Abstract
Angiotensin II (Ang II) is a potent vasoconstrictor and induces inflammation and end-organ injury through its activation of the proinflammatory transcription factor, nuclear factor–κB (NF-κB). Heat shock (HS) treatment with subsequent expression of heat shock proteins (Hsps) is an effective strategy for tissue protection against oxidative injuries. Recently, HS and Hsps have been shown to interact with NF-κB in tissue injury. In this study, we investigated whether HS could protect against Ang II–induced hypertension and inflammation by inhibiting NF-κB. Sprague-Dawley rats were divided into control and HS groups. Control and 24-hour post–heat shocked rats were treated with Ang II. At days 1, 3, 5, 7, 11, and 14 after Ang II administration, systolic blood pressures were measured by tail-cuff plethysmography, and aorta tissues were collected. Aorta NF-κB deoxyribonucleic acid–binding activity was measured by electrophoretic mobility shift assay, and NF-κB p65 subunit, Hsp70, Hsp27, and interleukin-6 (IL-6) expressions were measured by Western analysis. HS treatment significantly decreased Ang II–induced hypertension. The activation of NF-κB in aorta by Ang II was suppressed by HS treatment. The elevated expression of IL-6 induced by Ang II treatment was also decreased by HS treatment. Although Ang II treatment induced an increase in Hsp70 and Hsp27, HS treatment induced a greater elevation of Hsp70 and Hsp27 expression. HS treatment protects against Ang II–induced hypertension and inflammation. This protection may relate to the interaction of Hsps and the NF-κB pathway.
INTRODUCTION
The heat shock (HS) response is highly conserved and is associated with the expression of a spectrum of inducible proteins called heat shock proteins (Hsps). There are several Hsp families that are classified according to their molecular weight: Hsp110, Hsp90, Hsp70, Hsp60, and small Hsps such as Hsp27 and ubiquitin (Welch 1992). Among these families of Hsps, 2 proteins, Hsp70 and Hsp27, are highly inducible and have repeatedly been associated with a protective role in tissue injury and cell death (Currie and Plumier 1998; Mosser et al 2000; Krueger-Naug et al 2002). Hsps function as molecular chaperones and facilitate the refolding, assembling, and stabilization of denatured proteins (Hartl 1996; Rogalla et al 1999). In addition, Hsps play antiapoptotic roles by regulating the activation of caspase, C-jun NH2-terminal kinase, and nuclear factor–κB (NF-κB) pathways (Beere 2001). We have shown that HS induces the expression of Hsp70 and enhances postischemic ventricular recovery (Currie et al 1988; Karmazyn et al 1990). Similarly, overexpression of Hsp70 in transgenic mice provides improved postischemic myocardial function and suppresses neuronal cell death (Plumier et al 1995, 1997). Angiotensin II (Ang II), the effector peptide of the rennin-angiotensin system formed as a result of sequential proteolysis of the angiotensinogen precursor, plays an important role in the development of hypertension and regulation of body fluid homeostasis. Interestingly, Ang II induces expression of Hsps in smooth muscle cell culture, aorta, and kidney (Xu et al 1995; Aizawa et al 2000; Meier et al 2001; Ishizaka et al 2002), and all suggest a protective role of Hsps against Ang II–induced end-organ injury. However, there is no direct evidence for HS treatment having an effect on Ang II–induced hypertension and inflammation. Several signaling pathways are activated in Ang II–induced tissue injury. NF-κB, a proinflammatory transcription factor, is required for maximal transcription of many cytokines, including interleukin (IL)-6, IL-8, tumor necrosis factor–α (TNF-α), and intercellular adhesion molecule–1, and all are thought to be important in the generation of inflammation responses (Blackwell and Christman 1997). Recently, Ang II–induced cell and tissue injury has been related to the activation of NF-κB (Han et al 1999; Kranzhofer et al 1999; Ruiz-Ortega et al 2001). We hypothesized that HS-induced protection on Ang II–induced hypertension and inflammation might be mediated, at least in part, by the suppression of NF-κB activation. IL-6, an NF-κB downstream target gene, is a multifunctional cytokine that mediates the acute-phase response and lymphocyte activation (Le and Vilcek 1989; Han et al 1999) and was used as a marker of the proinflammatory response in this study.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Quebec, Canada) weighing 280–310 g were cared for in accordance with the Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care and were housed in a climate-controlled room with a 12:12 hour light-dark cycle and had free access to water and food. Rats were randomly assigned to either Ang II treatment group (Ang group, n = 36) or HS and Ang II treatment group (HS + Ang group, n = 36). For HS treatment, animals were anesthetized with ketamine (80 mg/kg intraperitoneally [ip]) and xylazine (10 mg/kg ip) and then placed on a heating pad (50°C) until their rectal temperature reached 42°C. Core body temperature was maintained between 42 and 42.5°C for 15 minutes. Animals in the Ang group were anesthetized with the same drug but not heated. Twenty-four hours after HS or sham-HS treatment, an osmotic minipump (Alzet model 2002; Durect Corporation, Cupertino, CA, USA) containing Ang II dissolved in 0.9% NaCl was implanted subcutaneously between scapulae. The Ang II infusion rate was 0.7 mg/kg/d. Some sham animals underwent an identical surgical procedure with an empty osmotic pump implanted for 7 days. Systolic blood pressures were measured in conscious rats by tail-cuff plethysmography (model 29; IITC Inc, Woodland Hills, CA, USA). In some experiments, the selective type-1 angiotensin (AT1) receptor antagonist eprosartan (Solvay Pharmaceuticals, Weesp, The Netherlands) was administrated (60 mg/kg/d) to rats through intraperitoneal implantation of an osmotic minipump (Alzet model 2ML2) or the nonspecific vasodilator hydralazine (Ishizaka et al 1997) was given (15 mg/kg/d) in drinking water, beginning 2 days before implantation and during the Ang II infusion. The dosage of eprosartan was on the basis of previous research demonstrating its renoprotective effects in rat (Wong et al 2000). We also examined the norepinephrine (NE) model of hypertension by infusing NE at a rate of 2.8 mg/kg/d with an osmotic minipump (Alzet model 2001) through polyethylene tubing that was placed in the superior vena cava via the right external jugular vein (Ishizaka et al 1997). Rats were killed with an overdose of sodium pentobarbital, 1, 3, 5, 7, 11, or 14 days after Ang II infusion or after sham surgery. The aorta was taken for analysis and frozen in liquid nitrogen and then stored at −70°C until analyzed.
