Abstract
In the past decade, stimuli-responsive hydrogels are increasingly studied as biomaterials for tissue engineering and regenerative medicine purposes. Smart hydrogels can not only replicate the physicochemical properties of the extracellular matrix but also mimic dynamic processes that are crucial for the regulation of cell behavior. Dynamic changes can be influenced by the hydrogel itself (isotropic vs anisotropic) or guided by applying localized triggers. The resulting swelling–shrinking, shape-morphing, as well as patterns have been shown to influence cell function in a spatiotemporally controlled manner. Furthermore, the use of stimuli-responsive hydrogels as bioinks in 4D bioprinting is very promising as they allow the biofabrication of complex microstructures. This perspective discusses recent cutting-edge advances as well as current challenges in the field of smart biomaterials for tissue engineering. Additionally, emerging trends and potential future directions are addressed.
1. Introduction
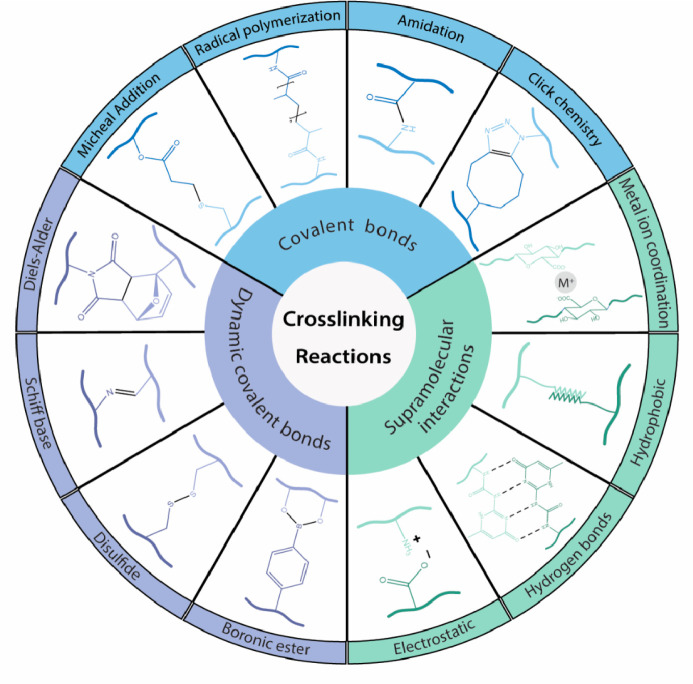
In modern medicine, the use of biomaterials has become a routine strategy for the treatment of several diseases and injuries.1 Biomaterials are essentially natural or synthetic materials that are used in medicine to restore proper physiological function of soft tissues, organs, and bones. One major challenge in tissue engineering applications is the development of biomimicking materials that are able to reproduce all of the natural properties and functions of the extracellular matrix (ECM).2 This includes biocompatibility and a variety of physicochemical properties such as stiffness, geometry, and topology.3 Furthermore, biomaterials need to support cell function and behavior such as cell signaling, adhesion, migration, proliferation, and differentiation, which are strongly regulated by the surrounding ECM.4,5 These cell–matrix interactions are highly dynamic and remain to this day challenging to reproduce by combining cells with traditional hydrogel-based biomaterials that are lacking adaptability.6 Hydrogels can be defined as three-dimensionally cross-linked, polymeric networks that are highly hydrophilic and retain high water contents. They present similar characteristics as compared to natural tissues such as their mechanical properties including viscoelasticity. In addition, they usually present large macropores (>50 nm) procuring good diffusion properties for essential (bio)molecules and waste products.7−9 Hydrogel properties can differ greatly according to their origin (natural or synthetic), their composition (homopolymer, copolymer, or interpenetrating networks of multipolymers), their morphology (amorphous, semicrystalline, or crystalline), or the type of cross-linking (noncovalent, covalent, or dynamic covalent).10 A resume of the most frequent cross-linking techniques of hydrogels for tissue engineering is presented in Figure 1.
Figure 1.
Most commonly used cross-linking techniques for stimuli-responsive hydrogels used in tissue engineering. Cross-linking can be achieved by covalent bonds (dynamic reversible or nondynamic irreversible links) or noncovalent supramolecular bonds. The dynamic covalent bonds are classified from the least to the most dynamic ones under physiological conditions (from top to bottom).
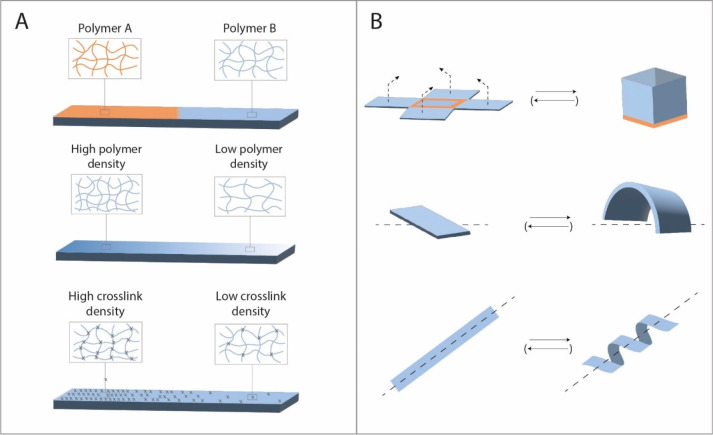
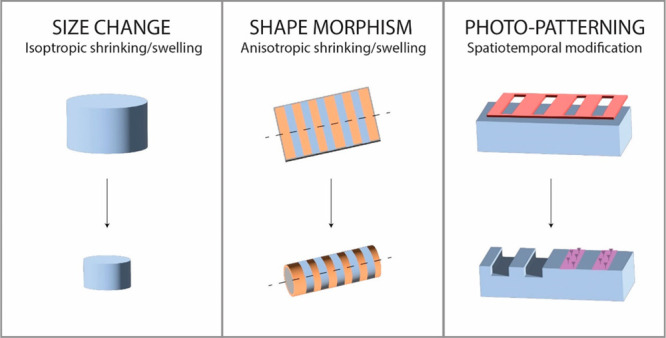
The combination of biocompatible hydrogels, as support scaffold, combined with cells and bioactive molecules has been extensively studied for soft tissue engineering purposes.11−13 To dynamically tune the properties of biomaterials and therefore bridge the gap to true ECM mimics, innovative stimuli-responsive smart hydrogel scaffolds are currently under development. These stimuli-responsive hydrogels can react to small changes in their microenvironment with very specific property changes.14 They are, for instance, subjected to dynamic responses via the degradation of a polymer in a spatiotemporally controlled manner or through stimuli-induced deformations. Dynamic changes can be triggered by a large variety of cues such as physical (e.g., temperature, light, sound, electric, or magnetic fields), chemical (e.g., pH, ionic strength, or humidity), or biological (e.g., cells or enzymes) cues.15 The induced deformations can be either isotropic, inducing a size increase/decrease, or anisotropic leading to shape-morphing such as rolling, twisting, bending, folding, or moving (Figure 2). The anisotropic swelling or shrinking of a hydrogel can be tuned by the used polymer (single or multi material),16−19 different cross-linking gradients,16,20 polymer densities,21 or application of layers or zones22−24 (Figure 2). Alternatively, the cross-linking of hydrogels can also be tuned via photoisomerization reactions influencing the length of the polymer chains and thus cross-linking density, which induces swelling/shrinking.25,26
Figure 2.
(A) Shape-morphing of anisotropic hydrogels can be tuned in function of the used polymer(s), the polymer density, or the cross-linking density. (B) Common shape deformations include folding, bending, twisting, and rolling and are generally triggered by (de)hydration.
Shape-morphing can be induced by various mechanisms. One possibility, albeit rare in the context of tissue engineering, is the use of shape-memory polymers.33,34 Another method relies on the use of self-assembling structures consisting of solid segments connected by thin flexure hinges.35,36 Yet by far the most used approach in this field relies on anisotropic hydrogels with distinct swelling rates. On the one hand, shape-morphing materials allow the fabrication of complex hierarchical microstructures such as hollow tubes, which are challenging to produce with ordinary biofabrication techniques such as micromolding, extrusion bioprinting, photolithography, electrodeposition, or microfluidics.27 The use of stimuli-responsive hydrogels in bioinks investigated as shape-morphing materials will be further discussed in the 4D bioprinting section. On the other hand, instructive dynamic materials can be used to steer cell responses toward a desired outcome.28 For instance, tuning of hydrogel stiffness has been linked to controlled changes in the cellular phenotype. This conversion of mechanical information from the microenvironment into biochemical signaling is called mechanotransduction.29 It can be of interest to guide natural processes such as inflammatory response and tissue repair (by influencing macrophages) or cell differentiation and maturation.30,31 In particular, photodegradation as well as the spatiotemporally controlled light-induced cross-linking and/or release of biomolecules has attracted a lot of attention. Overall, the development of smart materials has gained a lot of interest, particularly in the past decade, as their dynamic and/or stimuli responsive properties can be of use in a variety of biomedical applications including regenerative medicine and tissue engineering.8,32,33 Their tunability also makes them particularly interesting in the field of personalized medicine.34
In this perspective, recent cutting-edge advances are discussed in the field of tissue engineering related to dynamically shape-morphing, swelling–shrinking, as well as photoresponsive hydrogels. This includes a discussion of the current challenges and the status of different approaches aimed at tackling those issues. In particular, the use of stimuli-responsive hydrogels as bioinks in 4D bioprinting will be addressed. Indeed 4D bioprinting can be defined as 3D printing of cell-laden materials in which the printed structures are be able to respond dynamically over time to external stimuli or internal cell forces.35 Finally, currently emerging trends and future directions in the field of smart materials for tissue engineering purposes are addressed.
2. Shape-Morphing and Size Changes in Stimuli-Responsive Hydrogels
2.1. Hydration-Induced Shape-Morphing in Hydrogels
(De)hydration is one of the most used triggers for shape-morphing of hydrogels. The absorption of water can induce swelling of the cross-linked network, whereas dehydration leads to a decrease in volume. Hydration-induced swelling of isotropic gels and/or deformation of anisotropic gels can, for instance, be attained upon implantation of the biomaterial in vivo, naturally a high-water content environment. Recent studies have shown that this approach allows the biofabrication of small microstructures such as vasculature at physiologically relevant sizes containing homogeneously dispersed cells.22 The following examples highlight the extensive use of shape-morphing hydrogels to create hollow channels for the regeneration of vasculature24,36−38 or the trachea.23
Kirillova and colleagues showed that cell-containing bioinks can be printed on a flat surface, which is then rolled up on demand based on anisotropic cross-linking densities. The resulting self-folded hydrogel tubes, composed of alginate and hyaluronic acid (HA), supported the viability of mouse bone marrow stromal cells for at least 7 days. This technique enabled the production of hollow tubes with an internal diameter as small as 20 μm (similar to the diameter of small blood vessels).38 In addition, this approach allows homogeneous cell distribution even in complex structures.22 According to Kitana and colleagues, this approach can be extended to the biofabrication of perfusable T-shaped vascular junctions.37
Kim and colleagues have reported the creation of 3D printed self-foldable sheets made of glycidyl methacylate-modified silk fibroin (Silk-MA). The folding was based on the layering of sheets with different solid content, thickness, or pattern (e.g., narrow or large gaps). They biofabricated and implanted a trachea that contained different cell types in different areas of the construct. In vivo, integration into the host trachea was observed as well as the development of both epithelium and cartilage at the intended sites.23
Furthermore, Joshi and colleagues have demonstrated that shape deformations, in particular, the roll-up of sheets, can be influenced by tuning the printing pattern and infill angles. In addition, they successfully predicted the structures of 3D printed constructs computationally as a function of the printing properties.39 Similarly, the use of fillers, such as aligned stiff collagen fibrils, has been considered to induce directionality in the swelling process.40,41
In addition to the explained layering and patterning techniques, it is also possible to combine different polymers with characteristic swelling capacities. Multimaterial constructs have been largely used to induce selective swelling and thus predetermined deformations. Researchers took, for example, advantage of the swelling difference between oxidized and methacrylated alginate (OMA) and methacrylated gelatin (GelMA).17,18 The resulting hydrogels were biodegradable and supported cell viability at high cell densities (e.g., NIH3T3 mouse fibroblasts). Similarly, Hiendlmeier and collaborators investigated the use of superabsorbers such as sodium polyacrylate, which is known for its significant swelling capacity (up to 20 times in weight). They have combined these superabsorbers with flexible nonswelling hydrogels to create shape-morphing constructs.19
Besides vasculature, other interesting targets include nerves,39 muscle,42 neural, cardiac,42 and cartilage tissue.20,43 Diaz-Payno and colleagues have investigated the use of multilayered curved constructs to mimic native cartilage. The 4D biofabrication method is based on the differential swelling of layered tyramine-functionalized hyaluronan (HAT) presenting high swelling and alginate with HAT (AHAT) characterized by lower degrees of swelling due to its higher stiffness. Interestingly, the incorporation of human bone marrow cells in the curved construct allowed the formation of a cartilage-like matrix over time. Constante and colleagues decided to target tissues presenting a uniaxial orientation of cells such as skeletal muscle, cardiac, and neural tissues. They combined extrusion printed methacrylated alginate with melt-electrowritten polycaprolactone fibers to direct cell alignment. The first tests, with myoblasts cultured inside a scrolled bilayer scaffold, showed promising viability, proliferation, and directed alignment.42 Up to now, a large majority of the studies are limited to in vitro investigations. However, the remarkable work of Joshi and colleagues demonstrates a significant step toward the in vivo use of shape-morphing materials. They used 4D printed gels as sutureless nerve-guiding conduits for the repair of sciatic nerve defects. The alginate-methylcellulose-based hydrogels were able to roll-up into hollow tubes in vivo in a rat model. Histological evaluation and functional assessments showed that the use of those rolls successfully assisted in the healing of peripheral nerve damage.39
In conclusion, these studies highlight the increasing technical know-how to predict and control hydrogel shape changes by tuning their chemical composition and printing conditions. Hydration has been widely used as a trigger for rapid shape-morphing as it is perfectly biocompatible and can even be used in vivo. However, in this case, the shape deformation will be a one-time occurrence upon injection or implantation and is not reversible in an in vivo setting.
