Abstract
It is now generally accepted that CD4+CD25+ Treg cells play a major role in the prevention of autoimmunity and pathological immune responses. Their involvement in the pathogenesis of chronic arthritis is controversial, however, and so we examined their role in experimental antigen-induced arthritis in mice. Depletion of CD25-expressing cells in immunized animals before arthritis induction led to increased cellular and humoral immune responses to the inducing antigen (methylated bovine serum albumin; mBSA) and autoantigens, and to an exacerbation of arthritis, as indicated by clinical (knee joint swelling) and histological scores. Transfer of CD4+CD25+ cells into immunized mice at the time of induction of antigen-induced arthritis decreased the severity of disease but was not able to cure established arthritis. No significant changes in mBSA-specific immune responses were detected. In vivo migration studies showed a preferential accumulation of CD4+CD25+ cells in the inflamed joint as compared with CD4+CD25- cells. These data imply a significant role for CD4+CD25+ Treg cells in the control of chronic arthritis. However, transferred Treg cells appear to be unable to counteract established acute or chronic inflammation. This is of considerable importance for the timing of Treg cell transfer in potential therapeutic applications.
Keywords: arthritis, regulatory T cells
Introduction
Rheumatoid arthritis (RA) is the most common autoimmune disease in humans, affecting 1% of the population in western countries. Histologically, RA is characterized by hyperplasia and infiltration of the synovial membrane with mononuclear cells, development of an aggressive tissue called pannus and secretion of proteases, which are responsible for the destruction of articular cartilage and adjacent bone. It is well established that macrophages and synovial fibroblasts are effector cells of joint destruction, and it is presumed that autoreactive CD4+ T cells are involved in their activation [1]. There is now a large body of evidence that, in rodents, regulatory T cells (Treg) actively control the activation of autoreactive T cells and thus maintain immunological self-tolerance. Apart from adaptive Treg cells, which can be induced by antigen-specific stimulation of conventional peripheral T cells under tolerogenic conditions (for review [2]), there is no doubt that naturally occurring Treg cells exist in healthy mice as well as in humans and rats, and these are characterized by constitutive expression of CD25 [3-5]. Absence of these cells in vivo results in a multi-organ autoimmune syndrome [3,6]. These CD4+CD25+ Treg cells leave the thymus as committed 'professional' suppressor T cells [7-9], proliferate in the periphery, and acquire an effector/memory-like phenotype [10]. In unmanipulated mice, Treg cells can also be found in the CD25- compartment, based on the expression of the integrin αEβ7 [10,11], possibly reflecting differences in developmental stages of these cells.
The exact role played by naturally occurring CD4+CD25+ Treg cells in the pathogenesis of arthritis remains controversial. Arthritis is part of the autoimmune syndrome induced by transfer of CD25-depleted splenocytes into lymphopenic hosts [3], and CD4+CD25+ cells are protective in collagen-induced arthritis [12]. However, Bardos and coworkers [13] ruled out a role for naturally occurring CD4+CD25+ Treg cells in proteoglycan-induced arthritis.
To clarify this issue, we used the antigen-induced arthritis (AIA) model. AIA is a Tcell-dependent experimental arthritis that is induced by intra-articular injection of antigen (methylated bovine serum albumin [mBSA]) into knee joints of preimmunized mice [14,15]. This results in an acute inflammatory reaction, which is characterized by exudation of neutrophils and fibrin, which later proceeds to a chronic arthritis with synovial hyperplasia, infiltration of mononuclear cells, and cartilage and bone destruction – histopathological changes similar to those that occur in RA. Autoimmune responses against cartilage constituents such as collagen types I and II and proteoglycans are involved in rendering the disease chronic [16,17]. Beyond the 100% incidence of arthritis, another major advantage of the AIA model is that the time point of induction of arthritis is known, allowing manipulation of CD4+CD25+ Treg cell number in vivo at defined stages in the disease. Using depletion of CD25-expressing cells or transfer of CD4+CD25+ cells, in the present study we demonstrated that Treg cells modulate the onset of AIA but are ineffective at later stages, calling into question their value as a new therapeutic approach to established chronic arthritis.
Methods
Animals, arthritis induction and assessment
For all animal experiments, female C57Bl/6 mice (Charles River, Sulzfeld, Germany; age range 6–10 weeks) were used. Animals were kept under standard conditions, fed a standard diet and given free access to water. All animal studies were approved by the government commission for animal protection.
At 21 and 14 days before arthritis induction, mice were subcutaneously injected with 100 μg mBSA (Sigma, Deisenhofen, Germany), emulsified in complete Freund's adjuvant (Sigma) supplemented to 2 mg/ml heat-killed Mycobacterium tuberculosis (strain H37RA; Becton Dickinson [BD], Heidelberg, Germany). Simultaneously, mice received 5 × 108 heat-inactivated Bordetella pertussis (Chiron-Behring, Marburg, Germany) intraperitoneally. Arthritis was induced by intra-articular injection of 100 μg mBSA in 25 μl phosphate-buffered saline (PBS) into the right knee joint cavity.
Arthritis severity was monitored by measurement of lateral joint diameter using a vernier caliper (Oditest, Kroeplin Längenmesstechnik, Schlüchtern, Germany). Histological severity of arthritis was scored in a blinded manner by two investigators (PKP and MG) in frontal knee joint sections, stained with haematoxylin and eosin and prepared as described previously [14]. Briefly, at least four sections per knee joint were semiquantitatively examined on a 0–3 point scale for each of the following: extent of synovial hyperplasia, mononuclear infiltration, cartilage destruction and pannus formation.
Antibodies and reagents
The following antibodies were grown and purified from the culture supernatants in our laboratory: anti-CD25 (PC61), anti-CD3 (145 2C11), anti-CD4-FITC and FITC-labelled anti-CD4-F(ab) (GK1.5), anti-CD8 (TIB105), anti-CD28 (37.51) and anti-Mac-1 (M1/70). The following antibodies and secondary reagents were purchased from BD Pharmingen (Heidelberg, Germany): PE-Cy5-labelled anti-CD4 (H129.9), biotinylated anti-αEβ7 (M290), biotinylated anti-CD25 (7D4), allophycocyanine or FITC-conjugated anti-CD25 (PC61), streptavidin-allophycocyanine and streptavidin-PE, and matched antibody pairs for ELISPOT analysis of IFN-γ (R4-6A2 and biotinylated XMG1.2) and IL-4 (BVD4 1D11 and biotinylated BVD6-24G2) production.
