Abstract
Epithelial cells have been identified in the blood and bone marrow of patients with cancer and other diseases. However, the presence of normal epithelial cells in the blood and bone marrow of healthy individuals had yet to be identified in a consistent way. Presented here is a reproducible method for isolating epithelial cells from healthy human and murine blood and bone marrow (BM) using flow cytometry and immunofluorescence (IF) microscopy. Epithelial cells in healthy individuals were first identified and isolated via flow cytometry using epithelial cell adhesion molecule (EpCAM). These EpCAM positive cells were confirmed to express keratin using immunofluorescence microscopy and Krt1–14;mTmG transgenic mice. Human blood samples had 0.18% ± 0.0004 of all cells EpCAM+ (standard error of the mean [SEM]; n=7 biological replicates, 4 experimental replicates). In human BM, 3.53% ± 0.006 (SEM; n=3 biological replicates, 4 experimental replicates) of mononuclear cells were EpCAM+, regardless of the number of cells counted. In mouse blood, EpCAM+ cells constituted 0.45% ± 0.0006 (SEM; n=2 biological replicates, 4 experimental replicates) and in mouse BM, 5.17% ± 0.001 (SEM; n=3 biological replicates, 4 experimental replicates) were EpCAM+. In mice, virtually all the EpCAM+ cells were immunoreactive to pan-cytokeratin as determined by IF microscopy. Results were confirmed using Krt1–14;mTmG transgenic mice, with low (8.6 native GFP+ cells per 106 cells analyzed; 0.085% of viable cells), but significant numbers (p<0.0005) of GFP+ cells present in normal murine BM that were not the result of randomness compared with multiple negative controls. Further, EpCAM+ cells in mouse blood were more heterogeneous than CD45+ cells (0.58% in BM; 0.13% in blood). These observations conclude that cells expressing cytokeratin proteins are reproducibly detectable among mononuclear cells from human and murine blood and BM. This report demonstrates a method of tissue harvesting, flow cytometry, and immunostaining that can be used to identify and determine the function of these pan-cytokeratin epithelial cells in healthy individuals.
SUMMARY:
This paper will present a reproducible method with new findings on the presence of epithelial cells in normal human and mouse blood and bone marrow using flow cytometry and immunofluorescence microscopy. Krt1–14;mTmG transgenic mice were used as an in vivo method to confirm these findings.
INTRODUCTION:
Epithelial cells are found in the physical barriers between our bodies and the environment, and are able to recognize and respond to changes in their microenvironment8. They have a proliferating stem cell niche which provides a way to turn over new tissue and repair damage1. The Morris lab studies stem cells in the hair follicles of skin. Skin is a good model for studying epithelial tissue and stem cell proliferation because it is easily visible and there is a constant turnover of cells. Epithelial cancers are the most common form of cancer, possibly due to epithelial tissues, such as the skin, being the first line of defense against environmental carcinogens, leading to high turnover rates and proliferation of epithelial cells4. Most of the skin epidermis, or top protective layer, is composed of keratinocytes, which express different types of keratins to provide support and structure5. Patients with epithelial cancers often have epithelial cells present in their blood and bone marrow that also express keratins. Liquid biopsies are a non-invasive way to detect and monitor these epithelial cells in different bodily fluids13. Circulating epithelial cells, also called circulating tumor cells (CTCs), are found in peripheral blood and can be biomarkers for cancer prognosis, as well as guide individualized therapy treatments. CTCs can also indicate disease progression, treatment efficacy, and overall patient survival.
Epithelial cell adhesion molecule (EpCAM) is a clinically used marker for CTCs and can identify tumors of epithelial origin in cancer patients. EpCAM plays a role in cell adhesion, migration, signaling, proliferation, and differentiation. For a circulating epithelial cell to be classified as a CTC, it must also be positive for cytokeratins 8, 18, and 19 and negative for CD45, a common leukocyte marker6. CTCs are usually identified by first depleting CD45 with magnetic microbeads followed by testing for EpCAM and cytokeratin 19 using immunofluorescence microscopy11. The main limitation to the detection of CTCs is their rarity; they make up less than 0.01% of all cells in the blood, and very few survive in circulation to reach distant organs9,14,18. Consideration must go into designing experiments and techniques to isolate and identify these cells due to their rare nature. Currently, there is only one FDA-approved automated single cell sorter used for identifying CTCs, and it uses EpCAM as its biomarker. Other methods include magnetic bead separation and flow cytometry, or a combination of these methods. There is a need for new techniques that have higher sensitivity and specificity for the detection of rare CTCs17.