2.2. Thermoresponsive Shape- and Size-Morphing Hydrogels
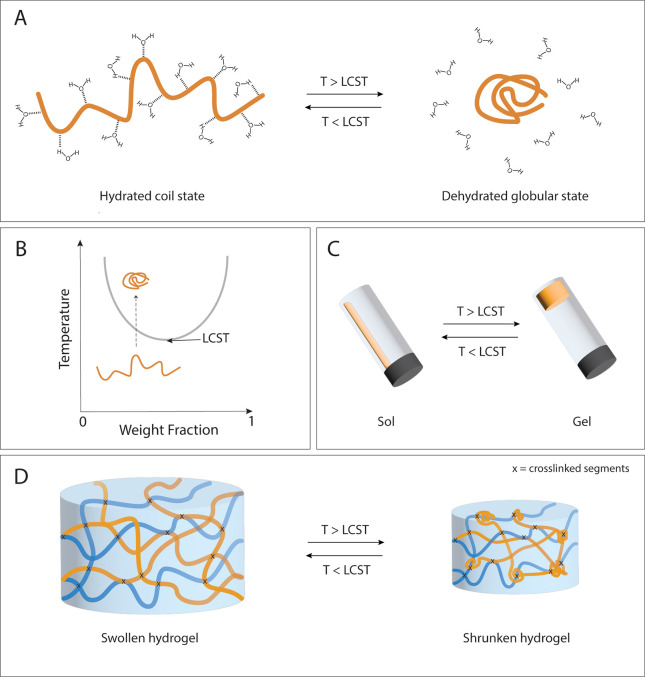
Thermoresponsive biomaterials are widely studied in smart valve systems or as delivery vehicles for cells, drugs, genes, and growth factors.15,44,45 They rely on polymers presenting temperature-dependent aqueous solubility, generally being soluble at low temperatures but precipitating when increasing the temperature. The temperature variation alters the hydrophilic–lipophilic balance (HLB) of the polymeric chain, which induces its collapse and eventually causes macroscopic precipitation.46 The temperature at which this phase transition occurs is known as the cloud point (CP), which depends on the polymer concentration: the lowest CP is called the lower critical solution temperature (LCST) (Figure 3).47 Polymers presenting an LCST in a physiological temperature window are particularly interesting in tissue engineering applications. In fact, hydrogels based on thermosensitive polymers such as poly(N-isopropylacrylamide) (PNIPAM, LCST of ∼32 °C), poly(N-vinylcaprolactam) (PVCL, LCST of 32–35 °C), poly(ethylene oxide)–poly(propylene oxide), or Pluronics (PEO–PPO or PEO–PPO–PEO, LCST of 12.5–52.5 °C) have been shown to respond to physiological temperature changes with important variations in physical properties including shape deformations, shrinking–swelling behavior, and stiffening.48,49
Figure 3.
(A) Coil–globular transition of thermosensitive polymers. (B) As a function of the temperature, the polymer will be more (below the LCST) or less hydrated (above the LCST). (C) Sol–gel transition of aqueous mixtures containing thermosensitive polymers. (D) Reversible swelling–shrinking behavior of thermoresponsive hydrogels.
Especially PNIPAM-based hydrogels have been extensively studied as they exhibit large reversible volume changes due to temperature-dependent conformational changes in the polymer network. Below its critical temperature, PNIPAM is predominantly hydrophilic and presents a hydrated coil structure in an aqueous environment, thus providing swollen hydrogels. Upon increase of the temperature above 32 °C, hydrogen bonds with water are broken, and the polymer dehydrates and transitions to a globular structure (coil–globule transition), which is accompanied by a significant water expulsion and thus shrinkage of the hydrogel. This transition is entirely reversible50,51 (Figure 3).
In addition to this thermoresponsive reversible shrinking–swelling behavior, PNIPAM-based hydrogels can also achieve particularly high strength and robustness,44 which is the reason why these hydrogels have been widely used as bioink in 4D bioprinting.44,51−54 To enhance printability, including shear-thinning behavior, shape fidelity, as well as its cytocompatibility, PNIPAM is often combined with natural polymers such as alginate,44,53,55 gelatin methacryloyl (GelMA),54 agarose,52 or methylcellulose55 to form interpenetrating networks. It has been reported that shrinking of hydrogels enables the creation of materials at higher resolution as compared to conventionally printed hydrogels. In fact, constructs have been shrunken postprinting (also referred to as contraction fabrication) and could thus replicate even small physiological structures such as blood vessels with a size below 100 μm. Li and colleagues reported the fabrication of microscale vasculature using cytocompatible scaffolds based on PNIPAM and GelMA. A minimum diameter of 70 μm was achieved after shrinking by using sacrificial alginate fibers.54
Also, Podstawczyk and colleagues investigated the use of an interpenetrating network of PNIPAM and alginate for the formation of hollow tubes. Their approach is based on the use of 3D honeycomb-patterned hydrogel discs, which are self-rolling into tubular constructs in response to a specific temperature stimulus. Depending on the used cross-linking strategy (single or dual photo-cross-linking), the sheets can roll-up at low temperatures (12 °C) and unfold upon increase of the temperature (to 42 °C) or vice versa.55 As explained in the previous section, this shape-morphing behavior can be generated during the 3D printing process by means of a shear-induced anisotropy as well as the internal stress generated during the polymerization.53 Han and colleagues reported that the shape-morphing of thermoresponsive PNIPAM gels could be controlled by the manufacturing conditions and the polymer resin composition, especially as a function of the molar ratio of cross-linker to NIPAM monomer. They also demonstrated that the incorporation of ionic monomers such as methacrylamidopropyltrimethylammonium chloride (MAPTAC) increases the swelling transition temperature of PNIPAM. The combination of materials with different transition temperatures can also be used for anisotropic deformations of printed constructs.51
Similarly, Xu and colleagues developed thermosensitive hydrogels capable of anisotropic deformations based on locally cross-linked P(NIPAM-co-NaMAc) (sodium methacrylate) hydrogels. Fe3+ cations were applied in a specific pattern on the hydrogel by means of ionoprinting. The coordination between these cations and the carboxyl groups in the polymeric network influences the mechanical properties inducing an internal stress but also the thermoresponsiveness allowing the anisotropic swelling–shrinking of the construct.56
Furthermore, de Almeida and colleagues have shown that thermosensitive synthetic polymers can also be used to mimic cytoskeletal stiffening. They developed a hybrid PNIPAM- polyisocyanide (PIC) network, which stiffens reversibly up to 50 times its original modulus as a function of the temperature. The stiffening is mainly caused by the pulling of one polymer network on the other.57
The use of thermosensitive hydrogels has attracted particular attention as wound-healing dressings. They often present favorable features for the in situ treatment of chronic wounds such as good biocompatibility, appropriate mechanical properties, LCST behavior, biodegradability, and the possibility to retain and absorb large amounts of water or wound exudate. They can not only deliver cells for skin regeneration (e.g., fibroblasts) but also bioactive molecules such as antimicrobial agents.55 Niziol and colleagues developed a hydrogel based on PNIPAM, alginate, and methylcellulose with accurate printability and shape fidelity. Their 3D-printed wound dressings can be fabricated and geometrically shrunken to perfectly fit the target wound. The patches could reversibly swell and shrink at least four times in cycles of 120 min (20–37 °C), reaching about 80% of their initial mass in the shrunken state. In addition, the antimicrobial agent Octenisept was successfully incorporated and delivered in vitro, showing higher release at 37 °C (swollen state) than at 20 °C (shrunken state). The shown reversibility is interesting for in vitro setups, however less relevant in vivo due to limited potential to change the temperature.55
Hydroxybutyl chitosan (HBC) is another thermosensitive polymer in aqueous environment with characteristics similar to those of PNIPAM in terms of the sol–gel transition at physiological temperatures as well as printability. Prior to the gelation, they both maintain low viscosity and thus low fluid shear stress, which is beneficial for the survival of cells in the bioink. At body temperature, their viscosity increases, which allows good shape retainment of the filament during extrusion printing.34 HBC-based materials have been investigated for personalized in situ bioprinting. It could be directly printed into the body and thus meet patient-specific needs.34 Luo and colleagues were able to enhance cell adhesion and significantly improve the mechanical properties of methacrylated hydroxylbutyl chitosan (MHBC) hydrogels by supplementing it with GelMA or (fish) collagen.34,58 The obtained composites presented a thermosensitive transition and could also be photocured. These properties make them promising candidates for high-resolution contraction fabrication (also referred to as postprinting-shrinking) of small-scale features.34,58 Che and colleagues even report the possibility of triple-conjugated photo-, temperature-, and pH-sensitive chitosan (N-succinyl hydroxybutyl methacrylated chitosan (NS-HBCMA)). The exhibited swelling behavior was tunable as a function of the pH, the temperature, and the degree of succinylation. Furthermore, NS-HBC-MA did not negatively affect the cell viability or growth of bovine ear fibroblast cells (BEFCs).59
More recently, Wang and colleagues have reported a series of cytocompatible, multiresponsive hydrogels with important stretchability, self-healing properties as well as a temperature-responsive shape memory effect. The cross-linking of their gelatin- and PEG-based material was achieved by means of dynamic covalent bonds (imine/Diels–Alder) (allowing the self-healing) as well as supramolecular H-bonding with a hyperbranched triethoxysilane reagent (HPASi) endowing its thermo-sensitivity.60
In conclusion, thermoresponsive hydrogels are a kind of shape-morphing materials that are particularly promising to enhance the printing resolution of small features by means of contraction fabrication. In addition, these recent studies highlight the potential of thermo-sensitive hydrogels in various domains of tissue engineering and regeneration. They allow the in situ formation and deformation of hydrogels, which is particularly interesting for personalized in situ bioprinting. However, once implanted in the body, the microenvironment will remain stable and prevent any further shape-morphing. Even though a large number of thermogels are currently under development, only few have been commercialized so far, mainly due to lacking long-term biocompatibility studies and patient compliance.61
2.3. Electrostatic and Ionic Induction of Hydrogel Deformation
The use of ions as cross-linking agents of hydrogels suitable for cell encapsulation has been extensively studied since the 1990s.62 Particularly alginate, characterized by its ionotropic gelation with bivalent cations such as Ca2+ or Ba2+, has attracted a lot of attention in soft tissue engineering.63,64 Recently, alginate-based materials have, for example, been studied in the regeneration of muscle or bone tissues65−67 as well as for drug delivery purposes.68 Interestingly, it has been shown that a prolonged incubation of alginate-based materials in a solution containing cationic species (e.g., Ca2+ or Ba2+) induces a certain degree of shrinkage due to electrostatic interactions.66 Furthermore, Cao and colleagues have shown that anisotropically methacrylated alginate can first be photopolymerized and then further cross-linked by Ca2+ cations or chitosan solutions to induce shape-morphing.69
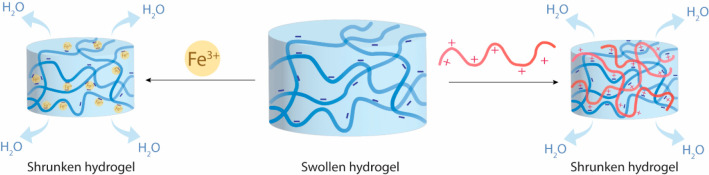
The incubation of a cross-linked polyionic polymer network (e.g., negatively charged methacrylated alginate (alg-MA) or methacrylated hyaluronic acid (HAMA)) within a solution of polyions of the opposite net charge (e.g., positively charged chitosan) induces complexation. The occurring charge compensation leads to the expulsion of water and thus shrinking of the hydrogel (Figure 4). This phenomenon can be used for shape-morphing purposes in anisotropic gels or for generating high-resolution hydrogel-based structures by postprinting shrinking.70 In contrast, the controlled swelling of materials has gained a lot of interest in the field of expansion microscopy. It involves the polymerization and cross-linking of charged monomers in the presence of a biological sample of interest. Subsequently, the sample is immersed in water, which induces swelling of the charged polymer network, resulting in the isotropic expansion of the sample, that enables imaging nanoscale biological structures.71
Figure 4.
