Abstract
People spend a remarkable 30–50% of awake life thinking about something other than what they are currently doing. These experiences of being “off-task” can be described as spontaneous thought when mental dynamics are relatively flexible. Here we review recent neuroscience developments in this area and consider implications for mental wellbeing and illness. We provide updated overviews of the roles of the default mode network and large-scale network dynamics, and we discuss emerging candidate mechanisms involving hippocampal memory (sharp-wave ripples, replay) and neuromodulatory (noradrenergic and serotonergic) systems. We explore how distinct brain states can be associated with or give rise to adaptive and maladaptive forms of thought linked to distinguishable mental health outcomes. We conclude by outlining new directions in the neuroscience of spontaneous and off-task thought that may clarify mechanisms, lead to personalized biomarkers, and facilitate therapy developments toward the goals of better understanding and improving mental health.
1.0: Spontaneous Thought and its links to Mental Health
The last several decades have brought a wealth of research into the neural underpinnings of processes evoked by experimentally-directed tasks. However, typical adults spend much of their waking life entertaining thoughts that extend beyond the task at hand (termed “off-task thought”), and/or that emerge spontaneously, relatively free from constraints that guide and stabilize cognition. For example, depending on how these types of experiences are sampled, being “off-task” has been estimated to occupy a striking ~30–50% of daily time awake in adults.1–5
Having spontaneous and off-task thoughts is a normal, and typically healthy, part of everyday life that can involve reminiscing, reflecting, imagining, fantasizing, problem-solving, or generating creative ideas.6–8 Yet such experiences—to which an enormous amount of daily time is dedicated—can also take on forms that are negative in character. For example, rather than reminiscing about the past, an individual may repetitively dwell on prior negative experiences; instead of adaptively planning for the future, one may engage in uncontrollable worry or suicidal ideation. With increasing prevalence rates of psychiatric symptoms and illness in recent years,9 it is likely that dysfunctional manifestations of inner experience have been increasing in frequency. Given that a significant proportion of psychiatric patients are resistant to currently available treatments,10 there is a need for novel therapies such as neuromodulation to correct disruptive patterns of thought. Thus, understanding neural mechanisms of spontaneous and off-task thought is becoming of increasing societal and clinical importance,11–13 an urgency mirrored by growing scientific interest (Figure 1).14–18
Figure 1: Number of publications on spontaneous thought and mental health over the last two decades.