In vivo depletion
Mice were injected with 0.5 mg purified anti-CD25 antibody (PC61) 4 and 2 days before intra-articular antigen injection. Polyclonal rat IgG, purified from normal rat serum, was used as control. The degree of depletion was determined by fluorescence-activated cell sorting, using a non-cross-reactive biotin-labelled anti-CD25, FITC-labelled anti-CD4 and streptavidin-conjugated allophycocyanine. Measurement was performed using FACSCalibur® (BD) and data were analyzed using WinMDI http://www.scripps.edu.
Preparation, pre-activation and transfer of regulatory T cells
Pooled spleen and lymph node cells from naive C57Bl/6 donors or, if indicated, from immunized mice were incubated with anti-CD4-FITC (clone GK1.5) and anti-CD25-biotin (clone 7D4; BD). CD4+ T cells were isolated using an anti-FITC-Multisort-Kit (Miltenyi Biotech, Bergisch-Gladbach, Germany) in accordance with the manufacturer's instructions. CD4+ T cells were sorted into CD25- and CD25+ cells using anti-biotin MicroBeads (Miltenyi Biotech). Purity was greater than 92% for CD4+CD25- and greater than 80% for CD4+CD25+ cells.
CD25-expressing and αEβ7-expressing subsets were sorted by FACS. Briefly, pooled spleen and lymph node cells from naive mice were stained with anti-CD25-FITC, anti-αEβ7-biotin and streptavidin-PE. The stained cells were enriched with anti-FITC and anti-PE MicroBeads, using the AutoMACS separation unit (Miltenyi Biotech). Thereafter, the cells were sorted into subsets according to their expression of CD25 or αEβ7 using a FACSDiVa cell sorter (BD). The purity was 90–95%, as determined by FACS.
For activation, cells were cultured for 24–72 hours in the presence of plate-bound anti-CD3 (3 μg/ml), anti-CD28 (10 μg/ml) and rhIL-2 (100 U/ml; Chiron, Ratingen, Germany) in RPMI 1640 containing 10% fetal calf serum (FCS; Gibco, Karlsruhe, Germany). Thereafter, cells were washed with PBS and transferred intravenously via lateral tail vein into mice at the time point of AIA induction or at later time points when indicated.
Delayed-type hypersensitivity reaction
Seven days after arthritis induction, mice were challenged by intradermal injection into their ears of 5 μg mBSA in 10 μl PBS. Ear thickness was measured before injection and 24 and 48 hours later using a vernier caliper (Kroeplin).
Proliferation assay and ELISPOT analysis
Single cell suspension from spleens and lymph nodes (inguinal, popliteal, axillary) were cultured at a density of 1 × 106/ml in RPMI 1640, containing 10% FCS, 2 mmol/l L-glutamine, 10 mmol/l Hepes, 1 mmol/l sodium pyruvate, 0.5 μmol/l 2-mercaptoethanol and antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin; all from Gibco) in the presence of medium alone or 25 μg/ml mBSA for 72 hours in 96-well tissue culture plates (Greiner Bio One, Nürtingen, Germany). Cells were pulsed with 0.5 μCi [3H]thymidine (Amersham-Buchler, Braunschweig, Germany) for the last 18 hours of culture. Thereafter, cells were harvested onto 96-well glass fibre filters (Packard Bioscience, Groningen, The Netherlands), and [3H]thymidine incorporation was measured with a scintillation counter (Top-Count; Packard Bioscience).
For ELISPOT analysis, PVDF-membrane 96-well microplates (Millipore, Eschborn, Germany) were coated overnight at 4°C with the primary antibody diluted in sterile PBS. After washing, plates were blocked for 2 hours with RPMI 1640 containing 10% FCS. Thereafter 2 × 105 (IL-2 and IFN-γ) or 1 × 106 (IL-4) cells were cultured in duplicate wells for 24 (IL-2 and IFN-γ) or 48 hours (IL-4). After washing again plates were incubated overnight at 4°C with the secondary antibody diluted in PBS/1% BSA. Extravidin–alkaline phosphatase conjugate (1:30,000 in PBS/1% BSA) and BCIP/NBT solution (bromochloroindolyle phophate/nitroblue tetrazolium; both from Sigma) were used for spot development. The number of spots was quantified using a KS-ELISPOT-Reader (Carl Zeiss, Oberkochen, Germany).
Determination of serum IgG by ELISA
Microplates (96-well; Greiner Bio One) were coated with antigen (0.125 μg/ml mBSA), collagen type I (from rat tail tendon) and type II (10 μg/ml), and proteoglycans (10 μg/ml both from bovine cartilage) and left overnight, as described previously [14]. After washing, plates were incubated with serially diluted serum samples and the amount of bound IgG was determined using anti-mouse IgG-peroxidase conjugate (ICN, Eschwege, Germany) and ortho-phenylendiamine (Sigma) as substrate. Extinction was measured at 492 nm against 620 nm with an ELISA reader (Tecan, Crailsheim, Germany).
Cell transfer for in vivo homing assay
For in vivo homing assay, cells were sorted with a modified protocol and labelled with 111indium, as described elsewhere [10]. Briefly, CD4+ cells were enriched by negative selection. Enriched CD4+ T cells were stained with FITC-conjugated anti-CD4-F(ab) and anti-CD25-allophycocyanine and sorted into CD4+CD25+ or CD4+CD25- cells by FACS (BD). Cells were labelled with 111In (Indiumoxin; Amersham-Buchler) for 20 min at room temperature; 1 × 106 labeled cells were injected intravenously, and 24 hours later mice were killed and the distribution of radioactivity in various organs and the rest of the body was measured in a γ-counter (Wallac Counter, Turku, Finnland).
Alternatively, a proportion of these cells was labelled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) by incubation with 5 μmol/l CFSE (Molecular Probes, Leiden, The Netherlands) in RPMI 1640 for 5 min at room temperature. After washing, 1 × 106 cells were injected intravenously. Twenty-four hours later single cell suspensions were prepared from the draining and nondraining peripheral and mesenteric lymph nodes, the spleen and the peripheral blood, and stained with anti-CD4 and analyzed by FACS. Dead cells were excluded using propidiumiodide.
