Abstract
The induction of heat shock proteins (Hsps) serves not only as a marker for cellular stress but also as a promoter of cell survival, which is especially important in the nervous system. We examined the regulation of the constitutive and stress-induced 70-kD Hsps (Hsc70 and Hsp70, respectively) after sciatic nerve (SN) axotomy in the neonatal mouse. Additionally, the prevention of axotomy-induced SN cell death by administration of several preparations of exogenous Hsc70 and Hsp70 was tested. Immunohistochemistry and Western blot analyses showed that endogenous levels of Hsc70 and Hsp70 did not increase significantly in lumbar motor neurons or dorsal root ganglion sensory neurons up to 24 hours after axotomy. When a variety of Hsc70 and Hsp70 preparations at doses ranging from 5 to 75 μg were applied to the SN stump after axotomy, the survival of both motor and sensory neurons was significantly improved. Thus, it appears that motor and sensory neurons in the neonatal mouse do not initiate a typical Hsp70 response after traumatic injury and that administration of exogenous Hsc/Hsp70 can remedy that deficit and reduce the subsequent loss of neurons by apoptosis.
INTRODUCTION
It is well established that expression of heat shock proteins (Hsps) is increased after cell stress. This stress protein response, which is a facet of the cellular reaction to damage, can be used as a marker for cells and tissues affected by insults including trauma, ischemia, diabetes, cancer, heart disease, viral infection, aging, and neurodegenerative disorders (Edgington 1995; Kiang and Tsokos 1998; Mattson 2000; Tytell and Hooper 2001). In the nervous system, as in other tissues, the induction of Hsps not only serves as a marker for stress but has a protective effect as well (Ohtsuka and Suzuki 2000; Rajdev and Sharp 2000; Sheller et al 1998; Yenari et al 1999). These proteins are known to stabilize other protein structures that are sensitive to denaturation and to help them to retain or to restore their native, functional conformations (Hartl 1996; Lindquist and Craig 1988; Morimoto et al 1997; Welch and Suhan 1986). Preventing protein aggregation and stabilizing protein structures are the foundations for overcoming the consequences of metabolic stresses in neurons as well as in other cell types.
The Hsp70 family contains a number of related protein isoforms ranging in molecular weight from 66 to 78 kD. Included in this family are the constitutive Hsp73, commonly referred to as Hsc70, and the inducible Hsp72, referred to as Hsp70. Most neurons contain high levels of the constitutive form and low levels of the inducible form (Brown 1991; Brown 1994). High levels of the constitutive Hsc70 in neurons may reflect their metabolic activity, which is one of the highest of all cells in the body. High levels of Hsc70 may also enable these postmitotic cells to counteract or buffer the initial effects of stress without resorting to the inducible Hsp70.
Several studies in adult and some neonatal animals have analyzed the role of Hsps, particularly Hsc/Hsp70, in the survival of neurons after injuries that affect peripheral nerves and their cell bodies. Previous work examining the upregulation of Hsp70 family members indicated that injury to peripheral nerves causes a peak in Hsp70 messenger ribonucleic acid levels between 1 and 2 hours, whereas protein levels peak at 12 hours and can remain elevated for days (Moreno-Flores et al 1997; New et al 1989). Hsp70 has been shown to increase in facial motor neurons (MNs) in the adult hamster after axotomy of the facial nerve, but it was found to decrease in the neonate (Newfry and Jones 1998).
The endogenous expression of Hsc/Hsp70 before and after sciatic nerve (SN) injury has been examined primarily in adult animals, and the expression patterns of Hsc/Hsp70 in adult may be different from those in neonatal mice. Mature lumbar MNs do not undergo cell death after axotomy, whereas many MNs are still dependent on trophic support in the neonate, resulting in apoptosis and the loss of approximately 50% of the MNs after axotomy of the SN (Li et al 1994; Li et al 1998). Similarly, there is a loss of 30–35% of sensory neurons in the lumbar dorsal root ganglia (DRG) after sciatic axotomy in the neonate (Houenou et al 1996; Snider et al 1992).
Although it is generally assumed that a cell must produce its own Hsps to be protected by them, there is also evidence that Hsps can pass from cell to cell. It was discovered in the late 1970s in the squid giant axon that many proteins made in glial cells are transferred into the axon by a mechanism that remains unknown (Lasek et al 1977). This observation led to the glia-neuron protein transfer hypothesis (Lasek et al 1977). Using the same system, Tytell and coworkers observed that Hsp70 produced in glia was included in the group of proteins transferred to the axoplasm (Tytell et al 1986). This was confirmed by 2 other reports on other invertebrate axons (Greenberg and Lasek 1985; Sheller et al 1998). Furthermore, locally synthesized proteins such as actin and Hsp70, presumably from glia or other nonneuronal cells, were found to be taken up and transported retrograde to the cell body in a study using in vitro regenerating adult frog sciatic sensory axons (Edbladh et al 1994). Finally, localized heating of the rat SN results in the production of Hsp70 in surrounding nucleated cells such as Schwann and endothelial cells but not in the axon (Hoogeveen et al 1993). Most recently, Bechtold and Brown observed indirect evidence for the transfer of Hsp27 and 32, 2 other Hsps, from presynaptic glia to postsynaptic neural processes in the rat brain after whole-body hyperthermic stress (Bechtold and Brown 2000).
It is generally assumed that the protective effects of Hsps are limited to the cell that produces them. However, in addition to the glia-axon transfer referred to above, an unconventional release of Hsp70 from cultured rat embryo cells was documented some time ago by Hightower and Guidon (1989). The first observation of the stress tolerance–enhancing activity of exogenous Hsc/Hsp70 was reported by Johnson and coworkers (Johnson et al 1990; Johnson et al 1995), who showed that Hsc/Hsp70 added to the culture medium can bind to cultured arterial smooth muscle cells and improve their resistance to nutrient deprivation stress. In cultured monocytes, chemically induced apoptosis was inhibited by the addition of Hsc/Hsp70 to the medium (Guzhova et al 1998). Furthermore, exogenous Hsp70 added to cultured lymphocytes was shown to be imported readily into both cytoplasmic and nuclear compartments (Fujihara and Nadler 1999). In neural tissues, there are 4 reports substantiating the protective activity of exogenous Hsc/Hsp70. Work from this laboratory showed that an Hsc/Hsp70 mixture administered to the cut end of the SN or injected intravitreally into the rat eye prevented apoptotic cell death of sensory neurons or protected light-damaged photoreceptors from degeneration, respectively (Houenou et al 1996; Yu et al 2001). Guzhova et al (2001) documented that cultured glioma cells released Hsp70 and that a mixture of Hsc70 and Hsp70 was taken up by cultured neuroblastoma cells, rendering them resistant to apoptosis. Lastly, Kelty et al (2002) showed in a rat brain slice preparation that addition of Hsp70 to the medium was as effective as hyperthermic induction of Hsp70 synthesis in preserving normal synaptic transmission. Thus, there is a growing body of evidence that Hsps made by 1 cell may be passed to its neighbors and that Hsps in the extracellular fluid can interact with cells to promote stress tolerance.
