Abstract
Neonates show considerable variation in growth that can be recognized through serial measurements of basic variables such as weight, length, and head circumference. If possible, measurement of subcutaneous and total body fat mass can also be useful. These biometric measurements at birth may be influenced by demographics, maternal and paternal anthropometrics, maternal metabolism, preconceptional nutritional status, and placental health. Subsequent growth may depend on optimal feeding, total caloric intake, total metabolic activity, genetic makeup, postnatal morbidities, medications, and environmental conditions. For premature infants, these factors become even more important; poor in utero growth can be an important reason for spontaneous or induced preterm delivery. Later, many infants who have had intrauterine growth restriction (IUGR) and are born small for gestational age (SGA) continue to show suboptimal growth below the 10th percentile, a condition that has been defined as extrauterine growth restriction (EUGR) or postnatal growth restriction (PNGR). More importantly, a subset of these growth-restricted infants may also be at high risk of abnormal neurodevelopmental outcomes. There is a need for well-defined criteria to recognize EUGR/PNGR, so that correctional steps can be instituted in a timely fashion.
Keywords: Body fat mass, Cohort of Indonesian PreTerm infants for long-term Outcomes study, Corrected gestational age, Delta-Z, Demographic factors, Extra-uterine growth restriction, Failure to thrive, Fenton growth chart, Genetic make-up, growth charts, Infant growth, Intra-uterine growth restriction, Intergrowth 21st charts, Infant feeding, Linear growth velocity, Maternal metabolism, Maternal and paternal anthropometrics, Medications, Newborn, Neonate, Neurodevelopmental outcomes, Neonatal morbidities, Placental health, Postnatal growth restriction, Postnatal malnutrition, Pre-conceptional nutritional status, Postnatal morbidities, Postnatal growth, Small for gestation, Term corrected age, Total caloric intake, Total metabolic activity, Weight gain velocity, Z-scores
Introduction
Advancements in the field of neonatology over last two decades has led to improved survival of premature and low birth weight infants.1,2 However, the growth of these premature and critically ill neonates remains a cause for concern. To define and monitor the growth faltering of these infants, many terminologies has been used in literature such as EUGR and PNGR,3,4 failure to thrive,4 and postnatal malnutrition.5 Many definitions have been suggested for these terminologies and attempts have been made to correlate these with neurodevelopmental and other clinical outcomes,3,6–8
Infant growth rates have been followed on charts such as the Fenton,9 British,10 and Italian.11 Growth parameters have been studied in greater detail at some time points such as at a postnatal age of 28 days, postnatal and/or corrected gestational ages of 36–40 weeks, or at the time of discharge from NICU. Similar to full-term infants, preterm infants also grow along these growth curves.12,13 The growth trajectories may get altered during periods of high-acuity illness, which might cause one or more anthropometric parameters to drop in terms of centile ranks at discharge or at term corrected age.14,15
Many studies have shown that drops in weight centiles may predict suboptimal neurodevelopmental outcomes.4,6,7 Altered length16,17 and head circumference14,18–20 at corrected 36 weeks, at discharge, and/or and poor weight gain post-discharge have also been associated with poor developmental outcomes. However, we still have not been able to identify critical thresholds of these parameters. There is a need to define EUGR in terms of weight alone or in combination with length, head circumference, body composition, and genetic markers, and genetic potential based on parental anthropometric indices. Growth monitoring is also important for interpretation of postnatal weight loss and loss of growth centiles during high-acuity illness; we currently interpret our findings by comparing with the reference fetus and arbitrary statistical growth percentile cut offs. The objective of this article is to extensively review the current literature and provide uniform definition of EUGR postnatally,21,22 while answering few important questions which lead the way.
We Still Need to Agree on a Single Definition of Extrauterine Growth Restriction
To assess the appropriate medical and nutritional interventions and to predict auxological long term outcomes, a consensus definition of EUGR is still needed. We recommend rectification, not only in the criteria to define EUGR but also the method and tool for growth monitoring. Until a consensus defines EUGR, the recommendations from “Identifying Malnutrition in Preterm and Neonatal Populations: Recommended Indicators” (Table 1)5 and “Extrauterine Growth Restriction: Definitions and Predictability of Outcomes in a Cohort of Very Low Birth Weight Infants or Preterm Neonates,”23 which defines EUGR as longitudinal (if the weight loss is more than one standard deviation (SD) between birth and a given t-time and cross sectional (if weight was below the 10th centile at a given t-time). A recent prospective cohort study from Jakarta, Indonesia [the Cohort of Indonesian PreTerm infants for long-term Outcomes (CIPTO) study] to study preterm infants born at the Cipto Mangunkusumo General Hospital has also provided important information.24 They defined EUGR (as in Table 1) as a decline in the weight-for-age Z-score of above or equal to 1.2 and have reported related outcomes.
