Abstract
Purpose
The potential disparities in palliative care delivery for underrepresented minorities with breast cancer are not well known. We sought to determine whether race and ethnicity impact the receipt of palliative care for patients with metastatic breast cancer (MBC).
Methods
We retrospectively reviewed the National Cancer Database for female patients diagnosed with stage IV breast cancer between 2010 and 2017 who received palliative care following diagnosis of MBC to assess the proportion of patients who received palliative care, including non–curative-intent local–regional or systemic therapy. Multivariable logistic regression analysis was performed to identify variables associated with receiving palliative care.
Results
60,685 patients were diagnosed with de novo MBC. Of these, only 21.4% (n = 12,963) received a palliative care service. Overall, there was a positive trend in palliative care receipt from 18.2% in 2010 to 23.0% in 2017 (P < 0.001), which persisted when stratified by race and ethnicity. Relative to non-Hispanic White women, Asian/Pacific Islander women (aOR 0.80, 95% CI 0.71–0.90, P < 0.001), Hispanic women (adjusted odds ratio [aOR] 0.69, 95% CI 0.63–0.76, P < 0.001), and non-Hispanic Black women (aOR 0.94, 95% CI 0.88–0.99, P = 0.03) were less likely to receive palliative care.
Conclusions
Fewer than 25% of women with MBC received palliative care between 2010 and 2017. While palliative care has significantly increased for all racial/ethnic groups, Hispanic White, Black, and Asian/Pacific Islander women with MBC still receive significantly less palliative care than non-Hispanic White women. Further research is needed to identify the socioeconomic and cultural barriers to palliative care utilization.
Keywords: Palliative care, Metastatic breast cancer, Health equity, Under-represented minorities
Introduction
In the US, patients with metastatic breast cancer (MBC) have a 5-year survival rate of 29% compared to 90% for all breast cancer patients [1]. The importance of palliative and supportive care interventions for improving short-term quality of life and symptom burden has been well studied in randomized trials and is supported by the American Society of Clinical Oncology (ASCO) [2-9]. The study of palliative care utilization is especially crucial for patient populations with metastatic cancer that experience worse survival outcomes. Previous studies have reported disparities in overall survival rates for underrepresented patients with MBC [10]. For example, Black women with MBC have an estimated 5-year survival rate of 26% compared to 35% to 40% for patients of all other races and ethnicities [11]. While biologic and nonbiologic factors contribute to these disparate outcomes, social and structural determinants of health are essential targets for designing and implementing health equity initiatives. Using a nationwide cancer registry, we examined racial/ethnic disparities in palliative care use during the past decade among patients with MBC to better illuminate care inequities.
Methods
To explore palliative care trends among patients with MBC in the US, we queried the National Cancer Database (NCDB). This extensive nationwide clinical oncology database captures nearly 70% of new US breast cancer diagnoses and was used to identify individuals diagnosed with de novo stage IV breast cancer between 2010 and 2017. Our study was approved by Roswell Park Comprehensive Cancer Center institutional review board (BDR-131220) and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The primary outcome was determining which patients received palliative care, defined in the NCDB as receiving a procedure to alleviate symptoms without curative intent, including surgery, radiation therapy, chemotherapy, and pain management therapy.
Variables of interest included the patient’s race and ethnicity, facility type, facility volume, income, insurance, education, residential setting, diagnosis age, Charlson-Deyo comorbidity score, diagnosis year, tumor grade, clinical T and N stage, metastasis location, tumor receptor types, tumor histology, surgery types, and treatment(s) (including chemotherapy, hormone therapy, immunotherapy, and radiation therapy). Stratification was performed based on the 2016 American Community Survey data for education and income levels. We chose to use binary income and education level (below or above median) in our analysis to simplify the interpretation of the results by providing easily understandable categories that allow assessment of the impact of area-level socioeconomic status factors on palliative care service utilization and differences between race and ethnic groups. For education level, measured as the percentage of individuals who did not graduate high school, the median value of 10.9% was used for stratification. For income, the median value of $50,353 annual income based on patient zip code was used for stratification. All missing values were coded as unknown.