The following experiments aimed to extend our earlier observations on the neuroprotective effects of Hsc/Hsp70 (Houenou et al 1996) and to relate them to the endogenous Hsc70 and Hsp70 responses of sensory neurons and MNs after axotomy. We show that different preparations of the Hsc70 and Hsp70, or mixtures of the two, similarly confer protection from axotomy-induced apoptosis and propose that the administration of the proteins may remedy deficits in the stress response observed in the neonatal sensory neurons and MNs.
Neuroanatomical abbreviations and terms used in this article: MN—motor neuron cell body, located in the ventral spinal cord, whose axons form part of the SN; DRG—dorsal root ganglion, a collection of sensory neurons cell located on either side of the vertebral column that have processes within the SN; axotomy—complete transaction of the axons of the SN.
MATERIALS AND METHODS
Axotomy
Sciatic nerves were severed in postnatal day 5 (P5) BalB/cByJ mice as described previously (Houenou et al 1996). Mice were anesthetized by hypothermia induced by contact with an ice-cold surface for approximately 2 minutes. Using a dissecting microscope and microsurgical tools, the SN was located and transected at the midthigh level. The wound was sutured closed with a P3 5-0 silk black braided suture (Ethicon). When this surgery was performed to assess neuronal survival, a 2-mm section of the nerve was removed to prevent regeneration and promote the maximal cell death response. Immediately after axotomy a dissolvable sterile Gel Foam pad containing 5 μL of either saline only or saline containing one of the several doses and preparations of Hsp was applied to the proximal nerve stump, and the wound sutured was closed. Recombinant Hsps were obtained from StressGen (Vancouver, BC, Canada) (human, rhHsp70 and bovine, rbHsc70), and purified bovine skeletal muscle Hsc/Hsp70 (mHsp), 2:3 ratio, was a gift from Dr B. A. Margulis, Russian Academy of Sciences (St Petersburg, Russia). Four days later, an intramuscular injection of 5 μL of saline only or saline plus the same dose and preparation of Hsp was administered to the wound site to prolong the availability of the proteins to the injured axons. Mice were sacrificed 3 days after the second treatment (7 days postaxotomy or at P12). Either MNs from the contralateral motor column or sensory neurons in the contralateral DRG were considered as positive controls, whereas neurons from the ipsilateral side treated with saline or bovine serum albumin (BSA) (Sigma, St Louis, MO, USA) were taken as negative controls. Treatment groups consisted of those that received (1) only 25 μg rbHsc70; (2) only rhHsp70 at 5 to 10, 15 to 20, 25 to 30, and 75 μg doses; (3) mHsp70 at 15 or 30 μg doses; (4) 30 μg of a 1:1 ratio of rbHsc and rhHsp70.
Concentration of Hsps and the luciferase refolding assay
Lyophilized proteins received from StressGen (Victoria, BC, Canada) were reconstituted in saline. Proteins supplied as dilute solutions were concentrated by centrifugation at 5000 × g at 4°C in tubes containing Microcon YM-30 regenerated cellulose centrifugable filters (Millipore Corp., Bedford, MA, USA) with a molecular weight cutoff of 30 000. This step allowed water to be removed from the protein solution while retaining the protein in a reduced volume above the filter. Hsp functional activity was determined using a luciferase refolding assay, a modification of that of Schumacher et al (1996). Luciferase (Sigma) at 100 nM was diluted 1:100 in stability buffer (details given below), heat denatured for 15 minutes at 42°C, and then chilled on ice for 10 minutes. This solution was diluted again 1:10 in Tris buffer containing 50% rabbit reticulocyte lysate (Sigma), 1.6 μg/mL YDJ (also known as Hsp40, a cochaperone of Hsp70, a gift from David Toft, Department of Biochemistry and Molecular Biology, Mayo Graduate School, Rochester, MN, USA), and 300 or 100 μg/mL rbHsc70, rhHsp70, or m-Hsp and incubated for up to 2 hours at 25°C. A small aliquot (20 μL) of this luciferase mixture was added to 100 μL of Steady-Glo luciferase assay buffer containing the substrate luciferin (Promega, Madison, WI, USA), in 8× 50-mm luminometer tubes and incubated for 2 hours at 25°C. Luciferase activity was measured at different times during the 2-hour incubation period, using a Turner Luminometer model 20e (Turner Designs, Sunnyvale, CA, USA). The activity was expressed as a percentage of control samples, which were handled identically but without denaturation at 42°C.
Buffers for the luciferase assay included stability buffer (25 mM Tricine–HCl, pH 7.8, 8 mM MgSO4, 0.1 mM ethylenediamine-tetraacetic acid [EDTA], 10 mg/mL BSA, 10% glycerol, and 0.25% Triton-X 100) and Tris buffer (10 mM Tris–HCl, pH 7.5, 3 mM MgCl2, 50 mM KCl, and 2 mM dithiothreitol).
Endogenous expression of Hsps after axotomy
Dissection
Mice were euthanatized by prolonged hypothermic treatment, similar to that described in the Axotomy section, but the treatment was continued until respiration and heartbeat ceased. The following tissues were collected at 6, 12, 24, and 48 hours after axotomy: portions of the ipsilateral SN proximal and distal to the cut, contralateral (intact) SN, ipsilateral and contralateral spinal cord, and 3–5 ipsilateral and contralateral lumbar DRG.
Methacarn fixation and paraffin embedding
Sciatic nerve and whole spinal cords were dissected and fixed overnight with methacarn solution (60% methanol, 30% 1,1,1-trichloroethane, 10% acetic acid (Mitchell et al 1985; Puchtler et al 1970)). Lumbar spinal cords were further dissected the next day. After dehydration through a graded series of mixtures of alcohol and 1,1,1-trichloroethane, tissues were embedded in paraffin. Sections from all tissues were prepared using a rotary microtome, quickly examined by microscopy for proper orientation, mounted onto ProbeOn Plus slides (Fisher Scientific, Suwanee, GA, USA), and dried on a slide warmer.