Ionic and electrostatic interaction leading to water expulsion and shrinkage of the hydrogel.
Zhao and colleagues studied gelatin/oxidized dextran hydrogels for wound-healing purposes. They demonstrated that these gels undergo different degrees of shrinkage after treatment with various kosmotropic ions following the Hofmeister series (CO32– > SO42– > S2O32– > H2PO4–). The appropriate ion can thus be selected on the basis of the shrinking requirements and biocompatibility.72
Also, Fe3+ cations have been studied in this context as they can cross-link polymers by means of dynamic coordination of carboxyl groups. Xu and colleagues reported a significant shrinkage of hyaluronic acid hydrogels by Fe3+cross-linking.73 Similarly, Zheng and colleagues studied Fe3+ cross-linked hydrogels composed of layers of poly(acrylic acid)-polyacrylamide and PNIPAM-polyacrylamide. They demonstrated significant shape-morphing upon incubation of the hydrogel in saline solution. This can be explained by the fact that the saline solution induces dehydration and a phase transition of PNIPAM resulting in the stiffening of the PNIPAM-containing segments of the construct.74
Furthermore, electrostatic interactions can also be influenced by the redox potential of a polymer or cross-linker.75 The most prominent example of this phenomenon are viologen cross-linkers. In the oxidized state, viologen is fully charged and thus stretched out. Upon reduction, the electrostatic repulsion decreases leading to intramolecular folding and thus shrinking.76−78
It is worth mentioning that also pH-triggered shape-morphing hydrogels are being developed.79,80 Their use as biomaterials in tissue engineering is however very limited as the incorporation of living cells within the hydrogel requires the strict maintenance of physiological pH.
2.4. Biotriggers Inducing Dynamic Material Changes
In the world of tissue engineering, researchers generally strive to mimic nature and physiological body functions as closely as possible. While some attempt to reproduce biological mechanisms using synthetic stimuli-responsive hydrogels, others focus exclusively on natural, biologically sourced materials.81 Furthermore, a large variety of biomaterials, which are sensitive to biological cues, have been developed. A large variety of cells,82−84 viruses85 and biomolecules such as proteins86−89 or DNA,90,91 have been investigated as potential triggers to induce dynamic responses in hydrogels. In the following paragraphs, we will highlight some of the most recent and cutting-edge findings in this field.
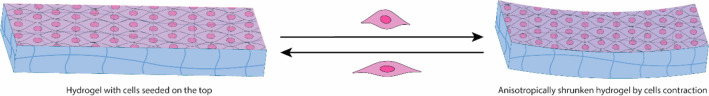
One research area, that particularly attracted interest in the past decade, studies the development of “biological machines” or “bio-bots”. We define biobots as the combination of living cells and a cell-instructive microenvironment interacting with each other to create a specific dynamic response enabling sensing, information processing, transport, protein expression, or mechanical actuation. Such biobots harness, for example, the contractile activity of cardiomyocytes to drive a movement such as locomotion or deformation (Figure 5).82,83 On the basis of this principle, Cui and colleagues reported the development of a 4D physiologically adaptable cardiac patch for the treatment of myocardial infarction. They combined cardiomyocytes with a matrix containing aligned fibers composed of polyethylene glycol diacrylate (PEGDA) and GelMA. The beating heart was simulated by shape-morphing hydrogels presenting anisotropic cross-linking densities, which were triggered by the contraction of cardiomyocytes under physiological mechanical stimulations. The combination of this self-morphing capacity and the expandable microstructure of the implant allowed the dynamic integration of the patch with the beating heart. A 4-months in vivo study revealed promising cell engraftment and vascular supply in a murine model with chronic myocardial infection.84
Figure 5.
Biologically triggered hydrogel deformation based on the contraction and relaxation of cells in a scaffold with aligned fibers.
It has been shown that this kind of shape-morphing in hydrogels can be induced not only by dynamically active cells but also by proteins. Bian and colleagues demonstrated the use of reversible denaturation of proteins as shape deformation actuator. The denaturation process is specific to a certain protein and causes unfolding and thus swelling. In this study, a bilayered hydrogel constituted of the modular elastomeric proteins (GB1)8 and (FL)8 was designed. The distinct folding–unfolding mechanisms of the different layers induce a reversible bidirectional bending deformation that can be tuned by the denaturant concentration and layer geometry.86 Furthermore, proteins can be of use for the enzymatic degradation of certain materials such as methacrylated bovine serum albumin (MA–BSA) or gelatin methacrylate-co-polyethylene glycol dimethacrylate (GelMA-co-PEGDMA).87,88 This biodegradation process will induce physicochemical changes in the hydrogel such as reduced stiffness, which can be beneficial to induce shape deformations.
Also, Devillard and colleagues attempted a new strategy of inducing biological activities in hydrogels making use of enzymes. On the one hand, they included alkaline phosphatase to trigger the calcification of specific regions in the hydrogel. On the other hand, the diffusion of thrombin led to the formation of a fibrin biofilm entrapping living cells (NIH3T3/GFP, a fibroblast line expressing green fluorescent protein). This study represents a nice example of how the entrapment of enzymes can be used to locally steer the properties of hydrogels toward their application as complex substrates in tissue engineering.89
Finally, it is worth mentioning that not only mammalian cells have been studied in stimuli-responsive hydrogels. Rivera-Tarazona and colleagues studied the use of genetically modified yeast within cross-linked acrylamide matrices. They used specific biomolecules such as l-tryptophan, l-leucine, or uracil to trigger the cellular proliferation of the yeast in specific regions. The resulting volumetric increase in the proliferative regions induced a locally controlled shape change.92
3. Hydrogel Patterning by Means of Photodegradation, Photorelaxation, and Photoconjugation
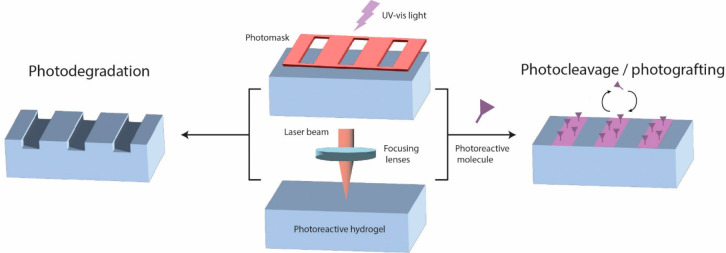
One of the biggest challenges in the development of biomaterials for tissue engineering is the creation of a microenvironment that is able to support, guide, and influence the entrapped cells. It is crucial to design instructive materials capable of steering cell responses on demand into a desired outcome. This can not only be achieved by making use of anisotropic hydrogels but also by applying localized triggers able to photopattern biomaterials on demand. Two main strategies have been extensively studied to gain instructive materials with spatiotemporal control: photomodulation of mechanical properties and photoconjugation (Figure 6).
Figure 6.
Photoreactive hydrogels can be patterned by means of spatially confined light irradiation to induce photodegradation or photoconjugation (photocleavage/photografting) of molecules to the gel surface. The light source can be locally applied by the use of photomasks or focused laser beams.
Both strategies can benefit from the use of light-sensitive hydrogels adapted for photodegradation and photopatterning. In particular, photolithography methods are used to modify light-sensitive materials. Photolithography techniques typically use UV light or of late also visible light to transfer a geometric pattern by means of photomasks or projections onto a photosensitive substrate.93,94 More recently, laser-based degradation of hydrogels has become more prominent as higher resolutions can be achieved. In addition, lasers allow the use of (near) infrared wavelength. Infrared light is characterized by lower frequencies (and is) less energetic as compared to UV light, which reduces the risk of damaging the cells during the irradiation process and allows higher in vivo penetration depth. More detailed information about different laser-based methods (continuous wave versus pulsed, single or multiphoton) can be found in Pradhan et al.95
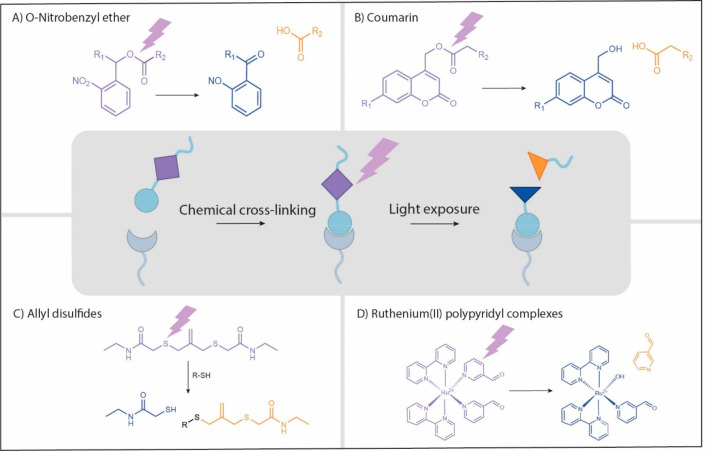
The cross-linking reaction of the used hydrogels must be orthogonal to the light-triggered reaction (using a different wavelength or nonlight-based cross-linking). Most common nonlight-based cross-linking methods include free radical polymerization,96,97 Michael-type additions,98−100 azide–alkyne cycloaddition,101−105 and amide bond formation106 (see Figure 1). The predominantly used materials are PEG-based hydrogels. They allow the straightforward incorporation of photolabile moieties within the macromer or at the cross-linking site. These photolabile moieties photocleave when exposed to a specific wavelength and reduce thus the cross-linking density of the gel at the illuminated zones. This kind of photodegradation avoids the use of small molecule catalysts or other potentially toxic compounds and thus provides generally a cytocompatible environment. A variety of photolabile moieties have been studied including o-nitrobenzyl ether derivates (o-NB),107,108 coumarin derivatives,104,109 allyl disulfides,110,111 or Ru(II) polypyridyl complexes.112 The respective degradation reactions of these molecules are described in Figure 7, and further details can be consulted in Hansen et al. and Truong et al.113,114
Figure 7.
Most commonly used classes of photolabile moieties for the photodegradation or photoconjugation of light-sensitive hydrogels in the field of tissue engineering (and the single-photon wavelengths required for degradation): (A) O-nitrobenzyl ether (365 nm and derivates up to 480 nm), (B) coumarin derivates (320–500 nm), (C) allyl disulfides (365 nm), and (D) ruthenium(II) polypyridyl complexes (400–500 nm).
3.1. Photomodulation of Mechanical Properties
Cells present mechanosensing properties that can be promoted by making use of photodegradation techniques. Indeed, cell migration and spreading can be guided following patterned hydrogels presenting geometrical cues and contact guidance. It is based on defined 3D architectures with zones of lower or higher stiffness.