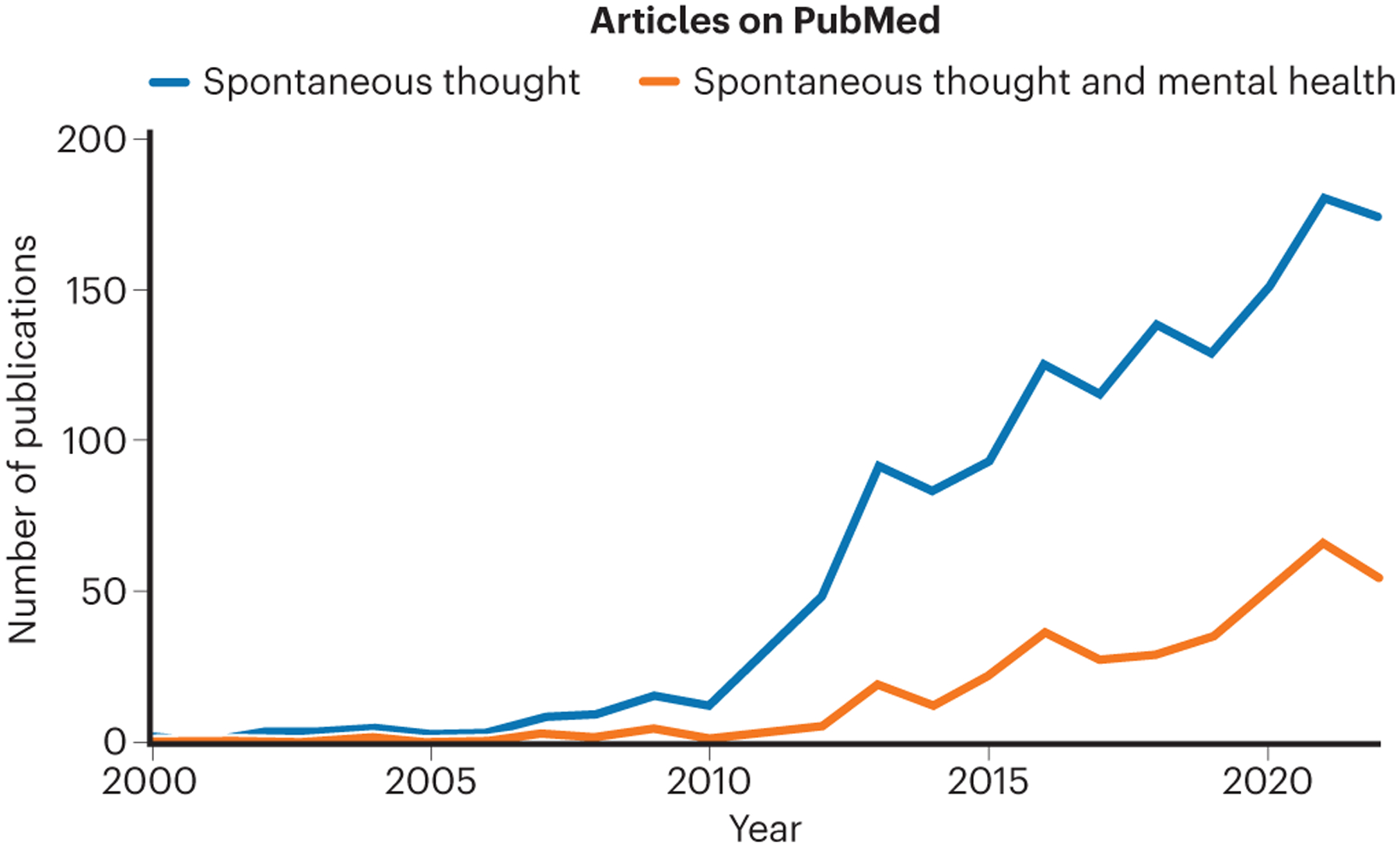
PubMed search query for Spontaneous Thought: (“spontaneous thought” OR “mind wandering” OR “task-unrelated thought” OR “stimulus-independent thought” OR “freely moving thought” OR “self-generated thought”). PubMed search query for Mental Health: (“mental health” OR “positive affect” OR “negative affect” OR “emotion” OR “mood” OR “sadness” OR “happiness” OR “distress” OR “emotional state” OR “depression” OR “anxiety” OR “ADHD” OR “schizophrenia”).
Advances in our understanding of spontaneous thought have linked it to dynamic patterns of brain activity that fluctuate across various time scales.14,15 The recently proposed Dynamic Framework of Thought (DFT)14,19 describes spontaneous thought as emerging in the course of interactions across large-scale brain networks such as the Default Mode Network (DMN), with its core and medial temporal lobe (MTL) subcomponents, as well as the salience and frontoparietal-control networks. Mapping the dynamic interactions across large-scale brain networks to the rich varieties of covert, subjective experience presents some unique scientific challenges that have led to novel developments in experimental paradigms. The neural basis of spontaneous thought, along with off-task, have so far largely been investigated with functional neuroimaging,14,15 but the field is rapidly maturing, incorporating insights from causal experiments (neurostimulation and lesion studies),20 neurophysiological mechanisms of memory,16,21 and dynamics of ascending neuromodulator systems.16,18,22
In this review, we cover the most recent developments in methodologies and advances in the study of the neural bases of spontaneous and off-task thought with an emphasis on the implications for mental health. We provide an updated overview of the DMN and broader, large-scale networks. Beyond those networks, we discuss candidate mechanisms that involve spontaneous activity in hippocampal memory and ascending neuromodulatory systems. Throughout, we explore how distinct brain states can be associated with and give rise to various forms of healthy and unhealthy thinking patterns. Finally, we outline new directions in the neuroscience of spontaneous and off-task thought that may significantly advance mental health research and clinical practice.
2.0: Conceptual and Methodological Considerations
Before reviewing the neural bases of spontaneous and off-task thought, we consider conceptual and methodological issues that provide background for interpretation of research findings. We cover some definitional issues surrounding the evolving concept of ‘spontaneous thought’, examine the evidence for how the related but distinct construct of off-task thought relates to mental health, and review some of the challenges when it comes to investigating spontaneous thought along with the emerging techniques that are beginning to help us overcome these challenges.