Table 1:
Currently available identification tools for EUGR
| Criteria | Mild EUGR | Moderate EUGR | Severe EUGR | When to apply? |
|---|---|---|---|---|
|
| ||||
| 1. Weight-for-age Z-scoresa | Decline of 0.8–1.2 SD | Decline of >1.2–2 SD | Decline >2 SD | Not appropriate for first 2 weeks of life |
| 2. Weight gain velocityb | <75% of expected weight gain for that particular age | <50% of expected weight gain for that particular age | <25% of expected weight gain for that particular age | Not appropriate for first 2 weeks of life |
| 3. ≥2 of the following | Not appropriate for first 2 weeks, can be used subsequently in conjunction with other parameters if accurate length measurement is available. | |||
| •Length-for-age Z-scoresa | Decline of 0.8–1.2 SD | Decline of >1.2–2 SD | Decline >2 SD | |
| •Length gain velocityb | <75% of expected length gain for that particular age 15–18 days | <50% of expected length gain for that particular age 19–21 days | <25% of expected length gain for that particular age | |
| •Days to regain birth weight (in conjugation with nutrient intake) | (>3–5 consecutive days of <75% intakes of estimated protein/calorie) | (>5–7 consecutive days of <75% intakes of estimated protein/calorie) | >21 days (>7 consecutive days of <75% intakes of estimated protein/calorie) | Preferred for first 2 weeks of life |
Expected Z-score for weight for age and length for age.
Weight gain velocity and linear growth velocity were estimated using online calculator (www.peditools.org). In this calculator, weight gain velocity is estimated by using the World Health Organization (WHO) methods; weight increments are classified by birth-weight category presented in 1-week and 2-weeks intervals from birth to 60 days.21
Are we Using Appropriate Metrics to Define Extrauterine Growth Restriction?
We are not sure if it is appropriate to assess growth using a single-point, single-parameter measurement such as weight. Weight measurements show high variability, and we have still not identified one growth chart as better than others for plotting growth of preterm infants. We are also not certain whether a specific set of growth charts can better assess the initial postnatal weight loss and post discharge catch-up. These questions are important in identification of EUGR.
Most studies still use a single point, single-parameter measurement such as weight at corrected 36 weeks’ gestation or at discharge.25–29 This is a convenient way to define EUGR but many studies have identified that there is a good catch-up between 36–40 weeks’ gestation;11,30,31 EUGR has been defined in literature using the following:
Percentile ranks (<3rd and <10th percentiles) at 36 weeks/discharge,32
The Z-scores (Z-score of 1 or 2) for weight loss from that at birth,33
The Z-score at discharge of < −1.5 of intrauterine growth or standard postnatal growth,26,34,35
Drop in Z-score (delta-Z) from birth to 36 weeks corrected gestational age or at discharge.36,37
Shah et al.6 compared the definitions related to both percentiles (<3rd and <10th percentiles) at 36 weeks’ discharge, and Z-score (1 or 2 Z-scores) losses from birth weight. Zozaya et al.7 compared Z-scores at discharge of below −1.5 of intrauterine growth or standard postnatal growth and drop in Z-score (delta-Z) from birth to corrected 36 weeks’ gestational age or at discharge. Both studies concluded that the drop in Z-scores has a higher predictive value in relation with neurodevelopment outcome than a single time point measurement of weight less than 10th percentile.
Currently there is no consensus about which growth monitoring tool is better for assessment of growth in preterm infants during postnatal period. There are two following standard growth charts available: (A) the Fenton growth chart is a reference chart based on a cross-sectional study population9 and (B) the Intergrowth 21st charts (IG-21)38 that are based on a postnatal longitudinal study population. In accordance with the available literature, growth failure defined as a Z-score drop of >1 from birth to discharge in terms of weight, length and head circumference7,23,39 has shown that that PNGR was less common with IG-21 as compared to Fenton40–45 and is strongly associated with poor long-term outcome. Larger studies are still required to identify the most optimal growth assessment tool.