Interestingly, Li and colleagues reported the possibility to efficiently photodegrade hydrophilic polymethacrylates without adding any external photolabile moieties. They demonstrate that UV irradiation of such polymethacrylates can cause photohomolysis of main-chain carbon–carbon bonds resulting in the degradation of the hydrogel.115 On the basis of this technology, they developed an inherently photodegradable poly(ethylene glycol) methacrylate (PEGMA)-based material, supplemented with GelMA to increase biocompatibility and make it suitable for 3D cell culture.116 Similarly, Applegate and colleagues developed another photopatterning technique completely without the use of any exogenous or chemical cross-linkers. They created 2D and 3D multiscale patterns in soft silk hydrogels by means of ultrafast laser pulses. The principle is based on the simultaneous or consecutive absorption of two or more photons by an atom, molecule, or ion. Multiphoton absorption can reach up to 1 cm of penetration depth in transparent silk gels.117 More information about this kind of laser-based multiphoton absorption for hydrogel patterning can be found in the review of Pradhan and colleagues.95
Watanabe and colleagues combined the use of photolabile o-nitrobenzyl moieties and laser-induced multiphoton excitation to fabricate hollow structures by means of photoerosion. They used a microfluidic setup to design blood vessels with a specific structure and size in the tens of micrometer scale. The obtained structures sustained the adhesion and growth of vascular endothelial cells (HUVECs).107 Furthermore, photodegradable hydrogels can be used in wound-healing applications.118 Villiou and colleagues developed a tissue glue that can adhere to tissues for a certain time period and then can be photodegraded on demand under cytocompatible conditions by means of photocleavable nitrobenzyl triazole groups.119 Other applications of photoresponsive hydrogels include their use as a synthetic hydrogel system to support and guide organoid development and expansion (e.g., intestinal), potentially replacing the need for animal-derived matrixes (Matrigel).111
Another concept worth mentioning is called photorelaxation. It is based on sequential photodegradation and photoinitiated cross-linking reactions leading to the respective softening and stiffening of hydrogels in a physiologically relevant range of moduli. This method can be used to influence the behavior of mechano-sensitive cells.120 Rosales and colleagues reported the development of a photosensitive polymer based on hyaluronic acid with dynamic mechanics and thus capable of mimicking dynamic aspects of physiological microenvironments.120 In addition, the concept of photorelaxation has been used to induce shape-morphing in hydrogels (e.g., rolling sheets).121,122
3.2. Photoconjugation
The second strategy is based on biochemical patterning via the controlled release of signaling molecules that were photoconjugated to the hydrogel. It has been shown that cell function can be controlled in space and time based on the availability of extrinsic signaling molecules.105 Bioactive molecules such as peptides and growth factors can be grafted or cleaved reversibly based on photochemically controlled click reactions.101 The spatially controlled immobilization or release of growth factors can, for example, be beneficial to guide stem cell differentiation in 3D hydrogels.123 Rana and colleagues have recently reported a GelMA-based biomaterial photopatterned with aptamer-tethered VEGF that spatiotemporally regulates vascular morphogenesis.124 Furthermore, Van der Putten and colleagues recently reported the use of UV-photopatterned materials for the development of cell culture substrates. They patterned polymeric materials (mainly PDMS, polydimethylsiloxane) with various proteins including collagen type 1, gelatin, and fibronectin by means of a UV lithography-based substrate microfabrication. Studies showed that cell attachment of primary human keratocytes and human dermal fibroblasts could be influenced by these protein contact-guidance cues but also geometrical cues such as the substrate curvature. The reported photopatterning technique in combination with digital masks enabled the formation of various geometries with a resolution as small as 1.5 μm.125 Recently, Falandt and colleagues reported the use of volumetric bioprinting to draw and imprint gradients and patterns of growth factors in any custom-designed geometry across a centimeter-scale.126
In conclusion, light-triggered methods are very interesting for the local modification of photoresponsive hydrogels as it can be delivered with very high precision and thus good resolution. In addition, they have been shown to be compatible with the use of cells, in particular when using visible or (near) infrared light. A shift from lower to higher wavelengths has been shown to be beneficial for cell compliance but also as it allows for slightly deeper in vivo penetration. In fact, one major challenge remains the limited tissue penetration depth of light as well as within the gels if lacking transparency. In consequence, most currently reported applications are limited to 2.5D surface patterning. Interestingly, the use of more sophisticated volumetric printing techniques has recently been reported as a potential solution for the fabrication and modification of actual 3D constructs in a spatiotemporally controlled manner.127
4. From 3D to 4D Printing Thanks to Stimuli-Responsive Hydrogels
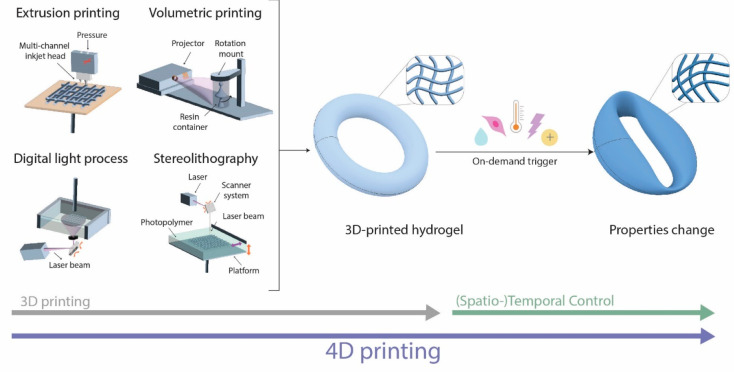
It has been shown that the function of biological tissues is highly dependent on their structure, size, and shape, not only on a macroscopic but also on a microscopic level.128 Accordingly, one major goal in tissue engineering is the generation of structures and shapes, which are perfectly mimicking the targeted physiological tissue. In this attempt, various biofabrication techniques have been explored. One of the most prominent examples in recent years has been the use of 3D bioprinting techniques, which allow the fabrication of complex constructs at higher resolution as compared to other biofabrication techniques.129−131 On the basis of medical imaging data, 3D models can be designed by means of computer-aided design (CAD) software.132,133 Subsequently, these 3D models can be printed by various 3D bioprinting techniques including light-based printing (e.g., digital light process (DLP), stereolithography (SLA), and volumetric) as well as extrusion-based additive manufacturing techniques. An extensive description of these techniques can be consulted from Moroni et al.134 By using stimuli-responsive hydrogels as bioink, the printed objects become capable of dynamic changes in size, shape, and functionalities, introducing a fourth dimension. Different triggers can be applied over time as a postprinting treatment to modify and adapt the properties of the printed construct (Figure 8). The fabrication of smart biomaterials by means of 3D printing techniques combined with stimuli-responsive inks is referred to as 4D (bio)printing.
Figure 8.
Various light-based and extrusion-based printing techniques can be used for 3D printing. In combination with stimuli-responsive hydrogels as (bio)inks, the printed construct can undergo further dynamic changes, which can be spatiotemporally controlled by various on-demand triggers (e.g., hydration, biological, temperature, light, or electrostatic). The fabrication of biomaterials by means of 3D printing techniques combined with stimuli-responsive inks is referred to as 4D (bio)printing.
A large variety of approaches have been reported to enhance the printability of smart hydrogels. Some rely on technological advances such as cryoprinting135 or ultrafast digital printing,136 while others investigate the use of additives or focus on the development of new kinds of hydrogels. Particularly, the use of fillers such as methylcellulose, carbomer, or nanoclay to adapt the material properties has been widely studied.21,52,137 For example, Lai and colleagues reported an alginate-based material supplemented with methylcellulose that displayed excellent rheological properties, extrudability, and shape fidelity of printed structures. This hydrogel was 4D printed in a series of modeled 2D architectures that were encoded with anisotropic stiffness and swelling behavior by strategically controlling the density gradients of the mesh vertically with respect to the orientation of the modeled strips.21 However, the incorporation of fillers increases the complexity of the bioink and thus the risk for uncontrolled effects on its bioactive and mechanical characteristics.
Alternatively, single-component 4D bioinks in the form of jammed microgels could allow straightforward fabrication of biomaterials with a defined composition. Because of their small size (1–1000 μm), they can be easily extruded and subsequently cross-linked. Ding and colleagues recently reported a single-component (oxidized and methacrylated alginate) jammed microflake hydrogel (MFH) system with shear-thinning, shear-yielding, and rapid self-healing properties.20 Similarly, Es Sayed and colleagues developed an innovative 4D printable granular hydrogel that combines reversible temperature-induced resolution enhancement and on-demand disintegration. The ink is composed of a jammed dispersion of submicrometer PNIPAM microgels bearing terpyridine (Tpy) ligands (MG-Tpy). The printed scaffold was cross-linked after printing via immersion in a solution containing iron(II) ions, which complexed the terpyridine moieties between the microgel particles. These supramolecular bonds can be disintegrated on demand by increasing the pH. The temperature-sensitive behavior of PNIPAM allowed the resolution to be reversibly increased up to 230 μm with an initial size of about 600 μm. As a consequence, this hydrogel allows the printing of multiresponsive constructs (pH and temperature). Combined with its cytocompatibility, as shown by high viability of glioblastoma cells, it makes for a promising 4D bioprinting material.138
In conclusion, these examples highlight the increasing interest to use smart materials as bioinks in 4D bioprinting applications and the important efforts to enhance their printability while maintaining good cytocompatibility.
5. Future Challenges and Promises of the Field
This perspective discusses the current challenges and potential solutions in the field of stimuli-responsive hydrogels for tissue engineering. Over the past decade, researchers were able to synthesize a large variety of smart biomaterials that are able to sense, respond, and adapt as a function of environmental changes. It has been shown that swelling–shrinking behavior or shape deformations in anisotropic gels can be triggered by various cytocompatible triggers. In particular, hydration or physiological temperature changes have been extensively studied as they are easily compatible with an in vivo environment. Recently, even the possibility of cell patterning or guidance by means of sound or magnetic cues has been reported.139,140 Yet also biological entities including cells and proteins have been used efficiently to trigger dynamic changes in hydrogels such as motion in biobots. Moreover, light-triggered methods are increasingly based on visible light sources instead of UV-light to avoid cell damage.99,107 In addition, light-based printing methods are attracting more attention. Recent papers report high precision and high resolution by targeted laser irradiation that does not affect the surrounding cells.95,99,107
To harvest the full potential of stimuli-responsive hydrogels and make them the ideal biomaterials of tomorrow, several challenges still require tackling.
5.1. Toward Physiologically Sized Features
One major challenge in the field of tissue engineering is the reproduction of small features in a physiologically relevant size range. In recent years, the influence of the size and shape of biological tissues on their function has become more evident.128 Accordingly, a large variety of promising solutions have been reported aiming at overcoming the dimension limitations of scaffold preparation. In particular, the production of thin hollow tubes comparable to blood vessels, kidney tubules, and nerves has been extensively studied. One promising method is based on the autonomous rolling-up of anisotropic hydrogel gels under the influence of a temperature or (de)hydration trigger.24,37−39,42 Alternatively, photoerosion has been investigated for the formation of thin hollow channels in hydrogels. This method is either based on the use of photomasks (downward erosion) or very precise multiphoton laser irradiation.94,107 Furthermore, postprinting shrinking procedures are very interesting candidates to reduce the dimensions of a printed construct. Both temperature and charge compensation triggers have been reported in the literature.34,54,70,138 With all three techniques (self-rolling sheets, photoerosion, and printing-shrinking), the formation of hollow channels in the tens of micrometer range has been reported.38,70,107 The smallest features have been reached by means of pulsed laser irradiation. However, the seeding of cells in channels of that small size can be very challenging. For that reason, the printing-shrinking approach or self-rolling sheets might be more interesting from a practical point of view. Cells can be seeded easily in relatively large channels (>200 μm141), followed by a shrinking step to reach the desired dimensions. Similarly, cells can also be seeded on top of hydrogel sheets and subsequently rolled-up to form tubes.
5.2. Toward Biocompatibility and Biomimicry
Currently, another main challenge lies in the biocompatibility of the used synthetic polymers toward the encapsulated cells but also toward the host body. Many efforts have been reported, aimed at increasing the cytocompatibility of materials based on synthetic polymers. Evidently, cell–matrix interactions play a crucial role in maintaining proper cell growth and function.3 It is thus necessary to provide a cell-friendly environment presenting all of the required biological cues. Several groups report the inclusion of natural biopolymers such as gelatin or collagen to promote long-term cell viability.4,5 Furthermore, cell binding domains such as adhesive peptides, most commonly RGD, have been successfully incorporated into stimuli-responsive hydrogels.6−8 Promotion of cell attachment to the biomaterial by binding to cellular integrin receptors has been reported.3 Also, the coupling of other bioactive molecules such as growth factors has been used to guide cell responses. Indeed, biochemical photopatterning presents a promising, flexible, and high throughput method to create multicue substrates.