2.1: The concept of spontaneous thought is still evolving
The term “spontaneous thought” has become prominent in the scientific literature on a broad range of inner experiences such as mind-wandering and dreaming.23 Yet notions of what spontaneous thought exactly is and how it is best defined continue to evolve. One important outstanding question remains on whether certain forms of repetitive thought (e.g. rumination and worry) should be viewed as spontaneous thought,24,25 or as the DFT argues, are better characterized as a distinct mode of thought that unfolds in a highly constrained, inflexible manner.14 There are also broader philosophical concerns regarding the spontaneity of thought.26 One proposed way to determine spontaneity is to inquire whether the experiencer could identify a cue or trigger.27 However, even in a context where a thought is not initiated consciously by an external stimulus or explicit cognitive control, factors that are typically unobserved, such as interoceptive inputs, may trigger experiences.28 Similar issues have been discussed regarding spontaneity in brain activity.29
Beyond considering how mental activity is initiated, it is also important to account for the dynamic processes that ensure the continuity of thought.30 The DFT14,19 offers an explicit definition of spontaneous thought that hinges on a crucial distinction that the DFT makes between mental contents and dynamic thought processes. Contents describe what someone is thinking about, including dimensions such as relevance to current performance of a task, affective valence (e.g. feels positive or negative), temporal orientation (e.g. past or future-oriented), and sensory imagery (e.g. mentation in the visual or auditory modality). In contrast, dynamics describe how thoughts change from one mental state to another. Within the DFT, spontaneous thought occurs when those dynamics are relatively flexible, and thoughts can change easily and freely (rather than being limited to repetitively recurring content). Conversely, according to the DFT, thought’s spontaneity is reduced when its flow is deliberately constrained (i.e., by cognitive control) or automatically constrained (i.e., by perceptual or affective salience mechanisms that lead to attentional capture). Thus, within the DFT, spontaneous thought is defined based on thought’s dynamics rather than its content, and mind-wandering is defined as a form of spontaneous thought that unfolds relatively freely, with low deliberate and automatic constraints.14 Dreaming and creative thinking are also defined as forms of spontaneous thought with distinct deliberate and automatic constraint profiles.
Despite the emphasis on thought dynamics within the DFT, the vast majority of empirical investigations have focused on measuring thought contents rather than dynamics.31 Mind-wandering, specifically, has been frequently operationalized based on thought contents, although recent empirical investigations have begun measuring subjective, dynamic features such as the extent to which thoughts shift around freely.31,32 Alternatively, thought dynamics have been characterized by quantifying how experiences change with different durations of time passing.33
Acknowledging ongoing philosophical debates and outstanding conceptual issues (see25,34 for detailed discussions), this article reviews a variety of mental experiences and is inclusive of off-task thoughts, experiences described by the DFT as “spontaneous thought,” and thoughts that arise and unfold in an automatically constrained or habitual manner. All of these classes of thought may be indirectly elicited by environmental cues in everyday life (e.g. by initiating mental associations),35,36 but they are not, by definition, directly initiated by explicit cues that instruct people to deliberately engage in specific thoughts. Shared features across classes are a focus on inner mental content (as opposed to immediate physical stimuli) and the involuntary nature of thought unfolding. According to the DFT, the category of automatically or habitually constrained thought is of particular interest because it includes phenomena such as rumination and obsessive thought that are of clinical significance.14 However, automatic constraints on thought and their clinical implications remain relatively understudied. Instead, most empirical investigations have focused on the related but distinct phenomenon of off-task (or task-unrelated) thought, which we turn to in the next section.
2.2: On the relationship between off-task thought and mental health
Research has overwhelmingly focused on deleterious cognitive and health effects of thoughts that are unrelated to the task at hand (i.e., off-task thoughts that can be spontaneous or deliberate). Studies suggest that off-task thought impairs cognitive performance in various experimental and real-world settings, including in executive control,37 sustained attention,38 memory encoding39 tasks, in classroom lectures,40 during reading,41 and during automobile driving.42 Off-task thought can lead to perseverative thinking such as rumination and worry,43,44 lowered mood,2,45 increased stress,46 and impaired sleep.47 Excessive off-task thought—particularly when initiated unintentionally—has been associated with symptoms of depression,48 obsessive-compulsive disorder,44 and eating disorders,49 and attention-deficit/hyperactivity disorder (ADHD)50 in certain task contexts.51
In contrast, positive impacts on mental wellbeing often become apparent when evaluating characteristics beyond the sheer presence of off-task thought, such as what people think about and how thought unfolds.8 For example, though off-task thoughts are often associated with less positive affect than on-task thoughts, off-task thoughts are still, on average, experienced as mildly pleasant, positive and enjoyable.52 In some situations, the content of off-task thought may lead to subsequent improvements in mood.53,54 While negative mood has been associated with thinking about past events,55–57 people tend to daydream more frequently about the future than the past.58 This “prospective bias” of thought can be adaptive, for example benefiting problem-solving and creative achievement6 or advancing social relationships59 (though positive content in future-oriented off-task thought is reduced in individuals with depression symptoms60). Past-related thoughts can also be adaptive, promoting meaningful connections with the current and future self.61 Engaging in off-task thought may promote insight—moments when one becomes abruptly aware of a solution to a problem, often associated with a pleasurable feeling of reward.62 Besides thought content, there is increasing recognition for the mental health relevance of dynamic dimensions. For example, thought described as freely-moving (i.e., unconstrained) has been associated with momentary positive affect.32,63
Context is another major factor that determines how off-task thought interacts with mental health.8,17 For example, the content and dynamics of off-task thought depend on whether a task is high or low demand64 and whether thoughts are sampled in the laboratory or in the real world.65 In one study, several patterns of off-task thought were identified based on distinct content, and among these patterns, emotional thoughts showed the least generalizability between laboratory and real-world environments across individuals.65 Overall, how off-task thought interacts with mental health is highly dependent on factors such as thought content, dynamics, and context. Importantly, there is tremendous variability between—and even within—individuals that is critical to consider when assessing impacts on mental health.