Preparation of nuclear protein extracts
For the isolation of nuclear protein extracts, a modified method (Pritts et al 2000) was used. Briefly, aorta tissue was rinsed with ice-cold phosphate-buffered saline (PBS) and homogenized on ice in 1 mL of ice-cold buffer A (10 mM N-2-hydroxythylpiperazine-N′-2-ethane-sulfonic acid [HEPES] [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 1 mM 1,4-dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 10 μg/mL aprotinin). After a 10-minute incubation on ice, the homogenates were centrifuged at 850 × g for 10 minutes at 4°C. The supernatants were discarded, and the pellets were suspended in 90 μL of ice-cold buffer A with 0.1% Triton X-100, incubated on ice for 10 minutes, and then centrifuged as above. The supernatants were stored as cytoplasmic extracts at −70°C. The pellets were resuspended in 750 μL buffer A and then centrifuged at 850 × g for 10 minutes. The supernatants were removed, and the purified nuclear pellets were resuspended in 50 μL buffer B (20 mM HEPES [pH 7.9], 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediamine-tetraacetic acid [EDTA], 1 mM DTT, 0.5 mM PMSF, 10 μg/mL leupeptin, 10 μg/mL aprotinin). The suspension was incubated for 30 minutes on ice. After centrifuging at 16 000 × g for 15 minutes at 4°C, the supernatants were transferred in aliquots to new tubes and stored at −70°C until analyzed. Protein concentrations were determined by the method of Lowry et al (1951).
Electrophoretic mobility shift assay
NF-κB consensus oligonucleotide sequence (5′-AGTGAGGGACTTTCCCAGGC-3′) (Promega, Madison, WI, USA) was end-labeled with [γ-32P] adenosine triphosphate (Amersham Pharmacia, Piscataway, NJ, USA) using T4 polynucleotide kinase (Promega) and purified in G-25 Sephadex columns (Amersham Pharmacia). Nuclear extracts (20 μg) were incubated with 2 μL binding buffer (20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 50 mM Tris-HCl [pH 7.5], 0.25 mg/mL poly(dI-dC)·poly(dI-dC)), then the labeled probe 1 μL (0.035 pmol) was added and incubated for 20 minutes at room temperature. Negative controls contained no nuclear extracts, and HeLa cell nuclear extracts were used as the positive control. To establish the specificity of the reaction, competition assays with 50 times excess of unlabeled NF-κB and SP-1 oligonucleotides (5′-ATTCGATCGGGGCGGGGCGAGC-3′) (Promega) were performed by adding unlabeled probes 10 minutes before the addition of the labeled probe. For supershift assays, 2 μg of anti-p65 and anti-p50 antibodies (Santa Cruz Biotechnology Inc, Santa Cruz, CA) was added to nuclear protein extracts and incubated for 1 hour after the addition of the labeled probe. The reaction was stopped by adding gel-loading buffer (250 mM Tris-HCl [pH 7.5], 0.2% bromophenol blue, 40% glycerol). The protein–deoxyribonucleic acid (DNA) complexes were separated on a nondenaturing 4% acrylamide gel in Tris-borate. Gels were dried onto Whatman 3MM paper and exposed to X-ray film with an intensifying screen at −70°C overnight.
Western analysis
Aorta tissue was thawed and rinsed with PBS and then homogenized on ice in 1 mL of buffer containing 50 mM HEPES (pH 7.5), 5 mM EDTA, 50 mM NaCl, 1 mM PMSF, 10 μg/mL leupetin, and 10 μg/mL aprotinin. The homogenates were stored at −70°C as whole-cell extracts. Protein samples (20 μg) were boiled for 10 minutes in sample buffer (250 mM Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate, 10% glycerol, 2% β-mercaptoethanol, and 0.003% bromophenol blue) at 100°C, then they were separated on denaturing 10% sodium dodecyl sulfate–polyacrylamide gels and transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). After blocking with 5% dry nonfat milk in Tris-buffered saline with 0.1% Tween 20 (TBS/T) for 1 hour at room temperature, membranes were washed 3 times for 5 minutes in TBS/T and then incubated overnight at 4°C with rabbit polyclonal anti–NF-κB p65 (1:1000, Santa Cruz Biotechnology Inc), goat polyclonal anti–IL-6 (1:1000, R&D Systems, Minneapolis, MN, USA), rabbit polyclonal anti-Hsp27 antibody (1:5000, StressGen, Victoria, Canada, catalog no. SPA 801), or mouse monoclonal anti-Hsp70 (1:1000, StressGen, catalog no. SPA 810), each in TBS/T containing 5% bovine serum albumin. Membranes were washed 3 times in TBS/T for 5 minutes and incubated with appropriate peroxidase-conjugated secondary antibodies in TBS. After another 3 washes with TBS/T for 5 minutes, membranes were reacted with the enhanced chemiluminescence system (Amersham Pharmacia) according to the manufacturer's protocol and then exposed to films. Protein levels were quantified by scanning densitometry using image-analysis systems (Bio-Rad, Hercules, CA, USA). The membranes were stained with amido black to ensure that equal amounts of protein were loaded on each lane.
