Abstract
Context:
Neonatal gut ultrasound (US) is an emerging clinical tool for quick diagnosis and prognosis in various abdominal pathologies. In this review, we summarize normal gut US findings and concentrate on the specifications of diagnosing necrotizing enterocolitis.
Evidence:
A comprehensive literature search was conducted across numerous sources with relevant keywords along with the specified age group of 0–28 days of life.
Findings:
This review describes the normal gut US picture with the basic technicalities needed to master the art of point-of-care (POC) abdominal US. This modality is gaining importance due to its accuracy, applicability, safety, and affordability. Key findings include altered bowel perfusion, decreased peristalsis, and bowel wall thickening with better precision compared to abdominal X-ray (AXR). Many meta-analyses and narrative reviews have already demonstrated their usefulness. The high specificity and positive predictive value could make this tool a guide for early identification and prompt surgical intervention in the dreaded diagnosis of necrotizing enterocolitis.
Conclusion:
Emerging evidence and expertise in the field of abdominal US will make it a valuable tool for early diagnosis and prognosis of necrotizing enterocolitis.
Keywords: Gut signature, Necrotizing enterocolitis, Point-of-care abdominal ultrasound
Introduction
Necrotizing enterocolitis (NEC), an acute inflammatory condition of the gut, is a dreaded complication in premature and critically ill term infants. It is seen in about 5–10% of very-low-birth-weight (VLBW) infants with mortality rates ranging between 20 and 40%.1–3 The mortality is inversely proportional to gestation and birth weight.3,4 There is some information that timely diagnosis of NEC can improve the outcomes in these patients.
Early diagnosis of NEC is often difficult because clinical features such as feeding intolerance, abdominal distension, and gastrointestinal hemorrhages are non-specific and so are the routine laboratory tests. Abdominal X-ray (AXR) evaluation has been considered a gold standard for diagnosing NEC and in assessing its severity, such as in the modified Bell’s staging.5,6 The ease of access, cost-effectiveness, and the short learning curve have made it an integral part of both the diagnosis and monitoring of NEC. However, AXR has important limitations; the findings of dilated gut loops, air-fluid levels, and ascites can be non-specific, and others such as pneumatosis, portal venous gas, and fixed bowel loops are seen only in about half of all cases with confirmed disease.7 Therefore, there is a need for diagnostic tools that are accurate, easy to apply, and yet affordable. Point-of-care (POC) abdominal ultrasound (US) can fulfill all these criteria; it is inexpensive, portable, not too uncomfortable for the patient, has a fast turnaround time, can be repeated for monitoring the course of the disease, and there is no radiation exposure.
The POC abdominal US was first proposed in the 1980s for diagnosis and subsequent monitoring of NEC.8,9 Since then, many studies have supported its role in the assessment/clinical management of NEC.10–15 Even though AXR remains a first-line modality with more than 90% of neonatologists still relying on it to diagnose and manage NEC,16 POC abdominal US is gaining importance. Ahle et al.16 conducted a survey and showed that POC abdominal US was used in combination with AXR for managing NEC in about 58% of all centers. Abdominal US was done most frequently when the AXR findings were inconclusive. Many studies have shown that POC abdominal US was better than AXR in diagnosing NEC.12,13,15,17 However, abdominal US remains underutilized and there is a need for better awareness and training of neonatologists for early diagnosis and treatment of NEC, which will likely improve the outcomes.7,18
Technique
The imaging of the gastrointestinal tract in POC abdominal US requires a standardized, structured format. Faingold19 published a detailed protocol for doing POC abdominal US for diagnosing NEC. High-frequency linear probes of 8–20 Hz are recommended for clear visualization of the gut wall. Lower-frequency curvilinear probes may be more useful for imaging free fluid in deeper spaces and in detecting portal venous gas.