We Need to Consider Body Composition in Addition to the Conventional Anthropometric Measurements to Define Extrauterine Growth Restriction/Postnatal Growth Restriction
Weight gain, as an isolated index, may not be the most appropriate method to assess growth because weight is an indirect indicator of body composition (lean body mass + fat tissue + body fluid).22 The lean body mass is likely to be more accurate as a predictor, and fat tissue may be the least important. In a systematic review and meta-analysis, Johnson et al.46 and Gianni et al.47 showed that preterm infants at corrected term gestational age of 40 weeks had a higher proportion of body fat than comparable full-term infants. The preterm infants in this cohort had less lean body mass, and this continued to be a matter of concern until 5 years of age. In another consideration, all preterm infants show a variable degree of physiological weight loss in the first few days after birth due to loss of extracellular fluid. This is reflected in growth charts as loss of growth percentiles.12 This fluid loss is important for hemodynamic and physiological stability of these infants.48,49 If a preterm infant is discharged from the hospital during this period of physiological weight loss, these changes will be documented as EUGR but Rochow et al.12 showed that they subsequently regain normal growth trajectories. The WHO recommends body mass index (BMI) and weight-for-length as better standards for monitoring growth during childhood,50 but these guidelines cannot be readily extrapolated for preterm infants. In these patients, the body composition will remain concerning even if they gain weight because the length will likely not change proportionately.51,52 We believe that instead of using a single-point, single-parameter measurement such as weight, following the three most commonly used anthropometric parameters (weight, length and head circumference) over time might be better.
The Impact of Suboptimal Growth goes Beyond Somatic Consequences
In premature infants, neurodevelopmental outcomes are an important indicator of the quality of care. Many recent studies emphasize the effect of nutrition on neurodevelopmental outcome of premature infants.53–55 Others suggest that preterm infants with multiple morbidities grow slower than controls who have been relatively healthy.14,15,56 Hence, optimization of nutrition can potentially improve both growth and developmental outcomes. However, weight below 10th percentile at a single time point (36 or 40 weeks’ corrected gestational age, or at discharge) is not the only growth indicator that is associated with poor developmental outcome;4,6,7 length16,17 and head circumference16,18–20,57–59 may also be important predictors.
Infants who are born SGA60 have shown slower growth during their hospital stay.7,14,57–59 They are at increased risk of multiple neonatal morbidities,5,56 neurological injury such as intraventricular hemorrhage and periventricular leukomalacia,14 and are at risk of developmental delay. However, these are complex issues that extend beyond infant health; parental cognitive capacity, involvement, education, and socioeconomic status are also important predictors of developmental outcome.61–63 A cursory look at the host–infections–environment–therapy–nutrition–systems management hexagon that we have been using in this journal to study disease pathogenesis suggests that there is a clear possibility of multifactorial origin (Fig. 1).64 There is a need for an in-depth, careful analysis to determine the relative weightage of each of these nodes.
Fig. 1:
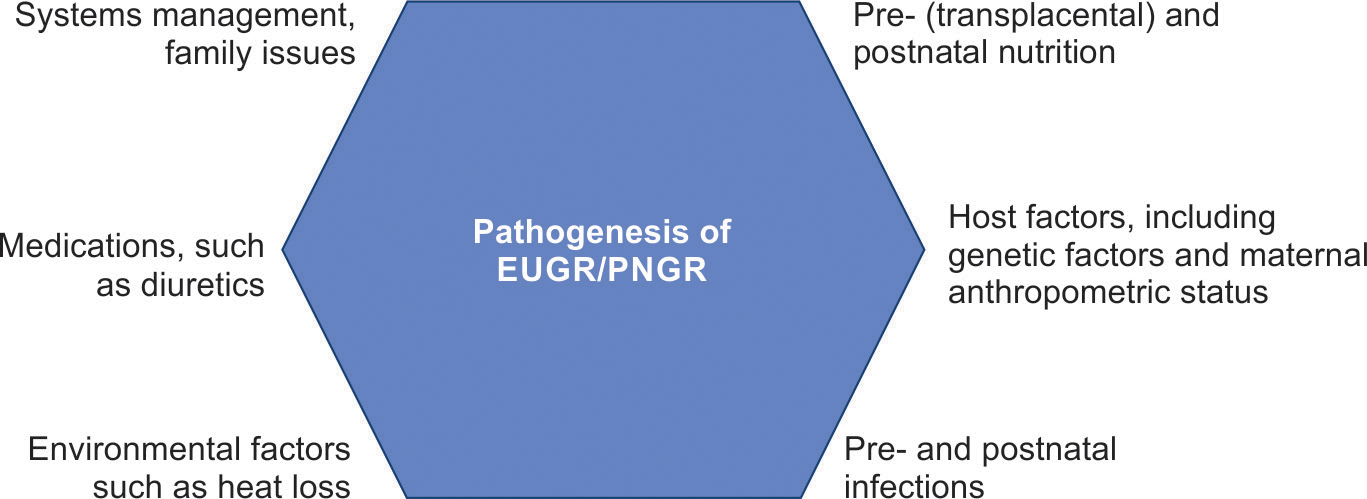
Multiple factors are likely involved in the pathogenesis of EUGR/PNGR
Conclusion