Baseline characteristics were compared using the chi-square test. A Cochran-Armitage test was performed to evaluate palliative care use trends from 2010 to 2017. Multivariable logistic regression was performed to identify variables associated with receiving palliative care. Multivariable Cox regression was performed to evaluate variables associated with overall survival (OS) rates, defined as the time from diagnosis to last follow-up or death. Multivariable models included all clinically relevant variables listed previously. To address immortal time bias, multivariable Cox regression was repeated among patients receiving chemotherapy with postdiagnosis survival of greater than 6 months. All P values were two-sided. Holm-Bonferroni corrections were used to adjust for three comparisons among racial and ethnic subgroups (non-Hispanic White [NHW] vs. Hispanic women [HW], NHW vs. non-Hispanic Black [NHB] women, and NHW vs. Asian or Pacific Islander [API] women). P values less than 0.05 were considered to be statistically significant. All analyses were performed using R software version 4.0.3 (R Project for Statistical Computing).
Results
A total of 60,685 women met the inclusion criteria, and 21.4% received palliative care (n = 12,963). Most patients in our study cohort were NHW women (n = 44,082), followed by NHB (n = 10,776), Hispanic (n = 3656), and API (n = 2171) women (Table 1). Each patient cohort by race and ethnicity differed significantly. Each variable was evaluated for patient demographics and tumor and treatment characteristics (Table 1).
Table 1.
Patient demographic, tumor, and treatment characteristics (N = 60,685)
| Variable | Racial/ethnic groups (number of patients, percentages) | P value | |||
|---|---|---|---|---|---|
| NHW (N = 44,082) | Hispanic (N = 3656) | NHB (N = 10,776) | API (N = 2171) | ||
| Palliative care | < 0.001 | ||||
| No | 34,305 (77.8) | 3083 (84.3) | 8551 (79.4) | 1783 (82.1) | |
| Yes | 9777 (22.2) | 573 (15.7) | 2225 (20.6) | 388 (17.9) | |
| Chemotherapy | < 0.001 | ||||
| No | 18,607 (42.2) | 1192 (32.6) | 3805 (35.3) | 762 (35.1) | |
| Yes | 24,653 (55.9) | 2358 (64.5) | 6733 (62.5) | 1355 (62.4) | |
| Not available | 822 (1.9) | 106 (2.9) | 238 (2.2) | 54 (2.5) | |
| Endocrine therapy | < 0.001 | ||||
| No | 17,214 (39.0) | 1742 (47.6) | 5547 (51.5) | 953 (43.9) | |
| Yes | 25,712 (58.3) | 1798 (49.2) | 4852 (45.0) | 1142 (2.6) | |
| No available | 1156 (2.6) | 116 (3.2) | 377 (3.5) | 76 (3.5) | |
| Immunotherapy | < 0.001 | ||||
| No | 39,073 (88.6) | 3140 (85.9) | 9519 (8.3) | 1818 (83.7) | |
| Yes | 4854 (11.0) | 501 (13.7) | 1209 (11.2) | 339 (15.6) | |
| Not available | 155 (0.4) | 15 (0.4) | 48 (0.4) | 14 (0.6) | |
| Radiation therapy | < 0.001 | ||||
| No | 28,210 (64.0) | 2320 (63.5) | 7107 (66.0) | 1370 (63.1) | |