2.3: How can spontaneous thought be studied in the brain?
The study of spontaneous thought in the brain faces a unique challenge: How can mental activity that is initiated without any instructive, external trigger—and that is unpredicted even by an experiencer themself—be measured or quantified, and attributed to brain events? While typical experimental paradigms in cognitive neuroscience employ structured tasks or stimuli to trigger brain responses that are time-locked to external events, distinct approaches are needed to generate insights into the neural basis of spontaneous thought.
Prior to the last two decades, hypotheses about neural mechanisms were largely limited to interpretive comments when authors observed patterns of elevated brain activity during task-free, waking rest, such as the EEG posterior alpha wave (reviewed in66).67,68 However, “resting state” paradigms alone cannot generate direct insights into the brain’s representation of subjective experiences during spontaneous thought, which require that brain activity be directly linked to self-reports of such experiences.14,15,69 Starting in the 1990’s, positron emission tomography (PET)68,70 and functional MRI (fMRI)71,72 studies began to examine relationships between brain activity and self-reported frequency of off-task thought, which participants estimated retrospectively after completing brain scans.
More recently, online experience-sampling during neuroimaging has been applied to examine finer temporal dynamics within individuals (Figure 2a)73 (see also74 for an earlier EEG example). Here people are intermittently presented with self-report prompts (“thought probes”) asking them to rate or describe their most immediate experience. Thought probes must be designed carefully and with the aim of identifying valid and reliable brain-experience relationships, as it has been shown that the specific phrasing and manner of presentation4,5,75 as well as the population being studied76 can influence participant responses. Despite such issues, experience sampling has been used successfully to identify brain-experience relationships in groups of participants on the order of seconds to minutes.77 Alternative approaches to intermittent experience sampling, such as self-caught reporting of experience may provide temporal insights into the arising of spontaneous thoughts and their neural antecedents (especially in trained individuals such as meditation practitioners).78,79 Moreover, free association-based thought sampling80 and think aloud paradigms81 have also been used, albeit more rarely, in combination with neuroimaging. In some cases, verbal or auditory cues have been used to elicit involuntary mental associations, thereby manipulating the occurrence of spontaneous thought.82–84
Figure 2: Linking spontaneous thought and off-task thought phenomenology to neural measures with experience sampling.
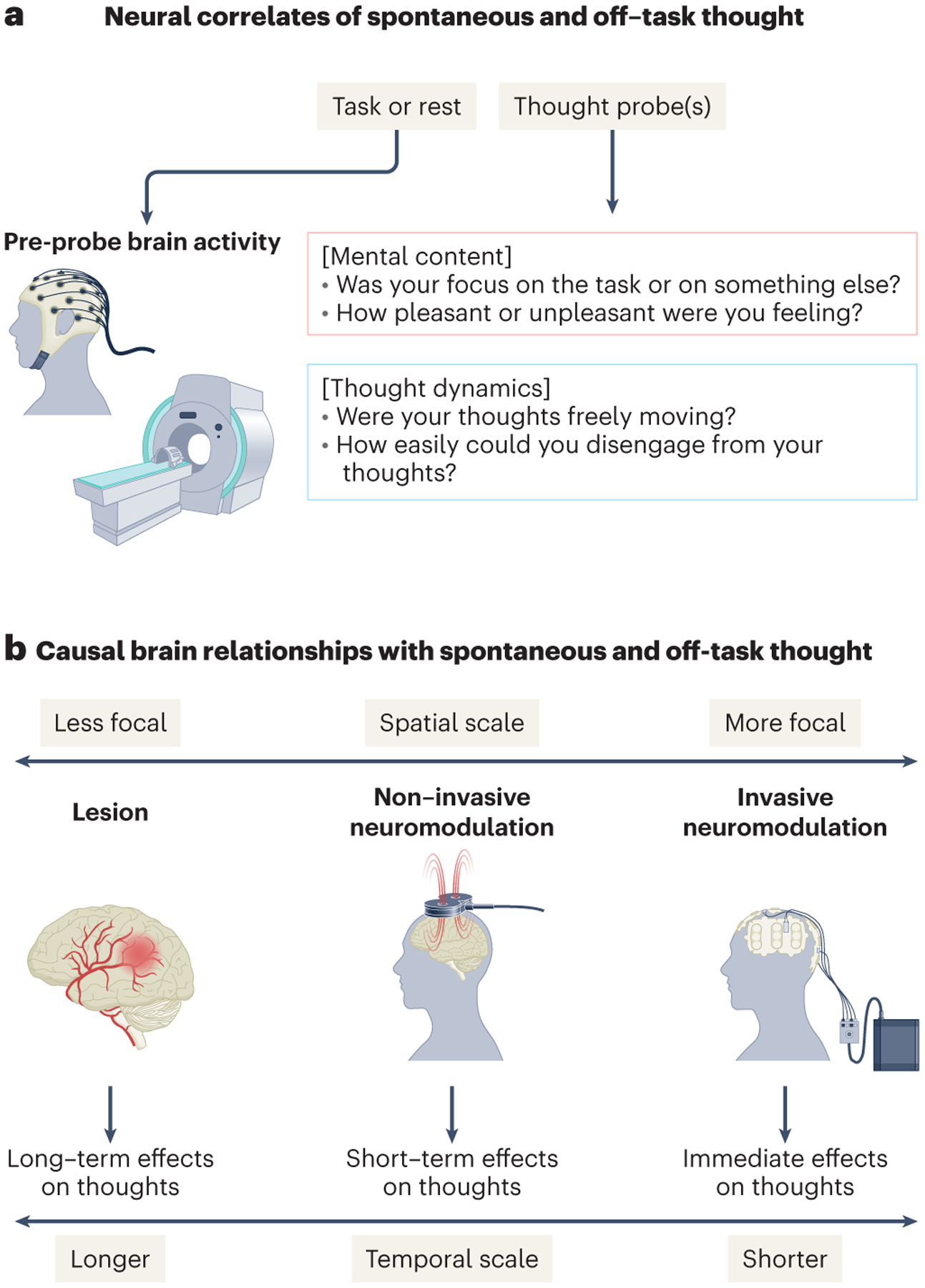