Extrauterine growth restriction/PNGR is an important problem in recovering premature/critically ill infants and cannot be ignored. To define EUGR appropriately is the need of the hour and growth monitoring of preterm infants using appropriate growth charts should be encouraged to identify early deviance in growth trajectories. Only then we will be able to institute relevant interventions in a timely fashion. The association with neurodevelopmental outcomes increases its importance even further; it may enrich our currently used single-point, single-parameter measurement at 36 weeks or at corrected term gestational age. If we do not have an optimum way of measurement, it will remain difficult to compare the impact of many prophylactic/therapeutic interventions.
Highlights.
Neonates show considerable variation in growth; assessment requires serial measurement of basic parameters such as weight, length, and head circumference.
Biometric parameters at birth may reflect demographic factors, maternal and paternal anthropometrics, maternal metabolism, preconceptional nutritional status, and placental health. Postnatal determinants of growth include feeding, total caloric intake, metabolic activity, genetic factors, morbidities, and environmental conditions.
Infants who have had intrauterine growth restriction (IUGR) and are born small for gestational age (SGA) may have low growth potential. Many have suboptimal neurodevelopmental outcomes.
There is a need to accurately define extrauterine growth restriction (EUGR) so that correctional steps can be instituted in a timely fashion.
Source of support:
This manuscript is based on the work done in the initial years of the National Institute of Health award HL124078 to AM.
Footnotes
Conflict of interest: Dr Nitasha Bagga, Dr Md Mozibur Rahman and Dr Akhil Maheshwari are associated as the Editorial board members of this journal and this manuscript was subjected to this journal’s standard review procedures, with this peer review handled independently of these Editorial board members and their research group.
References
- 1.Euser AM, de Wit CC, Finken MJ, et al. Growth of preterm born children. Horm Res 2008;70(6):319–328. DOI: 10.1159/000161862. [DOI] [PubMed] [Google Scholar]
- 2.Bagga N, Reddy KK, Mohamed A, et al. Quality improvement initiative to decrease extrauterine growth restriction in preterm neonates. Nutr Clin Pract 2021;36(6):1296–1303. DOI: 10.1002/ncp.10735. [DOI] [PubMed] [Google Scholar]
- 3.Fenton TR, Chan HT, Madhu A, et al. Preterm infant growth velocity calculations: A systematic review. Pediatrics 2017;139(3):e20162045. DOI: 10.1542/peds.2016-2045. [DOI] [PubMed] [Google Scholar]
- 4.Hack M, Merkatz IR, Gordon D, J et al. The prognostic significance of postnatal growth in very low birth weight infants. Am J Obstet Gynecol 1982;143(6):693–699. DOI: 10.1016/0002-9378(82)90117-x. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg DL, Becker PJ, Brigham K, et al. Identifying malnutrition in preterm and neonatal populations: Recommended indicators. J Acad Nutr Diet 2018;118(9):1571–1582. DOI: 10.1016/j.jand.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Shah PS, Wong KY, Merko S, et al. Postnatal growth failure in preterm infants: Ascertainment and relation to long-term outcome. J Perinat Med 2006;34(6):484–489. DOI: 10.1515/JPM.2006.094. [DOI] [PubMed] [Google Scholar]
- 7.Zozaya C, Diaz C, de Pipaon MS. How should we define postnatal growth restriction in preterm infants? Neonatology 2018;114(2):177–180. DOI: 10.1159/000489388. [DOI] [PubMed] [Google Scholar]
- 8.Tudehope DI, Burns Y, O’Callaghan M, et al. The relationship between intrauterine and postnatal growth on the subsequent psychomotor development of very low birthweight (VLBW) infants. Aust Paediatr J 1983;19(1):3–8. DOI: 10.1111/j.1440-1754.1983.tb02041.x. [DOI] [PubMed] [Google Scholar]
- 9.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59. DOI: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole TJ, Williams AF, Wright CM, et al. Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts. Ann Hum Biol 2011;38(1):7–11. DOI: 10.3109/03014460.2011.544139. [DOI] [PubMed] [Google Scholar]