| Yes | 14,480 (32.8) | 1172 (32.1) | 3292 (30.5) | 727 (33.5) | |
| Not available | 1392 (3.2) | 164 (4.5) | 377 (3.5) | 74 (3.4) | |
| Surgery | < 0.001 | ||||
| None | 32,577 (73.9) | 2627 (71.9) | 8186 (76.0) | 1546 (71.2) | |
| Lumpectomy | 3268 (7.4) | 264 (7.2) | 685 (6.4) | 143 (6.6) | |
| Mastectomy | 8021 (18.2) | 737 (20.2) | 1844 (17.1) | 466 (21.5) | |
| Other | 53 (0.1) | 2 (0.1) | 12 (0.1) | 6 (0.3) | |
| Not available | 163 (0.4) | 26 (0.7) | 49 (0.5) | 10 (0.5) | |
| Facility | < 0.001 | ||||
| Nonacademic | 28,969 (65.7) | 1734 (47.4) | 5560 (51.6) | 1089 (50.2) | |
| Academic | 12,815 (29.1) | 1444 (39.5) | 4263 (39.6) | 856 (39.4) | |
| Not available | 2298 (5.2) | 478 (13.1) | 953 (8.8) | 226 (10.4) | |
| Facility volume | < 0.001 | ||||
| Low | 4668 (10.6) | 227 (6.2) | 629 (5.8) | 185 (8.5) | |
| Intermediate | 11,178 (25.4) | 851 (23.3) | 227 (20.7) | 480 (22.1) | |
| High | 28,236 (64.1) | 2578 (70.5) | 7920 (73.5) | 1506 (69.4) | |
| Age | < 0.001 | ||||
| < 50 | 7247 (16.4) | 1228 (33.6) | 2705 (25.1) | 631 (29.1) | |
| 50–70 | 22,935 (52.0) | 1746 (47.8) | 5761 (53.5) | 1119 (51.5) | |
| > 70 | 13,900 (31.5) | 682 (18.7) | 2310 (21.4) | 421 (19.4) | |
| Charlson-Devo Score | < 0.001 | ||||
| 0 | 35,870 (81.4) | 3041 (83.2) | 8348 (77.5) | 1811 (83.4) | |
| 1 | 5768 (13.1) | 473 (12.9) | 1653 (15.3) | 266 (12.3) | |
| ≥ 2 | 2444 (5.5) | 142 (3.9) | 775 (7.2) | 94 (4.3) | |
| Year of diagnosis | < 0.001 | ||||
| 2010–2013 | 19,843 (45.0) | 1597 (43.7) | 4902 (45.5) | 842 (38.8) | |
| 2014–2017 | 24,239 (55.0) | 2059 (56.3) | 5874 (54.5) | 1329 (61.2) | |
| Histology | < 0.001 | ||||
| Ductal or lobular | 34,907 (79.2) | 2908 (79.5) | 8729 (81.0) | 1786 (82.3) | |
| Others | 9175 (20.8) | 748 (20.5) | 2047 (19.0) | 385 (17.7) | |
| Grade | < 0.001 | ||||
| Well diff | 3065 (7.0) | 174 (4.8) | 487 (4.5) | 107 (4.9) | |
| Moderately diff | 15,760 (35.8) | 1181 (32.3) | 3157 (29.3) | 721 (33.2) | |
| Poorly diff | 15,817 (35.9) | 1478 (40.4) | 5097 (47.3) | 922 (42.5) | |
| Other | 179 (0.4) | 26 (0.7) | 33 (0.3) | 14 (0.6) | |
| Not available | 9261 (21.0) | 797 (21.8) | 2002 (18.6) | 407 (18.7) | |
| cT | < 0.001 | ||||
| 1 | 6959 (15.8) | 454 (12.4) | 1282 (11.9) | 226 (10.4) | |
| 2 | 12,613 (28.6) | 957 (26.2) | 2657 (24.7) | 595 (27.4) | |
| 3 | 5823 (13.2) | 585 (16.0) | 1665 (15.5) | 357 (16.4) | |
| 4 | 12,808 (29.1) | 1080 (29.5) | 3840 (35.6) | 738 (34.0) | |
| Not available | 5879 (13.3) | 580 (15.9) | 1332 (12.4) | 255 (11.7) | |
| cN | < 0.001 | ||||
| 0 | 11,413 (25.9) | 735 (20.1) | 1997 (18.5) | 413 (19.0) | |
| 1 | 17,781 (40.3) | 1571 (43.0) | 4534 (42.1) | 925 (42.6) | |
| 2 | 4589 (10.4) | 392 (10.7) | 1375 (12.8) | 235 (10.8) | |
| 3 | 5248 (11.9) | 522 (14.3) | 1714 (15.9) | 378 (17.4) | |