Immunohistochemistry
After deparaffinization and rehydration of the tissues, slides were washed in phosphate-buffered saline containing 0.1% Tween and 0.01% thimerosal (PBS-T, pH 7.4), and nonspecific binding was blocked by PBS-T containing 10% normal goat serum (Vector Laboratories Inc., Burlingame, CA, USA). Next, slides were incubated overnight in PBS-T containing primary antibodies against Hsc70 (SPA-815, StressGen, Victoria, BC, Canada) or Hsp70 (SPA-810, StressGen, Victoria, BC, Canada). Mouse ascites fluid (Sigma) or rat IgG (Kirkegaard & Perry Labs [KPL], Gaithersburg, MD, USA) were used as controls for nonspecific binding of IgGs. Slides were then incubated with goat anti-mouse or goat anti-rat biotin-labeled secondary antibodies (KPL) in PBS-T for 1 hour. The presence of the secondary antibody was detected using peroxidase-conjugated streptavidin (KPL) and diaminobenzidene as the substrate (Vector). Slides were coverslipped using Permount. Digital images of the tissue sections were collected using a Sony color video camera attached to an Olympus BH-2 microscope and processed using Scion Image (version 1.62c, Scion Corp., Frederick, MD, USA). This software was also used for quantitatively comparing immunohistochemical staining intensities between the ipsilateral and contralateral lumbar MNs within each animal in the following way. The mean gray values in 3 random areas within the cytosol of large lumbar MNs in nonadjacent sections were measured. Ratios of the ipsilateral to the contralateral mean intensities of 5–22 lumbar MNs were calculated for each animal. These ratios were compared with the value of 1 using a 1-sample 2-tailed t-test, where a value of 1 would represent no difference in staining between the contralateral and ipsilateral MNs.
Western blots
Dissected tissues were homogenized in lysis buffer containing 50 mM Tris HCl, 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethyl sulfonyl fluoride, 2.5% sodium dodecyl sulfate (SDS), and 1 μg/mL each of leupeptin, aprotinin, and pepstatin and stored at −20°C. The Pierce BCA protein assay kit was used to determine homogenate protein concentrations (Pierce, Rockford, IL, USA). Proteins were resolved on a 12% SDS–polyacrylamide gel by electrophoresis and transferred onto nitrocellulose membranes (BioRad, Hercules, CA, USA). Recombinant human Hsp70 or recombinant bovine Hsc70 run on the same gels served as controls. After blocking the membranes with 5% dry milk in PBS-T overnight at 4°C, the membranes were incubated for 1 hour at room temperature with the appropriate primary antibodies (αHsp70 at 1:1000 and αHsc70 at 1:4000, StressGen, Victoria, BC, Canada) followed by peroxidase-conjugated secondary antibodies (donkey anti-mouse or anti-rat at 1:5000, Jackson Immuno Research Laboratories Inc., West Grove, PA, USA) and detected with enhanced chemiluminescence (Pierce) by exposure to X-ray film. Images of immunoreactive bands on the film were captured using an Agfa Arcus II color scanner and then analyzed using the Kodak Digital Science 1D Image Analysis software for densitometry (version 2.0, Kodak, Rochester, NY, USA). For blots analyzing DRG tissue homogenates, net intensities from the ipsilateral sample were divided by those of the contralateral sample collected at the same time points and compared with the value 1 in a 1-sample 2-tailed t-test, as described previously. Membranes were stained with India ink (1:1000 in PBS-T) to confirm that the samples were evenly loaded on the gels.
Neuronal survival determination
Whole lumbar spinal cords with attached vertebra and DRG were dissected and fixed in Bouin's solution (750 mL saturated aqueous picric acid, 250 mL concentrated formalin, 50 mL glacial acetic acid) for 2 weeks followed by 70% ethanol for 1–2 weeks and then changed to 70% ethanol 2–3 times during the following week. After paraffin embedding, the tissue was cut into 12-μm-thick serial sections, and the sections were stained with hematoxylin and eosin. Either MNs from the contralateral lateral motor column or sensory neurons in the contralateral DRG were considered as positive controls, whereas neurons from the ipsilateral side treated with saline or BSA were taken as negative controls. MN survival was determined by neuronal cell counts in every fifth section in the fourth lumbar segment (L4) of the lateral motor column of the spinal cord. Sensory neuron survival was determined by neuronal cell counts in every fifth section of the L4 DRG. Only neurons with a large nucleus containing at least 1 distinct nucleolus and well-stained cytoplasm were counted, as previously described (Li et al 1994; Houenou et al 1996). Digital images of these tissue sections were obtained using a spot camera (Diagnostic Instruments) attached to a Zeiss Axioplan microscope. Ipsilateral cell counts were analyzed and converted to a percentage of the matched contralateral cell counts. These data were analyzed by analysis of variance (ANOVA) and Fischer's protected t-test using the least squares difference (LSD) approach.