- 11.Bertino E, Coscia A, Mombro M, et al. Postnatal weight increase and growth velocity of very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2006;91(5):F349–F356. DOI: 10.1136/adc.2005.090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochow N, Raja P, Liu K, et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr Res 2016;79(6):870–879. DOI: 10.1038/pr.2016.15. [DOI] [PubMed] [Google Scholar]
- 13.Cole TJ, Statnikov Y, Santhakumaran S, et al. Birth weight and longitudinal growth in infants born below 32 weeks’ gestation: A UK population study. Arch Dis Child Fetal Neonatal Ed 2014;99(1):F34–F40. DOI: 10.1136/archdischild-2012-303536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franz AR, Pohlandt F, Bode H, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009;123(1):e101–e109. DOI: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999;104 (2 Pt 1):280–289. DOI: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 16.Belfort MB, Gillman MW, Buka SL, et al. Preterm infant linear growth and adiposity gain: Trade-offs for later weight status and intelligence quotient. J Pediatr 2013;163(6):1564.e2–1569.e2. DOI: 10.1016/j.jpeds.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dusick AM, Poindexter BB, Ehrenkranz RA, et al. Growth failure in the preterm infant: Can we catch up? Semin Perinatol 2003;27(4):302–310. DOI: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 18.Raghuram K, Yang J, Church PT, et al. Head growth trajectory and neurodevelopmental outcomes in preterm neonates. Pediatrics 2017;140(1):e20170216. DOI: 10.1542/peds.2017-0216. [DOI] [PubMed] [Google Scholar]
- 19.Georgieff MK, Hoffman JS, Pereira GR, et al. Effect of neonatal caloric deprivation on head growth and 1-year developmental status in preterm infants. J Pediatr 1985;107(4):581–587. DOI: 10.1016/s0022-3476(85)80028-7. [DOI] [PubMed] [Google Scholar]
- 20.Sammallahti S, Pyhala R, Lahti M, et al. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J Pediatr 2014;165(6):1109.e3–1115.e3. DOI: 10.1016/j.jpeds.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Bagga Nitasha, Panigrahay Nalinikant, Maheshwari Akhil. Extra-uterine growth restriction in preterm infants. Newborn 2022;1(1):67–73. DOI: 10.5005/jp-journals-11002-0019. [DOI] [Google Scholar]
- 22.Fenton TR, Cormack B, Goldberg D, et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J Perinatol 2020;40(5):704–714. DOI: 10.1038/s41372-020-0658-5. [DOI] [PubMed] [Google Scholar]
- 23.Peila C, Spada E, Giuliani F, et al. Extrauterine growth restriction: Definitions and predictability of outcomes in a cohort of very low birth weight infants or preterm neonates. Nutrients 2020;12(5):1224. DOI: 10.3390/nu12051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohsiswatmo R, Kaban RK, Sjahrulla MAR, et al. Defining postnatal growth failure among preterm infants in Indonesia. Front Nutr 2023;10:1101048. DOI: 10.3389/fnut.2023.1101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlomai NO, Reichman B, Lerner–Geva L, et al. Population-based study shows improved postnatal growth in preterm very-low-birthweight infants between 1995 and 2010. Acta Paediatr 2014;103(5):498–503. DOI: 10.1111/apa.12569. [DOI] [PubMed] [Google Scholar]
- 26.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1):986–990. DOI: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 27.Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: A universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed 2004;89(5):F428–F430. DOI: 10.1136/adc.2001.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: An inevitable consequence of current recommendations in preterm infants? Pediatrics 2001;107(2):270–273. DOI: 10.1542/peds.107.2.270. [DOI] [PubMed] [Google Scholar]