In addition to these biochemical cues, the mechanical properties of the hydrogel play a crucial role in cell behavior. Biomaterials need to present adequate stiffness, topology, and geometry to provide a cell-friendly environment.9 Several, herein reported, studies investigated photodegradation and photorelaxation techniques in an attempt to guide cell migration, proliferation, or differentiation by softening or stiffening the microenvironment.8,10,11 Furthermore, the use of dynamic covalent bonds becomes a more prominent solution to avoid the use of stiff hydrogels that hinder cell proliferation, migration, and development. The incorporation of reversible covalent bonds endows the hydrogels with an interesting adaptiveness inducing self-healing and stimuli-responsive capacities.12
5.3. Toward In Vivo Use and Clinical Applications
Currently, a large variety of stimuli-responsive hydrogels are investigated for their use in tissue engineering. The majority of the studies discussed in this review are limited to in vitro models. They highlight the potential of such engineered tissues for fundamental research, drug toxicity studies, or disease models. However, there are still hurdles to overcome to enable the translation to in vivo applications. In an in vivo microenvironment, with stable physiological conditions in terms of temperature, hydration, and pH, many of the discussed hydrogel responses would only be triggered once upon implantation. The dynamicity is thus mostly a unique event that is limited to one nonreversible shape or size change. Photosensitive hydrogels could be a promising alternative. However, their in vivo application is currently still challenging due to limited light penetration depths. Furthermore, extensive studies of biodegradation kinetics and mechanisms of smart hydrogels are required to enable their use in a clinical setup. It has to be assured that the degradation rate is controlled and that the debris does not cause any negative side effects.
5.4. Toward Personalized 4D Biofabrication
Furthermore, the lab-scale synthesis procedures need to be scaled-up and ultimately translated to large-scale biofabrication techniques. Currently, 4D bioprinting techniques remain quite wasteful and not all stimuli-responsive hydrogels are yet suitable as bioinks. A lot of research is thus focusing on the enhancement of the printability of various bioinks by means of supplements, fillers, and carrier-inks. Besides technological advancements, the use of printing-shrinking techniques significantly improved the resolution of the printed constructs. In addition, the tunability of 4D bioprinting techniques offers interesting opportunities in the field of personalized medicine.
In conclusion, this perspective gives an overview of the current challenges and recent advancements in the field of stimuli-responsive hydrogels for tissue engineering. By tackling current challenges such as poor resolution preventing the fabrication of small features or limited, nonreversible dynamicity, we could harvest the tremendous potential of those biomaterials in regenerative and personalized medical applications of tomorrow. The presented smart biomaterials are able to sense, respond, and adapt as a function of environmental changes. In the future, we might also be able to incorporate intelligent functions into those biomaterials. In this manner, they would not only be able to respond to a specific stimulus but also to acquire knowledge allowing self-testing, self-calibration, self-diagnose, self-validation, self-adaptation, or other intelligent functions.142,143
Acknowledgments
The Dutch Research Council (NWO/VICI 18673) is acknowledged for funding.
Biographies

Myriam Neumann is a postdoctoral researcher at the Utrecht University in the group of Prof. Tina Vermonden. She obtained her Ph.D. in 2022 at the University of Namur in the groups of Prof. Bao-Lian Su (Laboratory of Inorganic Materials Chemistry) and Prof. Thierry Arnould (Laboratory of Biochemistry and Cellular Biology). Her interdisciplinary research interests focus on the development of biomaterials for tissue engineering and regenerative medicine purposes.

Greta Di Marco received her M.Sc. degree in Pharmaceutical Sciences from the University of Trieste, Italy, in 2019. She joined the group of Prof. Tina Vermonden at Utrecht University, Netherlands, in 2021. During her current Ph.D. track, she is researching temperature-triggered shrinkable hydrogels for tissue engineering applications.

Dmitrii Iudin obtained his bachelor’s and master’s degrees from Saint Petersburg State University. Since 2021, he has been pursuing his Ph.D. degree at Utrecht University under the supervision of Prof. Tina Vermonden and Dr. Bas van Ravensteijn. His research interests include the synthesis of smart polymers and the investigation of hydrogels for tissue regeneration applications.

Martina Viola obtained her bachelor’s and master’s degrees in industrial chemistry from the University of Rome “La Sapienza”. She started her Ph.D. in 2019 at the University Medical Center/Utrecht University in the groups of Prof. Jos Malda and Prof. Tina Vermonden. In her project, she focuses on developing synthetic and natural materials applicable to the electrohydrodynamic printing technique for cartilage regeneration.

Cornelus (René) F. van Nostrum obtained his Ph.D. degree in 1995 at the University of Nijmegen. Afterward, he worked as a postdoctoral fellow at Philips Research Laboratories in Eindhoven and later as an assistant professor at the department of Polymer Chemistry and Coating Technology of the Eindhoven University of Technology. In 1999, he joined the department of Pharmaceutics of the Utrecht University as assistant professor and was promoted to associate professor in 2004. His present research activities include the design, synthesis, and characterization of polymers for hydrogels, micelles, microspheres, and nanoparticles, and their application as drug delivery devices and as absorbants for toxins.

Bas G. P. van Ravensteijn is an assistant professor at Utrecht University. He obtained his Ph.D. with Prof. Willem Kegel at the Van’t Hoff Laboratory for Physical & Colloid Chemistry. After completing a postdoctoral research project at the University of California – Santa Barbara in the groups of Prof. Hawker and Prof. Helgeson, he joined the Voets lab at Eindhoven University of Technology as a Marie-Skłodowska-Curie fellow. In 2021 he moved to the Utrecht Institute for Pharmaceutical Sciences where he combines fundamental physical and polymer chemistry with pharmaceutical science to rationally design and understand tomorrow’s biomaterials.

Tina Vermonden obtained her Ph.D. degree in Organic Chemistry at Wageningen University. Afterward, she joined the Department of Pharmaceutics at Utrecht University in The Netherlands, where she currently is professor in Biomaterials for Drug Delivery and Regenerative Medicine. She was awarded prestigious VIDI, VICI, and Aspasia-grants of Dutch Research Council for research on hydrogels for local drug delivery and shrinking printing technology. Since 2022, she is associate editor for the ACS journal Biomacromolecules.
The authors declare no competing financial interest.
References
- Chen F.-M.; Liu X. Advancing Biomaterials of Human Origin for Tissue Engineering. Prog. Polym. Sci. 2016, 53, 86–168. 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn L. T. Biomaterials. Introd. to Biomed. Eng. 2005, 255–312. 10.1016/B978-0-12-238662-6.50008-2. [DOI] [Google Scholar]
- Wang T.; Nanda S. S.; Papaefthymiou G. C.; Yi D. K. Mechano-Physical Cues in Extracellular Matrix Regulation of Cell Behaviors. Chembiochem 2020, 21 (9), 1254–1264. 10.1002/cbic.201900686. [DOI] [PubMed] [Google Scholar]
- Kutys M. L.; Doyle A. D.; Yamada K. M. Regulation of Cell Adhesion and Migration by Cell-Derived Matrices. Exp. Cell Res. 2013, 319 (16), 2434–2439. 10.1016/j.yexcr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cescon M.; Gattazzo F.; Chen P.; Bonaldo P. Collagen VI at a Glance. J. Cell Sci. 2015, 128 (19), 3525–3531. 10.1242/jcs.169748. [DOI] [PubMed] [Google Scholar]
- Yamada K. M.; Doyle A. D.; Lu J. Cell-3D Matrix Interactions: Recent Advances and Opportunities. Trends Cell Biol. 2022, 32 (10), 883–895. 10.1016/j.tcb.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinadha Rao M.; Phanindra C.; Yamini M.; Prasad C. H. Hydrogels the Three Dimensional Networks: A Review. Int. J. Curr. Pharm. Res. 2021, 13, 12–17. [Google Scholar]
- Bordbar-Khiabani A.; Gasik M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. 10.3390/ijms23073665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahiyan P.; Baradaran B.; de la Guardia M.; Oroojalian F.; Mokhtarzadeh A. Cutting-Edge Progress and Challenges in Stimuli Responsive Hydrogel Microenvironment for Success in Tissue Engineering Today. J. Controlled Release 2020, 328 (September), 514–531. 10.1016/j.jconrel.2020.09.030. [DOI] [PubMed] [Google Scholar]
- Ahmed E. M. Hydrogel : Preparation, Characterization, and Applications : A Review. J. Adv. Res. 2015, 6 (2), 105–121. 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-sherbiny I. M.; Yacoub M. H. Review Article Hydrogel Scaffolds for Tissue Engineering : Progress and Challenges 2013, 2013, 38. 10.5339/gcsp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulescu D.-M.; Neacsu; Ionela Andreea Grumezescu A.-M.; Andronescu E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers (Basel). 2022, 14 (799), 1–30. 10.3390/polym14040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama R.; Ulziibayar A.; Reinhardt J. W.; Watanabe T.; Kelly J.; Shinoka T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. 10.3390/biom13020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A. S.; Stayton P. S.. Applications of “Smart Polymers” as Biomaterials, 4th ed.; Elsevier: New York, 2020. [Google Scholar]
- Zhang X.; Chen L.; Lim K. H.; Gonuguntla S.; Lim K. W.; Pranantyo D.; Yong W. P.; Jian W.; Yam T.; Low Z.; Teo W. J. Pathway to Intelligence : Using Stimuli-Responsive Materials as Building Blocks for Constructing Smart and Functional Systems. 2019, 1804540, 1–48. 10.1002/adma.201804540. [DOI] [PubMed] [Google Scholar]
- Ding A.; Jeon O.; Tang R.; Lee Y. Bin; Lee S. J.; Alsberg E. Cell-Laden Multiple-Step and Reversible 4D Hydrogel Actuators to Mimic Dynamic Tissue Morphogenesis. Adv. Sci. 2021, 8 (9), 1–9. 10.1002/advs.202004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A.; Lee S. J.; Tang R.; Gasvoda K. L.; He F.; Alsberg E. 4D Cell-Condensate Bioprinting. Small 2022, 18, 36. 10.1002/smll.202202196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. Bin; Jeon O.; Lee S. J.; Ding A.; Wells D.; Alsberg E. Induction of Four-Dimensional Spatiotemporal Geometric Transformations in High Cell Density Tissues via Shape-Changing Hydrogels. Adv. Funct. Mater. 2021, 31, 1–9. 10.1002/adfm.202010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiendlmeier L.; Teshima T. F.; Zurita F.; Url H.; Rinklin P.; Wolfrum B. A Superabsorbent Sodium Polyacrylate Printing Resin as Actuator Material in 4D Printing. Macromol. Mater. Eng. 2022, 307 (10), 1–8. 10.1002/mame.202200306. [DOI] [Google Scholar]