Images of all four quadrants of the abdomen should be obtained (Fig. 1) with the probe placed in the two perpendicular planes—transverse and longitudinal. The procedure is often easier to perform by nesting or swaddling the infant, who should be monitored for vital signs during the evaluation. Gas reverberation artifacts (Fig. 2) are seen frequently while doing POC abdominal US. These can be reduced with gentle compression of the probe and turning the infant to one side.
Fig. 1:
Four quadrants of the abdomen are to be studied in POC abdominal US
LLQ, Left lower quadrant; LUQ, Left upper quadrant; RLQ, Right lower quadrant; RUQ, Right upper quadrant
Fig. 2:
Gas reverberation artifacts commonly seen in POC abdominal US
The infant should be carefully assessed before placing the sonographic probe(s) as NEC is frequently associated with abdominal tenderness; there might be a need for analgesics/conscious sedation. The abdomen should be examined for (A) fluid or free-air collection and portal venous gas using a lower-frequency curvilinear probe; and (B) findings in the gastrointestinal tract in grayscale and color Doppler mode. In the grayscale, the intestines should be evaluated for echotexture, gut wall thickness, dilatation, pneumatosis intestinalis, and peristaltic movements. These findings may each need to be examined for at least one minute in each view for changes with peristaltic movements. In the color Doppler mode, the gut wall vascularity should be assessed in comparison with adjacent bowel loops. The color Doppler may need to be examined at a velocity of 0.029–0.11 m/seconds with the lowest possible pulse repetition frequency without aliasing, and with a low wall filter and the highest Doppler gain settings without flash artifacts. These tools help in better delineation of intestinal blood flow.15,19 Observations of each quadrant should be documented; the inability to assess specific region(s) due to artifacts should be recorded for examination at later time points. Follow-up assessments are frequently performed every 12–24 hours in view of the dynamic nature of the disease and because gut wall abnormalities may be missed in some scans because of frequently seen artifacts.
Normal Gut Appearance in Point-of-care Abdominal Ultrasound
Gut Signature
The gut wall on POC abdominal US appears as alternate hyper- and hypo-echoic layers (Figs 3 and 4). The intestinal wall shows the following five layers from within outward: The mucosa (hyperechoic), muscularis mucosae (hypoechoic), submucosa (hyperechoic), muscularis propria (hypoechoic) and serosa (hyperechoic). This alternating hyperechoic and hypoechoic layers on abdominal US is called the “gut signature”.20,21 The appearance of these five layers depends on the resolution of the probe, the quality of the sonogram, and the depth of the gut being studied. In neonates, three layers are more easily discernible, the most prominent being the hypoechoic muscularis propria. The loss of this gut signature indicates bowel pathology.
Fig. 3:
Diagrammatic representation of alternating hypoechoic and hyperechoic layers of gut wall seen on POC abdominal US (gut signature)
Fig. 4:
Gut signature in POC abdominal US
Gut Wall Thickness
Gut wall thickness is age dependent.22 In neonates, the normal gut wall thickness varies between 1–2.6 mm (Fig. 5).15,23 In NEC, most inflamed intestinal segments show increased wall thickness. Some segments with ischemia and necrosis are relatively thin.7,24
Fig. 5:
Calipers measuring gut wall thickness. Normal gut wall thickness in neonates is 1–2.6 mm
Perfusion of Normal Gut
Gut wall perfusion can be assessed by placing color Doppler in the gut wall. Normally, 1–9 color signal dots can be detected per sq. cm at a color Doppler velocity of 0.029–0.11 m/s with the lowest possible pulse repetition frequency without aliasing. The highest Doppler gain settings without flash artifacts indicate normal physiological bowel wall perfusion (Fig. 6).15 Abnormalities of gut perfusion show no color dots even at the lowest velocity. However, some segments can show increased dots or there might be abnormal patterns.7,15,24
Fig. 6:
Normal gut perfusion was assessed by putting a color Doppler and counting the signal dots per sq. cm
Peristalsis in Normal Gut
When POC abdominal US is used for real-time monitoring, peristalsis can be seen as a worm-like motion; some segments may show displacement of echogenic fluid in the opening/closing gut lumen. Normal bowel usually shows at least 10 peristaltic movements per minute.15
Intraluminal Gas Shadows
In the normal intestine, intraluminal gas shadows show as echogenic dots (Fig. 7). These dots should be differentiated from the echogenic dots present in the intestinal wall indicating intramural pneumatosis intestinalis.7
Fig. 7:
Echogenic dots present in the lumen of the normal intestine
Point-of-care Abdominal Ultrasound in Necrotizing Enterocolitis
Probe Tenderness
When an US probe is placed, signs of tenderness, even though non-specific, may indicate early NEC. Administration of analgesics may provide some comfort to these patients.