RESULTS
Endogenous levels of Hsp70 and Hsc70 after axotomy
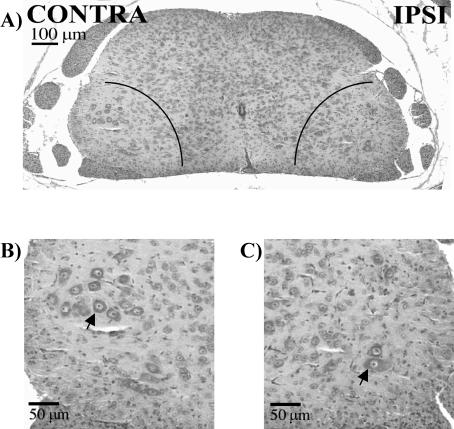
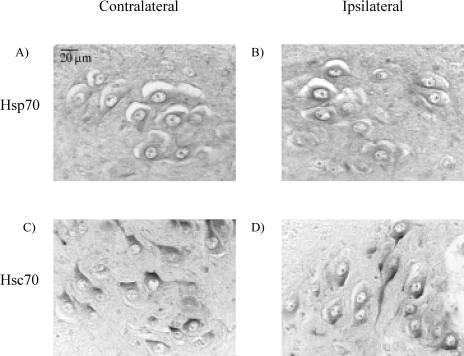
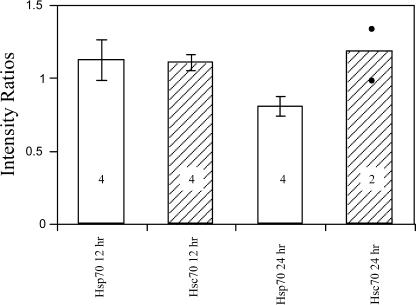
We assayed the SN, lumbar DRG, and lumbar MNs to determine whether the trauma of axotomy elicited an increase in endogenous levels of Hsp70, as would be expected in a typical stress response. This information helped us to interpret the impact on neuron survival of the administration of various preparations of the 70-kD Hsps. Figure 1 shows a typical example of L4 MNs in the lateral motor columns in a cross section of a P12 spinal cord after axotomy on P5, resulting in apoptosis and loss of the MNs. There is an obvious decrease in the number of MNs on the ispilateral (axotomized) vs the contralateral (intact) side. A similar axotomy-induced loss of sensory neurons in the lumbar DRGs has been documented in this model previously (Houenou et al 1996). Immunohistochemical staining of the lumbar spinal cord tissue sections from P5 mice was performed to detect Hsp70 and Hsc70. The Hsp70 and Hsc70 immunoreactivities (IRs) in the MNs subjected to axotomy (the half of the spinal cord ipsilateral to the axotomized nerve) were compared with those on the uninjured side (contralateral to the axotomized nerve) at 12 hours after axotomy, and no obvious changes in either protein were noted (Fig 2). To confirm that the protein levels were unaffected, the IR at 12 and 24 hours after axotomy was quantified by measuring the mean gray levels of IR of individual MNs in digitized images of the ipsilateral and contralateral sides of the spinal cords in each animal. The results from all the animals averaged for each group are summarized in Figure 3. Ratios of ipsilateral to contralateral IR were not significantly different from 1, which reflected no changes as a result of axotomy.
Fig 1.
Hematoxylin and eosin of lumbar-4 spinal cord in P12 mice (7 days after axotomy on P5). (A) The areas marked by quarter circles represent the lateral motor columns of the L4 spinal cord, where motor neurons (MNs) comprising the sciatic nerve are found. There is a decrease in the number of MNs on the ipsilateral (IPSI) vs the contralateral (CONTRA) side. These areas are enlarged in contralateral (B) and ipsilateral (C). Arrowheads point to examples of MNs.
Fig 2.
Immunohistochemistry of Hsp70 and Hsc70 in lumbar motor neurons in P5 mice 12 hours after axotomy. These are higher-magnification images taken from within areas comparable with those marked in Figure 1. No changes were observed in immunohistochemical staining for either Hsp70 or Hsc70 between the contralateral side (A, C) and the ipsilateral side (B, D)
Fig 3.
Evaluation of endogenous expression of Hsp70 and Hsc70 in lumbar motor neurons (MNs) in P5 mice 12 and 24 hours after axotomy. Data are represented as ratios of ipsilateral to contralateral staining intensity for each P5 animal, calculated from the mean intensities measured in 5–22 lumbar MNs in nonadjacent sections. The ratios were compared statistically with the value 1, where 1 represents no change, greater than 1 represents an increase, and less than 1 represents a decrease. The numbers in the bars are the number of animals in each group, and the bar for Hsc70 24 hours is the average of 2 animals represented by the dots. No significant changes were observed in immunostaining as a result of axotomy
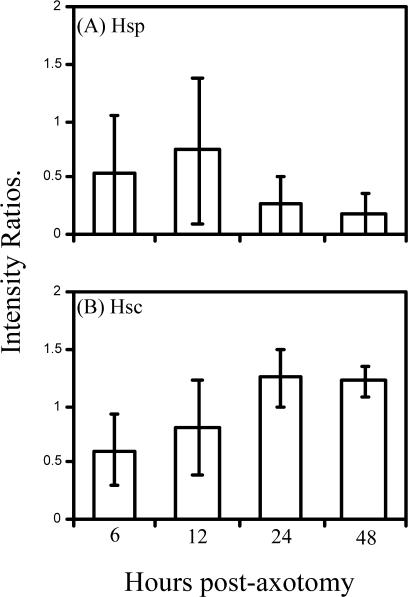
The endogenous expression of Hsc70 and Hsp70 in the dorsal root ganglion and SN after axotomy in neonatal mice was also examined by Western blotting. Tissues were collected from DRG and SNs at 6, 12, 24, and 48 hours after axotomy. Both Hsc70 and Hsp70 were detectable in the DRG, whereas only Hsc70 was detectable in the SNs (not shown). At none of the postaxotomy intervals were any significant changes in these proteins detected in the DRG (Fig 4) or SNs (not shown). However, the Hsp70 data in Figure 4A must be viewed cautiously because Hsp70 was undetectable in about half the 12 pairs of matched control and axotomized DRG samples (5 control and 6 axotomized DRG samples). This absence of detectable Hsp70 occurred despite the fact that twice as much total tissue protein was loaded on the gels processed for Hsp70 Western blotting as on those processed for Hsc70 (50 vs 25 μg, respectively). It also accounts for the large error bars in Figure 4A. Hsc70, in contrast, was more abundant in those tissue samples, being undetectable in only 1 control and 2 axotomized samples.
Fig 4.
Endogenous expression of Hsp70 and Hsc70 in lumbar dorsal root ganglia from P5 mice after axotomy. (A) and (B) are graphs of the mean ratios of ipsilateral to contralateral samples analyzed by densitometry. No changes were observed in minimally detectable amounts of Hsp70 (A). Hsc70 was readily detectable but also showed no changes (B). In some cases, especially for the Hsp70 blots, tissues from 2 animals were pooled in the attempt to detect the low levels of this protein
Luciferase assay of Hsp refolding activity
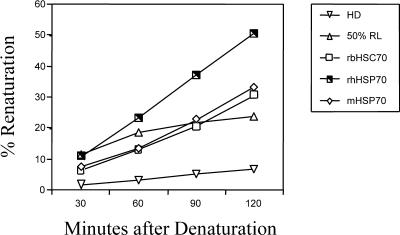
Proteins used in vivo were either recombinant or purified from bovine skeletal muscle. Some of the proteins from the manufacturer were provided at too low a concentration to be used as supplied for the in vivo experiments. Those proteins were concentrated, and the luciferase assay was used to confirm that the Hsps retained refolding activity. We tested 100 μL of Hsp solutions at concentrations of 100 and 300 μg/mL of rbHsc70, rhHsc70, and m-Hsp70 at incubation periods of 30–120 minutes after luciferase denaturation. Only the results from the higher concentrations of each Hsp preparation tested are shown (Fig 5). All 3 protein preparations were able to renature the heat-denatured luciferase.