Flow cytometry is a preferred method for the detection of rare cell populations in the blood, bone marrow, and other tissue samples. These rare cells can include stem cells, circulating endothelial cells, circulating tumor cells, and residual disease cells. Flow cytometry enables quantitative measurements of each cell type, and sorts those cells for further testing11,14. Incremental counts were performed to ensure accurate assessment of the rare cells. The ability to use gating to exclude cells from further analysis is a way to increase specificity when analyzing cells. The limitations of flow cytometry are the time required for the analysis of large samples and the lack of visual confirmation for cell identity. To overcome this, immunofluorescence microscopy was performed on the sorted cells to confirm their identities.
Previously, the Morris lab has shown in mice that bone marrow cells are recruited and contribute to skin tumors12. These bone marrow-derived cells are positive for pan-cytokeratin and epidermal cytokeratins. To elucidate further the role of epithelial stem cells, bone marrow-derived cells, and cytokeratin expressing cells in tumor progression, EpCAM+ cytokeratin positive cells in normal murine and human blood and bone marrow samples were looked for. As with most experiments, this method was developed through multiple iterations. In the 2018 Nature Communications paper by Heuijoon Park and associates, transgenic mice were used along with incremental counts to look for K14GFP-expressing cells in the bone marrow12. As more cells were counted, a representative population of rare cells was able to be identified as shown in Figure 1 in the representative results section. The rationale for investigating epithelial cells in normal blood and bone marrow was based on early literature on CTCs where normal healthy donors had background levels of EpCAM+ cells10. As mentioned previously, EpCAM characterization often begins with the depletion of CD45. This step was omitted because some hematopoietic cells have cytokeratin expression for unknown reasons. Therefore, cells were sorted for EpCAM and then stained for cytokeratin. The protocol below and the workflow shown in Figure 2, describe a technique that uses flow cytometry with compensation and controls, statistical methods, and immunofluorescence imaging to isolate and identify these rare populations of epithelial cells.
Figure 1. Krt14Cre;mTmG transgenic mice with incremental counts of bone marrow.
The bone marrow of Krt1–14;mTmG mice were counted incrementally. GFP positive cells indicate keratin 14 expression and were identified using flow cytometry. As more bone marrow cells were counted, this keratin 14 positive cell population was more easily identifiable.
Figure 2. Workflow for EpCAM+ and cytokeratin+ cells.
The bone marrow and blood cells were first sorted using fluorescence activated cell sorting (FACS) to separate EpCAM+ and EpCAM-cells. These cells were sorted into two different test tubes, as well as onto two different slides. The cells sorted into test tubes were spun onto slides using a cytocentrifuge. Slides were then stained using a pan-cytokeratin primary antibody, then stained with an AlexaFluor 488 secondary antibody. The slides were analyzed using fluorescence microscopy to observe pan-cytokeratin expression.
PROTOCOL
NOTE: All animal use protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee in accordance with NIH and federal guidelines.
1. Preparing solutions
NOTE: All solutions must be prepared in a biological hood with a sterile environment. Obtain BSL2 certification for work with human blood and bone marrow.
1.1. Prepare bone marrow harvest solution by adding 1 mL gentamicin and 5 mL fetal bovine serum (FBS) to 500 mL Hank’s balance salt solution (HBSS).
1.2. Prepare staining buffer solution by adding 50 mL FBS to 500 mL HBSS.
1.3. Prepare 1X lysis buffer by combining 10 mL 10X lysis buffer with 90 mL sterile water.
2. Preparing the hood
2.1. Turn on the hood and allow it to run a few minutes to obtain proper airflow.
2.2. Gather supplies to perform bone marrow harvest. Spray all supplies and inside of the hood with 70% ethanol to maintain a sterile environment. Spray hands every time before they enter the hood.
2.3. Place the autoclaved tray in the hood along with autoclaved scissors, assembled scalpel, tweezers, and curved forceps in a small cup filled with 70% ethanol to keep sterile.
2.4. Add 10 mL of the bone marrow harvest solution to one 50 mL centrifuge tube and label it “Limbs”. Label another 50 mL centrifuge tube “Bone Marrow”.
2.5. Draw up 10 mL of bone marrow harvest solution into a syringe and then attach a 26 gauge needle.
3. Harvesting bone marrow from mice
3.1. Euthanize the mouse using CO2 gas. Death is confirmed by absence of a heartbeat 123 and pinching the feet to ensure that there is no reaction. Cervical dislocation may 124 be performed as a secondary means of complete euthanasia.
3.2. Place the mouse in a 500 mL container and add enough iodine to cover half of the mouse. Gently shake and swirl the container to ensure thorough coverage. Rinse with deionized water. Perform one more iodine wash, and then two washes with 70% ethanol following the same steps.
3.3. Place the mouse in the hood and lay it on its back on the tray. Grab the hind legs and pull them apart until a pop is felt, this is to separate the legs from the spine.