- Ding A.; Jeon O.; Cleveland D.; Gasvoda K. L.; Wells D.; Lee S. J.; Alsberg E. Jammed Micro-Flake Hydrogel for Four-Dimensional Living Cell Bioprinting. Adv. Mater. 2022, 34 (15), 1–11. 10.1002/adma.202109394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.; Ye X.; Liu J.; Wang C.; Li J.; Wang X.; Ma M.; Wang M. 4D Printing of Highly Printable and Shape Morphing Hydrogels Composed of Alginate and Methylcellulose. Mater. Des. 2021, 205, 109699. 10.1016/j.matdes.2021.109699. [DOI] [Google Scholar]
- Cui C.; Kim D. O.; Pack M. Y.; Han B.; Han L.; Sun Y.; Han L. H. 4D Printing of Self-Folding and Cell-Encapsulating 3D Microstructures as Scaffolds for Tissue-Engineering Applications. Biofabrication 2020, 12, 045018. 10.1088/1758-5090/aba502. [DOI] [PubMed] [Google Scholar]
- Kim S. H.; Seo Y. B.; Yeon Y. K.; Lee Y. J.; Park H. S.; Sultan M. T.; Lee J. M.; Lee J. S.; Lee O. J.; Hong H.; Lee H.; Ajiteru O.; Suh Y. J.; Song S. H.; Lee K. H.; Park C. H. 4D-Bioprinted Silk Hydrogels for Tissue Engineering. Biomaterials 2020, 260 (January), 120281. 10.1016/j.biomaterials.2020.120281. [DOI] [PubMed] [Google Scholar]
- Wang S.; Zhao Q.; Li J.; Du X. Morphing-to-Adhesion Polysaccharide Hydrogel for Adaptive Biointerfaces. ACS Appl. Mater. Interfaces 2022, 14 (37), 42420–42429. 10.1021/acsami.2c10117. [DOI] [PubMed] [Google Scholar]
- Li L.; Scheiger J. M.; Levkin P. A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 26. 10.1002/adma.201807333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaso K.; Takashima Y.; Harada A. Fast Response Dry-Type Artificial Molecular Muscles with [C2]Daisy Chains. Nat. Chem. 2016, 8 (6), 625–632. 10.1038/nchem.2513. [DOI] [PubMed] [Google Scholar]
- Jansen K.; Schuurmans C. C. L.; Jansen J.; Masereeuw R.; Vermonden T. Hydrogel-Based Cell Therapies for Kidney Regeneration: Current Trends in Biofabrication and In Vivo Repair. Curr. Pharm. Des. 2017, 23 (26), 3845–3857. 10.2174/1381612823666170710155726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Kabb C. P.; Sims M. B.; Sumerlin B. S. Architecture-Transformable Polymers: Reshaping the Future of Stimuli-Responsive Polymers. Prog. Polym. Sci. 2019, 89, 61–75. 10.1016/j.progpolymsci.2018.09.006. [DOI] [Google Scholar]
- Caliari S. R.; Burdick J. A.; Terms B. O. X. K. E. Y. REVIEW A Practical Guide to Hydrogels for Cell Culture. Nat. Publ. Gr. 2016, 13, 405–414. 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T.; Tan B.; Zhu L.; Wang Y.; Liao J. A Review on the Design of Hydrogels With Different Stiffness and Their Effects on Tissue Repair. 2022, 10 (January), 1–18. 10.3389/fbioe.2022.817391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvendiren M.; Burdick J. A. Stiffening Hydrogels to Probe Short- and Long-Term Cellular Responses to Dynamic Mechanics. Nat. Commun. 2012, 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- Mahmood A.; Patel D.; Hickson B.; Desrochers J.; Hu X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. 2022, 23, 1415. 10.3390/ijms23031415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanashetti N. A.; Heggannavar G. B.; Kariduraganavar M. Y. Smart Biopolymers and Their Biomedical Applications. Procedia Manuf. 2017, 12 (December 2016), 263–279. 10.1016/j.promfg.2017.08.030. [DOI] [Google Scholar]
- Luo X.; Liu Y.; Pang J.; Bi S.; Zhou Z.; Lu Z.; Feng C.; Chen X.; Kong M. Thermo/Photo Dual-Crosslinking Chitosan-Gelatin Methacrylate Hydrogel with Controlled Shrinking Property for Contraction Fabrication. Carbohydr. Polym. 2020, 236 (February), 116067. 10.1016/j.carbpol.2020.116067. [DOI] [PubMed] [Google Scholar]
- Ashammakhi N.; Ahadian S.; Zengjie F.; Lorestani F.; Orive G.; Ostrovidov S.; Angeles L.; Lumpur K.; Lumpur K.; Angeles L.; Angeles L.; Arabia S. Advances and Future Perspectives in 4D Bioprinting Nureddin. 2018, 13 (12), 1–21. 10.1002/biot.201800148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Xiang Y.; Zhang H.; Cheng L.; Mao X.; An N.; Zhang L.; Zhou J.; Deng L.; Zhang Y.; Sun X.; Santos H. A.; Cui W. A Biomimetic 3D-Self-Forming Approach for Microvascular Scaffolds. Adv. Sci. 2020, 7, 9. 10.1002/advs.201903553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitana W.; Apsite I.; Hazur J.; Boccaccini A. R.; Ionov L. 4D Biofabrication of T-Shaped Vascular Bifurcation. Adv. Mater. Technol. 2023, 8, 2200429. 10.1002/admt.202200429. [DOI] [Google Scholar]
- Kirillova A.; Maxson R.; Stoychev G.; Gomillion C. T.; Ionov L. 4D Biofabrication Using Shape-Morphing Hydrogels. Adv. Mater. 2017, 29 (46), 1–8. 10.1002/adma.201703443. [DOI] [PubMed] [Google Scholar]
- Joshi A.; Choudhury S.; Baghel V. S.; Ghosh S. 4D Printed Programmable Shape-Morphing Hydrogels as Intraoperative Self-Folding Nerve Conduits for Sutureless Neurorrhaphy. 2023, 12, 1–34. 10.1002/adhm.202300701. [DOI] [PubMed] [Google Scholar]
- Sydney Gladman A.; Matsumoto E. A.; Nuzzo R. G.; Mahadevan L.; Lewis J. A. Biomimetic 4D Printing. Nat. Mater. 2016, 15 (4), 413–418. 10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- Fourmann O.; Hausmann M. K.; Neels A.; Schubert M.; Nyström G.; Zimmermann T.; Siqueira G. 3D Printing of Shape-Morphing and Antibacterial Anisotropic Nanocellulose Hydrogels. Carbohydr. Polym. 2021, 259, 1–11. 10.1016/j.carbpol.2021.117716. [DOI] [PubMed] [Google Scholar]
- Constante G.; Apsite I.; Alkhamis H.; Dulle M.; Schwarzer M.; Caspari A.; Synytska A.; Salehi S.; Ionov L. 4D Biofabrication Using a Combination of 3D Printing and Melt-Electrowriting of Shape-Morphing Polymers. ACS Appl. Mater. Interfaces 2021, 13 (11), 12767–12776. 10.1021/acsami.0c18608. [DOI] [PubMed] [Google Scholar]
- Díaz-Payno P. J.; Kalogeropoulou M.; Muntz I.; Kingma E.; Kops N.; D’Este M.; Koenderink G. H.; Fratila-Apachitei L. E.; van Osch G. J. V. M.; Zadpoor A. A. Swelling-Dependent Shape-Based Transformation of a Human Mesenchymal Stromal Cells-Laden 4D Bioprinted Construct for Cartilage Tissue Engineering. Adv. Healthc. Mater. 2023, 2201891, 2201891. 10.1002/adhm.202201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakarich S. E.; Gorkin R.; Panhuis M.; In Het; Spinks G. M. 4D Printing with Mechanically Robust, Thermally Actuating Hydrogels. Macromol. Rapid Commun. 2015, 36, 1211–1217. 10.1002/marc.201500079. [DOI] [PubMed] [Google Scholar]
- Liu X.; Gao M.; Chen J.; Guo S.; Zhu W.; Bai L.; Zhai W.; Du H.; Wu H.; Yan C.; Shi Y.; Gu J.; Qi H. J.; Zhou K. Recent Advances in Stimuli-Responsive Shape-Morphing Hydrogels. 2022, 2203323, 1–25. 10.1002/adfm.202203323. [DOI] [Google Scholar]
- Xue W.; Hamley I. W.; Huglin M. B. Rapid Swelling and Deswelling of Thermoreversible Hydrophobically Modified Poly(N-Isopropylacrylamide) Hydrogels Prepared by Freezing Polymerisation. Polymer (Guildf). 2002, 43 (19), 5181–5186. 10.1016/S0032-3861(02)00396-8. [DOI] [Google Scholar]
- Najafi M.; Hebels E.; Hennink W. E.; Vermonden T. Poly(N -Isopropylacrylamide): Physicochemical Properties and Biomedical Applications. Temp. Polym. 2018, 1–34. 10.1002/9781119157830.ch1. [DOI] [Google Scholar]
- Weng L.; Xie J. Smart Electrospun Nanofibers for Controlled Drug Release : Recent Advances and New Perspectives Smart Electrospun Nanofibers for Controlled Drug Release : Recent Advances and New Perspectives. 2015, 21 (March), 1944. 10.2174/1381612821666150302151959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscornea C.; David A.; Ioan L.; Teodorescu M. Effect of Additives upon the Phase Transition Temperature of α,ω-(2-Hydroxyethoxy) Oligo(Propylene Oxide) in Aqueous Solutions. Mater. Plast. 2013, 50, 163–166. [Google Scholar]
- Wu C.; Wang X. Globule-to-Coil Transition of a Single Homopolymer Chain in Solution. Phys. Rev. Lett. 1998, 80 (18), 4092–4094. 10.1103/PhysRevLett.80.4092. [DOI] [Google Scholar]
- Han D.; Lu Z.; Chester S. A.; Lee H. Micro 3D Printing of a Temperature-Responsive Hydrogel Using Projection Micro-Stereolithography. Sci. Rep. 2018, 8 (1), 1–10. 10.1038/s41598-018-20385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.; Zhang R.; Zhang L.; Cao X. 4D Printing of Robust Hydrogels Consisted of Agarose Nanofibers and Polyacrylamide. ACS Macro Lett. 2018, 7 (4), 442–446. 10.1021/acsmacrolett.7b00957. [DOI] [PubMed] [Google Scholar]
- Podstawczyk D.; Nizioł M.; Szymczyk-Ziółkowska P.; Fiedot-Toboła M. Development of Thermoinks for 4D Direct Printing of Temperature-Induced Self-Rolling Hydrogel Actuators. Adv. Funct. Mater. 2021, 31 (15), 1–10. 10.1002/adfm.202009664. [DOI] [Google Scholar]
- Li S.; Wang W.; Li W.; Xie M.; Deng C.; Sun X.; Wang C.; Liu Y.; Shi G.; Xu Y.; Ma X.; Wang J. Fabrication of Thermoresponsive Hydrogel Scaffolds with Engineered Microscale Vasculatures. Adv. Funct. Mater. 2021, 31, 27. 10.1002/adfm.202102685. [DOI] [Google Scholar]
- Nizioł M.; Paleczny J.; Junka A.; Shavandi A.; Dawiec-Liśniewska A.; Podstawczyk D. 3D Printing of Thermoresponsive Hydrogel Laden With an Antimicrobial Agent Towards Wound Healing Applications. Bioengineering 2021, 8 (6), 1–18. 10.3390/bioengineering8060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Xu Z.; Fu J. Programmable and Reversible 3D-/4D-Shape-Morphing Hydrogels with Precisely Defined Ion Coordination. ACS Appl. Mater. Interfaces 2020, 12 (23), 26476–26484. 10.1021/acsami.0c06342. [DOI] [PubMed] [Google Scholar]
- de Almeida P.; Jaspers M.; Vaessen S.; Tagit O.; Portale G.; Rowan A. E.; Kouwer P. H. J. Cytoskeletal Stiffening in Synthetic Hydrogels. Nat. Commun. 2019, 10 (1), 2–9. 10.1038/s41467-019-08569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Luo X.; Wu W.; Zhang A.; Lu B.; Zhang T.; Kong M. Dual Cure (Thermal/Photo) Composite Hydrogel Derived from Chitosan/Collagen for in Situ 3D Bioprinting. Int. J. Biol. Macromol. 2021, 182, 689–700. 10.1016/j.ijbiomac.2021.04.058. [DOI] [PubMed] [Google Scholar]
- Che Q. T.; Charoensri K.; Seo J. W.; Nguyen M. H.; Jang G.; Bae H.; Park H. J. Triple-Conjugated Photo-/Temperature-/PH-Sensitive Chitosan with an Intelligent Response for Bioengineering Applications. Carbohydr. Polym. 2022, 298 (August), 120066. 10.1016/j.carbpol.2022.120066. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Gu J.; Zhang D.; Zhang Y.; Chen J. Structurally Dynamic Gelatin-Based Hydrogels with Self-Healing, Shape Memory, and Cytocompatible Properties for 4D Printing. Biomacromolecules 2023, 24 (1), 109–117. 10.1021/acs.biomac.2c00924. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Xue K.; Loh X. J. Thermo-Responsive Hydrogels : From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. 10.3390/gels7030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti P. M.; Aebischer P.; Lysaght M. J. The Dawn of Biotechnology in Artificial Organs. ASAIO J. 1995, 41 (1), 49–57. 10.1097/00002480-199541010-00009. [DOI] [PubMed] [Google Scholar]