Fig 5.
Refolding activity of heat shock proteins. Activity is expressed as a percentage of renaturation after heating at 42°C compared with unheated control samples. The different proteins, rbHSC70, rhHSP70, and mHSP70, were analyzed using 300 μg/mL of each and sampled at the times indicated. The mHSP70 corresponds to the protein purified from skeletal muscle containing both Hsp70 and Hsc70. Rabbit reticulocyte lysate, RL, was used as a positive control for the luciferase assay system
Exogenous application of Hsps and neuronal survival
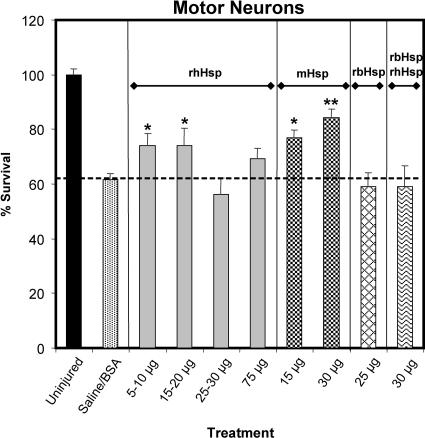
It was previously observed that treatment of the severed SN with 5–25 μg of a preparation of bovine brain Hsc70 caused a trend toward increased MN survival but did not achieve statistical significance (Houenou et al 1996). This effect prompted us to consider that preparations of Hsps with different proportions of Hsc70 and Hsp70 might have greater survival-promoting activity on MNs. A variety of Hsc70 and Hsp70 preparations and combinations were used to address this question, and Figure 6 illustrates the results. Without Hsp or with the control protein, BSA, 35–42% of the lumbar MNs degenerated 1 week after axotomy. The lower doses of rhHsp70, those between 5 and 20 μg, significantly improved MN survival, so that only an average of 26% of the MNs degenerated (P < 0.05). Surprisingly, the higher doses of rhHsp70 had no significant effect on survival. However, the mixture of Hsc70 and Hsp70 purified from bovine skeletal muscle (mHsp) also promoted survival, such that only 23% (P < 0.05) and 16% (P < 0.01) of the MNs degenerated after treatment with the 15- and 30-g doses, respectively. The positive effect of the mHsp preparation made us wonder whether the mixture of the 2 isoforms had greater benefit than either protein alone. We attempted to mimic the composition of the mHsp preparation by mixing the rhHsp70 and rbHsc70 at a 1:1 ratio. That artificially created mixture had no effect on survival of the injured MNs at 30 μg Fig 6, rbHsp-rhHsp. Similarly, 25 μg of rbHsp70 alone provided no MN survival–promoting effect (Fig 6).
Fig 6.
Lumbar-4 motor neuron (MN) survival with Hsp70 or Hsc70 treatment (or both). The ipsilateral MN cell counts were expressed as a percentage of their matching contralateral cell counts. These data were analyzed by analysis of variance (P = 0.0001) and Fischer's protected t-test using the least squares difference approach. Axotomy in the saline-treated control group caused a significant loss of MNs compared with the number in the ventral horn of the lumbar segments of the uninjured group (P < 0.01, not indicated for the sake of clarity). Treatment of axotomized mice with 30 μg bovine serum albumin had no effect on this loss of neurons. In contrast, the death of MNs was significantly inhibited after postaxotomy treatment with the 2 lower-dose ranges of rhHsp (5–10 μg or 15–20 μg of rhHsp70, *P < 0.05) or with either 15 or 30 μg of mHsp (*P < 0.05, **P < 0.01, respectively), resulting in greater numbers of MNs in those groups compared with the saline-treated group. Lastly, rbHsc alone or mixed 1:1 with rhHsp had no significant effect on MN survival
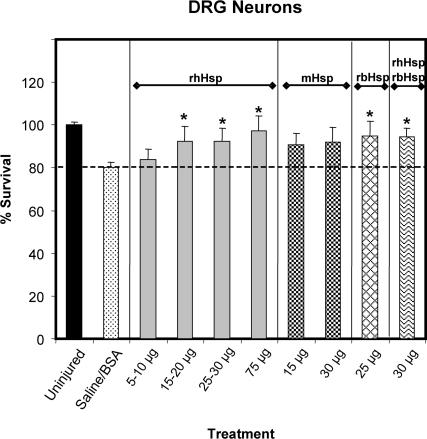
Sensory neuron survival was also examined after axotomy because it has been shown to be significantly improved by treatment using 5 μg or more of a preparation of bovine brain Hsc70 that consisted of about 95% Hsc70 (Houenou et al 1996). Although the loss of DRG neurons after axotomy in the group that received saline or BSA was about half that of MNs, being 19.7% rather than 38.5%, certain doses of some of the Hsp preparations did significantly improve survival. Intriguingly, the effective treatments were different from those seen for the MNs (Fig 7). The 3 higher doses of rhHsp70 all significantly increased survival, reducing the loss of DRG neurons to between 2.8% and 7.6% (P < 0.05). Additionally, the 25-μg dose of rbHsc70 and the rbHsc-rhHsp70 mixture also significantly improved survival, with these groups showing an average loss of only 5.1% and 5.4% of the DRG neurons, respectively, after axotomy (P < 0.05). Unexpectedly, both doses of mHsp70 fell just short of significantly improving survival, with losses of 9.4 and 8.1% of DRG neurons for the 15- and 30-μg treatments, respectively.
Fig 7.