(a) Schematic example of the use of online experience sampling to examine neural correlates and predictors of spontaneous thought and off-task thought. (b) Distinct techniques for examining causal brain relationships with spontaneous thought that provide insights at multiple spatial and temporal scales.
Researchers have only recently begun to characterize the brain bases of spontaneous thought’s more granular features that have relevance to mental health. Multi-dimensional experience-sampling17 during neuroimaging has been used to examine thoughts that vary in terms of emotion,85 self-relevance,28 level of detail,86 modality and perceptual features of sensory imagery,87 and dynamic properties.88 Recent work has also explored the neural basis of “mind-blanking,” an absence of reportable thought (Box 1).89,90 Studies involving online experience-sampling with brain activity measures are beginning to emerge in patients diagnosed with ADHD91,92 and treatment-resistant depression.93 Correlative neuroimaging findings are being complemented with insights from interventions94 as well as causal approaches in neuroscience such as transcranial direct current stimulation (tDCS), intracranial electrical stimulation, and lesion studies, providing insight into the emergence of spontaneous thought across a wide range of time scales (Figure 2b). Taken together, the study of spontaneous thought in the brain is quickly expanding in terms of paradigms used, phenomenology explored, clinical populations examined, and neuroscience techniques applied. With these emerging developments, it is timely to examine their potential to yield new insights, given what is currently known about spontaneous thought in the brain.
Box 1: Neural basis of mind blanking and implications for mental health.
When people are disengaged from the immediate environment, they are usually engaged in their own thoughts, but there are also moments of “mind blanking” when no mental contents can be recalled. Though less studied with respect to mental health than mind-wandering and spontaneous thought, studies suggest that mind blanking is elevated in ADHD199 and impairs cognitive performance in depression.200 The neural correlates of mind blanking have recently been explored. When people were instructed to intentionally engage in mind blanking, deactivation of the DMN was found (i.e., opposite to the typical pattern for off-task thought).201 Online experience-sampling with EEG and fMRI have further confirmed that mind blanking has distinct neural correlates compared to mind-wandering or off-task thought.89,90 These findings should motivate inclusion of mind blanking response options in experience-sampling protocols in mental health contexts. Notably, protocols should carefully distinguish between absence of thought versus absence of all conscious experience, as these distinct features may have been conflated in prior works.202
3.0: Recent Neuroscientific Findings with Relevance to Spontaneous Thought and their Links to Mental Health
3.1: Default Mode Network: An Update.