| Not available | 5051 (11.5) | 436 (11.9) | 1156 (10.7) | 220 (10.1) | |
| Tumor receptor | < 0.001 | ||||
| HR+/HER2− | 27,806 (63.1) | 2035 (55.7) | 5675 (52.7) | 1253 (57.7) | |
| HR−/HER2+ | 3744 (8.5) | 440 (12.0) | 1073 (10.0) | 271 (12.5) | |
| HR+/HER2+ | 6975 (15.8) | 636 (17.4) | 1691 (15.7) | 397 (18.3) | |
| HR−/HER2− | 5557 (12.6) | 545 (14.9) | 2337 (21.7) | 250 (11.5) | |
| Metastasis to bone | < 0.001 | ||||
| No | 13,500 (30.6) | 1249 (34.2) | 3994 (37.1) | 764 (35.2) | |
| Yes | 30,227 (68.6) | 2376 (65.0) | 6705 (62.2) | 1384 (63.7) | |
| Not available | 355 (0.8) | 31 (0.8) | 77 (0.7) | 23 (1.1) | |
| Metastasis to brain | < 0.001 | ||||
| No | 40,031 (90.8) | 3253 (89.0) | 9745 (90.4) | 1996 (91.6) | |
| Yes | 3342 (7.6) | 332 (9.1) | 889 (8.2) | 148 (6.8) | |
| Not available | 709 (1.6) | 71 (1.9) | 142 (1.3) | 27 (1.2) | |
| Metastasis to liver | < 0.001 | ||||
| No | 32,766 (74.3) | 2790 (76.3) | 7744 (71.9) | 1597 (73.6) | |
| Yes | 10,674 (24.2) | 805 (22.0) | 2900 (26.9) | 546 (25.1) | |
| Not available | 642 (1.5) | 61 (1.7) | 132 (1.2) | 28 (1.3) | |
| Metastasis to lungs | < 0.001 | ||||
| No | 30,649 (69.5) | 2455 (67.1) | 6920 (64.2) | 1419 (65.4) | |
| Yes | 12,608 (28.6) | 1128 (30.9) | 3695 (34.3) | 715 (32.9) | |
| Not available | 825 (1.9) | 73 (2.0) | 161 (1.5) | 37 (1.7) | |
| Education | < 0.001 | ||||
| Above median | 23,353 (53.0) | 838 (22.9) | 2715 (25.2) | 1015 (46.8) | |
| Below median | 16,651 (37.8) | 2524 (69.0) | 7099 (65.9) | 974 (44.9) | |
| Not available | 4078 (9.3) | 294 (8.0) | 962 (8.9) | 182 (8.4) | |
| Income | < 0.001 | ||||
| Above median | 25,458 (57.8) | 1671 (45.7) | 3308 (30.7) | 1501 (69.1) | |
| Below median | 14,459 (32.8) | 1686 (46.1) | 6497 (60.3) | 486 (22.4) | |
| Not available | 4165 (9.4) | 299 (8.2) | 971 (9.0) | 184 (8.5) | |
| Insurance | < 0.001 | ||||
| None | 1577 (3.6) | 541 (14.8) | 859 (8.0) | 152 (7.0) | |
| Private | 18,594 (42.2) | 1179 (32.2) | 3776 (35.0) | 990 (45.6) | |
| Government | 23,366 (53.2) | 1860 (50.9) | 5971 (55.4) | 998 (46.0) | |
| Not available | 545 (1.2) | 76 (2.1) | 170 (1.6) | 31 (1.4) | |
| Residence | < 0.001 | ||||
| Metropolitan | 36,193 (82.1) | 3465 (94.5) | 9716 (90.2) | 2007 (92.4) | |
| Urban | 5925 (13.4) | 136 (3.7) | 740 (6.9) | 98 (4.5) | |
| Rural | 755 (1.7) | 9 (0.2) | 105 (1.0) | 16 (0.7) | |
| Not available | 1209 (2.7) | 55 (1.5) | 215 (2.0) | 50 (2.3) | |
N number, NHW non-Hispanic White, NHB non-Hispanic Black, API Asian/Pacific Islander, diff differentiated, cT tumor grade, cN node, HR hormone-receptor, HER2 human epidermal growth factor receptor 2
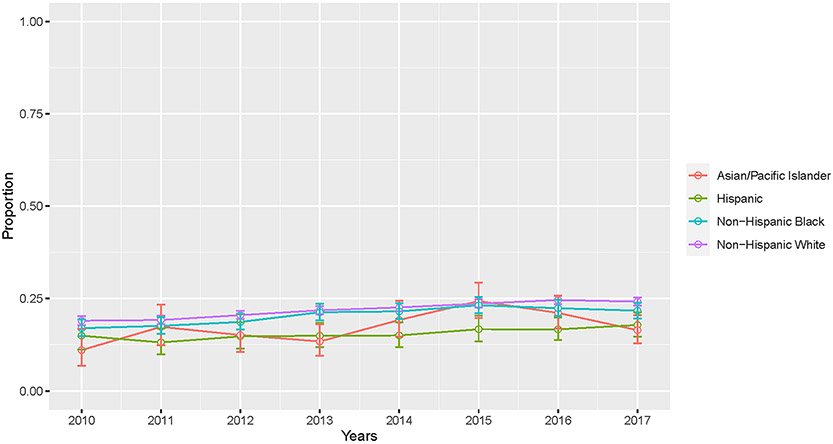