Statistical analysis
Data are expressed as mean ± SEM. The significance of differences was determined by analysis of variance and post hoc multiple comparison test by using SPSS 10.0 software (SPSS, IL, USA). P < 0.05 was considered to be statistically significant.
RESULTS
HS treatment protected against Ang II–induced hypertension
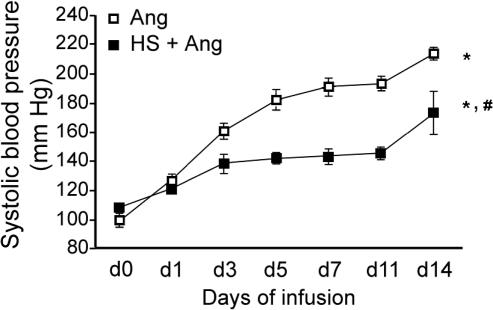
Blood pressure was significantly increased by the infusion of Ang II (0.7 mg/kg/d) (Fig 1). HS treatment suppressed the Ang II–induced elevation of blood pressure. This apparent protective role of HS treatment was significant from day 3 to day 14 of Ang II infusion (P < 0.01).
Fig 1.
Effect of heat shock (HS) treatment on angiotensin II (Ang II)–induced hypertension in rats. HS was administered 24 hours before initiation of Ang II infusion. Systolic blood pressure was measured at days 0, 1, 3, 5, 7, 11, and 14 of Ang II infusion. Data points represent mean ± SEM (n = 6). *P < 0.01, d0 vs d1 to d14 Ang; P < 0.05, d0 vs d1 HS + Ang; P < 0.01, d0 vs d3 to d14 HS + Ang; #P < 0.01, d3 to d14, Ang vs HS + Ang
HS suppressed Ang II–induced activation of NF-κB in aorta
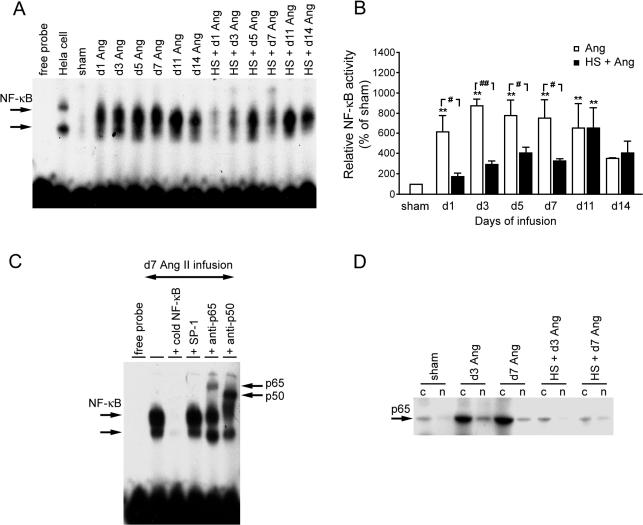
Ang II significantly increased the activation of the transcription factor NF-κB by 24 hours after infusion (Fig 2A,B). Specificity of NF-κB complexes was examined by using 50 times concentration of unlabeled NF-κB or SP-1 oligonucleotides (Fig 2C). Specific competitor NF-κB inhibited the formation of NF-κB–DNA complexes, whereas the same excess of unrelated SP-1 had no effect. Supershift assay using p65 and p50 antibodies shifted the migration characteristics of the complexes and showed that these complexes were specific to NF-κB. Peak activation occurred at day 3, and then decreased gradually but was still elevated at day 11 (Fig 2A,B). At day 14, although there was an apparent increase in the activation of NF-κB after Ang II treatment, this change was not statistical different between Ang II–treated and sham-treated rats. HS treatment significantly suppressed the activation of NF-κB induced by Ang II. Interestingly, by days 11 and 14, the HS treatment no longer suppressed the activation of NF-κB, ie, the activation of NF-κB appeared to be similar, and there was no statistical difference between Ang and HS + Ang groups, suggesting that the protective role of HS was declining by this time. Sham animals showed low-level expression of NF-κB p65 subunit in the cytoplasm (Fig 2D). After Ang II infusion, the p65 subunit was detected at increased levels in both the cytoplasmic and nuclear fractions. HS treatment suppressed the Ang II–induced expression of p65 in the cytoplasmic fraction and appeared to block its translocation to the nuclear fraction.
Fig 2.