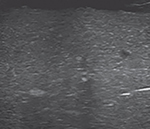
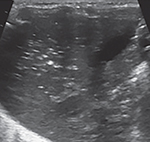
Portal Venous Gas
Echogenic intestinal intramural gas can often be seen escaping from the mesenteric vessels into the portal venous system in hepatic parenchyma (Fig. 8). On real-time POC abdominal US assessment, these echogenic foci are typically seen moving distally. These are seen as sharp bidirectional spikes superimposed on the underlying waveform on spectral Doppler. Even though these echogenic foci are usually transient and have low sensitivity, these are highly specific for the recognition of NEC.23 The observers should note that insertion/manipulation of an umbilical venous catheter(s) can also be seen as echogenic foci.
Fig. 8:
Portal venous gas—echogenic foci in the portal vein system
Free Fluid Collection
Neonates with NEC may have ascites, which show as anechoic areas in the abdominal cavity between the gut and the surrounding intraperitoneal structures. It can be of two types—simple and complex (Fig. 9). Small amounts of simple free fluid can also present physiologically.15 Complex ascites are more often echogenic and can show loculations. These findings may indicate intestinal perforation and a need for surgical intervention.25
Figs 9A and B:
Free fluid collection. (A) Simple free fluid—anechoic area between the gut walls; (B) Complex free fluid collection—echogenic material is seen in between the anechoic areas suggestive of debris
Free Air Collection
Pneumoperitoneum, a sign of intestinal perforation, is seen on US as a hyperechoic line posterior to the anterior abdominal wall. It is easiest to visualize between the anterior surface of the liver and the abdominal wall (Fig. 10). However, the examiners should be cautious and not compress the probe as this might displace any air present in the space between the liver and the abdominal wall.
Fig. 10:
Pneumoperitoneum—hyperechoic line can be seen between the anterior surface of the liver and the abdominal wall
Loss of Gut Signature
Changes in gut wall echotexture with the loss of gut signature usually indicate bowel wall pathology. Increased intestinal echogenicity with a loss of the hypoechoic muscularis signal is a characteristic sign (Fig. 11). This should be differentiated from the pneumatosis intestinalis, which is usually associated with posterior shadowing.26
Fig. 11:
Loss of gut signature
Gut Wall Thickness
The gut should be visualized with measurements of bowel wall thickness in all four quadrants (Fig. 12A). In the initial stages of NEC, inflammation and edema are seen as increased bowel wall thickness (>2.5–2.7 mm) (Fig. 12B).15,23 Later, disease progression with ischemia and necrosis may reduce bowel wall thickness (typically <1 mm) (Fig. 12C).7,15,23,24 In a meta-analysis, neonates with low gut wall thickness were at higher risk of requiring surgery or of death [odds ratio (OR): 7.1, 1.6–32.3] compared to those with increased thickness (OR: 3.9, 2.4–6.1).25
Figs 12A to C:
Gut wall thickness. (A) Normal gut wall thickness; (B) Increased gut wall thickness; (C) Decreased gut wall thickness
Loss of Peristalsis
The normal gut has peristaltic movements. Gut pathology in NEC leads to decreased or absent peristaltic movements (<10/min).15 These findings might be missed if the bowel is not examined for adequate periods of time.