Statistical analysis
Data are expressed as mean ± standard error of mean, unless otherwise indicated. Experimental groups were tested for statistically significant differences with the Mann–Whitney U-test using SPSS 10.0 (SPSS Inc, Chicago, IL, USA).
Results
Depletion of CD25-expressing cells exacerbates antigen-induced arthritis
Mice were injected intraperitoneally with 0.5 mg anti-CD25 (PC61) 4 and 2 days before induction of arthritis (i.e. 19 and 17 days after first immunization). Depletion of CD25-expressing cells was confirmed using FACS at the time of AIA induction (day 0) using an antibody that recognizes a different epitope on the CD25 molecule. In the PC61-treated group there was a 70.9 ± 11.4% (n = 3) reduction in CD4+CD25+ cells in the spleens as compared with control mice injected with rat IgG (Fig. 1). Of note, the anti-CD25 treatment almost completely depleted cells with high expression of CD25, which are considered Treg cells, in contrast to CD4+ T cells with low or intermediate levels of CD25 expression.
Figure 1.
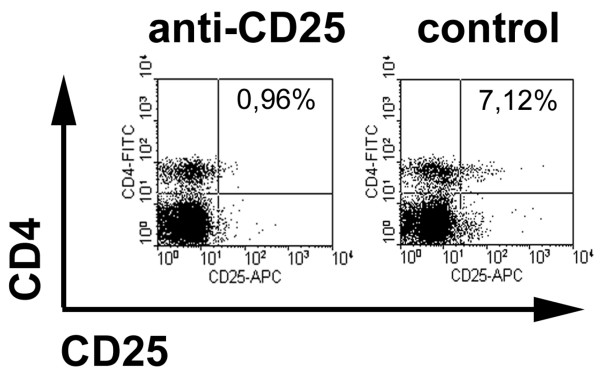
Depletion of CD25-expressing cells by anti-CD25 treatment. Mice immunized with methylated bovine serum albumin (mBSA) were injected intraperitoneally with 0.5 mg PC61 (anti-CD25) or rat IgG as control 4 and 2 days before arthritis induction. Representative example for flow-cytometric assessment of depletion in spleen cells, using a non-cross-reactive anti-CD25 antibody (7D4) at the time of arthritis induction (day 0).
After intra-articular antigen injection, knee joint swelling of the CD25-depleted mice was significantly greater from day 3 onward than in the control group injected with rat IgG (Fig. 2a). Histological examination of knee joint sections 14 days after AIA induction revealed increased hyperplasia and infiltration of the synovial membrane, as well as increased articular damage in those animals (Fig. 2b–d). In summary, this indicates a marked exacerbation of AIA by depletion of CD25-expressing cells.
Figure 2.
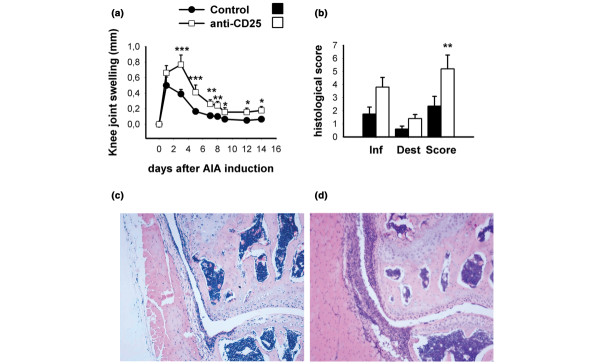
Clinical and histological severity of antigen-induced arthritis (AIA) in CD25-depleted mice. (a) Knee joint swelling (difference in mediolateral joint diameters of arthritic minus nonarthritic knee joints) during the time course of arthritis was higher in CD25-depleted mice. (b) Haematoxylin and eosin stained frontal knee joint sections were scored on a 0–3 point scale at day 14 of AIA for each of the following: severity of synovial hyperplasia, cellular infiltration, cartilage destruction and pannus formation. A score for inflammatory changes (Inf) was calculated by adding the points for synovial hyperplasia and infiltration, and for joint destruction (Dest) by adding the points for cartilage damage and pannus formation. Total arthritis score (Score) was calculated by adding scores for inflammatory changes and joint destruction, giving a maximal AIA score of 12 points. Representative photomicrographs of (c) a control (rat IgG-injected) and (d) a knee joint from an anti-CD25-treated mouse. Ten animals were included in each group in two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, versus control.
Increased cellular and humoral immune responsiveness in CD25-depleted mice
To assess how in vivo cellular immune responses against mBSA are influenced by depletion of CD25-expressing cells, delayed-type hypersensitivity (DTH) reaction against the same antigen was tested by intradermal injection of mBSA into the ears of mice at day 7 after induction of AIA. Anti-CD25 treated mice mounted a significantly stronger DTH response than did rat IgG-treated controls (Fig. 3a).
Figure 3.