- Lee S. J.; Seok J. M.; Lee J. H.; Lee J.; Kim W. D.; Park S. A. Three-Dimensional Printable Hydrogel Using a Hyaluronic Acid/Sodium Alginate Bio-Ink. Polymers (Basel). 2021, 13 (5), 1–8. 10.3390/polym13050794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo D. R.; Biswal T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3 (1), 1–19. 10.1007/s42452-020-04096-w. [DOI] [Google Scholar]
- Zhao L.; Zhang X.; Luo Q.; Hou C.; Xu J.; Liu J. Engineering Nonmechanical Protein-Based Hydrogels with Highly Mechanical Properties: Comparison with Natural Muscles. Biomacromolecules 2020, 21 (10), 4212–4219. 10.1021/acs.biomac.0c01002. [DOI] [PubMed] [Google Scholar]
- Liu S.; Hu Y.; Zhang J.; Bao S.; Xian L.; Dong X.; Zheng W.; Li Y.; Gao H.; Zhou W. Bioactive and Biocompatible Macroporous Scaffolds with Tunable Performances Prepared Based on 3D Printing of the Pre-Crosslinked Sodium Alginate/Hydroxyapatite Hydrogel Ink. Macromol. Mater. Eng. 2019, 304 (4), 1–11. 10.1002/mame.201800698. [DOI] [Google Scholar]
- Lee K. Y.; Mooney D. J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37 (1), 106–126. 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Miao Y.; Zhang J.; Wu J. P.; Kirk T. B.; Xu J.; Ma D.; Xue W. Three-Dimensional Printing of Shape Memory Hydrogels with Internal Structure for Drug Delivery. Mater. Sci. Eng., C 2018, 84 (November 2017), 44–51. 10.1016/j.msec.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Cao P.; Tao L.; Gong J.; Wang T.; Wang Q.; Ju J.; Zhang Y. 4D Printing of a Sodium Alginate Hydrogel with Step-Wise Shape Deformation Based on Variation of Crosslinking Density. ACS Appl. Polym. Mater. 2021, 3 (12), 6167–6175. 10.1021/acsapm.1c01034. [DOI] [Google Scholar]
- Gong J.; Schuurmans C. C. L.; Genderen A. M. van; Cao X.; Li W.; Cheng F.; He J. J.; López A.; Huerta V.; Manríquez J.; Li R.; Li H.; Delavaux C.; Sebastian S.; Capendale P. E.; Wang H.; Xie J.; Yu M.; Masereeuw R.; Vermonden T.; Zhang Y. S. Complexation-Induced Resolution Enhancement of 3D-Printed Hydrogel Constructs. Nat. Commun. 2020, 11 (1), 1–14. 10.1038/s41467-020-14997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassie A. T.; Zhao Y.; Boyden E. S. Expansion Microscopy: Principles and Uses in Biological Research. Nat. Methods 2019, 16 (1), 33–41. 10.1038/s41592-018-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B.; Zhang Y.; Li D.; Mo X.; Pan J. Hofmeister Effect-Enhanced Gelatin/Oxidized Dextran Hydrogels with Improved Mechanical Properties and Biocompatibility for Wound Healing. Acta Biomater. 2022, 151, 235–253. 10.1016/j.actbio.2022.08.009. [DOI] [PubMed] [Google Scholar]
- Xu C.; Hung C.; Cao Y.; Liu H. H. Tunable Crosslinking, Reversible Phase Transition, and 3D Printing of Hyaluronic Acid Hydrogels via Dynamic Coordination of Innate Carboxyl Groups and Metallic Ions. ACS Appl. Bio Mater. 2021, 4 (3), 2408–2428. 10.1021/acsabm.0c01300. [DOI] [PubMed] [Google Scholar]
- Zheng S. Y.; Shen Y.; Zhu F.; Yin J.; Qian J.; Fu J.; Wu Z. L.; Zheng Q. Programmed Deformations of 3D-Printed Tough Physical Hydrogels with High Response Speed and Large Output Force. Adv. Funct. Mater. 2018, 28 (37), 1–8. 10.1002/adfm.201803366. [DOI] [Google Scholar]
- Che Y.; Zschoche S.; Obst F.; Appelhans D.; Voit B. Double-Crosslinked Reversible Redox-Responsive Hydrogels Based on Disulfide-Thiol Interchange. J. Polym. Sci. Part A Polym. Chem. 2019, 57 (24), 2590–2601. 10.1002/pola.29539. [DOI] [Google Scholar]
- Greene A. F.; Danielson M. K.; Delawder A. O.; Liles K. P.; Li X.; Natraj A.; Wellen A.; Barnes J. C. Redox-Responsive Artificial Molecular Muscles: Reversible Radical-Based Self-Assembly for Actuating Hydrogels. Chem. Mater. 2017, 29 (21), 9498–9508. 10.1021/acs.chemmater.7b03635. [DOI] [Google Scholar]
- Aramoto H.; Osaki M.; Konishi S.; Ueda C.; Kobayashi Y.; Takashima Y.; Harada A.; Yamaguchi H. Redox-Responsive Supramolecular Polymeric Networks Having Double-Threaded Inclusion Complexes. Chem. Sci. 2020, 11 (17), 4322–4331. 10.1039/C9SC05589D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Tahara H.; Sagara T. Driving Quick and Large Amplitude Contraction of Viologen-Incorporated Poly- l -Lysine-Based Hydrogel by Reduction. ACS Appl. Mater. Interfaces 2018, 10 (42), 36415–36424. 10.1021/acsami.8b12530. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Wang Z.; Jin D.; Zhang C.; Sun R.; Li Z.; Hu K.; Ni J.; Cai Z.; Pan D.; Wang X.; Zhu W.; Li J.; Wu D.; Zhang L.; Chu J. Botanical-Inspired 4D Printing of Hydrogel at the Microscale. Adv. Funct. Mater. 2020, 30 (4), 1–10. 10.1002/adfm.201907377. [DOI] [Google Scholar]
- Zhang Y.; Liao J.; Wang T.; Sun W.; Tong Z. Polyampholyte Hydrogels with PH Modulated Shape Memory and Spontaneous Actuation. Adv. Funct. Mater. 2018, 28 (18), 1–9. 10.1002/adfm.201707245. [DOI] [Google Scholar]
- Mulakkal M. C.; Trask R. S.; Ting V. P.; Seddon A. M. Responsive Cellulose-Hydrogel Composite Ink for 4D Printing. Mater. Des. 2018, 160, 108–118. 10.1016/j.matdes.2018.09.009. [DOI] [Google Scholar]
- Chan V.; Park K.; Collens M. B.; Kong H.; Saif T. A.; Bashir R. Development of Miniaturized Walking Biological Machines. Sci. Rep. 2012, 2, 1. 10.1038/srep00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Fu F.; Yu Y.; Wang H.; Shang Y.; Zhao Y. Cardiomyocytes-Actuated Morpho Butterfly Wings. Adv. Mater. 2019, 31 (8), 1–7. 10.1002/adma.201805431. [DOI] [PubMed] [Google Scholar]
- Cui H.; Liu C.; Esworthy T.; Huang Y.; Yu Z. X.; Zhou X.; San H.; Lee S. J.; Hann S. Y.; Boehm M.; Mohiuddin M.; Fisher J. P.; Zhang L. G. 4D Physiologically Adaptable Cardiac Patch: A 4-Month in Vivo Study for the Treatment of Myocardial Infarction. Sci. Adv. 2020, 6, 26. 10.1126/sciadv.abb5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriantsilefisoa R.; Nie C.; Parshad B.; Pan Y.; Bhatia S.; Haag R. Double Trouble for Viruses: A Hydrogel Nanocomposite Catches the Influenza Virus While Shrinking and Changing Color. Chem. Commun. 2020, 56 (24), 3547–3550. 10.1039/C9CC09069J. [DOI] [PubMed] [Google Scholar]
- Bian Q.; Fu L.; Li H. Engineering Shape Memory and Morphing Protein Hydrogels Based on Protein Unfolding and Folding. Nat. Commun. 2022, 13 (1), 1–8. 10.1038/s41467-021-27744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narupai B.; Smith P. T.; Nelson A. 4D Printing of Multi-Stimuli Responsive Protein-Based Hydrogels for Autonomous Shape Transformations. Adv. Funct. Mater. 2021, 31 (23), 1–9. 10.1002/adfm.202011012. [DOI] [Google Scholar]
- Athas J. C.; Nguyen C. P.; Zarket B. C.; Gargava A.; Nie Z.; Raghavan S. R. Enzyme-Triggered Folding of Hydrogels: Toward a Mimic of the Venus Flytrap. ACS Appl. Mater. Interfaces 2016, 8 (29), 19066–19074. 10.1021/acsami.6b05024. [DOI] [PubMed] [Google Scholar]
- Devillard C. D.; Mandon C. A.; Lambert S. A.; Blum L. J.; Marquette C. A. Bioinspired Multi-Activities 4D Printing Objects: A New Approach Toward Complex Tissue Engineering. Biotechnol. J. 2018, 13, 12. 10.1002/biot.201800098. [DOI] [PubMed] [Google Scholar]
- Cangialosi A.; Yoon C. K.; Liu J.; Huang Q.; Guo J.; Nguyen T. D.; Gracias D. H.; Schulman R. DNA Sequence-Directed Shape Change of Photopatterned Hydrogels via High-Degree Swelling. Science (80-.). 2017, 357 (6356), 1126–1130. 10.1126/science.aan3925. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Wang C.; Yan H.; Liu Y. Soft Robotics Programmed with Double Crosslinking DNA Hydrogels. Adv. Funct. Mater. 2019, 29 (45), 1–10. 10.1002/adfm.201905911. [DOI] [Google Scholar]
- Rivera-Tarazona L. K.; Shukla T.; Singh K. A.; Gaharwar A. K.; Campbell Z. T.; Ware T. H. 4D Printing of Engineered Living Materials. Adv. Funct. Mater. 2022, 32 (4), 1–12. 10.1002/adfm.202106843. [DOI] [Google Scholar]
- Rothschild M. Projection Optical Lithography. Mater. Today 2005, 8 (2), 18–24. 10.1016/S1369-7021(05)00698-X. [DOI] [Google Scholar]
- DeForest C. A.; Anseth K. S. Cytocompatible Click-Based Hydrogels with Dynamically Tunable Properties through Orthogonal Photoconjugation and Photocleavage Reactions. Nat. Chem. 2011, 3 (12), 925–931. 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S.; Keller K. A.; Sperduto J. L.; Slater J. H. Fundamentals of Laser-Based Hydrogel Degradation and Applications in Cell and Tissue Engineering. Adv. Healthc. Mater. 2017, 6 (24), 1–28. 10.1002/adhm.201700681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkar P. M.; Kiick K. L.; Kloxin A. M. Designing Degradable Hydrogels for Orthogonal Control of Cell Microenvironments. Chem. Soc. Rev. 2013, 42 (17), 7335–7372. 10.1039/C3CS60040H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K. M. C.; Annabi N.; Ercole F.; Zhou K.; Karst D. J.; Li F.; Haynes J. M.; Evans R. A.; Thissen H.; Khademhosseini A.; Forsythe J. S. Facile One-Step Micropatterning Using Photodegradable Gelatin Hydrogels for Improved Cardiomyocyte Organization and Alignment. Adv. Funct. Mater. 2015, 25 (6), 977–986. 10.1002/adfm.201403124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K.; Tomatsu I.; Van Den Broek B.; Cui C.; Korobko A. V.; Van Noort J.; Meijer A. H.; Spaink H. P.; Kros A. Dextran Based Photodegradable Hydrogels Formed via a Michael Addition. Soft Matter 2011, 7 (10), 4881–4887. 10.1039/c1sm05291h. [DOI] [Google Scholar]
- Lunzer M.; Shi L.; Andriotis O. G.; Gruber P.; Markovic M.; Thurner P. J.; Ossipov D.; Liska R.; Ovsianikov A. A Modular Approach to Sensitized Two-Photon Patterning of Photodegradable Hydrogels. Angew. Chemie - Int. Ed. 2018, 57 (46), 15122–15127. 10.1002/anie.201808908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vlies A. J.; Barua N.; Nieves-Otero P. A.; Platt T. G.; Hansen R. R. On Demand Release and Retrieval of Bacteria from Microwell Arrays Using Photodegradable Hydrogel Membranes. ACS Appl. Bio Mater. 2019, 2 (1), 266–276. 10.1021/acsabm.8b00592. [DOI] [PubMed] [Google Scholar]
- DeForest C. A.; Anseth K. S. Photoreversible Patterning of Biomolecules within Click-Based Hydrogels. Angew. Chemie - Int. Ed. 2012, 51 (8), 1816–1819. 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azagarsamy M. A.; Anseth K. S. Wavelength-Controlled Photocleavage for the Orthogonal and Sequential Release of Multiple Proteins. Angew. Chemie - Int. Ed. 2013, 52 (51), 13803–13807. 10.1002/anie.201308174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong V. X.; Li F.; Forsythe J. S. Photolabile Hydrogels Responsive to Broad Spectrum Visible Light for Selective Cell Release. ACS Appl. Mater. Interfaces 2017, 9 (38), 32441–32445. 10.1021/acsami.7b11517. [DOI] [PubMed] [Google Scholar]