Pneumatosis Intestinalis
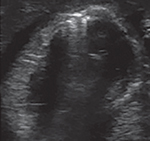
The term pneumatosis intestinalis refers to gas entrapped in the damaged layers of the bowel wall in NEC. These gases are produced due to bacterial fermentation of static gut contents in the inflamed bowel and are seen as echogenic dots in the intestinal wall.27 These dots can range from a few, interspersed hyperechoic lesions in the gut wall to a large circumferential cloud-like layer that surrounds the bowel wall (circle sign) (Fig. 13).
Fig. 13:
Pneumatosis intestinalis—echogenic dots in the wall of the gut
Pneumatosis is often a transient finding in NEC and may require repeated POC abdominal US scans.23 In addition to low diagnostic sensitivity, it also does not aid in predicting the need for surgery or the eventual outcome in these patients.25 As mentioned above, the intramural dots of pneumatosis should be differentiated from the normally seen intraluminal echogenic dots (Fig. 7). Unlike the intramural pneumatosis that can be seen anywhere along the bowel wall, the intraluminal gas shadows are usually seen in the non-dependent part of the intestine and get displaced on changing the position of the baby or on the application of pressure with the probe.
Abnormal Bowel Perfusion
After assessing the gut on a grayscale, further evaluation of vascularity and bowel perfusion can be performed using color Doppler. Inflamed segments of the bowel in NEC frequently show hyperemia with increased vascularity. These changes show characteristic US signatures such as the zebra pattern (increased vascularity at the mucosal folds of the small intestine resulting in multiple, parallel color lines) (Fig. 14A), Y-shaped patterns (subserosal and mesenteric vessels) (Fig. 14B), ring-shaped patterns (increased blood flow over the entire circumference of the bowel wall) (Fig. 14C), or as increased color dots, more than the normal (Fig. 14D).7,15,24,26 The ensuing bowel ischemia and necrosis result in decreased and then absent perfusion of the gut (Fig. 14E). The loss of perfusion frequently indicates a need for surgery or a fatal outcome.25 Comparison of the diseased segments of the intestine with the surrounding normal gut might provide important insights into the extent of altered perfusion.
Figs 14A to E:
Pathological perfusion of gut wall. (A) Zebra pattern; (B) Y-shaped pattern; (C) Ring-shaped pattern; (D) Increased color dots; (E) Decreased perfusion of gut wall in NEC
Utility of Point-of-care Abdominal Ultrasound in Necrotizing Enterocolitis
We know that AXR has limitations in the diagnosis of NEC.7,28 The diagnosis is frequently feasible only when pneumatosis and/or portal venous gas are seen. The sensitivity of these radiological markers can be even lower in mildly-afflicted cases.29 Bowel wall dilation is a sensitive finding but is not specific to NEC.7 Other non-specific findings include ascites, air-fluid levels, and bowel wall thickening. An important clinical difficulty arises in the low specificity of the sensitive signs and low sensitivity for the specific signs.7,28,30,31
Many meta-analyses and narrative reviews have shown that abdominal US is useful in the diagnosis of NEC.7,12,25,32,33 Compared to AXR, US seems to be more sensitive for early detection of NEC; it can detect early markers such as altered bowel perfusion, decreased peristalsis, and bowel wall thickening, and helps in the timely initiation of management before the onset of advanced disease marked by ileus, loss of bowel perfusion, and bowel wall thinning (Tables 1 and 2).10,11,25 Even though abdominal US may not detect NEC in every single case, it can confirm the absence of findings of NEC where AXR is equivocal.34,35 The detection of pneumatosis and/or portal venous gas can also help in differentiating perforated NEC from other causes of intestinal perforation like spontaneous intestinal perforation.36 Compared to abdominal radiography, US is more sensitive in detecting pneumatosis and/or portal venous gas.37