Effect of heat shock (HS) treatment on angiotensin II (Ang II)–induced nuclear factor–κB (NF-κB) activation in rat aorta. (A) Electrophoretic mobility shift assay (EMSA) of NF-κB deoxyribonucleic acid–binding activity. (B) Semiquantitative densitometry of NF-κB activity. (C) Specific NF-κB–binding activity and supershift assay showing identification of p50 and p65 subunits in the NF-κB complex. (D) HS suppressed NF-κB p65 subunit expression and its nuclear translocation, particularly at day 3. **P < 0.01 vs sham; #P < 0.05, ##P < 0.01 Ang vs HS + Ang. Cytoplasmic and nuclear fractions are indicated by c and n, respectively. Data are representative of 3 separate experiments
Comparison of HS, vasodilators, and NE on NF-κB activity in aorta
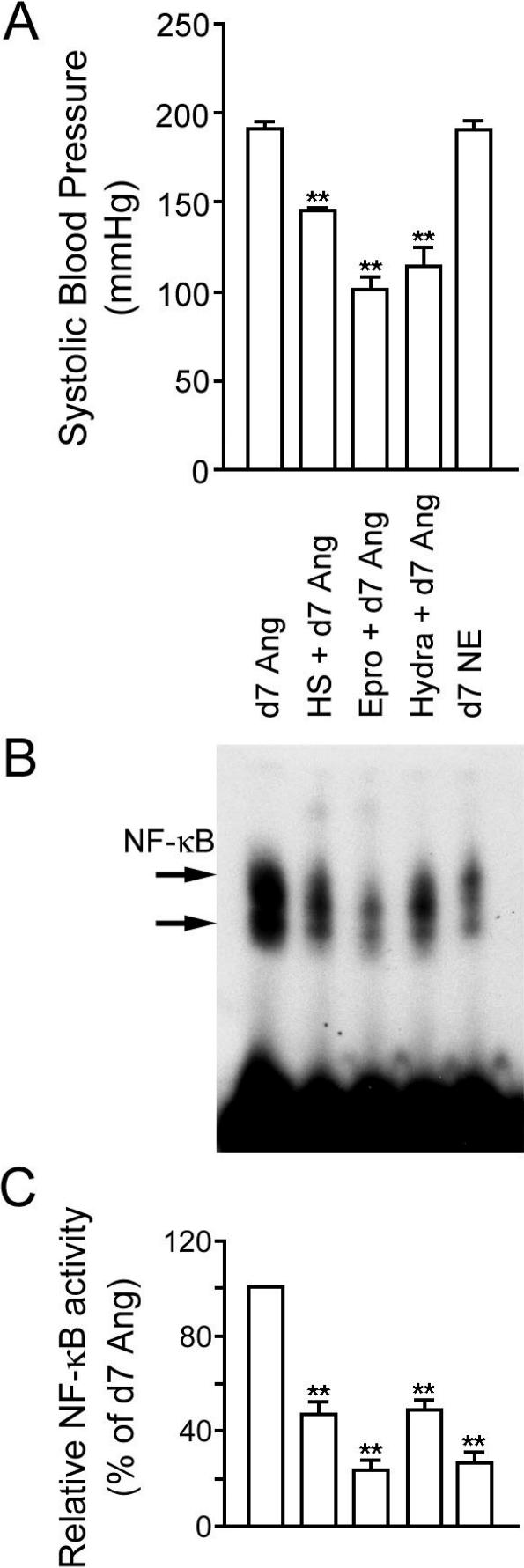
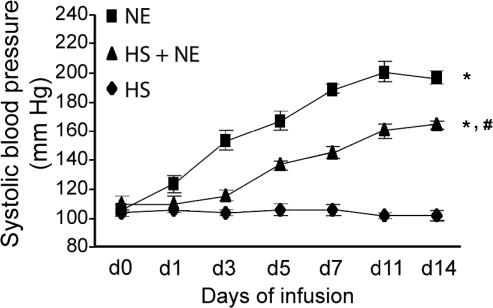
To examine whether NF-κB activation induced by Ang II was mediated through the activation of the AT1 receptor or was dependent of the elevation of blood pressure, we examined the effects of the specific AT1 receptor inhibitor eprosartan and the nonspecific vasodilator hydralazine on NF-κB activation on day 7 of Ang II infusion (Fig 3). Both antihypertensive agents suppressed the Ang II–induced increase in blood pressure (Fig 3A) and blocked the activation of NF-κB in aorta (Fig 3B,C), suggesting that the Ang II–induced activation of NF-κB is a pressordependent event. However, in another hypertension model using NE infusion, NF-κB was not activated, although blood pressure was increased similar to Ang II infusion. Although both eprosartan and hydralazine suppressed the elevation of blood pressure caused by Ang II infusion, there was a significant difference between the suppressing effects of eprosartan and hydralazine on the activation of NF-κB (P < 0.01). HS treatment decreased hypertension and suppressed the activation of NF-κB induced by Ang II, similar to the antihypertensive agent hydralazine. In another set of experiments, we investigated whether HS treatment could suppress the NE-induced increase in blood pressure. Although HS treatment alone had no long-term effect on blood pressure, the HS treatment significantly suppressed the NE-induced hypertension from day 3 (Fig 4).
Fig 3.
Comparison of blood pressure and nuclear factor–κB (NF-κB) activity after 7 days treatment with angiotensin II (Ang II) (day 7 Ang), heat shock (HS) and 7 days treatment with Ang II (HS + d7 Ang), vasodilator eprosartan and 7 days treatment with Ang II (Epro + d7 Ang), vasodilator hydralazine and 7 days treatment with Ang II (Hydra + d7 Ang), and 7 days treatment with norepinephrine (d7 NE). (A) Systolic blood pressure. **P < 0.01 vs d7 Ang II infused. Each bar represents the data from 6 rats. (B) NF-κB activity in aorta. (C) Semiquantitative densitometry of NF-κB activity in aorta. **P < 0.01 vs d7 Ang II infused. There are also significant differences (P < 0.01) between HS + d7 Ang vs Epro + d7 Ang and d7 NE and between Hydra + d7 Ang vs Epro + d7 Ang and d7 NE. Data are representative of 3 separate experiments
Fig 4.