The DMN has by far received the most frequent attention in reference to the neural basis of spontaneous and off-task thought. The DMN has also been studied extensively in relation to mental health and illness. We provide updates on different features of the DMN, including its activation and deactivation profiles, functional connectivity, subsystems, and the effects of perturbation. We describe how these features inform the neural basis of spontaneous thought and the implications for mental health.
3.1.1: Activation and Deactivation.
Regions comprising the default mode network–including large portions of the posteromedial, medial prefrontal cortex, and lateral parietal cortex (among others)–were initially largely appreciated for showing relative decreases in blood flow during (external) cognitive tasks compared to passive control conditions.95 The “default mode” network96,97 has since been shown to be recruited across a variety of internally-guided processes such as remembering, envisioning the future, and social inference98–experiences that are common during spontaneous thought.68,99 Moreover, the DMN’s activity is often anticorrelated with, or fluctuates independently from, networks involved in externally-oriented attention.100–102 Such observations led to the hypothesis that the DMN could be “the core brain system associated with spontaneous cognition.”103 Subsequently, fMRI studies with online experience-sampling identified trial-by-trial associations between off-task thought and increased DMN activation (or decreased deactivation), which was replicated across multiple contexts.73,99,104,105
The implications of such findings for mental health immediately became a topic of interest. In particular, researchers explored the idea that DMN over-activation underlies an inability to disengage from unwanted, off-task thoughts. For example, in attention and psychotic disorders, it was suggested that an inability to suppress the DMN could give rise to attention lapses, performance variability, and cognitive impairments.106,107 In disorders of mood and anxiety, DMN hyper-activation was hypothesized to underlie maladaptive rumination and worry.107 On the other hand, DMN activation may support healthy forms of thought such as creative idea generation and evaluation.108 Overall, findings highlighted that nuanced interpretations are needed when evaluating DMN activation/deactivation as a potential biomarker or treatment target in mental health contexts. Adding complexity, recent experience-sampling fMRI studies question the notion of a simple one-to-one relationship between DMN activation and off-task thought,86,109,110 while other paradigms have linked DMN activation to the use of working memory to guide task-related behavior.111,112 To better clarify the DMN’s role, it may be important to consider other network features.
3.1.2: Functional Connectivity.
A distinct defining feature of the DMN is correlated spontaneous activity between its different regions.97 Such “functional connectivity” has been shown to persist during resting states, task performance, and even sleep and unconscious states. As within-network functional connectivity appears to be largely preserved across cognitive states,113 it may not purely reflect current thoughts.15 Instead, the strength of correlation within intrinsic networks may be shaped by an intricate combination of factors such as prior life experience, genetic traits, current cognitive state, and other “intrinsic” neuronal processes.114
Resting state fMRI studies have reported correlations between individual off-task thought tendencies and DMN functional connectivity strength.115,116 Moreover, DMN connectivity abnormalities have been extensively reported within various populations in which mental health is impacted.107,117 Based on the widespread idea that DMN activation signifies off-task thought, authors have sometimes attributed connectivity abnormalities to disruptions in spontaneous thought or rumination that may arise at rest. Interestingly, distinct recurring brain states can be identified at rest,118 and the occurrence rate of a DMN-activation state was recently associated with individual differences in post-rest retrospective reports of unintentional intrusive thoughts about the past.119 However, to disentangle state (intra-individual) from trait (inter-individual) relationships, it is critical to examine experiential fluctuations within individuals.
To do so, studies have combined online experience-sampling with intra-individual analyses of pre-thought probe functional connectivity.92,105,110,115,120 These “dynamic functional connectivity”121 studies have so far focused almost exclusively on off-task thought. A complex set of findings have emerged, where intra-individual off-task thought has been associated with both increased and decreased within-DMN functional connectivity. Potentially reconciling those findings, recent work revealed both positive and negative correlations with off-task thought depending on the precise DMN subregions involved.92 Taken together with additional evidence linking DMN connectivity fluctuations with ongoing cognition and behavior, it is becoming clear that DMN dynamics reflect a complex combination of state factors (e.g. experience/thought, arousal and attentional fluctuations) and traits of individuals.123,124 However, as resting state functional connectivity studies in clinical populations do not typically include measures of online experience, it has been difficult to directly link alterations of spontaneous thought (or other state factors) to functional connectivity abnormalities. Further investigations that combine resting state paradigms, dynamic functional connectivity approaches, and online multi-dimensional experience-sampling may have an important role in clarifying the role of disrupted spontaneous thought in DMN connectivity abnormalities observed in mental illness.69,123
3.1.3: Subsystems.