3.4. Make a 1 cm incision on the mouse’s skin near the groin area. Insert a closed pair of scissors into the incision and then open the scissors under the skin to separate it from the peritoneum.
3.5. Cut the skin on the leg around the thigh, and then cut down the leg to expose the muscles and bones.
-
3.6. Remove the hind legs by cutting around the hip joint, being sure not to cut through the femur. Add the limbs to the tube labeled “Limbs.” When both legs are removed, place the mouse into a carcass bag and put it in the freezer.
NOTE: Feel where the hip bone is to help guide the scissors and avoid cutting the femur as that is where most of the bone marrow is.
3.7. With one of the limbs, begin cutting away the muscle, tissue, and fat. Start by using the scissors and cut parallel to the bone. Then use the scalpel and remove remaining fat or muscle using a perpendicular scraping motion against the bone.
3.8. Once cleaned down to the bone, separate the femur and tibia at the knee. Use the scalpel to make cuts on both ends of the femur and tibia where there is no visible bone marrow.
3.9. Insert the prepared syringe and needle into the bone. If there is resistance, cut a little more off the end of the bone until there is no longer resistance. While holding the bone above the “Bone Marrow” tube, expel bone marrow harvesting solution through the bone to flush the bone marrow into the tube. Repeat on the other end of the bone to obtain the rest of the bone marrow, until the bone appears all white or empty.
3.10. Repeat steps 3.7–3.9 for all remaining bones and limbs; all marrow will be flushed into the same conical tube.
3.11. Using an empty 10 mL syringe and a 20-gauge needle, break up the clumps of bone marrow in the tube by drawing the bone marrow up and down in the syringe 5–10 times.
3.12. Add a sterile 40 μm filter to a clean 50 mL centrifuge tube and rinse it with 1 mL of bone marrow harvest solution. Then filter the bone marrow to remove any remaining clumps. Label this tube “Filtered Bone Marrow.”
-
3.13. Store the bone marrow in the refrigerator or on ice until ready to use. It should be used within the same day as harvesting. If not used on the same day, freeze the cells using a cryopreservative such as DMSO.
CAUTION: All waste from these experiments should be disposed of properly in a biohazard waste container. All sharps should go in a properly labeled biohazard sharps container.
4. Red blood cell lysis
4.1. Centrifuge the cells at 170 x g for 10 minutes. Vacuum and dispose of the supernatant. Resuspend the cells in 10 mL of 1X red blood cell lysis buffer and incubate for 4 minutes.
4.2. Add 30 mL of Dulbecco’s phosphate buffered saline (dPBS) to stop the reaction of the lysis buffer. Centrifuge the cells for 8 minutes at 170 x g. Vacuum and dispose of the supernatant. Resuspend in 10–20 mL of staining buffer.
5. Cell count
-
5.1. Make a 1:20 dilution using the bone marrow harvest solution.
NOTE: This will be used for cell counts and does not need to be kept sterile.
5.2. Draw out 200 μl of cells, add them to 200 μl of trypan blue and mix well.
-
5.3. Add the mixture to a hemocytometer and count the alive and dead cells on both sides. Calculate the original cell suspension using this table and equations:
Alive Dead Total Side 1 Side 2 Total Cells per mL:Total Cells: 5.4. Spin down the cells at 170 x g for 10 minutes and resuspend to a concentration of 10 × 106 per 1 mL (1 × 106 cells per 100 μL) based on the manufacturer’s suggestions for antibody dilutions.
5.5. Proceed to flow cytometry (see step 8).
6. Harvesting blood from mice
6.1. Euthanize the mouse using CO2 gas or cervical dislocation.
6.2. Perform a cardiac blood draw at a 45-degree angle with a 26 gauge needle attached to a 1 mL syringe coated with 0.5 M EDTA.
-
6.3. Spin down the blood and resuspend in media or staining buffer.
NOTE: Cells will be spun down and resuspended to final concentration in staining buffer after red blood cell lysis and cell count.
6.4. Perform red blood cell lysis (see step 4).
6.5. Perform cell count (see step 5).
6.6. Proceed to flow cytometry (see step 8).
7. Processing human blood and bone marrow samples
7.1. Perform all procedures under BSL2-certified hood while wearing personal protective equipment.
7.2. Fresh blood and bone marrow was purchased from Lonza (1M-125D and 3W-370). Samples were collected with an approved IRB protocol from donors negative for HIV, Hepatitis B, Hepatitis C, and screened for COVID-19.
7.3. Samples were collected the night before and red blood cell lysis was performed by Lonza. All samples were de-identified and anonymized by Lonza before shipping the mononuclear samples overnight on ice.
-
7.4. Upon arrival, spin down live cells at 170 x g for 10 minutes.
NOTE: These are BSL2 samples which must follow local guidelines.