Effect of heat shock (HS) and norepinephrine (NE) on blood pressure in rats. HS was administered 24 hours before implantation of a minipump containing either NE or vehicle (saline). Some rats were anesthetized but not heat shocked before implantation of a minipump containing NE. Systolic blood pressure was measured at days 0, 1, 3, 5, 7, 11, and 14 after minipump implantation. Data points represent mean ± SEM (for HS, n = 6; NE, n = 5; HS + NE, n = 5). *P < 0.01, d1 to d14, NE vs HS; d5 to d14, HS + NE vs HS. #P < 0.05, d1; P < 0.01, d3 to d14, HS + NE vs NE
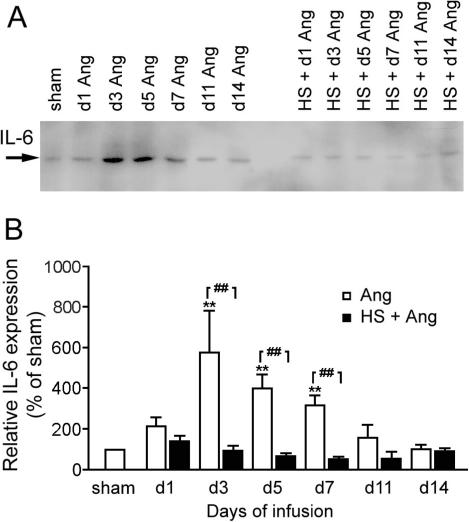
HS suppressed Ang II–induced IL-6 expression in aorta
The expression of IL-6 was significantly elevated by Ang II that peaked at day 3 and remained significantly higher than the basal level from day 3 to day 7 of Ang II infusion (Fig 5). HS treatment 24 hours before Ang II infusion suppressed the IL-6 expression level to near normal levels. Although HS appeared to suppress IL-6 below the basal level from day 3 to day 11, and returned to the basal level at 14 days of Ang II infusion, this decrease was not statistical significant.
Fig 5.
Effect of heat shock (HS) treatment on angiotensin II (Ang II)–induced interleukin-6 (IL-6) expression in rat aorta. **P < 0.01 vs sham; ##P < 0.01 Ang vs HS + Ang. Data are representative of 3 separate experiments
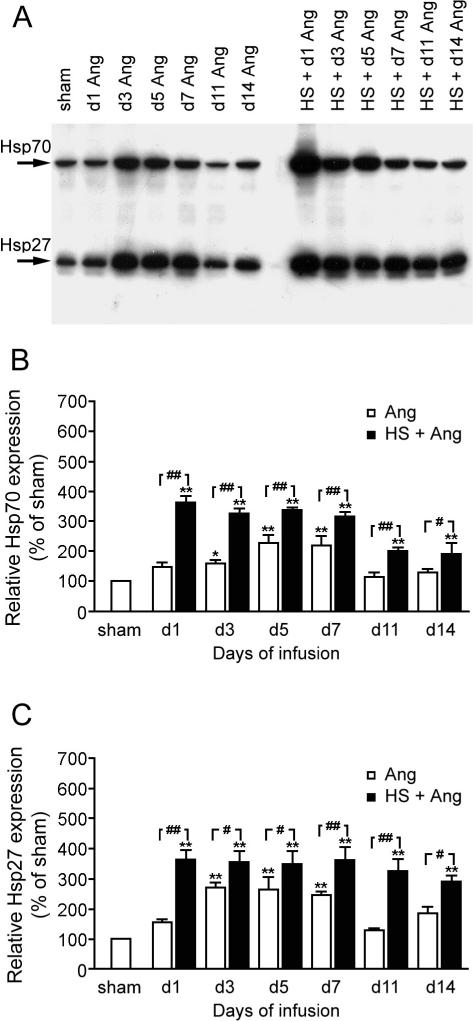
Ang II and HS treatment induced expression of Hsp70 and Hsp27
Interestingly, 3 to 5 days of Ang II treatment induced elevated expression of Hsp70 and Hsp27 (Fig 6). HS treatment induced a more rapid elevation in the expression of Hsp70 and Hsp27 that was associated with the HS-induced suppression of Ang II–induced hypertension, NF-κB activation, and IL-6 expression. The HS-induced expression of Hsp70 and Hsp27 peaked at 24 hours and declined slowly till day 14 but was still above basal levels (Fig 6).
Fig 6.
Expression of heat shock protein (Hsp)70 and Hsp27 in rat aorta after angiotensin II (Ang II) infusion and heat shock (HS) + Ang II infusion. *P < 0.05, **P < 0.01 vs sham; #P < 0.05, ##P < 0.01 Ang vs HS + Ang. Data are representative of 3 separate experiments
DISCUSSION
In this study, we have shown that HS treatment significantly suppresses the increase in blood pressure induced by chronic Ang II infusion. Although the suppression of NF-κB and blood pressure by HS, eprosartan, and hydralazine suggested that the NF-κB activation was dependent on hypertension, treatment with norepinephrine indicated that NF-κB activation was independent of hypertension. This suggests that the activation of NF-κB by Ang II infusion is related to the proinflammatory role of Ang II, as indicated by the elevated expression of IL-6. Finally, the suppression of NF-κB activation and its downstream gene expression, IL-6 by HS treatment, suggests an anti-inflammatory role for Hsps.