Table 1:
Summary of POC abdominal US findings in a normal gut and NEC
| Probe tenderness | Normal gut |
NEC |
||
|---|---|---|---|---|
| Absent | Present | |||
|
| ||||
| Gut wall echotexture | 5 layers of alternating hyperechoic and hypoechoic layers – gut signature |

|
Loss of gut signature |

|
| Gut wall thickness | 1–2.6 mm |

|
Increased thickness – >2.6 mm Later stages – decreased thickness <1 mm |

|
| Peristalsis | At least 10 peristaltic movements per minute | Less than 10 peristaltic movements per minute | ||
| Gut wall perfusion | 1–9 color signal dots detected per sq. cm |

|
Increased perfusion – >9 color signal dots detected per sq. cm Specific patterns – zebra pattern Y-shaped pattern Ring-shaped pattern Later stages – decreased or absent perfusion |

|
| Pneumatosis intestinalis | Absent (intraluminal gas may be present) |

|
Present |
|
| Portal venous gas | Absent |
|
Present |
|
| Fluid collection | Absent (small amount of simple free fluid can be present physiologically) | Present Complex free fluid is always pathological |

|
|
| Free air collection | Absent | Hyperechoic line posterior to the anterior abdominal wall – easiest to visualize between the anterior surface of the liver and the abdominal wall |

|
|
Table 2:
Format for POC abdominal US
| LLQ | LUQ | RUQ | RLQ | |
|---|---|---|---|---|
|
| ||||
| Phase I (using lower frequency curvilinear probe – 4–8 Hz) | ||||
|
| ||||
| Portal venous gas | ||||
| Tenderness | ||||
| Fluid collection | ||||
| Free air collection | ||||
|
| ||||
| Phase II (using high-frequency linear probe – 8–20 Hz) | ||||
|
| ||||
| Gut wall echotexture | ||||
| Gut wall thickness | ||||
| Peristalsis* | ||||
| Pneumatosis intestinalis | ||||
| Perfusion | ||||
For assessing peristalsis, the gut must be observed for at least 1 minute. For each quadrant, the examination should be done in two planes—transverse and longitudinal. LLQ, left lower quadrant; LUQ, left upper quadrant; RLQ, right lower quadrant; RUQ, right upper quadrant
Unfortunately, both AXR and abdominal US seem to be inadequate as stand-alone diagnostic tests for NEC. A non-diagnostic AXR cannot nullify a suspicious abdominal US in a preterm infant with suspicion of NEC. Abdominal US is more informative than AXR in the evaluation for NEC, but its sensitivity and negative predictive value are low. A negative abdominal US scans should be interpreted with caution, particularly in at-risk preterm infants. In a cohort of 100 infants with suspected NEC and a hypothetical overall prevalence of about 50%, Cuna et al.23 showed that 25–40 infants with a negative US evaluation could have had NEC (false negatives).
A meta-analysis23 of studies of infants with probable NEC showed US as having high specificity and positive predictive value for NEC diagnosis. Classic signs of NEC on abdominal US (portal venous gas, pneumatosis, and free air) had pooled sensitivities ranging from 0.27 to 0.48 and pooled specificities ranging from 0.91 to 0.99. Bowel wall thinning and absent peristalsis had overall low sensitivity (0.22 and 0.30) but high specificity (0.96 and 0.96) for NEC. Detection of abdominal fluid, which included ascites and focal fluid collection, also had overall low sensitivity and high specificity (simple ascites—0.45 and 0.92; focal fluid collection—0.19 and 0.98). Hence, sonographic evaluation may have low sensitivity and high specificity for the diagnosis of NEC. The absence of the findings in a single sonographic examination does not exclude NEC in a patient.
Abdominal US can help in prognostication in NEC. Focal fluid collections, complex ascites, absent peristalsis, pneumoperitoneum, bowel wall echogenicity, bowel wall thinning, absent perfusion, bowel wall thickening, and dilated bowel have been associated with the combined outcome of the need for surgery or death.25 Therefore, when positive, US can help in earlier, timely decision-making for surgical management in NEC, when AXRs might still be equivocal.