-
7.5. Resuspend cells in media or staining buffer.
NOTE: Cells will be spun down and resuspended to final concentration after cell count with staining buffer.
7.6. Perform cell count (see step 5).
7.7. Proceed to flow cytometry (step 8).
8. Flow cytometry
8.1. Staining
-
8.1.1. Divide the cells into individually labeled tubes based on the staining panel used, including compensation controls (unstained, isotype controls, single stained, and Fluorescence Minus Ones [FMOs]). Freeze any remaining cells or use them for fluorescence-activated cell sorting. See Table 1 for an example of a staining panel with antibody dilutions used.
NOTE: For every additional fluorophore added, FMOs in combination with remaining fluorophores must be added along with a single stain of that fluorophore. Isotype controls may be used to block non-specific staining. Each new antibody used should be titrated with the samples for optimal dilution.
-
8.1.2. Add antibodies according to manufacturer’s recommended dilutions and mix well by flicking the bottom of the tubes. Antibody concentrations are usually given as 1×106 cells per 100 μL. Dilutions with antibodies and controls are shown in Table 1. Antibodies are conjugated with fluorophores and must be protected from light.
8.1.2.1. It is best to use EpCAM conjugated to phycoerythrin (PE) to enable visualizing low populations of cells (Tritrate at 3–5 uL per 1 million cells as indicated in Table 1). PE-CD49f was used as a single stain PE control (20 uL per 1 million cells) because these cells are more prevalent than PE-EpCAM and thus easier to use for compensation.
8.1.3. Incubate for 30 minutes at 4 °C in the dark.
8.1.4. After incubation, bring tubes back to the BSL2 hood and add 1 mL of staining buffer to each tube.
8.1.5. Then centrifuge the tubes at 170 x g for 5–10 minutes.
8.1.6. After centrifugation, bring the capped tubes with cells back to the BSL2 hood and aspirate out the supernatant. Be sure to change tips on the aspiration pipet between each tube to prevent cross-contamination.
8.1.7. Resuspend the cell pellet in 1 mL of staining buffer and flick the bottom of the tubes to mix.
-
8.1.8. Repeat the wash of the tubes twice with aspiration of the media and 250 resuspending each time by flicking the bottom of the tubes.
NOTE: Do not use pipettes to resuspend to avoid losing cells.
-
8.1.9. Resuspend in 500 μL staining buffer for the final resuspension before flow cytometry.
8.1.9.1. Add DAPI or dead cell discriminator according to the manufacturer’s recommendations.
Table 1. Flow cytometry staining panel.
An example of a flow cytometry staining panel for blood or bone marrow mononuclear cells. Controls included are DAPI only, unstained control, and PE single stain control (PE-CD49f was used as a better positive control). Fluorescence Minus One are not included in this panel as it is only a single color (PE). When adding more fluorophore colors, such as FITC or APC, make sure to include FMOs by excluding one fluorophore for each FMO control.
| Human Bone Marrow or Blood | |||
|---|---|---|---|
| Antibody | Tube # | # of Cells | AB Conc |
| Unstained | 1 | 1x106 | X |
| DAPI only | 2 | 1x106 | 1uL/mL |
| PE isotype control | 3 | 1x106 | 1 uL |
| CD49f-PE Single Stain Control | 4 | 1x106 | 20 uL in 100uL per 1x106 |
| EpCAM-PE Low Titration | 5 | 1x106 | 3 uL in 100uL per 1x106 |
| EpCAM-PE Medium Titration | 6 | 1x106 | 4 uL in 100uL per 1x106 |
| EpCAM-PE High Titration | 7 | 1x106 | 5 uL in 100uL per 1x106 |
| EpCAM-PE High Sort on Slides | 8 | 10x106 | 50 uL in 1 mL |
8.2. Flow cytometer
-
8.2.1. After setting up the machine, use the controls and FMOs to set the 258 compensation and gates. Hank’s balanced salt solution (HBSS) is used as sheath fluid to help support the fragile cells. PBS can be used if HBSS is not available.
NOTE: Flow cytometer used was BD Aria 2 with DIVA software.
-
8.2.2. Load samples one at a time and collect data points for 50,000, 100,000, 500,000, and 1,000,000 events. Keep tubes on ice or refrigerated until use.
NOTE: A time limit can be set when collecting to ensure sample viability.
8.2.3. Collect cells into 3 mL of RPMI with 10% FBS in 15 mL centrifuge tubes.
8.2.4. If performing immunofluorescence on EpCAM+ cells, use the flow cytometer to sort 800 to 1,000 cells into each well of an 8-well slide.
-
8.2.5. Sort out the EpCAM- cell population onto separate slides as a negative control to use when staining for immunofluorescence.
NOTE: Cells can also be sorted into 15 mL tubes containing FBS and spun onto a slide using the cytocentrifuge or a pipette to prevent cells from popping.