In the current study, HS treatment 24 hours before the initiation of Ang II infusion suppressed the Ang II–induced increase in blood pressure between days 3 and 14. We have previously shown that at 24 to 96 hours after HS treatment, when hearts were subjected to ischemia and then reperfusion, coronary artery pressure was significantly decreased compared with control hearts or hearts 192 hours after HS treatment (Karmazyn et al 1990). Coincidentally, the expression of Hsp70 was associated with enhanced postischemic myocardial recovery at 24 and 48 hours after HS treatment. In hearts from transgenic mice overexpressing the human inducible Hsp70, coronary artery pressure was also significantly lower during postischemic reperfusion compared with the coronary artery pressure of nontransgenic hearts (Plumier et al 1995). Because HS treatment induces high-level expression of Hsp70 and Hsp27 and because overexpression of Hsp70 in transgenic mice is associated with decreased arterial pressure, we propose that the effect of HS on hypertension is related to the role of Hsp70 and possibly Hsp27 acting as molecular chaperones. As molecular chaperones, Hsp70 and Hsp27 regulate refolding and renaturation of damaged proteins, which may occur after Ang II infusion. In fact, Ang II induces the production and release of vascular superoxide and oxygen free radicals, which contribute to the alterations of vasomotor tone (Rajagopalan et al 1996; Landmesser et al 2002). The Hsps may interact with smooth muscle proteins such as AT1 receptor to regulate the tone of blood vessels. Alternately, HS may modulate endothelium-dependent vascular relaxation by the anti–oxygen free radical role of Hsps (Pittet et al 2002; Suzuki et al 2002). Another possible mechanism of HS having an effect on blood pressure regulation is through the role of Hsps in the central nervous system. After hyperthermic treatment, select neurons of the hippocampal formation, of the hypothalamus, such as paraventricular nucleus and dorsomedial hypothalamic nucleus, and of the circumventricular organs, such as subfornical organ and area postrema, expressed high levels of Hsp27. This suggests that some Hsp27-positive cells are involved in physiological responses to body fluid homeostasis (Krueger-Naug et al 2000). In addition, these regions of the brain are involved in the modulation of blood pressure by Ang II (Lenkei et al 1997).
Ang II is more than just a regulator of vascular tone, it is also a mediator affecting the local biology of the arterial wall (Han et al 1999; Kranzhofer et al 1999), the heart (Dechend et al 2001; Sano et al 2001), and the kidney (Mervaala et al 2000; Ruiz-Ortega et al 2001) by triggering inflammatory pathways including NF-κB, AP-1, extracellular regulated kinase, and p38 mitogen-activated protein kinase. Among them, NF-κB pathway is particularly interesting. NF-κB is a ubiquitous, inducible transcription factor that is involved in the inflammatory process and promotes transcription of multiple inflammatory factors and cytokines. Usually, NF-κB is in its constitutive form in the cytoplasm and is held in an inactive state by its inhibitor, I-κB. When NF-κB is stimulated by outside signals, such as reactive oxygen intermediates, ultraviolet light, lipopolysaccharide, or TNF-α, it disengages I-κB, translocates into the nucleus, and binds to DNA (Ghosh et al 1998). The most common form of activated NF-κB is the p50/p65 heterodimer. In our study, p50/p65 heterodimer was activated and translocated. Ang II–induced NF-κB activation may relate to the effect of Ang II on the release of oxygen free radicals. Recently, several studies have reported that NF-κB inhibition improves tissue injury induced by Ang II. For example, Muller et al (2001) found that aspirin inhibits NF-κB and protects Ang II–induced end-organ damage in heart and kidney, independent of blood pressure. Similarly, enalapril, pioglitazone, rosiglitazone, 3-hydroxy-3-methylglutaryl coenzyme A, and cyclosporine A are all protective against Ang II–induced inflammation and tissue injury by suppressing NF-κB activity (Mervaala et al 2000; Dechend et al 2001; Diep et al 2002; Ortiz et al 2002).
Endogenous cellular protection is thought to be mediated by Hsps functioning as molecular chaperones to prevent inappropriate protein aggregation, to mediate transport of immature proteins to the target organelles for final packaging, to repair damaged protein, or to target damaged proteins for degradation. Recently, several studies report that the heat stress response protects against tissue injury by increasing the expression of Hsps and suppressing the activation of NF-κB. Yoo et al (2000) found that the anti-inflammatory effect of Hsp70 by HS treatment in respiratory epithelial cells is related to the stabilization of I-κBα and hence the inhibition of NF-κB. Pritts et al (2000) first reported the downregulation of NF-κB activity by stress response in vivo. They induced Hsp70 expression by hyperthermia (42°C) or injection with sodium arsenite (10 mg/kg). The increase of NF-κB–DNA-binding activity and the decrease of I-κBα after endotoxin injection were inhibited by previous HS stress. Hsps protection by way of the NF-κB pathway has also been shown in heart and in liver ischemia injury (Shimizu et al 2002; Uchinami et al 2002). In this study, we report that HS treatment protects against Ang II–induced tissue inflammation by suppressing the inflammatory transcription factor NF-κB. This protection is related to high expression of Hsp70 and Hsp27 induced by HS. The HS protection against Ang II–induced inflammation appears to be independent of blood pressure. In this study, we found that although HS treatment suppressed the hypertension induced by Ang II, the systolic blood pressures were still higher than normal. Although NE increased blood pressure similar to Ang II, it did not activate NF-κB. Interestingly, NE-induced hypertension was not associated with an increase in vascular superoxide production and did not alter endothelial regulation of vasomotion as Ang II did (Rajagopalan et al 1996). This data suggest that Ang II may have a unique vascular effect that is not shared by other forms of hypertension. Our finding that HS treatment suppressed the NE-induced increase in blood pressure suggests that the cells of the aorta responsible for the increased blood pressure may be in a generally unresponsive state, ie, they are not responding to the signaling stimuli involved in establishing the hypertensive state of the aorta. In fact, Hsp70 and Hsp27 are reported to block apoptotic signaling pathways (Mosser et al 2000; Gabai et al 2002; Paul et al 2002), providing precedence for thinking that Hsps may block other kinds of signaling pathways as well.