Limitations
Further work is needed for increasing the acceptance of abdominal US as a standard modality for the diagnosis of NEC. There is limited experience in settings with low incidence of NEC. Also, the steep learning curve, the higher financial burden of the machine, interobserver variability, the perception of neonatologists toward US, and the common artifacts faced, hinder its widespread use. Clinical instability due to overzealous use of abdominal US leading to desaturation, apnea, and bradycardia can be present. These can be overcome by training and emphasis on the protocol-based approach of the POC abdominal US examination with special emphasis on the most important diagnostic pointers toward NEC. Using a large quantity of gel with minimum possible pressure on the abdomen may prevent stress to the neonate.
Conclusion
Necrotizing enterocolitis, a nightmare for neonatologists, continues to have poor outcome. Early diagnosis of NEC and timely surgical intervention can hugely improve the prognosis of these neonates. POC abdominal US along with clinical evaluation and AXR has the potential to become the gold standard for the evaluation of these neonates, which not only can diagnose early signs of NEC but also may differentiate NEC from other causes of feed intolerance. With the growing evidence about the benefits of abdominal US, and the clinicians getting accustomed to sonography, it will go a long way in managing such babies.
Highlights.
Neonatal gut ultrasound (US) is an emerging tool for quick diagnosis and prognosis in various abdominal pathologies such as necrotizing enterocolitis.
The abdomen is examined for fluid or free-air collection, portal venous gas, intestinal echotexture, gut wall thickness, pneumatosis intestinalis, and peristaltic movements.
The normal intestine shows a characteristic gut signature in abdominal US with alternating hyperechoic and hypoechoic layers. Peristalsis can be seen as a worm-like motion. In necrotizing enterocolitis (NEC), the inflamed intestine shows increased wall thickness due to ischemia and necrosis.
Intestinal intramural gas escaping through the mesenteric vessels to the portal veins can be detected as echogenic foci in the portal venous system or the distal parenchymal liver tissue.
The term “pneumatosis intestinalis” refers to gas entrapped in the damaged layers of the bowel wall in NEC. Most gas bubbles are seen as echogenic dots in the intestinal wall.
Source of support:
National Institutes of Health Awards: HL124078 and HL133022 (to AM).
Footnotes
Conflict of interest: Dr Akhil Maheshwari is associated as Editor-in-Chief of this journal and this manuscript was subjected to this journal’s standard review procedures, with this peer review handled independently of the Editor-in-Chief and his research group.
References
- 1.Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: A systematic review and meta-analysis. BMC Pediatr 2020;20(1):344. DOI: 10.1186/s12887-020-02231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zozaya C, González IG, Avila–Alvarez A, et al. Incidence, treatment, and outcome trends of necrotizing enterocolitis in preterm infants: A multicenter cohort study. Front Pediatr 2020;8:188. DOI: 10.3389/fped.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han SM, Hong CR, Knell J, et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J Pediatr Surg 2020;55(6):998–1001. DOI: 10.1016/j.jpedsurg.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009;44(6):1072–1075; Discussion 1075–1076. DOI: 10.1016/j.jpedsurg.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187(1):1–7. DOI: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh MC, Kliegman RM. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr Clin North Am 1986;33(1):179–201. DOI: 10.1016/s0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epelman M, Daneman A, Navarro OM, et al. Necrotizing enterocolitis: Review of state-of-the-art imaging findings with pathologic correlation. Radiographics 2007;27(2):285–305. DOI: 10.1148/rg.272055098. [DOI] [PubMed] [Google Scholar]