8.2.5.1. Fix slides in a 50% methanol/50% acetone mixture at −20 °C for 10 minutes.
8.2.5.2. Store slides at −20 °C until staining with immunofluorescence.
8.3. Flow cytometry analysis
8.3.1. Open licensed flow cytometry analysis software.
8.3.2. Load FCS files acquired from flow cytometer with controls and samples.
8.3.3. Click create group and use keywords to place samples and controls into a group.
-
8.3.4. Use the no stain control to gate in flow cytometry analysis software as shown in Figure 3.
8.3.4.1. Double click on the no stain control sample to open an ungated graph.
8.3.4.2. Set the graph y-axis to SSC-A and x-axis to FSC-A (side scatter area by forward scatter area) which will indicate cell size and internal complexity.
8.3.4.3. Click on the polygon shape in the top left panel and create a gate around the cells. Make sure to exclude the cells on the peripheral as these are most likely debris. Label this gate “Cells” as shown in Figure 3A.
8.3.4.4. Once the cells are gated, double click inside the polygon shape to open another graph in a new window.
8.3.4.5. Set the y-axis of the new graph to SSC-A and x-axis to SSC-W for doublet discrimination. Click the polygon shape and create a rectangle around the single cells. Label this “Single Cells” as shown in Figure 3B.
8.3.4.6. Once the cells are gated, double click inside the polygon shape to open another graph in a new window.
8.3.4.7. Set the y-axis to FSC-A and x-axis to DAPI-A (or whichever dead cell discriminator was used).
8.3.4.8. Create a polygon gate around the DAPI negative cells on the lefthand side of the graph. Label these “Live Cells” as shown in Figure 3C.
8.3.4.9. Once the cells are gated, double click inside the polygon shape to open another graph in a new window.
8.3.4.10. Set the y-axis to SSC-A and x-axis to EpCAM-PE (or whichever fluorophore is used to identify the EpCAM cells). Cells on the left are EpCAM negative. If any cells are shown on the right, they are EpCAM positive.
-
8.3.4.11. Use the EpCAM-PE graph to create a polygon gate which excludes the cells on the left and only includes the empty space on the right. Label the empty area on the right inside the polygon “EpCAM” as shown in Figure 3D.
NOTE: This is a no stain control so there should not be EpCAM expression. Use the no stain control to determine which cells are EpCAM positive.
8.3.4.12. This completes the gating strategy for compensation so all graphs may be closed at this time.
8.3.4.13. On the workspace page, highlight the no stain lineage with “Cells”, “Single Cells”, “Live Cells”, and “EpCAM”. Right click and select copy analysis to group. This will copy the gating strategy to all other loaded samples within the previously created group. If a group was not created initially, it can be created now by selecting Create Group in the top left.
8.3.4.14. Go through the samples within the group and check the polygon gates drawn previously to make sure they fit with all samples and controls. This will provide percentage number and number of cells.
8.3.4.15. Click the Layout Editor button “L” in the top left corner above “Workspace”. These graphs may then be arranged and exported using the layout editor.
Figure 3. Flow cytometry analysis software.
When analyzing flow cytometry data using analysis software, use the no stain control to select the cells of interest. Cells are first selected with SSC-A and FSC-A which show the internal complexity and size of the cells. A polygon gate is drawn around the cells as shown in A. Acquire single cells by gating SSC-A by SSC-W as shown in B. Acquire live cells by gating FSC-A vs DAPI as shown in C. Exclude EpCAM negative cells by gating to the right of the EpCAM negative cells as shown in D.
8.4. Preparation of immunofluorescence reagents
8.4.1. Prepare the antibody diluent by combining 1 g bovine serum albumin (BSA), 2 g non-fat milk, 10 mL 10X tris-buffered saline (TBS), 100 μL Tween, and 90 mL distilled water.
8.4.2. Prepare TBST by combining 50 mL 20x TBS, 950 mL distilled water, and 200 μL Tween.
8.4.3. Prepare 10% NHS by combining 1 mL NHS with 9 mL antibody diluent (see step 8.4.1).
8.4.4. Prepare 1% NHS by combining 1 mL of 10% NHS with 9 mL TBST.
8.5. Immunofluorescence staining
8.5.1. Remove slides from −20 °C and allow them to warm up to room temperature.
8.5.2. Wash slides 3 times in distilled water for 5 minutes each.
8.5.3. Block slides for 1 hour at room temperature in 10% NHS in antibody diluent (see step 8.4.3).
8.5.4. Dilute the primary antibody (Pan-cytokeratin) to 1:750 in 1% NHS in TBST. (see step 8.4.4). Incubate slides in the diluted primary antibody overnight at 4 °C.