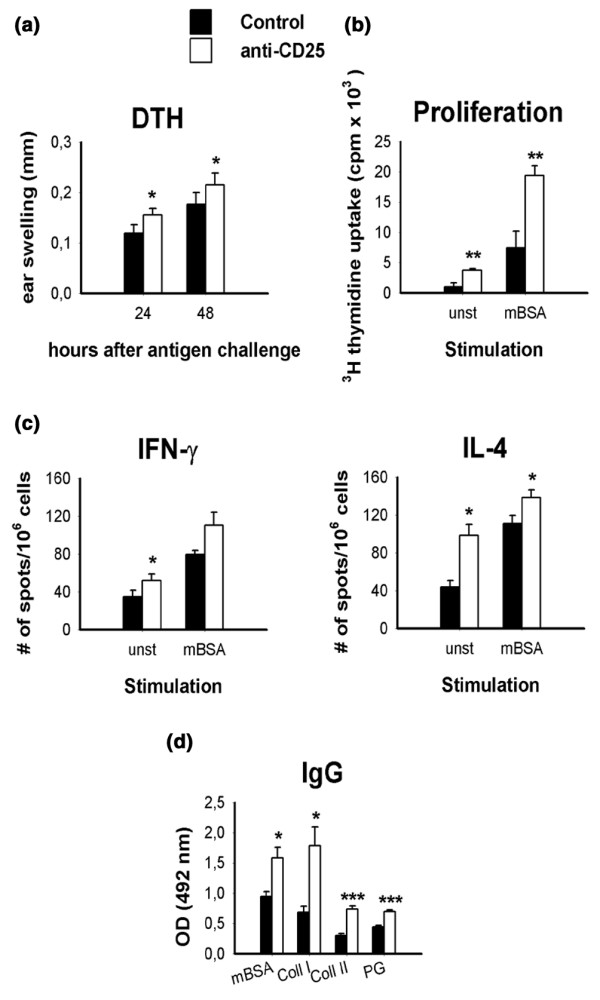
Analysis of in vivo and ex vivo immune responses in CD25-depleted mice. (a) In vivo delayed-type hypersensitivity (DTH) response against methylated bovine serum albumin (mBSA) as a marker for cellular immune response was measured as the increase in ear thickness after intradermal antigen challenge on day 7 of antigen-induced arthritis (AIA). (b) Proliferation, measured as [3H]thymidine incorporation of unstimulated (unst) or mBSA-stimulated (mBSA) draining lymph node cells at day 14 of AIA. (c) Cytokine production was measured with ELISPOT. (d) Serum levels of IgG against mBSA, collagen type I, collagen type II and cartilage proteoglycans were measured using ELISA after 14 days of AIA. Proliferation, DTH reaction and serum IgG titres were tested in 10 animals per group; cytokine production was measured in six animals per group. Data are from one out of two similar experiments. *P < 0.05, **P < 0.01, ***P < 0.001, versus control.
For analysis of the cellular immune responses ex vivo, draining lymph node cells of arthritic animals were harvested 14 days after AIA induction, restimulated with mBSA, and analyzed for proliferative response and cytokine production. As expected from the increased DTH reaction, the proliferative response to mBSA was significantly increased in cells from CD25-depleted mice as compared with that in rat IgG-treated controls (Fig. 3b). Importantly, even without antigenic stimulation the lymph node cells from CD25-depleted mice proliferated fourfold as much as cells from mice treated with control IgG. These data imply that a substantial proportion of the T-cell compartment is still activated 14 days after intra-articular antigen challenge in the absence of Treg cells.
Compatible with these findings is that the production of cytokines in response to mBSA was greater in CD25-depleted mice. Importantly, both T-helper-1 (IFN-γ) and T-helper-2 (IL-4) responses were aggravated by depletion of Treg cells, indicating that both types of response are subject to suppression by Treg cells (Fig. 3c). Again, cytokine secretion from Treg-depleted animals was increased even without antigenic stimulus. In accordance with this, serum levels of IgG directed against mBSA as well as levels of the cartilage-specific autoantigens collagen type I, collagen type II and proteoglycans, were found to be increased in CD25-depleted mice (Fig. 3d).
Taken together, these data clearly demonstrate that CD4+CD25+ Treg cells regulate the severity of arthritis by limiting the cellular and humoral immune responses against the inducing antigen mBSA as well as some arthritis-related autoantigens.
Transfer of CD4+CD25+ cells
To further characterize the suppressive potential of CD4+CD25+ Treg cells, we performed cell transfer studies. In a first set of experiments we transferred Treg cells freshly isolated from naive (Fig. 4a) or mBSA/CFA immunized (Fig. 4b) mice into mBSA-immunized recipients at the time of intra-articular antigen challenge (day 0). With this protocol, a slight decrease in the severity of clinical arthritis (knee joint swelling) could be induced. Accordingly, the histological severity of AIA was also found to be reduced, albeit not statistically significantly (Fig. 4a, b).
Figure 4.
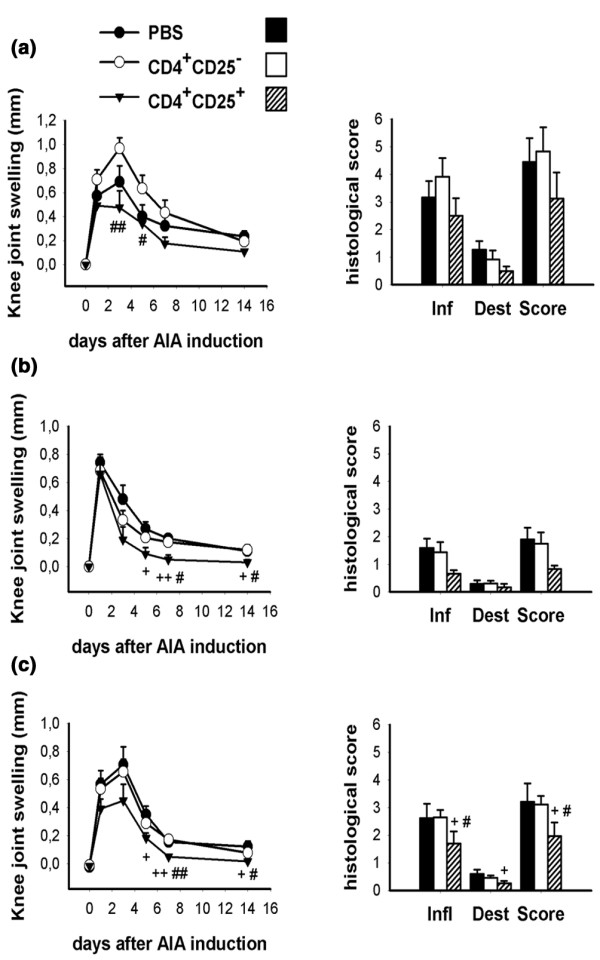
Modulation of antigen-induced arthritis (AIA) by transfer of regulatory T cells (Treg cells). Amelioration of clinical and histological severity of AIA by transfer of 2 × 106 CD4+CD25+ cells freshly isolated from (a) naive or (b) immunized mice at the time of AIA induction (day 0; n = 6 per group). (c) Transfer of 1 × 106 in vitro pre-activated cells at the time of AIA induction (n = 6). #P < 0.05, ##P < 0.01 for CD4+CD25+ versus CD4+CD25-; +P < 0.05, ++P < 0.01 for CD4+CD25+ versus phosphate-buffered saline. Dest, joint destruction; Inf, inflammatory changes; Score; total arthritis score
It is known that Treg cells must be activated via their T-cell receptor to exert their suppressive function. Because we were unable to use antigen-specific (i.e. T-cell receptor transgenic) Treg cells, we opted to pre-activate the CD4+CD25+ cells by in vitro culture in the presence of anti-CD3, anti-CD28 and IL-2 in order to increase their suppressive potential. Transfer of 1 × 106 pre-activated cells significantly suppressed both knee joint swelling and histological arthritis score (Fig. 4c). This effective suppression of AIA development was a consistent finding in different experiments, even with the use of lower cell numbers (for instance 2 × 105 cells; data not shown).