- Azagarsamy M. A.; Mckinnon D. D.; Alge D. L.; Anseth K. S. Coumarin-Based Photodegradable Hydrogel: Design, Synthesis, Gelation, and Degradation Kinetics. 2014, 3, 515. 10.1021/mz500230p. [DOI] [PubMed] [Google Scholar]
- Gandavarapu N. R.; Azagarsamy M. A.; Anseth K. S. Photo-Click Living Strategy for Controlled, Reversible Exchange of Biochemical Ligands. Adv. Mater. 2014, 26 (16), 2521–2526. 10.1002/adma.201304847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa F.; Sugiura S.; Takagi T.; Sumaru K.; Camci-Unal G.; Patel A.; Khademhosseini A.; Kanamori T. Activated-Ester-Type Photocleavable Crosslinker for Preparation of Photodegradable Hydrogels Using a Two-Component Mixing Reaction. Adv. Healthc. Mater. 2015, 4 (2), 246–254. 10.1002/adhm.201400180. [DOI] [PubMed] [Google Scholar]
- Watanabe U.; Sugiura S.; Kakehata M.; Yanagawa F.; Takagi T.; Sumaru K.; Satoh T.; Tamura M.; Hosokawa Y.; Torizuka K.; Kanamori T. Fabrication of Hollow Structures in Photodegradable Hydrogels Using a Multi-Photon Excitation Process for Blood Vessel Tissue Engineering. Micromachines 2020, 11 (7), 1–14. 10.3390/mi11070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R. G.; De Bon F.; Pereira P.; Carvalho F. M.; Freitas M.; Tavakoli M.; Serra A. C.; Fonseca A. C.; Coelho J. F. J. Photo-Degradable, Tough and Highly Stretchable Hydrogels. Mater. Today Bio 2022, 15, 100325. 10.1016/j.mtbio.2022.100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gracia Lux C.; Lux J.; Collet G.; He S.; Chan M.; Olejniczak J.; Foucault-Collet A.; Almutairi A. Short Soluble Coumarin Crosslinkers for Light-Controlled Release of Cells and Proteins from Hydrogels. Biomacromolecules 2015, 16 (10), 3286–3296. 10.1021/acs.biomac.5b00950. [DOI] [PubMed] [Google Scholar]
- Fairbanks B. D.; Singh S. P.; Bowman C. N.; Anseth K. S. Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction. Macromolecules 2011, 44 (8), 2444–2450. 10.1021/ma200202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavitt F. M.; Brown T. E.; Hushka E. A.; Brown M. E.; Dempsey P. J.; Lutolf M. P.; Anseth K. S. The Effect of Thiol Structure on Allyl Sulfide Photodegradable Hydrogels and Their Application as a Degradable Scaffold for Organoid Passaging. Adv. Mater. 2020, 32 (30), 1–23. 10.1002/adma.201905366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp A. T.; Highley C.; Manor B.; Burdick J.; Dmochowski I. J. Ruthenium-Crosslinked Hydrogels with Rapid, Visible-Light Degradation. Chem. - A Eur. J. 2018, 24, 2328. 10.1002/chem.201704580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. J.; Velema W. A.; Lerch M. M.; Szymanski W.; Feringa B. L. Wavelength-Selective Cleavage of Photoprotecting Groups: Strategies and Applications in Dynamic Systems. Chem. Soc. Rev. 2015, 44 (11), 3358–3377. 10.1039/C5CS00118H. [DOI] [PubMed] [Google Scholar]
- Truong V. X. Break Up to Make Up: Utilization of Photocleavable Groups in Biolabeling of Hydrogel Scaffolds. ChemPhotoChem. 2020, 4 (8), 564–570. 10.1002/cptc.202000072. [DOI] [Google Scholar]
- Li L.; Scheiger J. M.; Tronser T.; Long C.; Demir K.; Wilson C. L.; Kuzina M. A.; Levkin P. A. Inherent Photodegradability of Polymethacrylate Hydrogels: Straightforward Access to Biocompatible Soft Microstructures. Adv. Funct. Mater. 2019, 29 (33), 1–8. 10.1002/adfm.201902906. [DOI] [Google Scholar]
- Rosenfeld A.; Göckler T.; Kuzina M.; Reischl M.; Schepers U.; Levkin P. A. Designing Inherently Photodegradable Cell-Adhesive Hydrogels for 3D Cell Culture. Adv. Healthc. Mater. 2021, 10 (16), 1–10. 10.1002/adhm.202100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate M. B.; Coburn J.; Partlow B. P.; Moreau J. E.; Mondia J. P.; Marelli B.; Kaplan D. L.; Omenetto F. G.; Weitz D. A. Laser-Based Three-Dimensional Multiscale Micropatterning of Biocompatible Hydrogels for Customized Tissue Engineering Scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (39), 12052–12057. 10.1073/pnas.1509405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. A.; Kim S.; Jin Y.; Cho S.-W.; Yang K.; Hwang N. S.; Kim H. D. In Situ Microenvironment Remodeling Using a Dual-Responsive System: Photodegradable Hydrogels and Gene Activation by Visible Light. Biomater. Sci. 2022, 10 (14), 3981. 10.1039/D2BM00617K. [DOI] [PubMed] [Google Scholar]
- Villiou M.; Paez J. I.; Del Campo A. Photodegradable Hydrogels for Cell Encapsulation and Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12 (34), 37862–37872. 10.1021/acsami.0c08568. [DOI] [PubMed] [Google Scholar]
- Rosales A. M.; Vega S. L.; DelRio F. W.; Burdick J. A.; Anseth K. S. Hydrogels with Reversible Mechanics to Probe Dynamic Cell Microenvironments. Angew. Chemie - Int. Ed. 2017, 56 (40), 12132–12136. 10.1002/anie.201705684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Tan Y.; He C.; Ji S.; Xu H. Unconstrained 3D Shape Programming with Light-Induced Stress Gradient. Adv. Mater. 2021, 33 (42), 1–9. 10.1002/adma.202105194. [DOI] [PubMed] [Google Scholar]
- Käpylä E.; Delgado S. M.; Kasko A. M. Shape-Changing Photodegradable Hydrogels for Dynamic 3D Cell Culture. ACS Appl. Mater. Interfaces 2016, 8 (28), 17885–17893. 10.1021/acsami.6b05527. [DOI] [PubMed] [Google Scholar]
- Wylie R. G.; Ahsan S.; Aizawa Y.; Maxwell K. L.; Morshead C. M.; Shoichet M. S. Spatially Controlled Simultaneous Patterning of Multiple Growth Factors in Three-Dimensional Hydrogels. Nat. Mater. 2011, 10 (10), 799–806. 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- Rana D.; Padmanaban P.; Becker M.; Stein F.; Leijten J.; Koopman B.; Rouwkema J. Spatial Control of Self-Organizing Vascular Networks with Programmable Aptamer-Tethered Growth Factor Photopatterning. Mater. Today Bio 2023, 19 (January), 100551. 10.1016/j.mtbio.2023.100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten C.; D’urso M.; Bril M.; Woud T. E.; Bouten C. V. C.; Kurniawan N. A. Generation of Multicue Cellular Microenvironments by UV-Photopatterning of Three-Dimensional Cell Culture Substrates. J. Vis. Exp. 2022, 2022 (184), 1–25. 10.3791/63988. [DOI] [PubMed] [Google Scholar]
- Falandt M.; Bernal P. N.; Dudaryeva O.; Florczak S.; Größbacher G.; Schweiger M.; Longoni A.; Greant C.; Assunção M.; Nijssen O.; van Vlierberghe S.; Malda J.; Vermonden T.; Levato R. Spatial-Selective Volumetric 4D Printing and Single-Photon Grafting of Biomolecules within Centimeter-Scale Hydrogels via Tomographic Manufacturing (Adv. Mater. Technol. 15/2023). Adv. Mater. Technol. 2023, 8, 15. 10.1002/admt.202370071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W.; Gehlen J.; Bernero M.; Gehre C.; Nutal Schädli G.; Müller R.; Qin X.-H.; Qiu W.; Gehlen J.; Bernero M.; Gehre C.; Schädli G. N.; Müller R.; Qin X. A Synthetic Dynamic Polyvinyl Alcohol Photoresin for Fast Volumetric Bioprinting of Functional Ultrasoft Hydrogel Constructs. bioRxiv 2023, 33, 2022.10.20.513079. 10.1002/adfm.202214393. [DOI] [Google Scholar]
- Levato R.; Jungst T.; Scheuring R. G.; Blunk T.; Groll J. From Shape to Function : The Next Step in Bioprinting. 2020, 1906423. 10.1002/adma.201906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. V.; Atala A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32 (8), 773–785. 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- Gu B. K.; Choi D. J.; Park S. J.; Kim M. S.; Kang C. M.; Kim C. H. 3-Dimensional Bioprinting for Tissue Engineering Applications. Biomater. Res. 2016, 20 (1), 1–8. 10.1186/s40824-016-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H. J.; Khalmuratova R.; Park S. A.; Chung E. J.; Shin H. W.; Kwon S. K. Serial Analysis of Tracheal Restenosis After 3D-Printed Scaffold Implantation: Recruited Inflammatory Cells and Associated Tissue Changes. Tissue Eng. Regen. Med. 2017, 14 (5), 631–639. 10.1007/s13770-017-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimar A.; Palermo A.; Innocenti B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 1–10. 10.1155/2019/5340616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvendiren M.; Molde J.; Soares R. M. D.; Kohn J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2 (10), 1679–1693. 10.1021/acsbiomaterials.6b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni L.; Boland T.; Burdick J. A.; De Maria C.; Derby B.; Forgacs G.; Groll J.; Li Q.; Malda J.; Mironov V. A.; Mota C.; Nakamura M.; Shu W.; Takeuchi S.; Woodfield T. B. F.; Xu T.; Yoo J. J.; Vozzi G. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36 (4), 384–402. 10.1016/j.tibtech.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Béduer A.; Piacentini N.; Aeberli L.; Da Silva A.; Verheyen C. A.; Bonini F.; Rochat A.; Filippova A.; Serex L.; Renaud P.; Braschler T. Additive Manufacturing of Hierarchical Injectable Scaffolds for Tissue Engineering. Acta Biomater. 2018, 76, 71–79. 10.1016/j.actbio.2018.05.056. [DOI] [PubMed] [Google Scholar]
- Huang L.; Jiang R.; Wu J.; Song J.; Bai H.; Li B.; Zhao Q.; Xie T. Ultrafast Digital Printing toward 4D Shape Changing Materials. Adv. Mater. 2017, 29 (7), 1–6. 10.1002/adma.201605390. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Wu J.; Mu X.; Chen H.; Qi H. J.; Fang D. Desolvation Induced Origami of Photocurable Polymers by Digit Light Processing. Macromol. Rapid Commun. 2017, 38 (13), 1–6. 10.1002/marc.201600625. [DOI] [PubMed] [Google Scholar]
- Es Sayed J.; Khoonkari M.; Oggioni M.; Perrin P.; Sanson N.; Kamperman M.; Włodarczyk-Biegun M. K. Multi-Responsive Jammed Micro-Gels Ink: Toward Control over the Resolution and the Stability of 3D Printed Scaffolds. Adv. Funct. Mater. 2022, 10.1002/adfm.202207816. [DOI] [Google Scholar]
- Petta D.; Basoli V.; Pellicciotta D.; Tognato R.; Barcik J.; Arrigoni C.; Bella E. Della; Armiento A. R.; Candrian C.; Richards R. G.; Alini M.; Moretti M.; Eglin D.; Serra T. Sound-Induced Morphogenesis of Multicellular Systems for Rapid Orchestration of Vascular Networks. Biofabrication 2021, 13, 015004. 10.1088/1758-5090/abbb9c. [DOI] [PubMed] [Google Scholar]
- Tognato R.; Armiento A. R.; Bonfrate V.; Levato R.; Malda J.; Alini M.; Eglin D.; Giancane G.; Serra T. A Stimuli-Responsive Nanocomposite for 3D Anisotropic Cell-Guidance and Magnetic Soft Robotics. Adv. Funct. Mater. 2019, 29, 9. 10.1002/adfm.201804647. [DOI] [Google Scholar]
- Lin N. Y. C.; Homan K. A.; Robinson S. S.; Kolesky D. B.; Duarte N.; Moisan A.; Lewis J. A. Renal Reabsorption in 3D Vascularized Proximal Tubule Models. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (12), 5399–5404. 10.1073/pnas.1815208116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya C.; Du Y.; Gianforcaro A. L.; Orrego S.; Lelkes P. I. On the road to smart biomaterials for bone research: definitions, concepts, advances, and outlook. Bone Res. 2021, 9. 10.1038/s41413-020-00131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurish S. Y.Sensors: Smart vs Intelligent. Sensors Transducers J. 2010, 114. [Google Scholar]