8.5.5. The next day, wash the slides 3 times with a 1X wash buffer (PBS or TBST) for 5 minutes each.
8.5.6. Dilute the secondary antibody to 1:1000 in 1% NHS in TBST (see step 8.4.4). Incubate slides in the diluted secondary antibody for 1 hour at room temperature.
8.5.7. Wash slides 3 times with 1x wash buffer for 5 minutes each. Mount and coverslip slides with hardset mounting media with DAPI. Use a cotton swab to gently roll out any bubbles under the coverslip.
REPRESENTATIVE RESULTS:
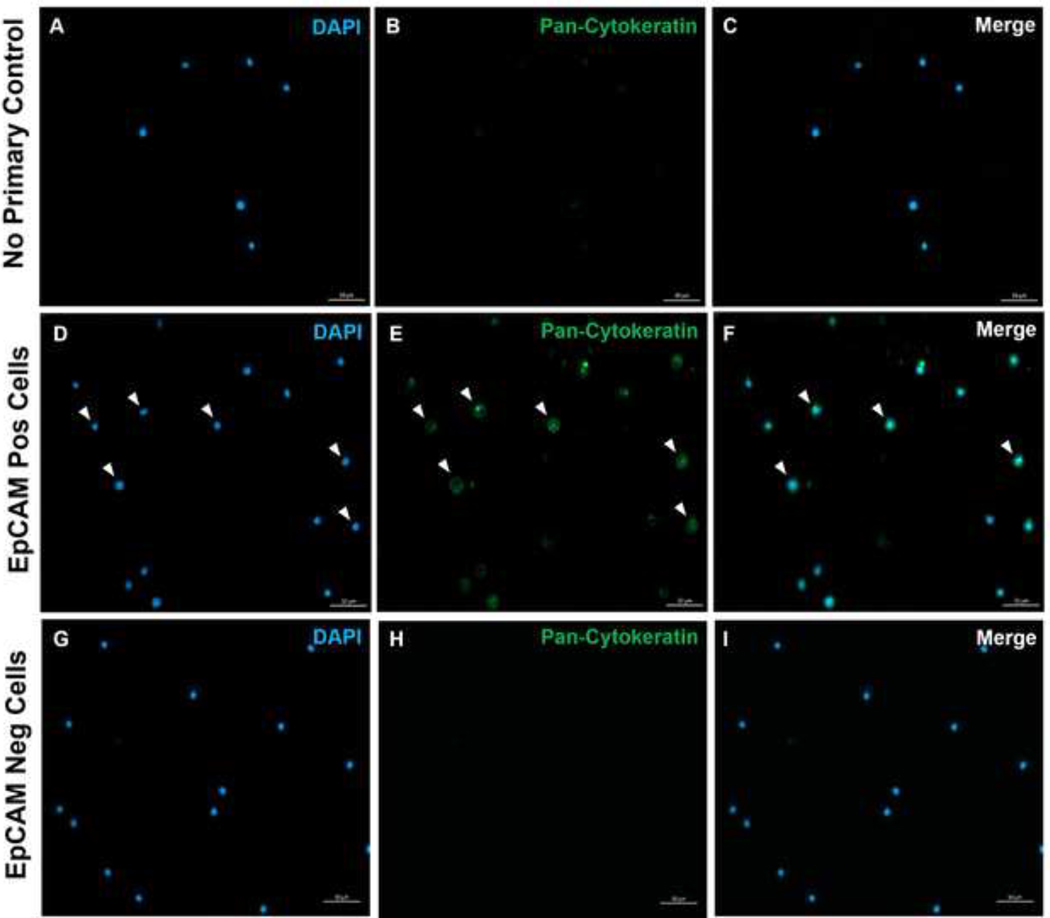
Using these methods, rare populations of epithelial cells in the blood and bone marrow of normal humans and mice were visualized. With the proper compensations and controls as described, the results consistently showed 4–5% of cells in murine bone marrow were EpCAM+, regardless of how many cells were counted as shown in Figures 4 and 5. In murine blood samples, less than 0.5% of cells were EpCAM+ as shown in Figure 6. In human bone marrow samples, 2–5% of cells were EpCAM+ as shown in Figures 7 and 8. While 2–5% is a big range, percentages within each individual donor were consistent as incrementally more cells were counted. In human blood samples, around 0.3% of cells were EpCAM+ as shown in Figure 9. Our control samples (no stain, isotype control, and FMOs) yielded very few false-positive EpCAM+ results as shown in Figures 4 and 7. Cells from the EpCAM+ and EpCAM- groups that were sorted onto slides showed positive staining for pan-cytokeratin in EpCAM+ samples and were negative for pan-cytokeratin in EpCAM- samples as shown in Figure 10. These results indicate that the experiments were appropriately designed and reproducible.