- 29.Roggero P, Gianni ML, Amato O, et al. Postnatal growth failure in preterm infants: recovery of growth and body composition after term. Early Hum Dev 2008;84(8):555–559. DOI: 10.1016/j.earlhumdev.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Fenton TR, Nasser R, Eliasziw M, et al. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr 2013;13:92. DOI: 10.1186/1471-2431-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niklasson A, Albertsson–Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr 2008;8:8. DOI: 10.1186/1471-2431-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry MA, Abrahamowicz M, Usher RH. Factors associated with growth of extremely premature infants during initial hospitalization. Pediatrics 1997;100(4):640–646. [PubMed] [Google Scholar]
- 33.Carlson SJ, Ziegler EE. Nutrient intakes and growth of very low birth weight infants. J Perinatol 1998;18(4):252–258. [PubMed] [Google Scholar]
- 34.Martin CR, Brown YF, Ehrenkranz RA, et al. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics 2009;124(2):649–657. DOI: 10.1542/peds.2008-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villar J, Giuliani F, Barros F, et al. Monitoring the postnatal growth of preterm infants: A paradigm change. Pediatrics 2018;141(2):e20172467. DOI: 10.1542/peds.2017-2467. [DOI] [PubMed] [Google Scholar]
- 36.Horbar JD, Ehrenkranz RA, Badger GJ, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 2015;136(1):e84–e92. DOI: 10.1542/peds.2015-0129. [DOI] [PubMed] [Google Scholar]
- 37.Radmacher PG, Looney SW, Rafail ST, et al. Prediction of extrauterine growth retardation (EUGR) in VVLBW infants. J Perinatol 2003;23(5):392–395. DOI: 10.1038/sj.jp.7210947. [DOI] [PubMed] [Google Scholar]
- 38.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. The INTERGROWTH-21st fetal growth standards: Toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol 2018;218(2S):S630–S640. DOI: 10.1016/j.ajog.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Salas AA, Bhatia A, Carlo WA. Postnatal growth of preterm infants 24 to 26 weeks of gestation and cognitive outcomes at 2 years of age. Pediatr Res 2021;89(7):1804–1809. DOI: 10.1038/s41390-020-01158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuzun F, Yucesoy E, Baysal B, et al. Comparison of INTERGROWTH-21 and Fenton growth standards to assess size at birth and extrauterine growth in very preterm infants. J Matern Fetal Neonatal Med 2018;31(17):2252–2257. DOI: 10.1080/14767058.2017.1339270. [DOI] [PubMed] [Google Scholar]
- 41.Kim YJ, Shin SH, Cho H, et al. Extrauterine growth restriction in extremely preterm infants based on the Intergrowth-21st Project Preterm Postnatal Follow-up Study growth charts and the Fenton growth charts. Eur J Pediatr 2021;180(3):817–824. DOI: 10.1007/s00431-020-03796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy KV, Sharma D, Vardhelli V, et al. Comparison of Fenton 2013 growth curves and Intergrowth-21 growth standards to assess the incidence of intrauterine growth restriction and extrauterine growth restriction in preterm neonates ≤32 weeks. J Matern Fetal Neonatal Med 2021;34(16):2634–2641. DOI: 10.1080/14767058.2019.1670795. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Metcalfe A, Leon JA, et al. Evaluation of the INTERGROWTH-21st project newborn standard for use in Canada. PLoS One 2017;12(3):e0172910. DOI: 10.1371/journal.pone.0172910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samarani M, Restom G, Mardini J, et al. Comparative study between Fenton and intergrowth 21 charts in a sample of Lebanese premature babies. BMC Pediatr 2020;20(1):74. DOI: 10.1186/s12887-020-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yitayew M, Chahin N, Rustom S, et al. Fenton vs. Intergrowth-21st: Postnatal growth assessment and prediction of neurodevelopment in preterm infants. Nutrients 2021;13(8):2841. DOI: 10.3390/nu13082841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson MJ, Wootton SA, Leaf AA, et al. Preterm birth and body composition at term equivalent age: A systematic review and meta-analysis. Pediatrics 2012;130(3):e640–e649. DOI: 10.1542/peds.2011-3379. [DOI] [PubMed] [Google Scholar]