In the next step, we attempted to cure established arthritis by transfer of Treg cells. Surprisingly, 1 × 106 pre-activated CD4+CD25+ cells had no influence on either knee joint swelling or histological arthritis score when transferred at day 1 (Fig. 5a) or day 7 (Fig. 5b) after induction of arthritis. Also, the transfer of 1 × 106 pre-activated αEβ7-expressing Treg cells, which are highly effective in preventing AIA [10], had no effect on disease at this time point (Fig. 5c).
Figure 5.
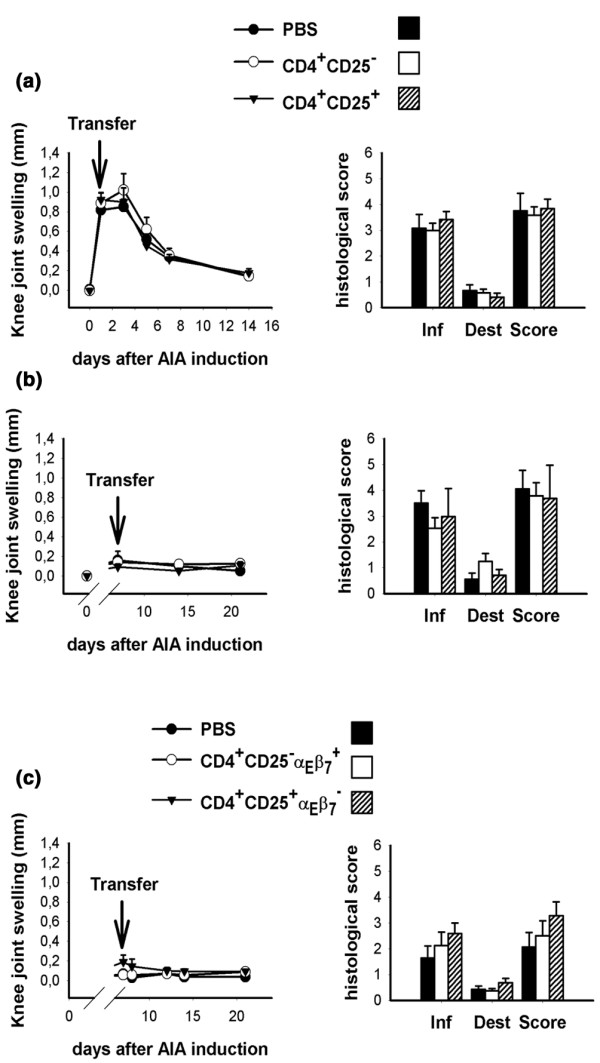
Transfer of regulatory T cells (Treg cells) cannot cure established arthritis. Pre-activated CD4+CD25+ cells (1 × 106) were transferred on (a) day 1 or (b) day 7 of antigen-induced arthritis (AIA). Arthritis severity was monitored by measurement of knee joint swelling and by histological assessment 14 days after cell transfer (n = 6–7 per group). (c) Also, 1 × 106 pre-activated αEβ7-expressing Treg cells have no curative effect in AIA (n = 8 per group).
Taken together, our data demonstrate that Treg cells can inhibit arthritis development when transferred at the time of arthritis induction. However, we were unable to demonstrate any therapeutic effect of Treg cell transfer (in numbers that are effective in prevention) when performed after disease onset.
Transferred CD4+CD25+ Treg cells do not suppress humoral or cellular immune responses
Because CD25-depletion caused a substantial increase in both cellular and humoral immunoreactivity against mBSA, we examined whether transfer of CD4+CD25+ Treg cells can suppress these responses. Neither DTH reactivity against mBSA (analyzed 7 days after AIA induction; Fig. 6a) nor mBSA-induced proliferation (Fig. 6b) and cytokine production by draining lymph node cells (Fig. 6c) at day 14 after induction of AIA were found to be suppressed in the recipients of 1 × 106 pre-activated CD4+CD25+ cells. Thus, transfer of Treg cells into immunized animals does not eliminate or induce functional modification to the previously primed mBSA-specific immune response. In contrast, transfer of CD4+CD25- cells did significantly enhance the proliferation as well as the cytokine production in the recipients. Accordingly, serum levels of IgG directed against mBSA and the cartilage-specific autoantigens collagen type I and type II, and proteoglycans were also not significantly diminished in Treg cell recipients compared with the saline-treated control group. Recipients of CD4+CD25- cells had higher levels of IgGs (Fig. 6d).
Figure 6.