Lumbar-4 sensory neuron survival with Hsp70 or Hsc70 treatment (or both). These data were analyzed in the same manner as that for the motor neurons in Figure 6. The overall analysis of variance was significant (P = 0.0002), and comparison of the mean number of sensory neurons in the L4-DRG of the saline-treated group with that of the contralateral, uninjured group showed that there was a significant loss of sensory neurons caused by axotomy (P < 0.01, not indicated for the sake of clarity). This axotomy-induced neuronal death was inhibited after treatment with only the higher doses of rhHsp (25–30 μg), with the 25-μg dose of rbHsc, or the 1:1 mix of rhHsp-rbHsp (*P < 0.05 compared with the saline-treated group)
DISCUSSION
Endogenous expression of Hsc70 and Hsp70 after injury
Although Hsc70 and Hsp70 were present in MNs and DRG neurons of neonatal mice, no changes were detected within the 48-hour period of analysis (Figs 2–4). Furthermore, in the SN, only Hsc70 was detected irrespective of whether it was severed (data not shown). The fact that Hsp70 was undetectable in many of these samples means that we cannot discount the possibility of axotomy-related changes that were below the sensitivity of detection in our Western blots. Nonetheless, the suggestion that these neurons in the developing mouse do not mount the expected Hsp70 response to physical trauma is supported by our observations of cultured MNs from the chick embryo, in which there was no increase in Hsp70 after hyperthermic stress or during apoptosis induced by withdrawal of the trophic factor (in preparation). Furthermore, 2 groups recently reported similar observations for MNs in neonatal rats and in culture from embryonic mice, showing an impairment of the ability of these neurons to accumulate Hsp70 after nerve injury in vivo or to activate heat shock factor 1 in vitro after hyperthermic or excitotoxic stress, or expression of mutant SOD-1 (Kalmar et al 2002a; Batulan et al 2003). However, the Hsp70 response system of MNs is not completely unresponsive. Using BRX-220, an analog of the hydroxylamine derivative bimoclomol, reported to amplify Hsp synthesis in other systems (Vigh et al 1997), Kalmar et al (2002b) demonstrated increased accumulation of Hsp70 in axotomized MNs of the neonatal rat, along with greater survival. That group also found that MNs showed a marked increase of another member of the Hsp family, Hsp27 (Kalmar et al 2002a).
Interpretation of changes in endogenous expression of Hsps in injured nervous tissue must be done with caution because induction may be dependent on the duration and type of injury. Additionally, the glial response may also be a source of increased amounts of the Hsps and may confer a protective effect on adjacent neurons (Tytell et al 1986; Sheller et al 1998; Guzhova et al 2001; unpublished observations).
Exogenous Hsc70 or Hsp70 (or both) promote neuron survival
Our results confirm and extend earlier work that showed that exogenous bovine brain–derived Hsc70 prevented apoptosis of sensory neurons after axotomy (Houenou et al 1996). In contrast to that report, we used 3 different preparations of the 70-kD Hsps and found that they had different effects on MN and sensory neuron survival after SN axotomy. In the case of MNs, recombinant bovine Hsc70 by itself had no survival-promoting activity, which was similar to the observation of Houenou et al (1996) using purified bovine brain Hsc70. However, recombinant human Hsp70 did increase MN survival at the smaller 2 of the 4 amounts administered (5–20 μg). Why the higher doses were ineffective remains to be determined, but the apparent negative effects of higher concentrations of exogenous Hsc70 have been observed previously in cultured smooth muscle cells treated with bovine brain–derived Hsc70 (Johnson et al 1995). It is possible that these differential effects of exogenous Hsp70 are a result of its ability to interact with membranes and alter their permeability characteristics in unexpected ways. This effect was first demonstrated by Alder et al (1990), who showed that human Hsp70 could interact with artificial lipid bilayers to form ion-conducting channels. Since then, several reports of membrane-based activity of different extracellular Hsc/Hsp70 preparations have been reported. These include increased Ca2+ efflux from Aplysia neurons (Smith et al 1995), activation of K+ channels in cultured U937 human promonocytes (Negulyaev et al 1996), and formation of cation-conducting channels in liposomes (Arispe and De Maio 2000). Furthermore, Arispe et al (2002) showed that Hsc70 and Hsp70 promote fusion of liposomes in distinct ways. Thus, the ability of the 2 Hsps to interact differently with membranes may be part of the reason for the different antiapoptotic effects seen here.
We also found that a mixture of Hsc70 and Hsp70 (approximately 2:3), derived from bovine skeletal muscle, had survival-promoting activity. However, when we attempted to duplicate the effect of the skeletal muscle–derived Hsc/Hsp70 mixture by combining the recombinant bovine Hsc70 with recombinant human Hsp70 in a 1:1 ratio, MN survival after axotomy was not affected. This discrepancy is unlikely to be the result of trace amounts of cochaperones like Hip, Hop, and Hsp40 (Frydman and Hohfeld 1997; Muchowski et al 2000) contained in the Hsc/Hsp70 from skeletal muscle because the final step in the purification process involves binding of the Hsc/Hsp70 to adenosine triphosphate (ATP) conjugated to agarose by way of a C8 linkage. The ATP-bound form of Hsc/Hsp70 does not associate with other binding proteins, and no traces of Hip, Hop, or Hsp40 have been detected in the preparation (B. Margulis, personal communication). It is possible that there may be differences in biological activity of the muscle-derived material compared with that produced from recombinant Escherichia coli. In this sense the muscle-derived Hsc/Hsp70 may match more closely the mixture of Hsps that occurs naturally in injured cells.
The survival of sensory neurons after axotomy was also significantly increased by treatment with the different forms of the 70-kD Hsps but in a manner distinct from that observed with MNs. Both recombinant Hsp70 and Hsc70 promoted sensory neuron survival when applied separately but not when applied as a mixture in the form of the skeletal muscle–derived preparation. This is in contrast to the positive effect of that mixture on MNs. A further distinction between the 2 classes of neurons was observed in the survival-promoting effects of rhHsp70. In contrast to MNs, sensory neuron survival was better with the higher doses of rhHsp70. These contrasting effects of the 2 forms of the 70-kD Hsps on sensory neurons and MNs suggests that each form interacts with and contributes uniquely to neuron survival. Furthermore, the results imply that different types of neurons may have distinct requirements for the differing functions of Hsc70 and Hsp70.
Another consideration in understanding the differential effects of the 70-kD Hsps on sensory neurons and MNs is the possibility that Hsp-specific receptors may be involved. Recent work has documented that such receptors exist on antigen-presenting cells like monocytes and macrophages (Asea et al 2000, 2002; Binder et al 2000). To our knowledge the Toll-like receptors identified as the Hsp70-binding entities in monocytes or macrophages (Asea et al 2002) are not expressed on neurons. However, it is intriguing that the quantities of Hsps we found to inhibit cell death here and in other reports (Johnson et al 1995; Houenou et al 1996; Guzhova et al 2001; Yu et al 2001) and those shown to bind specifically to the antigen-presenting cells and elicit cytokine responses seem to fall within a range of 0.1–100 μg/mL (about 0.001–1.0 μM), which is not as divergent as one would expect given the differences in the systems and end points used to evaluate these effects. Thus, it will be important for future studies to specifically address the possibility of the presence of Hsp receptors on neuronal cells.
Further work in this area also must assess the contributions of cochaperones and of other major Hsps like Hsp27 and Hsp90. For example, endogenously produced Hsp27 has recently been shown to be required for both sensory neuron and MN survival after axotomy in the neonatal rat (Benn et al 2002).