While the DMN as a whole may support a domain-general function, there is a wealth of fMRI evidence for specialized subsystems.98,125–127 According to one neurocognitive model,128 a DMN subsystem anchored in dorsomedial prefrontal cortex may support high-level abstract and verbal forms of cognition such as mentalizing and language comprehension (a “mind’s mind” form of thought), whereas a subsystem anchored in the posterior MTL may support more contextually-specific and imagery-based forms of cognition such as episodic memory and episodic future thinking (a “mind’s eye” form of thought). Recent findings illustrate individual-level, precise delineation of DMN subsystems based on intrinsic functional connectivity129 and activation during deliberate self-generated thoughts with distinct content.130 A common finding is that subsystems can be distinguished based on coupling with MTL structures including the hippocampus.131 The MTL-anchored DMN subsystem has also been termed the “posterior-medial memory network”132 and is consistently implicated in episodic thought about the past and future.
Understanding the specific roles of DMN subsystems in thought content and dynamics is an active area of investigation. Regarding content, it is likely that subsystems are regularly activated spontaneously during the occurrence of similar categories of thought that have been mapped in task-activation studies. For example, a ventromedial prefrontal region that has been implicated in social cognition (based on task-evoked activation) also shows increased activation with off-task thought involving episodic social content (as detected with experience sampling).133 Indirect support also comes from studies of individual differences showing that functional connectivity of the MTL, but not dorsomedial prefrontal, subsystem is associated with daydreaming frequency115 and with retrospectively reported thoughts about the past and future.99 Moreover, greater cortical thickness of MTL regions within DMN has been associated with individual tendencies for higher level of detail in off-task thought (in both laboratory and real-world settings).65,134 In terms of thought dynamics, it has been hypothesized that the MTL subsystem initiates spontaneous thought during conditions when deliberate constraints are weak.14 This idea is supported by fMRI findings within mindfulness practitioners, where self-caught spontaneous thoughts were preceded by MTL activation.78 We further discuss the potential role of the MTL in initiating spontaneous thought in Section 3.3.
How might DMN subsystems shed light on the role of spontaneous thought in mental health? A promising avenue involves characterizing relationships with phenomenological experiences, beyond off-task thought. A recent fMRI study provides insight into the role of the MTL subsystem in the self-relevance and affective valence of spontaneous thought,80 features known to be important to long-term health.2,45,135 In a free-association thought sampling paradigm,136 participants generated conceptual associations that varied in self-relatedness and negative affectivity. Multivoxel pattern analyses revealed that the self-relevance and affective valence of concepts could be predicted from a variety of brain networks, including key contributions of MTL and other DMN regions.80 Such findings suggest that DMN subsystems provide insight into the neural bases of spontaneous phenomenological experiences that are closely tied to mental health.
3.1.4: Effects of Perturbation.
So far we have reviewed neuroimaging studies that offer correlational evidence. Recently, investigators have begun to explore the possible causal role of the DMN based on lesion studies as well as perturbation with tDCS and invasive stimulation to localized DMN regions (Box 2). Beyond being critical to establishing causal mechanisms of spontaneous thought, these studies have direct implications for neuromodulation therapies for mental health.
Box 2: Intracranial neuromodulation of the DMN and spontaneous thought.
Intracranial electrical stimulation203 is a neuromodulation technique that offers more focality compared to tDCS and is similar to deep brain stimulation approaches.204 A short pulse of high-frequency (50–100 Hz) intracranial stimulation, often applied for the clinical purpose of functional mapping in neurosurgical patients, can immediately change subjective experience or behavior. The change may involve a wide range of perceptual, motor, cognitive, and affective effects, including spontaneous vivid experiences of past memories.205 Recently, intracranial stimulation effects have been functionally mapped to large-scale intrinsic networks. Interestingly, the elicitation rate of changes in subjective experience or behavior was lower in the DMN (~20%) than in any other intrinsic network.206 This finding may seem surprising, but it is notable that the context of intracranial stimulation is typically comparable to a resting state, when internally-guided cognition may already be high, thus limiting the potential to elicit effects. When DMN stimulation effects are found, they often involve vivid and highly specific phenomenological experiences. In a striking recent example, stimulation of a DMN region within posteromedial cortex caused a patient to report a sense of observing their own thoughts, an experience comparable to self-dissociation in neuropsychiatric illness.207 Beyond immediate experiential effects, a recent study showed that intracranial DMN stimulation reduced the capacity to generate creative thoughts.208 Such findings provide preliminary insights into the potential short- and long-term impacts that invasive, deep brain stimulation therapies (e.g. for treatment-resistant psychiatric illness) may have on spontaneous thought patterns and their associated mental health outcomes.