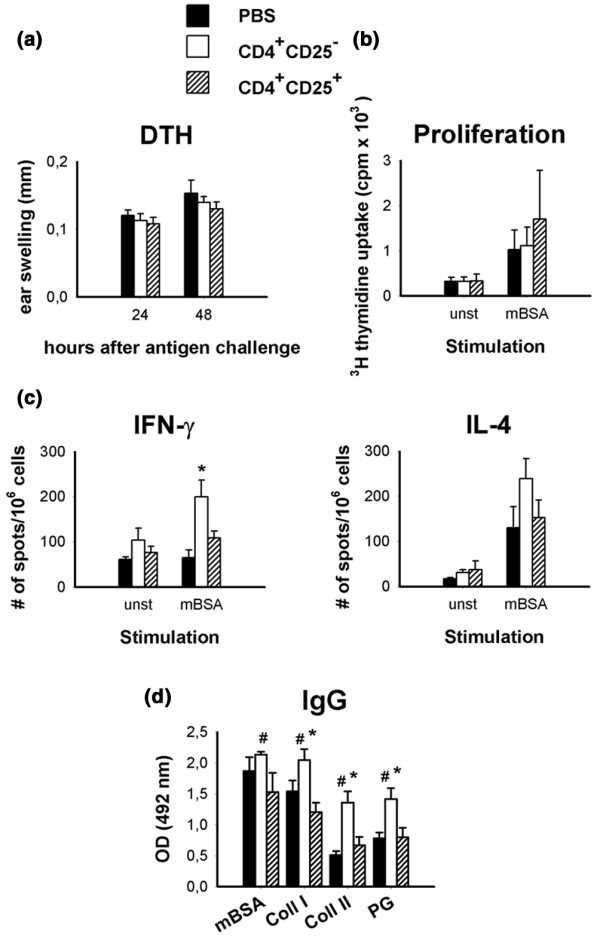
There is no suppression of cellular or humoral methylated bovine serum albumin (mBSA)-specific immunity with transfer of Treg cells. Pre-activated CD4+CD25+ cells (1 × 106) were transferred at the time of antigen-induced arthritis (AIA) induction. (a) Delayed-type hypersensitivity (DTH) reactivity against mBSA in vivo was tested 7 days later by an intradermal antigen challenge into the ears. (b) Antigen-specific proliferation ([3H]thymidine incorporation) and (c) cytokine production (ELISPOT) of draining lymph node cells was measured 14 days after AIA induction. (d) Serum levels of IgG against mBSA, collagen type I, collagen type II and cartilage proteoglycans were measured with ELISA after 14 days of AIA. Proliferation, DTH reaction, cytokine production, and serum IgG titres were tested in six animals per group. #P < 0.05 for CD4+CD25+ versus CD4+CD25-; *P < 0.05 for CD4+CD25- versus phosphate-buffered saline.
Homing properties of CD4+CD25+ Treg cells
Because the mechanism of suppression of Treg cells in vitro is cell contact dependent, localization of cells might be important for their regulatory activity. Therefore, we investigated the migration behaviour of CD4+CD25+ and CD4+CD25- cells in vivo. For these experiments CD4+ cells were enriched by negative selection and sorted by FACS into CD4+CD25+ and CD4+CD25- populations with preferential use of F(ab)-fragments or antibodies, which do not interfere with migration in vivo. Cells were labelled with 111In and injected intravenously into AIA mice 7 days after induction of arthritis. After 24 hours radioactivity was measured in different organs.
Compared with CD4+CD25- cells, CD4+CD25+ Treg cells were less abundant in secondary lymphoid organs such as lymph nodes and spleen. Thus, CD4+CD25+ cells recirculate through these organs less than do CD4+CD25- cells. In the liver, more radioactivity was recovered in recipients of CD4+CD25+ cells as compared with CD4+CD25- cells. Importantly, CD4+CD25+ cells also had a significantly better capacity to enter the inflamed joint than did CD4+CD25- cells (Fig. 7a). The level of radioactivity detected in the arthritic joints was low but similar to levels found in transfer experiments with effector T cells [18]. As a control, some mice were injected with CFSE-labelled cells. FACS analysis of the secondary lymphoid organs revealed the presence of viable cells 24 hours after transfer and excluded the possibility that the difference in migration pattern is due to leakage of radioactivity (Fig. 7b). The migration behaviour of CD4+CD25+ Treg cells does reflect their more activated phenotype, and their ability to enter inflamed joints makes it possible that they act directly at the site of inflammation.
Figure 7.
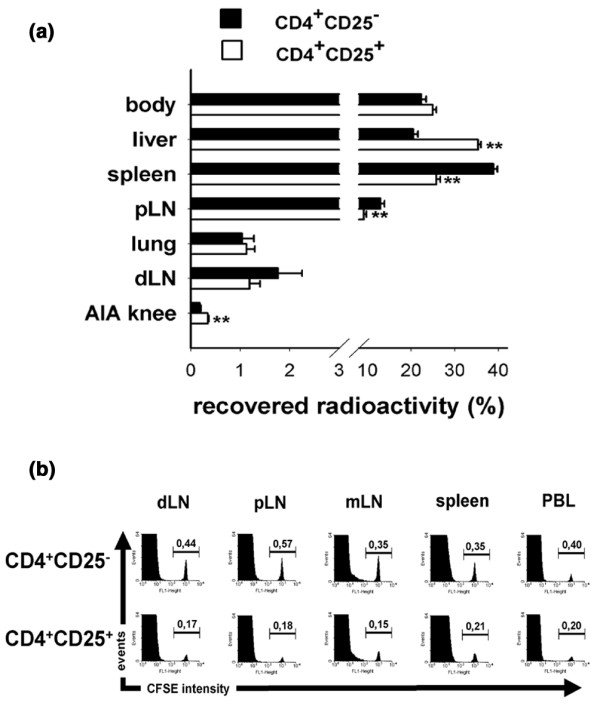
Migration behaviour of regulatory T cells (Treg cells). (a) CD4+CD25+ and CD4+CD25- cells were purified by fluorescence-activated cell sorting (FACS) and labelled with 111In. Cells (1 × 106) were injected intravenously into antigen-induced arthritis (AIA) mice at day 7. After 24 hours radioactivity in isolated organs and the rest of the body was determined using a γ-counter. Thereafter, the total radioactivity recovered per animal was calculated by adding the counts of the organs and the rest of the body. (a) The proportion of radioactivity found in the isolated organs is shown here as a percentage of total recovered radioactivity (n = 6; mean ± standard error of the mean; one representative out of two independent experiments; **P < 0.01). (b) FACS-purified cells were labelled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and injected intravenously. After 24 hours single-cell suspensions from draining lymph node (dLN), nondraining peripheral lymph node (pLN), mesenteric lymph node (mLN), spleen and peripheral blood lymphocytes (PBL) were analyzed by FACS. The percentage of CFSE+ cells of the total CD4+ cells was measured. Histogram plots are gated on CD4+ cells after propidium–iodide exclusion of dead cells (n = 3 per group). Higher numbers of CFSEhigh cells are found in the secondary lymphoid organs in the recipients of CD4+CD25- cells.