The responses to injury are critical in the nervous system because it is a tissue composed of postmitotic cells with limited self-renewal and repair mechanisms. Thus, understanding the biology of normal responses to injury will help in the development of more effective approaches to preserve damaged neurons, as well as glia, thereby reducing the loss of function after injury. The results presented here support the hypothesis that immediate application of exogenous Hsc70 or Hsp70, or both, may be physiologically similar to induced synthesis of endogenous Hsp70 and can increase survival of damaged neurons. Thus, it is likely that Hsc70 and Hsp70 have important therapeutic potential in the prevention of neurodegeneration as a result of trauma or disease.
Acknowledgments
This work was submitted in partial fulfillment of the requirements for completion of the PhD degree by J. Lille Tidwell. We would like to thank R. W. Oppenheim for his comments and suggestions about this work, David Toft for his expert technical advice and generous supplies for the luciferase assays, David Prevette for his technical expertise in tissue preparation, Deyrick O. Dean and Carol Kent for assisting with immunohistochemistry, and Jennifer Harris for assisting with luciferase assays. This work was supported by a grant from the Muscular Dystrophy Association to L.J.H., a Wake Forest University School of Medicine Venture Grant to M.T., and a gift from Emily and Frederick Windoe to M.T.
REFERENCES
- Alder GM, Austen BM, Bashford CL, Mehlert A, Pasternak CA. Heat shock proteins induce pores in membranes. Biosci Rep. 1990;10:509–518. doi: 10.1007/BF01116611.0144-8463(1990)010<0509:HSPIPI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem. 2000;275:30839–30843. doi: 10.1074/jbc.M005226200.0021-9258(2000)275<30839:AAAMAC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7:330–338. doi: 10.1379/1466-1268(2002)007<0330:lidtca>2.0.co;2.1466-1268(2002)007<0330:LIDTCA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697.1078-8956(2000)006<0435:HSCPTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular Hsp70. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200.0021-9258(2002)277<15028:NSTPUB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, Nalbantoglu J, Strong MJ, Durham HD. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003.0270-6474(2003)023<5789:HTFIOT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold DA, Brown IR. Heat shock proteins Hsp27 and Hsp32 localize to synaptic sites in the rat cerebellum following hyperthermia. Brain Res Mol Brain Res. 2000;75:309–320. doi: 10.1016/s0169-328x(99)00323-x.0169-328X(2000)075<0309:HSPHAH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Benn S, Perrelet D, Kato A, Scholz J, Decosterd I, Mannion R, Bakowska J, Woolf C. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/s0896-6273(02)00941-8.0896-6273(2002)036<0045:HUAPIR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Binder RJ, Harris ML, Ménoret A, Srivastava P. Saturation, competition, and specificity in interaction of heat shock proteins (hsp) gp96, hsp90, and hsp70 with CD11b+ cells. J Immunol. 2000;165:2582–2587. doi: 10.4049/jimmunol.165.5.2582.0022-1767(2000)165<2582:SCASII>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brown IR. Expression of heat shock genes (hsp70) in the mammalian nervous system. Results Probl Cell Diff. 1991;17:217–229. doi: 10.1007/978-3-540-46712-0_15.0080-1844(1991)017<0217:EOHSGH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brown IR 1994. Chapter 2. Induction of heat shock genes in the mammalian brain by hyperthermia and tissue injury. In: Heat Shock Proteins in the Nervous System, ed Brown IR. Academic Press, Inc., San Diego, CA, 31–53. [Google Scholar]
- Edbladh M, Ekstrom PAR, Edstrom A. Retrograde axonal transport of locally synthesized proteins, e.g. actin and heat shock protein 70, in regenerating adult frog sciatic sensory axons. J Neurosci Res. 1994;38:424–432. doi: 10.1002/jnr.490380408.0360-4012(1994)038<0424:RATOLS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Edgington SM. Therapeutic applications of heat shock proteins. Biotechnology (NY) 1995;13:1442–1444. doi: 10.1038/nbt1295-1442.0733-222X(1995)013<1442:TAOHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Frydman J, Hohfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0.0376-5067(1997)022<0087:CGITTH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fujihara SM, Nadler SG. Intranuclear targeted delivery of functional NF-kB by 70 kDa heat shock protein. EMBO. 1999;18:411–419. doi: 10.1093/emboj/18.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SC, Lasek RJ. Comparison of labelled heat shock proteins in neuronal and nonneuronal cells of Aplysia californica. Neuroscience. 1985;5:1239–1245. doi: 10.1523/JNEUROSCI.05-05-01239.1985.0306-4522(1985)005<1239:COLHSP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3.0006-8993(2001)914<0066:IVSSTH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Arnholdt ACV, and Darieva ZA. et al. 1998 Effects of exogenous stress protein 70 on the functional properties of human promonocytes through binding to cell surface and internalization. Cell Stress Chaperones. 3:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0.0028-0836(1996)381<0571:MCICPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206.0021-9541(1989)138<0257:SRFCMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hoogeveen JF, Van Der Kracht AHW, Wondergem J, Haveman J. Heat shock proteins (HSP-72kd) in thermotolerant rat sciatic nerves. Int J Hyperthermia. 1993;9:361–368. doi: 10.3109/02656739309005036.0265-6736(1993)009<0361:HSPHIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Houenou LJ, Li L, Lei M, Kent CR, Tytell M. Exogenous heat shock cognate protein Hsc70 prevents axotomy-induced death of spinal sensory neurons. Cell Stress Chaperones. 1996;1:161–166. doi: 10.1379/1466-1268(1996)001<0161:ehscph>2.3.co;2.1466-1268(1996)001<0161:EHSCPH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Berberian PA, Bond MG. Effect of heat shock proteins on survival of isolated aortic cells from normal and atherosclerotic cynomologous macaques. Atherosclerosis. 1990;84:111–119. doi: 10.1016/0021-9150(90)90080-3.0021-9150(1990)084<0111:EOHSPO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnson AD, Berberian PA, Tytell M. Differential distribution of 70-kD heat shock protein in atherosclerosis: its potential role in arterial SMC survival. Arterioscler Thromb Vasc Biol. 1995;15:27–36. doi: 10.1161/01.atv.15.1.27.1079-5642(1995)015<0027:DDOKHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kalmar B, Burnstock G, Vrbova G, Greensmith L. The effect of neonatal nerve injury on the expression of heat shock proteins in developing rat motoneurones. J Neurotrauma. 