There are 3 possible mechanisms for the HS inhibition of NF-κB activation. First, HS may increase cytoplasmic I-κB proteins. HS increases I-κB expression and decreases NF-κB activation (Wong et al 1997a, 1997b; Pritts et al 2000). Human I-κBα promoter contains a contiguous 20-bp segment that matches with the heat shock element (Wong et al 1997b); therefore, I-κBα may be regarded as a stress protein. Second, Hsps induced by HS may sense conformational changes of I-κB proteins by their role as molecular chaperones and interact with modified I-κB to prevent the subsequent phosphorylation, degradation, and disassociation from NF-κB complexes. By immunoprecipitation and double-labeling immunofluorescence, Shimizu et al (2001) detected Hsp70–I-κBα immunocomplexes and suggested that I-κBα stabilization through formation of complexes with Hsp70 attenuates NF-κB activation. Third, Guzhova et al (1997) have shown that Hsp70 could chaperone NF-κB and inhibit its activation directly, as a partial substitute for I-κB. In addition, it is also possible that Hsps or the Hsp–I-κB complex may inhibit the I-κB kinase directly or indirectly. Further studies to differentiate the roles played by Hsp70 and Hsp27 in Ang II–induced inflammation and hypertension are necessary to reveal the precise mechanism of the interaction of HS/Hsps and NF-κB pathway in Ang II–induced tissue injury.
Acknowledgments
This work was funded by grants from the Heart and Stroke Foundation of New Brunswick and the Canadian Stroke Network. Y.C. was supported by a scholarship from the Killam Trusts (2000–2003) and is currently supported by a scholarship from Nova Scotia Health Research Foundation (2003–2005).
REFERENCES
- Aizawa T, Ishizaka N, Taguchi J, Nagai R, Mori I, Tang SS, Ingelfinger JR, Ohno M. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: possible role in renoprotection. Hypertension. 2000;35:800–806. doi: 10.1161/01.hyp.35.3.800.0194-911X(2000)035<0800:HOIUIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Beere HM. Stressed to death: regulation of apoptotic signaling pathways by the heat shock proteins. Sci STKE. 2001;31:RE1. doi: 10.1126/stke.2001.93.re1.1525-8882(2001)031<RE1:STDROA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132.1044-1549(1997)017<0003:TRONFB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Currie RW, Karmazyn M, Kloc M, Mailer K. Heat-shock response is associated with enhanced post-ischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543.0009-7330(1988)063<0543:HRIAWE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Currie RW, Plumier J-CL 1998 The heat shock response and tissue protection. In: Delayed Preconditioning and Adaptive Cardioprotection, ed Baxter GF, Yellon DM. Kluwer Academic Publishers, Dordrecht, The Netherlands, 135–153. [Google Scholar]
- Dechend R, Fiebeler A, and Park JK. et al. 2001 Amelioration of angiotensin II-induced cardiac injury by a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Circulation. 104:576–581. [DOI] [PubMed] [Google Scholar]
- Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23.0009-7322(2002)105<2296:SEFCGA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22:3415–3424. doi: 10.1128/MCB.22.10.3415-3424.2002.0270-7306(2002)022<3415:HASKCN>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225.0732-0582(1998)016<0225:NBARPE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2.1466-1268(1997)002<0132:MSPHIW>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res. 1999;84:695–703. doi: 10.1161/01.res.84.6.695.0009-7330(1999)084<0695:AIIITI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0.0028-0836(1996)381<0571:MCICPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Aizawa T, and Ohno M. et al. 2002 Regulation and localization of HSP 70 and HSP 25 in the kidney of rats undergoing long-term administration of Angiotnesin II. Hypertension. 39:122–128. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, De Leon H, Laursen JB, Fukui T, Wilcox JN, De Keulenaer G, Griendling KK, Alexander RW. Angiotensin II-induced hypertension increase heme oxygenase-1 expression in rat aorta. Circulation. 1997;96:1923–1929. doi: 10.1161/01.cir.96.6.1923.0009-7322(1997)096<1923:AIHIHO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Mailer K, Currie RW. Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 1990;259:H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424.0002-9513(1990)259<H424:AADOHP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kranzhofer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kubler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1623–1629. doi: 10.1161/01.atv.19.7.1623.1079-5642(1999)019<1623:AIIAOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krueger-Naug AM, Hopkins DA, Armstrong JN, Plumier J-CL, Currie RW. Hyperthermic induction of the 27-Kda heat shock protein (Hsp27) in neuroglia and neurons of the rat central nervous system. J Comp Neurol. 2000;428:495–510. doi: 10.1002/1096-9861(20001218)428:3<495::aid-cne7>3.0.co;2-4.0021-9967(2000)428<0495:HIOTKH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krueger-Naug AM, Plumier J-CL, Hopkins DA, and Currie RW 2002 Hsp27 in the nervous system: expression in pathophysiology and in the aging brain. In: Small Stress Proteins, ed Arrigo AP, Muller WEG. Prog Mol Subcell Biol. 28:235–251. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98.0194-911X(2002)040<0511:ROPIVO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le JM, Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Investig. 1989;61:588–602.0023-6837(1989)061<0588:IAMCRI>2.0.CO;2 [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155.0091-3022(1997)018<0383:EOATAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275.0021-9258(1951)193<0265:PMWTFP>2.0.CO;2 [PubMed] [Google Scholar]