Utilizing the Cochran Armitage test, there was an overall positive trend in palliative care receipt from 2010 (18.2%) to 2017 (23.0%) (P < 0.001). Stratified by race and ethnic groups, there was a similar positive trend in palliative care receipt for NHW (19.0% in 2010 to 24.2% in 2017; P < 0.001), Hispanic (15.0% in 2010 to 17.8% in 2017; P = 0.04), NHB (17.0% in 2010 to 21.7% in 2017; P < 0.001), and API (11.0% in 2010 to 16.4% in 2017; P = 0.01; Fig. 1). Multivariable logistic regression confirmed this (Table 2). More recent years of diagnosis were found to be a statistically significant predictor of palliative care receipt for all patients (adjusted odds ratio [aOR] 1.04, 95% confidence interval [CI] 1.03–1.05, P < 0.001). Relative to NHW women, Hispanic (aOR 0.69, 95% CI 0.63–0.76, P < 0.001), NHB (aOR 0.94, 95% CI 0.88–0.99, P = 0.03), and API women (aOR 0.80, 95% CI 0.71–0.90, P < 0.001) were less likely to receive palliative care (Table 2).
Fig. 1.
Overall trends in palliative care utilization by race and ethnicity
Table 2.
Logistic multivariable analysis of palliative care receipt
| Variable | aOR | 95% CI | P value |
|---|---|---|---|
| Race | |||
| NHW | Reference | ||
| Hispanic | 0.69 | 0.63–0.76 | < 0.001 |
| NHB | 0.94 | 0.88–0.99 | 0.03 |
| API | 0.8 | 0.71–0.90 | < 0.001 |
| Chemotherapy | |||
| No | Reference | ||
| Yes | 0.93 | 0.88–0.97 | 0.002 |
| Endocrine therapy | |||
| No | Reference | ||
| Yes | 1.16 | 1.10–1.23 | < 0.001 |
| Immunotherapy | |||
| No | Reference | ||
| Yes | 1.10 | 1.02–1.18 | 0.01 |
| Radiation therapy | |||
| No | Reference | ||
| Yes | 3.91 | 3.74–4.09 | < 0.001 |
| Surgery | |||
| None | Reference | ||
| Lumpectomy | 0.40 | 0.36–0.44 | < 0.001 |
| Mastectomy | 0.36 | 0.33–0.38 | < 0.001 |
| Other | 0.52 | 0.26–0.96 | 0.05 |
| Facility | |||
| Nonacademic | Reference | ||
| Academic | 0.88 | 0.84–0.93 | < 0.001 |
| Facility volume | |||
| Low | Reference | ||
| Intermediate | 1.02 | 0.94–1.11 | 0.65 |
| High | 1.05–1.22 | 0.002 | |
| Age | |||
| < 50 | Reference | ||
| 50–70 | 1.07 | 1.01–1.15 | 0.03 |
| > 70 | 0.98 | 0.90–1.06 | 0.61 |
| Charlson-Deyo Score | |||
| 0 | Reference | ||
| 1 | 1.11 | 1.05–1.18 | < 0.001 |
| ≥ 2 | 1.27 | 1.17–1.39 | < 0.001 |
| Year of diagnosis | |||
| Per year increase | 1.04 | 1.03–1.05 | < 0.001 |
| Histology | |||
| Ductal or lobular | Reference | ||
| Other | 0.99 | 0.94–1.05 | 0.74 |
| Grade | |||
| Well diff | Reference | ||
| Moderately diff | 0.99 | 0.91–1.09 | 0.88 |
| Poorly diff | 1.03 | 0.94–1.12 | 0.59 |
| Other | 0.76 | 0.52–1.08 | 0.14 |
| cT | |||
| 1 | Reference | ||
| 2 | 1.04 | 0.97–1.11 | 0.31 |
| 3 | 1.04 | 0.96–1.13 | 0.29 |
| 4 | 1.15 | 1.07–1.23 | < 0.001 |
| cN | |||
| 0 | Reference | ||
| 1 | 0.95 | 0.90–1.01 | 0.08 |
| 2 | 1.00 | 0.92–1.08 | 0.96 |
| 3 | 0.98 | 0.90–1.05 | 0.51 |
| Tumor receptor | |||
| HR+/HER2− | Reference | ||
| HR−/HER2+ | 1.01 | 0.92–1.11 | 0.81 |
| HR+/HER2+ | 0.93 | 0.87–1.00 | 0.04 |
