubMed] [Google Scholar]
- Duan J.-W.; Lu Z.; Jiang B.; Yang B. V.; Doweyko L. M.; Nirschl D. S.; Haque L. E.; Lin S.; Brown G.; Hynes Jr J.; Tokarski J. S.; et al. Discovery of pyrrolo [1, 2-b] pyridazine-3-carboxamides as Janus kinase (JAK) inhibitors. Bioorg. Med. Chem. Lett. 2014, 24 (24), 5721–5726. 10.1016/j.bmcl.2014.10.061. [DOI] [PubMed] [Google Scholar]
- Ayati A.; Moghimi S.; Salarinejad S.; Safavi M.; Pouramiri B.; Foroumadi A. A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg. Chem. 2020, 99, 103811 10.1016/j.bioorg.2020.103811. [DOI] [PubMed] [Google Scholar]
- Aghcheli A.; Toolabi M.; Ayati A.; Moghimi S.; Firoozpour L.; Bakhshaiesh T. O.; Nazeri E.; Norouzbahari M.; Esmaeili R.; Foroumadi A. Design, synthesis, and biological evaluation of 1-(5-(benzylthio)-1, 3, 4-thiadiazol-2-yl)-3-phenylurea derivatives as anticancer agents. Med. Chem. Res. 2020, 29, 2000–2010. 10.1007/s00044-020-02616-2. [DOI] [Google Scholar]
- Toolabi M.; Moghimi S.; Bakhshaiesh T. O.; Salarinejad S.; Aghcheli A.; Hasanvand Z.; Nazeri E.; Khalaj A.; Esmaeili R.; Foroumadi A. 6-Cinnamoyl-4-arylaminothienopyrimidines as highly potent cytotoxic agents: Design, synthesis and structure-activity relationship studies. Eur. J. Med. Chem. 2020, 185, 111786 10.1016/j.ejmech.2019.111786. [DOI] [PubMed] [Google Scholar]
- Faraji A.; Motahari R.; Hasanvand Z.; Bakhshaiesh T. O.; Toolabi M.; Moghimi S.; Firoozpour L.; Boshagh M. A.; Rahmani R.; Ketabforoosh S. H.; Bijanzadeh H. R.; et al. Quinazolin-4 (3H)-one based agents bearing thiadiazole-urea: Synthesis and evaluation of anti-proliferative and antiangiogenic activity. Bioorg. Chem. 2021, 108, 104553 10.1016/j.bioorg.2020.104553. [DOI] [PubMed] [Google Scholar]
- Ayati A.; Moghimi S.; Toolabi M.; Foroumadi A. Pyrimidine-based EGFR TK inhibitors in targeted cancer therapy. Eur. J. Med. Chem. 2021, 221, 113523 10.1016/j.ejmech.2021.113523. [DOI] [PubMed] [Google Scholar]
- Aiello F.; Carullo G.; Giordano F.; Spina E.; Nigro A.; Garofalo A.; Tassini S.; Costantino G.; Vincetti P.; Bruno A.; Radi M. Identification of Breast Cancer Inhibitors Specific for G Protein-Coupled Estrogen Receptor (GPER)-Expressing Cells. ChemMedChem 2017, 12 (16), 1279–1285. 10.1002/cmdc.201700145. [DOI] [PubMed] [Google Scholar]
- Kim J.; Park M.; Choi J.; Singh D. K.; Kwon H. J.; Kim S. H.; Kim I. Design, synthesis, and biological evaluation of novel pyrrolo [1, 2-a] pyrazine derivatives. Bioorg. Med. Chem. Lett. 2019, 29 (11), 1350–1356. 10.1016/j.bmcl.2019.03.044. [DOI] [PubMed] [Google Scholar]
- Ito K.; Kinoshita K.; Tomizawa A.; Inaba F.; Morikawa-Inomata Y.; Makino M.; Tabata K.; Shibakawa N. Pharmacological profile of novel acid pump antagonist 7-(4-fluorobenzyloxy)-2, 3-dimethyl-1-{[(1S, 2S)-2-methyl cyclopropyl] methyl}-1H-pyrrolo [2, 3-d] pyridazine (CS-526). J. Pharmacol. Exp. Ther. 2007, 323 (1), 308–317. 10.1124/jpet.107.121350. [DOI] [PubMed] [Google Scholar]
- Riley H. A.; Gray A. R. Phenylglyoxal. Org. Synth. 1935, 2, 509. [Google Scholar]
- Cai Q.; Li D. K.; Zhou R. R.; Shu W. M.; Wu Y. D.; Wu A. X. Acid-catalyzed multicomponent Tandem cyclizations: Access to polyfunctional dihydroindolizino [8, 7-b] indoles. Org. Lett. 2016, 18 (6), 1342–1345. 10.1021/acs.orglett.6b00281. [DOI] [PubMed] [Google Scholar]
- Xiang J. C.; Wang Z. X.; Cheng Y.; Ma J. T.; Wang M.; Tang B. C.; Wu Y. D.; Wu A. X. AC–H oxidation/two-fold cyclization approach to imidazopyridoindole scaffold under mild oxidizing conditions. J. Org. Chem. 2017, 82 (24), 13671–13677. 10.1021/acs.joc.7b02448. [DOI] [PubMed] [Google Scholar]
- Foroumadi A.; Emami S.; Rajabalian S.; Badinloo M.; Mohammadhosseini N.; Shafiee A. N-Substituted piperazinyl quinolones as potential cytotoxic agents: Structure–activity relationships study. Biomed. Pharmacother. 2009, 63 (3), 216–220. 10.1016/j.biopha.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Huang X. C.; Wang M.; Pan Y. M.; Yao G. Y.; Wang H. S.; Tian X. Y.; Qin J. K.; Zhang Y. Synthesis and antitumor activities of novel thiourea α-aminophosphonates from dehydroabietic acid. Eur. J. Med. Chem. 2013, 69, 508–520. 10.1016/j.ejmech.2013.08.055. [DOI] [PubMed] [Google Scholar]
- Wu Z. L.; Fang Y. L.; Tang Y. T.; Xiao M. W.; Ye J.; Li G. X.; Hu A. X. Synthesis and antitumor evaluation of 5-(benzo [d][1, 3] dioxol-5-ylmethyl)-4-(tert-butyl)-N-arylthiazol-2-amines. MedChemComm 2016, 7 (9), 1768–1774. 10.1039/C6MD00234J. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.