2002a;19:667–679. doi: 10.1089/089771502753754127.0897-7151(2002)019<0667:TEONNI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kalmar B, Burnstock G, Vrbova G, Urbanics R, Csermely P, Greensmith L. Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats. Exp Neurol. 2002b;176:87–97. doi: 10.1006/exnr.2002.7945.0014-4886(2002)176<0087:UOHSPR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kelty J, Noseworthy P, Feder M, Robertson R, Ramirez J. Thermal preconditioning and heat-shock protein preserve synaptic transmission during thermal stress. J Neurosci. 2002;22:193. doi: 10.1523/JNEUROSCI.22-01-j0004.2002.0270-6474(2002)022<0193:TPAHPP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x.0163-7258(1998)080<0183:HSPKMB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Gainer H, Barker JL. Cell-to-cell transfer of glial proteins to the squid giant axon: The glia-neuron protein transfer hypothesis. J Cell Biol. 1977;74:501–523. doi: 10.1083/jcb.74.2.501.0021-9525(1977)074<0501:CTOGPT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Houenou LJ, Wu W, Lei M, Prevette DM, Oppenheim RW. Characterization of spinal motoneuron degeneration following different types of peripheral nerve injury in neonatal and adult mice. J Comp Neurol. 1998;396:158–168.0021-9967(1998)396<0158:COSMDF>2.0.CO;2 [PubMed] [Google Scholar]
- Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702.0022-3034(1994)025<0759:NAPMDF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022<0631:THP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mattson M. Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Res. 2000;886:47–53. doi: 10.1016/s0006-8993(00)02790-6.0006-8993(2000)886<0047:NSATAB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mitchell D, Ibrahim S, Gusterson BA. Improved immunohistochemical localization of tissue antigens using methacarn fixation. J Histochem Cytochem. 1985;33:491–495. doi: 10.1177/33.5.3921605.0022-1554(1985)033<0491:IILOTA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moreno-Flores MT, Olazabal UE, Kreutzberg GW. Axotomy increases the expression of glucose-related protein 78 kDa in rat facial nucleus. Exp Neurol. 1997;146:10–16. doi: 10.1006/exnr.1997.6526.0014-4886(1997)146<0010:AITEOG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29.0071-1365(1997)032<0017:THRRAF>2.0.CO;2 [PubMed] [Google Scholar]
- Muchowski P, Schaffar G, Sittler A, Wanker E, Hayer-Hartl M, Hartl F. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. PNAS. 2000;97:7841–7846. doi: 10.1073/pnas.140202897.0027-8424(2000)097<7841:HAHCCI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA, Kinev AV, Voronin AP. Exogenous heat shock protein hsp70 activates potassium channels in U937 cells. Biochim Biophys Acta. 1996;1282:156–162. doi: 10.1016/0005-2736(96)00055-7.0006-3002(1996)1282<0156:EHSPHA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- New GA, Hendrickson BR, Jones KJ. Induction of heat shock protein 70 mRNA in adult hamster facial nuclear groups following axotomy of the facial nerve. Metab Brain Dis. 1989;4:273–279. doi: 10.1007/BF00999773.0885-7490(1989)004<0273:IOHSPM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Newfry GA, Jones KJ. Differential effects of facial nerve transection on heat shock protein 70 expression in the developing and adult hamster facial nucleus. Metab Brain Dis. 1998;13:253–257. doi: 10.1023/a:1023280110386.0885-7490(1998)013<0253:DEOFNT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Suzuki T. Roles of molecular chaperones in the nervous system. Brain Res Bull. 2000;53:141–146. doi: 10.1016/s0361-9230(00)00325-7.0361-9230(2000)053<0141:ROMCIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Puchtler H, Waldrop FS, Meloan SN, Terry MS, Conner HM. Methacarn (methanol-Carnoy) fixation: practical and theoretical considerations. Histochemie. 1970;21:97–116. doi: 10.1007/BF00306176.0018-2222(1970)021<0097:MMFPAT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rajdev S, Sharp FR. Stress proteins as molelcular markers of neurotoxicity. Toxicol Pathol. 2000;28:105–112. doi: 10.1177/019262330002800113.0192-6233(2000)028<0105:SPAMMO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schumacher RJ, Hansen WJ, Freeman BC, Alnemri E, Litwak G, Toft DO. Cooperative action of Hsp70, Hsp90, and DnaJ proteins in protein renaturation. Biochemistry. 1996;35:14889–14898. doi: 10.1021/bi961825h.0006-2960(1996)035<14889:CAOHHA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sheller RA, Smyers ME, Grossfeld RM, Ballinger ML, Bittner GD. Heat-shock proteins in axoplasm: high constitutive levels and transfer of inducible isoforms from glia. J Comp Neurol. 1998;396:1–11. doi: 10.1002/(sici)1096-9861(19980622)396:1<1::aid-cne1>3.0.co;2-4.0021-9967(1998)396<0001:HPIAHC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith PJS, Hammar K, Tytell M. Effects of exogenous heat shock protein (hsp70) on neuronal calcium flux. Biol Bull. 1995;189:209–210. doi: 10.1086/BBLv189n2p209.0006-3185(1995)189<0209:EOEHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Snider WD, Elliott JL, Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992;23:1231–1246. doi: 10.1002/neu.480230913.0022-3034(1992)023<1231:ANDDD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tytell M, Greenberg SG, Lasek RJ. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986;363:161–164. doi: 10.1016/0006-8993(86)90671-2.0006-8993(1986)363<0161:HSPITF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tytell M, Hooper PL. Heat shock proteins: new keys to the development of cytoprotective therapies. Emerging Therapeutic Targets. 2001;5:267–287. doi: 10.1517/14728222.5.2.267. [DOI] [PubMed] [Google Scholar]
- Vigh L, Literati PN, and Horvath I. et al. 1997 Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 3:1150–1154. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Suhan JP. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986;103:2035–2052. doi: 10.1083/jcb.103.5.2035.0021-9525(1986)103<2035:CABEIM>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroportective potential of heat shock protein 70 (HSP70) Mol Med Today. 1999;5:525–531. doi: 10.1016/s1357-4310(99)01599-3.1357-4310(1999)005<0525:TNPOHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yu Q, Kent CR, Tytell M. Retinal uptake of intravitreally injected Hsc/Hsp70 and its effect on susceptibility to light damage. Mol Vis. 2001;7:48–56.1090-0535(2001)007<0048:RUOIIH>2.0.CO;2 [PubMed] [Google Scholar]