| HR−/HER2− | 1.22 | 1.13–1.32 | < 0.001 |
| Metastatic to bone | |||
| No | Reference | ||
| Yes | 1.84 | 1.75–1.94 | < 0.001 |
| Metastatic to brain | |||
| No | Reference | ||
| Yes | 1.04 | 0.97–1.12 | 0.27 |
| Metastatic to liver | |||
| No | Reference | ||
| Yes | 1.19 | 1.13–1.25 | < 0.001 |
| Metastatic to lungs | |||
| No | Reference | ||
| Yes | 1.18 | 1.12–1.23 | < 0.001 |
| Education | |||
| Above median | Reference | ||
| Below median | 0.93 | 0.88–0.98 | 0.004 |
| Income | |||
| Above median | Reference | ||
| Below median | 1.1 | 1.04–1.16 | < 0.001 |
| Insurance | |||
| None | Reference | ||
| Private | 0.91 | 0.83–1.00 | 0.06 |
| Government | 0.96 | 0.87–1.06 | 0.43 |
| Residence | |||
| Metropolitan | Reference | ||
| Urban | 1.16 | 1.09–1.24 | < 0.001 |
| Rural | 1.14 | 0.96–1.34 | 0.13 |
aOR adjusted odds ratio, CI confidence interval, NHW non-Hispanic White, NHB non-Hispanic Black, API Asian/Pacific Islander, diff differentiated, cT tumor grade, cN node, HR hormone-receptor, HER2 human epidermal growth factor receptor 2
Upon multivariable Cox regression analyses, with a median follow-up of 39.8 months (interquartile range 26.0–59.9), NHB women had significantly worse OS rates relative to NHW (adjusted hazards ratio [aHR] 1.10, 95% CI 1.07–1.13, P < 0.001) while Hispanic (aHR 0.79, 95% CI 0.75–0.83, P < 0.001) and API (aHR 0.85, 95% CI 0.79–0.91, P < 0.001) women had significantly improved OS rates. Among 31,767 patients receiving chemotherapy with at least 6 months of survival after diagnosis, similar findings were observed (NHB: aHR 1.15, 95% CI 1.10–1.20, P < 0.001; Hispanic: aHR 0.87, 95% CI 0.81–0.94, P < 0.001; API: aHR 0.90, 95% CI 0.81–0.99, P = 0.03).
Discussion
Despite a positive trend for palliative care utilization by women with de novo MBC in the US, less than 25% received palliative care with persistent disparities in palliative care for underrepresented patients. Specifically, all underrepresented patient groups received significantly less palliative care than NHW-matched counterparts from 2010 to 2017 (HW, API, Black; aOR range: 0.66–0.94). This disparity in quality care serves as a significant opportunity for future intervention.
Heterogeneity in the needs of patients with advanced cancer calls for further study of specific patient populations and the implementation of effective palliative care interventions [12]. For example, a recent retrospective cohort study reported that non-White patients with metastatic cancer were more likely to receive low-value, aggressive end-of-life interventions [13]. There is an urgent need to evaluate palliative care access for historically marginalized patients, the timing of palliative care integration into multidisciplinary oncologic care, and patient perspectives on the value of palliative care.