Discussion
Our findings provide clear evidence that CD4+CD25+ Treg cells are critical for regulating the severity of AIA in mice. We showed this by manipulating the Treg cell numbers using two different approaches: depletion of CD25-expressing cells and transfer of purified CD4+CD25+ Treg cells. It is important to stress that we depleted CD25-expressing cells in the interval between immunization and AIA induction, because CD25-depletion before immunization profoundly increases the resulting humoral and cellular immune responses [3,12]. These data are consistent with studies conducted in collagen-induced arthritis; however, in these experiments CD25-expressing cells were depleted before immunization with collagen type II, and the resulting more severe arthritis could be interpreted as the result of stronger immunization state [12]. With our experimental design, we were able to examine the effect of Treg cells in ongoing joint inflammation directly. Because CD25 is also expressed on activated conventional T cells, it could be assumed that injection of an anti-CD25 antibody would deplete not only Treg cells but also effector T cells, but the exacerbated AIA in CD25-depleted mice argues against such a depletion of effector T cells. Accordingly, in control experiments lymph node cells from CD25-depleted mice isolated at the time of induction of AIA were able to mount a similar anti-mBSA response in vitro as compared with control mice (data not shown). Furthermore, CD4+CD25+ cells isolated from immunized donors can suppress development of AIA (Fig. 4b). Taken together, these data imply that the CD25+ compartment in immunized mice largely consists of Treg cells.
AIA induction in CD25-depleted mice resulted in a much more severe arthritis in the acute and chronic stages of disease. We recently showed, with the use of a depleting anti-CD4 antibody, that this acute stage of AIA is already under the control of T cells [15]. Nevertheless, early AIA is dominated by cells of the innate immune system [19], and the exacerbation of arthritis in CD25-depleted mice could be due to a lack of suppression of these cells by Treg cells. In accordance with this view, CD4+CD25+ Treg cells are able to suppress innate immune cells in a model of bacteria-induced colitis [20].
In later stages exacerbated arthritis in CD25-depleted mice is accompanied by increased mBSA-specific proliferation and IgG production. This enhanced responsiveness emerged during arthritis development and is due to sustained T cell activation. Such prolonged T cell activation in the absence of CD4+CD25+ cells has also been described in other disease models [21] and is probably the cause of the increased AIA severity. Moreover, the PC61 antibody used in our study has a half-life of approximately 3 weeks in vivo (Sutmuller R, personal communication), which makes it possible that Treg cell function is not only impaired by depletion but also by blockade of IL-2 binding to CD25 by the PC61 antibody. IL-2 or IL-2 signalling via CD25 has been shown to be critical to the regulatory action of Treg cells [22,23]. Also, activation-induced cell death of pathogenic T cells, which is regulated by IL-2, could be impaired by withdrawal of IL-2 signalling and therefore contribute to the observed high levels of cellular immune responses in our study [24].
The fact that depletion of CD4+CD25+ Treg cells enhances the immune response against the foreign antigen mBSA clearly demonstrates that their suppressive effect is not strictly limited to autoreactive T cells. Taking into consideration that Treg cells are also critically involved in the control of immune responses against pathogens [25,26], their physiological function is not just to prevent autoimmunity but also to control the extent of inflammatory reactions in order to prevent tissue damage to the host. Further support for the influence of CD4+CD25+ Treg cells on arthritis development came from the transfer experiments. When transferred at the time of induction of AIA, CD4+CD25+ cells were able to ameliorate ongoing disease. Analysis of the recipients did not reveal a remarkable long-lasting suppression of systemic mBSA-specific immune reactions. Thus, prevention of AIA appears to be possible without inducing anergy or abrogating previously induced T-cell effector functions [27]. In contrast to this, transferred CD4+CD25- cells significantly enhance cell-mediated and humoral immune responses.
Furthermore, the homing data presented here demonstrate that CD4+CD25+ cells can migrate into the arthritic knee joint. Functional Treg cells have repeatedly been found within such effector sites and/or draining lymph nodes, for instance in tolerated allografts [28], in Langerhans islets and pancreatic lymph nodes in inflammation-induced diabetes [29], in chronically inflamed skin in a Leishmania infection model [30], and in the mucosa and mesenteric lymph nodes in inflammatory colitis in severe combined immunodeficient (SCID) mice [31]. Interestingly, two recent papers [32,33] reported an accumulation of functional Treg cells in the inflamed joints of patients with RA, juvenile arthritis and other rheumatic diseases.
It is most likely that the transferred CD4+CD25+ Treg cells act in the draining lymph node as well as in the inflamed tissue. Within such a scenario, it could be possible that Treg cells inhibit the activation of effector T cells and their subsequent migration to the joints. Such a mechanism was recently speculated in modulation of virally induced immunopathology by T cells [26].
Huehn and colleagues [11] recently demonstrated that CD4+CD25+ Treg cells can be divided into subsets based on the expression of the integrin αEβ7. Moreover, this marker identifies CD25- Treg cells [34]. Both αEβ7-expressing subsets had better capacity to reach the inflamed joint and to prevent arthritis in the AIA model, as compared with αEβ7- Treg cells [10]. Thus, suppression at the site of inflammation is also an important part of the activity of Treg cells. How this effect is mediated is unclear but an involvement of IL-10 or transforming growth factor-β is possible [20,35,36].
If these hypotheses are correct, then they could explain why the transfer of Treg cells after arthritis induction is not effective. On the one hand, transfer of Treg cells 24 hours after intra-articular antigen challenge might be too late to inhibit activation of effector T cells and their migration to the joint. Indeed, T-cell activation is an early event in AIA because CD4+ T cell depletion ameliorates the acute stage of the model [15]. On the other hand, it could be possible that the suppressive function of regulatory T cells is switched off under the inflammatory conditions present in the inflamed tissue by factors such as IL-6 or glucocorticoid-induced tumor necrosis factor family-related gene (GITR) and GITR-ligand interactions, abrogating the suppressive effect of Treg cells [37]. With this in mind, it could be interesting to investigate whether the accumulated Treg cells in patients with arthritis function properly in vivo and whether these patients could really benefit from a therapeutic enhancement of Treg function, as suggested by some enthusiastic investigators in this field.