- 8.Merritt CR, Goldsmith JP, Sharp MJ. Sonographic detection of portal venous gas in infants with necrotizing enterocolitis. AJR Am J Roentgenol 1984;143(5):1059–1062. DOI: 10.2214/ajr.143.5.1059. [DOI] [PubMed] [Google Scholar]
- 9.Robberecht EA, Afschrift M, De Bel CE, et al. Sonographic demonstration of portal venous gas in necrotizing enterocolitis. Eur J Pediatr 1988;147(2):192–194. DOI: 10.1007/BF00442221. [DOI] [PubMed] [Google Scholar]
- 10.Garbi–Goutel A, Brévaut–Malaty V, Panuel M, et al. Prognostic value of abdominal sonography in necrotizing enterocolitis of premature infants born before 33 weeks gestational age. J Pediatr Surg 2014;49:508–13. DOI: 10.1016/j.jpedsurg.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Zhong Y, Yu J, et al. Ultrasonography and radiography findings predicted the need for surgery in patients with necrotising enterocolitis without pneumoperitoneum. Acta Paediatr 2016;105:e151–5. DOI: 10.1111/apa.13315. [DOI] [PubMed] [Google Scholar]
- 12.Dilli D, Suna Oğuz S, Erol R, et al. Does abdominal sonography provide additional information over abdominal plain radiography for diagnosis of necrotizing enterocolitis in neonates? Pediatr Surg Int 2011;27:321–7. DOI: 10.1007/s00383-010-2737-8. [DOI] [PubMed] [Google Scholar]
- 13.Shebrya NH, Amin SK, El-Shinnawy MA, et al. Abdominal ultrasonography in preterm necrotizing enterocolitis. Is it superior to plain radiography? Egyptian J Rad Nuclear Med 2012;43(3):457–463. DOI: 10.1016/j.ejrnm.2012.06.001. [DOI] [Google Scholar]
- 14.Kim WY, Kim WS, Kim IO, et al. Sonographic evaluation of neonates with early-stage necrotizing enterocolitis. Pediatr Rad 2005;35(11):1056–1061. DOI: 10.1007/s00247-005-1533-4. [DOI] [PubMed] [Google Scholar]
- 15.Faingold R, Daneman A, Tomlinson G, et al. Necrotizing enterocolitis: Assessment of bowel viability with color Doppler US. Radiology 2005;235(2):587–594. DOI: 10.1148/radiol.2352031718. [DOI] [PubMed] [Google Scholar]
- 16.Ahle M, Ringertz HG, Rubesova E. The role of imaging in the management of necrotising enterocolitis: A multispecialist survey and a review of the literature. Eur Radiol 2018;28(9):3621–3631. DOI: 10.1007/s00330-108-5362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Hu Y, Liu Q, et al. Comparison of abdominal radiographs and sonography in prognostic prediction of infants with necrotizing enterocolitis. Pediatr Surg Int 2018;34(5):535–541. DOI: 10.1007/s00383-018-4256-y. [DOI] [PubMed] [Google Scholar]
- 18.Abdullah F, Zhang Y, Camp M, et al. Necrotizing enterocolitis in 20,822 infants: Analysis of medical and surgical treatments. Clin Pediatr 2010;49(2):166–171. DOI: 10.1177/0009922809349161. [DOI] [PubMed] [Google Scholar]
- 19.Faingold R Technical aspects of abdominal ultrasound and color Doppler assessment of bowel viability in necrotizing enterocolitis. Pediatr Radiol 2018;48(5):617–619. DOI: 10.1007/s00247-018-4077-0. [DOI] [PubMed] [Google Scholar]
- 20.Heyder N, Kaarmann H, Giedl J. Experimental investigations into the possibility of differentiating early from invasive carcinoma of the stomach by means of ultrasound. Endoscopy 1987;19(06):228–232. DOI: 10.1055/s-2007-1013019. [DOI] [PubMed] [Google Scholar]
- 21.Wilson SR. Gastrointestinal tract sonography. Abdom Imaging 1996;21(1):1–8. DOI: 10.1007/s002619900001. [DOI] [PubMed] [Google Scholar]