The increase in the utilization of palliative care between 2010 and 2017 corresponds with the timing of a 2012 update in the Commission on Cancer (COC) requirements on palliative care. The COC definition of palliative care is broad, including support for symptom management, spiritual and psychosocial needs, communication and medical decision-making, and bereavement. COC accreditation requires access to palliative care services, either on-site or by referral, as well as a minimum of two palliative care team members, including at least one physician. The guideline requires a policy and procedure outlining the available palliative care services and referral criteria [14, 15]. Support from national institutions and accreditation bodies is an important component affecting best practices, including palliative care utilization.
Our findings confirm worse mortality for Black women with MBC. Between 2010 and 2016, the survival rate for Black patients was 82% compared to 91% for White patients [16, 17]. Notably, this disparity persists for patients with metastatic breast cancer, as Black patients have an estimated five-year survival rate of 26% compared to 35–40% for patients of all other races/ethnicities [11]. In a SEER analysis, the excess risk of breast cancer specific mortality mediated by socioeconomic factors for Black women ages 18 to 64 ranged from 51.4 to 53.6%, compared to tumor characteristics (33.9–40.7%) and metastatic pattern (3.1–15.8%) [10]. This previous research demonstrated no racial difference in breast cancer specific survival rates among patients 65 or greater. Furthermore, among women with metastatic breast cancer, non-Hispanic Black women were more likely to be uninsured, unmarried, and reside in the lowest socioeconomic status neighborhoods compared to other racial/ethnic groups [10]. Relative to all other racial/ethnic patient groups, Black women with metastatic breast cancer also had the shortest follow-up duration (mean 20.1 months, with 95% CI that did not overlap with other patient groups) [10]. Thus, while biologic and nonbiologic factors contribute to these disparate outcomes, social and structural determinants of health are important targets for designing and implementing health equity initiatives.
Study limitations include those inherent in using the NCDB as a primary data source, including lack of cancer outcomes such as local control and disease-free survival rates. In addition, it lacks details on quality of life outcomes which are vital for patients with metastatic disease. Additionally, NCDB solely includes patients treated at COC-accredited cancer programs, thus capturing only 70% of all cancer patients nationwide. Considering recent randomized studies evaluating palliative care interventions, these limitations are relevant and important areas for further research [18].
Conclusions
Our study reveals that more than 75% of women with MBC in the US do not receive palliative care services. Despite an overall increase in palliative care utilization from 2010 to 2017 and for historically marginalized patient populations, palliative care receipt disparities for racially and ethnically underrepresented groups with MBC over the past decade remain concerning. Further research is needed to identify the systemic, socioeconomic, and cultural barriers to palliative care utilization and evaluate physician bias as a mechanism for lower utilization. Such research would support early access to supportive care through healthcare institutions and patient-centered initiatives for individuals with metastatic breast cancer.
Acknowledgements
The authors wish to thank Jessica Kirwan, Amy Carrao-Tackett, and Sean Hess for editorial assistance that greatly improved the manuscript.
Funding
This research was supported by the National Cancer Institute Cancer Center Support Grant (P30CA016056). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication; Mailhot Vega is supported by NCATS UL1TR001427 KL2 award.
Abbreviations
- aHR
Adjusted hazards ratio
- aOR
Adjusted odds ratio
- API
Asian or Pacific Islander
- ASCO
American society of clinical oncology
- HW
Hispanic White
- MBC
Metastatic breast cancer
- NCDB
National cancer database
- NHB
Non-Hispanic Black
- NHW
Non-Hispanic White
- OS
Overall survival
- STROBE
Strengthening the reporting of observational studies in epidemiology
- US
United States
Footnotes
Data responsibility Drs. Singh and Ma had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interest Bradley—ASCO/Pfizer research grant & Florida Breast Cancer Foundation research grant; Oladeru—Bristol Myers Squibb Foundation Grant, Radiation Oncology Institute and NRG Oncology; Singh—Astra-Zeneca-National Comprehensive Cancer Network grant, Department of Defense Translational Science Grant, P30 National Cancer Institute Grant; All other authors report no conflict of interest.