Figure 4. Flow cytometric analysis of EpCAM+ cells in mouse bone marrow.
Controls of No stain, Isotype, and FMOs are shown in the top panel with the incremental counts of 50,000, 100,000, and 500,000 cells shown in the bottom panel. These charts visualize the consistency in percentages across counts, despite the overall increase in total cells counted. The panels on the right indicate the gating strategy as discussed previously in the flow cytometry analysis section and as shown in Figure 3.
Figure 5. EpCAM+ cells in mouse bone marrow comprise 5.17% ± 0.001 of the population.
The bone marrow cells of three individual mice were analyzed. The appropriate controls were included for proper scientific rigor. The percentage of positive cells remained consistent across samples due to mice being genetically identical.
Figure 6. EpCAM+ cells in mouse blood comprise 0.45% ± 0.0006 of the population.
The blood cells of two individual mice were analyzed. Controls were included to show proper procedure was followed to produce conclusive results.
Figure 7. Flow cytometric analysis on EpCAM+ human bone marrow.
Controls of No stain, Isotype, and FMOs are shown in the top panel and the incremental counts of 50,000, 100,000, and 500,000 cells shown in the bottom panel. These charts visualize the consistency in percentages across counts, despite the overall increase of total cells counted. The panels on the right indicate the gating strategy as discussed previously in the flow cytometry analysis section and as shown in Figure 3.
Figure 8. EpCAM+ cells in human bone marrow comprise 3.53% ± 0.006 of the population.
Three different human bone marrow samples were analyzed. Appropriate controls for scientific rigor were included. The percentage of positive cells varies due to genetic heterogeneity among humans.
Figure 9. EpCAM+ cells human blood comprise 0.18% ± 0.0004 of the population.
Three different human blood samples were analyzed. Appropriate controls for scientific rigor were included.
Figure 10. Immunofluorescence of EpCAM+ and EpCAM- slides.
FACS was used to separate EpCAM+ and EpCAM- cells. Pan-cytokeratin was stained for using the DAKO Pan-cytokeratin antibody. A no primary control in normal serum was used as the pan-cytokeratin is a polyclonal antibody. These results confirm the accuracy of FACS.
DISCUSSION:
There is some evidence in the literature on the presence of epithelial cells in the bone marrow. These papers typically investigate the role of epithelial cells within the context of disease and injury, such as in the liver, lung, GI tract, thymus, and skin2,3,7,16. However, not much is known about the presence of these epithelial cells in the bone marrow of healthy individuals. This paper seeks to establish a reproducible method with which to identify and isolate epithelial cells from normal blood and bone marrow. This method will drive the field forward to identify why epithelial cells are present and what is their role in the blood and bone marrow in the absence of disease. Perhaps these cells are part of normal tissue maintenance or activated at times of injury. The bone marrow is a repository of stem cells. However, it is unclear what the lineage of these epithelial cells may be. A recent paper discusses bone marrow-derived epithelial cells in the thymus that first express EpCAM and the hematopoietic marker, CD45, and then lose their CD45 expression over time after injury3. Experiments within the Morris Lab have also confirmed the presence of CD45+ EpCAM+ cells within healthy blood and bone marrow in the absence of injury. However, the role of these cells is yet to be determined.
There was a need for a reproducible method to examine epithelial cells within healthy bone marrow. The method described will help in characterizing these cells further in their normal state. There are important steps in this method to maintain reproducibility. One of the most critical steps in this protocol is maintaining a sterile environment in the hood while harvesting the bone marrow from mice. If harvesting from multiple mice, avoid cross-contamination between samples by using new needles and syringes for each mouse. This will also ensure that the sample is clean and free of any contaminants that might affect the results. If harvesting bone marrow from more than one mouse, increase the number of prepared syringes and labeled conical tubes for each additional mouse. Another important step involves flushing the bones until a clean white color is evident to ensure that most of the bone marrow cells have been removed. The chances of detecting rare cells increases in a purer sample. A modification was made to optimize the red blood cell lysis protocol. Several lysis buffers were tested in attempt to find one that gave consistently high viability as almost half the cells were being lost during this step. Different reagents and incubation periods may need to be optimized for improved results in other settings. The most significant limitation of this protocol is using flow cytometry for finding rare cell populations. As discussed previously, the addition of controls and incremental counts helped increase the specificity and accuracy of the analysis. Another limitation is appropriate markers for the population of interest must be identified in advance. Thus, one needs to know about key markers, antibodies for flow cytometry, and antibodies compatible with the target species.
These methods are an improvement over existing methods because they allow for single cell analysis at a fraction of the cost of the existing automatic CTC isolator and single cell RNA sequencing. Additionally, flow cytometry is more readily available. FACS maintained higher viability for the cells compared with prior reported results using magnetic microbead separation. Lastly, these techniques allow for the separation of cells for downstream analyses such as bulk RNA sequencing, scRNA sequencing, or cell culture.