In this regard, data on the curative effects of Treg cells in experimental disease models are conflicting. To best of our knowledge, a curative effect of CD4+CD25+ Treg cells has only been demonstrated in the colitis model induced by transfer of CD45RBhigh T cells into SCID mice [31,38]. In contrast, other authors were unable to demonstrate such an inhibitory effect of Treg cells on SCID colitis when they were transferred 1 week after administration of pathogenic CD45RBhigh T cells [39]. Because arthritis in the AIA model has a hyperacute onset, it could be assumed that the time window for an ameliorative effect of Treg cell transfer ends very shortly after intra-articular injection of antigen. However, further studies on the role of Treg cells in other arthritis models are clearly needed to clarify whether enhancement in Treg cell function might be beneficial in experimental arthritis and perhaps in human disease.
Conclusion
Our data show that Treg cells are critically involved in the control of immune responses that are responsible for the pathogenesis of chronic arthritis. Transfer of such cells can modulate the severity of ongoing inflammatory arthritis but they cannot suppress established disease. Thus, timing of Treg cell transfer for therapeutic purposes is of considerable importance.
Abbreviations
AIA = antigen-induced arthritis; CFSE = 5,6-carboxyfluorescein diacetate succinimidyl ester; DTH = delayed-type hypersensitivity; ELISA = enzyme-linked immunosorbent assay; FACS = fluorescence-activated cell sorting; FCS = fetal calf serum; IFN = interferon; IL = interleukin; mBSA = methylated bovine serum albumin; PBS = phosphate-buffered saline; RA = rheumatoid arthritis; SCID = severe combined immunodeficient; Treg = regulatory T cell.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
OF purified the anti-CD25 hybridoma and purified the monoclonal antibodies from the supernatant; planned and conducted all animal experiments, including ELISA and ELISPOT analysis; and drafted the manuscript. PKP and MG scored the histological changes in arthritic joints. KS, JH and AH conducted the migration experiments, as well as the αEβ7 transfer experiments. RB supervised the project and participated together with AS and AR in the design of the study and its coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgments
We thank T Kaiser and K Raba for FACS sorting; M Schinz and A Kaufmann for help with ELISPOT; H Börner, C Hüttich and R Stöckigt for their excellent technical assistance; and KW Pratt and D Szczawinska for critical comments on the manuscript. This work was supported by the Kompetenznetz Rheuma (Grant 01 GI 0344), Deutsche Forschungsgemeinschaft (Grant Br 1372/5-1) and the Interdisciplinary Center for Clinical Research (IZKF) Jena.
Contributor Information
Oliver Frey, Email: oliver.frey@med.uni-jena.de.
Peter K Petrow, Email: peter.petrow@med.uni-jena.de.
Mieczyslaw Gajda, Email: mieczyslaw.gajda@med.uni-jena.de.
Kerstin Siegmund, Email: siegmund@drfz.de.
Jochen Huehn, Email: huehn@drfz.de.
Alexander Scheffold, Email: scheffold@drfz.de.
Alf Hamann, Email: hamann@drfz.de.
Andreas Radbruch, Email: radbruch@drfz.de.
Rolf Bräuer, Email: rolf.braeuer@med.uni-jena.de.
References
- Kinne RW, Palombo-Kinne E, Emmrich F. T-cells in the pathogenesis of rheumatoid arthritis villains or accomplices? Biochim Biophys Acta. 1997;1360:109–141. doi: 10.1016/s0925-4439(96)00079-8. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4+CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Natl Acad Sci USA. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- Bardos T, Czipri M, Vermes C, Finnegan A, Mikecz K, Zhang J. CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis. Arthritis Res Ther. 2003;5:R106–R113. doi: 10.1186/ar624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrow PK, Thoss K, Katenkamp D, Bräuer R. Adoptive transfer of susceptibility to antigen-induced arthritis into severe combined immunodeficient (SCID) mice: role of CD4+ and CD8+ T cells. Immunol Invest. 1996;25:341–353. doi: 10.3109/08820139609059316. [DOI] [PubMed] [Google Scholar]
- Pohlers D, Nissler K, Frey O, Simon J, Petrow PK, Kinne RW, Bräuer R. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen-induced arthritis: influence on T helper cell activation. Clin Exp Immunol. 2004;135:409–415. doi: 10.1111/j.1365-2249.2003.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrow PK, Thoss K, Henzgen S, Katenkamp D, Bräuer R. Limiting dilution analysis of the frequency of autoreactive lymph node cells isolated from mice with antigen-induced arthritis. J Autoimmun. 1996;9:629–635. doi: 10.1006/jaut.1996.0082. [DOI] [PubMed] [Google Scholar]
- Bräuer R, Kittlick PD, Thoss K, Henzgen S. Different immunological mechanisms contribute to cartilage destruction in antigen-induced arthritis. Exp Toxicol Pathol. 1994;46:383–388. doi: 10.1016/S0940-2993(11)80121-1. [DOI] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Lohning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Simon J, Surber R, Kleinstauber G, Petrow PK, Henzgen S, Kinne RW, Brauer R. Systemic macrophage activation in locally-induced experimental arthritis. J Autoimmun. 2001;17:127–136. doi: 10.1006/jaut.2001.0534. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- De La Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- Martin B, Banz A, Bienvenu B, Cordier C, Dautigny N, Becourt C, Lucas B. Suppression of CD4+ T lymphocyte effector functions by CD4+CD25+ cells in vivo. J Immunol. 2004;172:3391–3398. doi: 10.4049/jimmunol.172.6.3391. [DOI] [PubMed] [Google Scholar]
- Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4+CD25+ Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16:183–191. doi: 10.1016/S1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Cao D, Vollenhoven Rv R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–R346. doi: 10.1186/ar1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, de Jager W, Pugayung G, Giannoni F, Rijkers G, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- Banz A, Peixoto A, Pontoux C, Cordier C, Rocha B, Papiernik M. A unique subpopulation of CD4+ regulatory T cells controls wasting disease, IL-10 secretion and T cell homeostasis. Eur J Immunol. 2003;33:2419–2428. doi: 10.1002/eji.200324205. [DOI] [PubMed] [Google Scholar]
- Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25- T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta–TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- Foussat A, Cottrez F, Brun V, Fournier N, Breittmayer J-P, Groux H. A comparative study between T regulatory type 1 and CD4+CD25+ T cells in the control of inflammation. J Immunol. 2003;171:5018–5026. doi: 10.4049/jimmunol.171.10.5018. [DOI] [PubMed] [Google Scholar]