- 22.Haber HP, Stern M. Intestinal ultrasonography in children and young adults: Bowel wall thickness is age dependent. J Ultrasound Med 2000;19(5):315–321. [PubMed] [Google Scholar]
- 23.Cuna AC, Lee JC, Robinson AL, et al. Bowel ultrasound for the diagnosis of necrotising enterocolitis. Ultrasound Q 2018; 34:113–118. DOI: 10.1097/RUQ.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 24.van Druten J, Khashu M, Chan SS, et al. Abdominal ultrasound should become part of standard care for early diagnosis and management of necrotising enterocolitis: A narrative review. Arch Dis Child Fetal Neonatal Ed 2019;104(5):F551–F559. DOI: 10.1136/archdischild-2018-316263. [DOI] [PubMed] [Google Scholar]
- 25.Cuna AC, Reddy N, Robinson AL, et al. Bowel ultrasound for predicting surgical management of necrotizing enterocolitis: A systematic review and meta-analysis. Pediatr Radiol 2018;48(5):658–666. DOI: 10.1007/s00247-017-4056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexander KM, Chan SS, Opfer E, et al. Implementation of bowel ultrasound practice for the diagnosis and management of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2021;106(1):96–103. DOI: 10.1136/archdischild-2019-318382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priyadarshi A, Rogerson S, Hinder M, et al. Neonatologist performed point-of-care bowel ultrasound: Is the time right? Australas J Ultrasound Med 2018;22(1):15–25. DOI: 10.1002/ajum.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buonomo C The radiology of necrotizing enterocolitis. Radiol Clin North Am 1999;37(6):1187–1198. DOI: 10.1016/s0033-8389(05)70256-6. [DOI] [PubMed] [Google Scholar]
- 29.Tam AL, Camberos A, Applebaum H. Surgical decision making in necrotizing enterocolitis and focal intestinal perforation: Predictive value of radiologic findings. J Pediatr Surg 2002;37(12):1688–1691. DOI: 10.1053/jpsu.2002.36696. [DOI] [PubMed] [Google Scholar]
- 30.Kosloske AM, Musemeche CA, Ball WS, et al. Necrotizing enterocolitis: Value of radiographic findings to predict outcome. AJR Am J Roentgenol 1988;151(4):771–774. DOI: 10.2214/ajr.151.4.771. [DOI] [PubMed] [Google Scholar]
- 31.Daneman A, Woodward S, de Silva M. The radiology of neonatal necrotizing enterocolitis (NEC). A review of 47 cases and the literature. Pediatr Radiol 1978;7(2):70–77. DOI: 10.1007/BF00975674. [DOI] [PubMed] [Google Scholar]
- 32.Bohnhorst B Usefulness of abdominal ultrasound in diagnosing necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98(5):F445–F450. DOI: 10.1136/archdischild-2012-302848. [DOI] [PubMed] [Google Scholar]
- 33.Lok JM, Miyake H, Hock A, et al. Value of abdominal ultrasound in management of necrotizing enterocolitis: A systematic review and meta-analysis. Pediatr Surg Int 2018;34(6):589–612. DOI: 10.1007/s00383-018-4259-8. [DOI] [PubMed] [Google Scholar]
- 34.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123(1):58–66. DOI: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD neonatal research network. Pediatrics 2002;110(2 Pt 1):285–291. DOI: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 36.Pumberger W, Mayr M, Kohlhauser C, et al. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg 2002;195:796–803. DOI: 10.1016/s1072-7515(02)01344-3. [DOI] [PubMed] [Google Scholar]
- 37.Prithviraj D, Sandeep B, Suresh A, et al. Comparison between X-ray and abdominal ultrasound findings of necrotizing enterocolitis, its usefulness in early diagnosis, prognosis, and to assess, is this is the time to change our view of surgeon’s intervention according to the Bell’s criteria. Int J Sci Study 2015;3(4):119–130. DOI: 10.17354/ijss/2015/319. [DOI] [Google Scholar]
