- 47.Gianni ML, Roggero P, Piemontese P, et al. Boys who are born preterm show a relative lack of fat-free mass at 5 years of age compared to their peers. Acta Paediatr 2015;104(3):e119–e123. DOI: 10.1111/apa.12856. [DOI] [PubMed] [Google Scholar]
- 48.Heimler R, Doumas BT, Jendrzejczak BM, et al. Relationship between nutrition, weight change, and fluid compartments in preterm infants during the first week of life. J Pediatr 1993;122(1):110–114. DOI: 10.1016/s0022-3476(05)83502-4. [DOI] [PubMed] [Google Scholar]
- 49.Lorenz JM, Kleinman LI, Ahmed G, et al. Phases of fluid and electrolyte homeostasis in the extremely low birth weight infant. Pediatrics 1995;96(3 Pt 1):484–489. [PubMed] [Google Scholar]
- 50.de Onis M The use of anthropometry in the prevention of childhood overweight and obesity. Int J Obes Relat Metab Disord 2004;28 (Suppl. 3):S81–S85. DOI: 10.1038/sj.ijo.0802810. [DOI] [PubMed] [Google Scholar]
- 51.Lorch SA. The clinical and policy implications of new measures of premature infant growth. Pediatrics 2015;135(3):e703–e704. DOI: 10.1542/peds.2014-3774. [DOI] [PubMed] [Google Scholar]
- 52.Al-Theyab NA, Donovan TJ, Eiby YA, et al. Fat trajectory after birth in very preterm infants mimics healthy term infants. Pediatr Obes 2019;14(3):e12472. DOI: 10.1111/ijpo.12472. [DOI] [PubMed] [Google Scholar]
- 53.O’Connor DL, Gibbins S, Kiss A, et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A randomized clinical trial. JAMA 2016;316(18):1897–1905. DOI: 10.1001/jama.2016.16144. [DOI] [PubMed] [Google Scholar]
- 54.Makrides M, Gibson RA, McPhee AJ, et al. Ef fect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010;304(15):1675–1683. DOI: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 55.Keim SA, Boone KM, Klebanoff MA, et al. Effect of docosahexaenoic acid supplementation vs placebo on developmental outcomes of toddlers born preterm: A randomized clinical trial. JAMA Pediatr 2018;172(12):1126–1134. DOI: 10.1001/jamapediatrics.2018.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Regev RH, Arnon S, Litmanovitz I, et al. Association between neonatal morbidities and head growth from birth until discharge in very-low-birthweight infants born preterm: A population-based study. Dev Med Child Neurol 2016;58(11):1159–1166. DOI: 10.1111/dmcn.13153. [DOI] [PubMed] [Google Scholar]
- 57.Belfort MB, Rifas–Shiman SL, Sullivan T, et al. Infant growth before and after term: Effects on neurodevelopment in preterm infants. Pediatrics 2011;128(4):e899–e906. DOI: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006;117(4):1253–1261. DOI: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 59.Ong KK, Kennedy K, Castaneda–Gutierrez E, et al. Postnatal growth in preterm infants and later health outcomes: A systematic review. Acta Paediatr 2015;104(10):974–986. DOI: 10.1111/apa.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corpeleijn WE, Kouwenhoven SM, van Goudoever JB. Optimal growth of preterm infants. World Rev Nutr Diet 2013;106:149–155. DOI: 10.1159/000342584. [DOI] [PubMed] [Google Scholar]
- 61.Weisglas–Kuperus N, Hille ET, Duivenvoorden HJ, et al. Intelligence of very preterm or very low birthweight infants in young adulthood. Arch Dis Child Fetal Neonatal Ed 2009;94(3):F196–F200. DOI: 10.1136/adc.2007.135095. [DOI] [PubMed] [Google Scholar]
- 62.Pineda RG, Stransky KE, Rogers C, et al. The single-patient room in the NICU: Maternal and family effects. J Perinatol 2012;32(7):545–551. DOI: 10.1038/jp.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benavente–Fernandez I, Synnes A, Grunau RE, et al. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw Open 2019;2(5):e192914. DOI: 10.1001/jamanetworkopen.2019.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maheshwari A, Lui K, Motta M. Understanding the impact of maternal health on neonatal disease: A new horizon. Newborn 2023;1(4):iv–vi. DOI: 10.5005/newborn-1-4-iv. [DOI] [Google Scholar]