- Meier M, King GL, Clermont A, Perez A, Hayashi M, Feener EP. Angiotensin AT(1) receptor stimulates heat shock protein 27 phosphorylation in vitro and in vivo. Hypertension. 2001;38:1260–1265. doi: 10.1161/hy1201.096573.0194-911X(2001)038<1260:AARSHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mervaala E, Muller DN, and Park JK. et al. 2000 Cyclosporin A protects against angiotensin II induced end-organ damage in double transgenic rats harboring human rennin and angiotensionogen genes. Hypertension. 35:360–366. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000.0270-7306(2000)020<7146:TCFOHI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DN, Heissmeyer V, and Dechend R. et al. 2001 Aspirin inhibits NF-kappaB and protects from angiotensin II-induced organ damage. FASEB J. 15:1822–1824. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Champion HC, and Lasky JA. et al. 2002 Enalapril protects mice from pulmonary hypertension by inhibiting TNF-mediated activation of NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol. 282:L1209–L1221. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002.0270-7306(2002)022<0816:HAANRO>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet JF, Lu LN, Geiser T, Lee H, Matthay MA, Welch WJ. Stress preconditioning attenuates oxidative injury to the alveolar epithelium of the lung following haemorrhage in rats. J Physiol. 2002;538:583–597. doi: 10.1113/jphysiol.2001.013102.0022-3751(2002)538<0583:SPAOIT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier J-CL, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2.1466-1268(1997)002<0162:TMETHI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier J-CL, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved postischemic myocardial recovery. J Clin Investig. 1995;95:1854–1860. doi: 10.1172/JCI117865.0021-9738(1995)095<1854:TMETHH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritts TA, Wang Q, Sun X, Moon MR, Fischer DR, Fischer JE, Wong HR, Hasselgren PO. Induction of the stress response in vivo decreases nuclear factor-kappa B activity in jejunal mucosa of endotoxemic mice. Arch Surg. 2000;135:860–866. doi: 10.1001/archsurg.135.7.860.0004-0010(2000)135<0860:IOTSRI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munze T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation—contribution to alterations of vasomotor tone. J Clin Investig. 1996;97:1916–1923. doi: 10.1172/JCI118623.0021-9738(1996)097<1916:AIHITR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, and Preville X. et al. 1999 Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 274:18947–18956. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol. 2001;158:1743–1756. doi: 10.1016/s0002-9440(10)64130-2.0002-9440(2001)158<1743:SIOAII>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Fukuda K, and Sato T. et al. 2001 ERK and p38 MAPK, but not NF-kB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 89:661–669. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tamamori-Adachi M, Arai H, Tanaka H, Sunamori M. Lipopolysaccharide pretreatment attenuates myocardial infarct size: a possible mechanism involving heat shock protein 70-inhibitory kBα complex and attenuation of nuclear factor kB. J Thorac Cardiovasc Surg. 2002;124:933–941. doi: 10.1067/mtc.2002.122305.0022-5223(2002)124<0933:LPAMIS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, and Sammut IA. et al. 2002 Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 106:I270–I276. [PubMed] [Google Scholar]
- Uchinami H, Yamamoto Y, and Kume M. et al. 2002 Effect of heat shock preconditioning on NFkappaB/I-kappaB pathway during I/R injury of the rat liver. Am J Physiol Gastrointest Liver Physiol. 282:G962–G971. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063.0031-9333(1992)072<1063:MSRCPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wong HR, Ryan M, Wispe JR. The heat shock response inhibits inducible nitric oxide synthase gene expression by blocking IkB degradation and NF-kB nuclear translocation. Biochem Biophys Res Commun. 1997a;231:257–263. doi: 10.1006/bbrc.1997.6076.0006-291X(1997)231<0257:THSRII>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wong HR, Ryan M, Wispe JR. Stress response decreases NF-kB nuclear translocation and increases I-kBa expression in A549 cells. J Clin Investig. 1997b;99:2423–2428. doi: 10.1172/JCI119425.0021-9738(1997)099<2423:SRDNNT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VY, Laping NJ, Contino LC, Olson BA, Grygielko E, Brooks DP. Gene expression in rats with renal disease treated with angiotensin II receptor antagonist, eprosartan. Physiol Genomics. 2000;4:35–42. doi: 10.1152/physiolgenomics.2000.4.1.35.1531-2267(2000)004<0035:GEIRWR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu Q, Li D, Holbrook NJ, Udelsman R. Acute hypertension induces heat-shock protein 70 gene expression in rat aorta. Circulation. 1995;92:1223–1229. doi: 10.1161/01.cir.92.5.1223.0009-7322(1995)092<1223:AHIHPG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–5423. doi: 10.4049/jimmunol.164.10.5416.0022-1767(2000)164<5416:AEOHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]