ACKNOWLEDGMENTS:
Josh Monts, Core Facility Flow Cytometer Technician, The Hormel Institute
Todd Schuster, Core Facility Manager, The Hormel Institute
Derek Gordon, Statistician, Rutgers University
We would like to thank Karen Klein from Clarus Editorial Services, Santa Fe, NM for her editorial assistance.
This work was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21 AR075281, and a Grant-in-Aid of Research, Artistry and Scholarship, Office of the Vice President for Research, University of Minnesota [Proposal #324240]. We gratefully acknowledge support from The Hormel Institute.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/65118.
DISCLOSURES:
There are no known conflicts of interest.
Contributor Information
Stephanie Holtorf, The Hormel Institute, University of Minnesota
Jennifer Boyle, The Hormel Institute, University of Minnesota
Rebecca Morris, The Hormel Institute, University of Minnesota
REFERENCES:
- 1.Blanpain C, Fuchs E. Plasticity of epithelial stem cells in tissue regeneration. Science. 344 (6189), 1242281 (2014). 10.1126/science.1242281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borue X. et al. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. American Journal of Pathology. 165 (5), 1767–1772 (2004). doi: 10.1016/S0002-9440(10)63431-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti S. et al. Bone marrow-derived cells contribute to the maintenance of thymic stroma including TECs. Journal of Immunology Research. 2022, 6061746 (2022). 10.1155/2022/6061746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coradini D, Casarsa C, Oriana S. Epithelial cell polarity and tumorigenesis: New perspectives for cancer detection and treatment. Acta Pharmacologica Sinica, 32 (5), 552–564 (2011). 10.1038/aps.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmello C, Srivastava SS, Tiwari R, Chaudhari PR, Sawant S, Vaidya MM Multifaceted role of keratins in epithelial cell differentiation and transformation. Journal of Biosciences. 44 (2), 33 (2019). 10.1007/s12038-019-9864-8 [DOI] [PubMed] [Google Scholar]
- 6.Eslami-S Z, Cortés-Hernández LE, Alix-Panabières C. Epithelial cell adhesion molecule: an anchor to isolate clinically relevant circulating tumor cells. Cells. 9 (8), E1836 (2020). 10.3390/cells9081836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause DS et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 105 (3), 369–377 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Larsen SB, Cowley CJ, Fuchs E. Epithelial cells: liaisons of immunity. Current Opinion in Immunology. 62, 45–53 (2020). doi: 10.1016/j.coi.2019.11.004. PMID: 31874430; PMCID: PMC7067656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin D, et al. Circulating tumor cells: biology and clinical significance. Signal Transduction and Targeted Therapy. 6 (1), 404 (2021). doi: 10.1038/s41392-021-00817-8. PMID: 34803167; PMCID: PMC8606574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsman WA et al. Epithelial cells in bone marrow: do they matter?. Gut, 54 (12), 1821–1822 (2005). 10.1136/gut.2005.078774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris RJ Circulating tumor cells: quintessential precision oncology presenting challenges for biology. NPJ Precision Oncology. 1 (1), 16 (2017). 10.1038/s41698-017-0019-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H, et al. Bone marrow-derived epithelial cells and hair follicle stem cells contribute to development of chronic cutaneous neoplasms. Nature Communications. 9 (1), 5293 (2018). doi: 10.1038/s41467-018-07688-8. PMID: 30546048; PMCID: PMC6294255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulet G, Massias J, Taly V. Liquid biopsy: General concepts. Acta Cytologica. 63 (6), 449–455 (2019). doi: 10.1159/000499337. PMID: 31091522. [DOI] [PubMed] [Google Scholar]
- 14.Spence M, Adai S, Landon T, Richter G. Few and far between: Tools and strategies for rare-event detection using flow cytometry. Bioprobes Journal of Cell Biology. 71, 14–18 (2015). [Google Scholar]
- 15.Talasaz AH et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proceedings of the National Academy of Sciences. 106 (10), 3970–3975 (2009). 10.1073/pnas.0813188106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong AP et al. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. American Journal of Physiology-Lung Cellular and Molecular Physiology. 293 (3), L740–L752 (2007). doi: 10.1152/ajplung.00050.2007 [DOI] [PubMed] [Google Scholar]
- 17.Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Molecular Cancer 18, 114 (2019). 10.1186/s12943-019-1043-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu M, Stott S, Toner M, Maheswaran S, Haber DA Circulating tumor cells: approaches to isolation and characterization. Journal of Cell Biology, 192 (3), 373–382 (2011). 10.1083/jcb.201010021 [DOI] [PMC free article] [PubMed] [Google Scholar]