Ethical approval Roswell Park Comprehensive Cancer Center institutional review board (BDR-131220) approved our study.
Disclaimer The National Cancer Database is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The Commission on Cancer National Cancer Database and the hospitals participating in the program are the sources of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Data availability
The primary data set (National Cancer Database) is available publicly for investigators associated with the Commission on Cancer-accredited programs through the American College of Surgeons (https://www.facs.org/quality-programs/cancer/ncdb).
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA: Cancer J Clin 71(1):7–33 [DOI] [PubMed] [Google Scholar]
- 2.Geels P, Eisenhauer E, Bezjak A, Zee B, Day A (2000) Palliative effect of chemotherapy: objective tumor response is associated with symptom improvement in patients with metastatic breast cancer. J Clin Oncol 18(12):2395–2405 [DOI] [PubMed] [Google Scholar]
- 3.Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J et al. (2016) Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 316(20):2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J et al. (2009) Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 302(7):741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z et al. (2015) Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 33(13):1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A et al. (2014) Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 383(9930):1721–1730 [DOI] [PubMed] [Google Scholar]
- 7.Vanbutsele G, Pardon K, Van Belle S, Surmont V, De Laat M, Colman R et al. (2018) Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol 19(3):394–404 [DOI] [PubMed] [Google Scholar]
- 8.Grudzen CR, Richardson LD, Johnson PN, Hu M, Wang B, Ortiz JM et al. (2016) Emergency department-initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol 2(5):591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM et al. (2017) Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J Clin Oncol 35(1):96–112 [DOI] [PubMed] [Google Scholar]
- 10.Ren JX, Gong Y, Ling H, Hu X, Shao ZM (2019) Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res Treat 173(1):225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A (2017) Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 67(6):439–448 [DOI] [PubMed] [Google Scholar]
- 12.Nickolich MS, El-Jawahri A, Temel JS, LeBlanc TW (2016) Discussing the evidence for upstream palliative care in improving outcomes in advanced cancer. Am Soc Clin Oncol Educ Book 35:e534–e538 [DOI] [PubMed] [Google Scholar]
- 13.Deeb S, Chino FL, Diamond LC, Tao A, Aragones A, Shahrokni A et al. (2021) Disparities in care management during terminal hospitalization among adults with metastatic cancer from 2010 to 2017. JAMA Netw Open 4(9):e2125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Commision on Cancer (2023) American college of surgeons optimal resources for cancer care 2020 standards. Feb 2023, pp 1–112. https://accreditation.facs.org/accreditationdocuments/CoC/Standards/Optimal_Resources_for_Cancer_Care_Feb_2023.pdf?_gl=1*2tohlz*_ga*Mjg0ODgwOTMwLjE2ODE0MzYyOTY.*_ga_KBB21NPQBH*MTY4MTQzNjI5NS4xLjAuMTY4MTQzNjMwNS4wLjAuMA..*_ga_6C8S73MC87*MTY4MTQzNjI5NS4xLjAuMTY4MTQzNjMwNS4wLjAuMA..&_ga=2.218035606.167832602.1681436296-1355094836.1681436296. Accessed on 13 Apr 2023 [Google Scholar]
- 15.Sheldon LK (2014) Implementing the new commission on cancer standard on palliative care services. Clin J Oncol Nurs 18(Suppl):37–38 [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society (2021) Cancer Facts & Figures 2021. Atlanta: American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf. Accessed on 13 Apr 2023 [Google Scholar]
- 17.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2020) SEER cancer statistics review, 1975–2017, National Cancer Institute. Bethesda, Apr 2020. https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site. Accessed on 13 Apr 2023 [Google Scholar]
- 18.Greer JA, Moy B, El-Jawahri A, Jackson VA, Kamdar M, Jacobsen J et al. (2022) Randomized trial of a palliative care intervention to improve end-of-life care discussions in patients with metastatic breast cancer. J Natl Compr Canc Netw 20(2):136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data set (National Cancer Database) is available publicly for investigators associated with the Commission on Cancer-accredited programs through the American College of Surgeons (https://www.facs.org/quality-programs/cancer/ncdb).