Several investigators have applied tDCS to DMN regions and tested the short-term (presumably reversible) effects on frequency of off-task thought.137–139 However, these studies have so far yielded mixed findings that may depend on the targeted region, stimulation parameters, outcome measure, and other factors.20 Further research is needed to determine whether non-invasive DMN neuromodulation can reliably alter off-task thought frequency and whether effects may have clinical utility in conditions such as ADHD where off-task thought can occur excessively.140
Interestingly, both lesion and tDCS studies provide preliminary converging support for the causal importance of the MTL subsystem in the content of spontaneous thought. Patients with lesions to regions within this subsystem (ventromedial prefrontal cortex141 and hippocampus142) and with neurodegeneration affecting this subsystem122 report a reduced frequency of spontaneous thoughts about the past and future. In a recent study in healthy adults, tDCS to the posterior inferior parietal lobule, an MTL subsystem region, resulted in reduced frequency of thoughts about the past involving negative affect.143 Given the associations between past-related thought, negative affect and rumination,55,56 these findings preliminarily suggest that targeting the MTL subsystem may improve mood via altering the temporal focus of spontaneous thought.
3.2: Large-Scale Network Dynamics Beyond the DMN
Despite the field’s emphasis on the DMN, it is now widely appreciated that multiple other large-scale brain networks support the diverse content and dynamic qualities of spontaneous and off-task thought. In this section, we provide brief updates on networks beyond DMN, focusing primarily on the frontoparietal control (FPCN) and salience networks. We then highlight select, novel findings that have mapped network dynamics to features of spontaneous thought that are important to mental health.
The FPCN, a network that interacts closely with both the DMN and externally-oriented systems,144 has repeatedly been implicated in off-task thought.14,20 The FPCN is hypothesized to impose deliberate constraints and to cooperate with the DMN to control the course of thought.14,145 Recent tDCS studies have shown both increases and decreases in off-task thought after stimulation of a dorsolateral prefrontal area within the FPCN.146,147 Despite diverging results that may have depended on task context,20 these studies hint that modulation of the FPCN may alter deliberate constraints on thought, which can be beneficial in contexts where it is desirable to increase cognitive control and reduce off-task thought.
The salience network is often engaged when orienting attention toward salient stimuli or mental events148 and is hypothesized to impose automatic constraints on thought.14 Cortical areas of the salience network (e.g. anterior insula, mid-cingulate cortex) are tightly associated with subcortical arousal and noradrenergic systems.149,150 Though the salience network is not usually activated during off-task thought in fMRI studies (though see79), future studies are needed to determine its relevance for thought content and dynamics. Resting state fMRI combined with separately conducted experience-sampling in real-world settings have shown that individual tendencies to engage in negative thinking are associated with functional connectivity between the salience network, FPCN and DMN.151,152 These findings may be consistent with the salience network participating in thoughts with strong automatic constraints and arousal qualities, such as rumination and worry.
Various other large-scale networks appear to be involved in spontaneous thought content and dynamics. Sensory and motor networks have been linked to processes such as internally-generated imagery87 and perceptual decoupling.153 Within the context of reading, one study revealed that individuals who had more frequent off-task thoughts displayed reduced resting state functional connectivity between the DMN and early visual regions (potentially supporting perceptual decoupling during reading).154 The dorsal attention network (DAN) may compete with the DMN to support the movement of attention between spontaneous thought and external stimuli.14 Experience sampling across multiple task conditions has revealed that areas of the DAN (in parietal cortex) are more activated for on-task relative to off-task thought regardless of context.64 Subcortical systems that control ascending neuromodulatory signals likely contribute to the regulation and flow of spontaneous thought.16
Overall, spontaneous and off-task thoughts involve complex, dynamic orchestration of activity across distinct networks at the whole-brain level. These findings have motivated recent applications of data-driven, predictive modeling analyses155 that take into account a rich variety of brain network features. In recent work,92 connectome-based predictive modeling156 was combined with fMRI and online experience-sampling. In healthy adults, a pattern of whole-brain functional connectivity predicted trial-by-trial off-task thought and involved interactions within and between virtually all intrinsic networks to some degree (Figure 3a). A prominent feature was decreased anticorrelation between the DMN and FPCN during off-task thought, a finding in line with research implicating DMN-FPCN interactions in controlling the course of thought.145 A second prominent feature was decreased coupling between DMN and sensorimotor regions, a process that may underlie perceptual decoupling.153 Importantly, the network pattern also predicted off-task thought in adults with ADHD. These patients reported a heightened frequency of off-task thought and over-expressed the network pattern. As excessive off-task thought is strongly associated with adverse clinical outcomes in ADHD,140 these findings give clues into complex network interactions that could be considered as therapeutic targets.
Figure 3: Large-scale brain network predictive models of spontaneous fluctuations in experience sampling ratings.
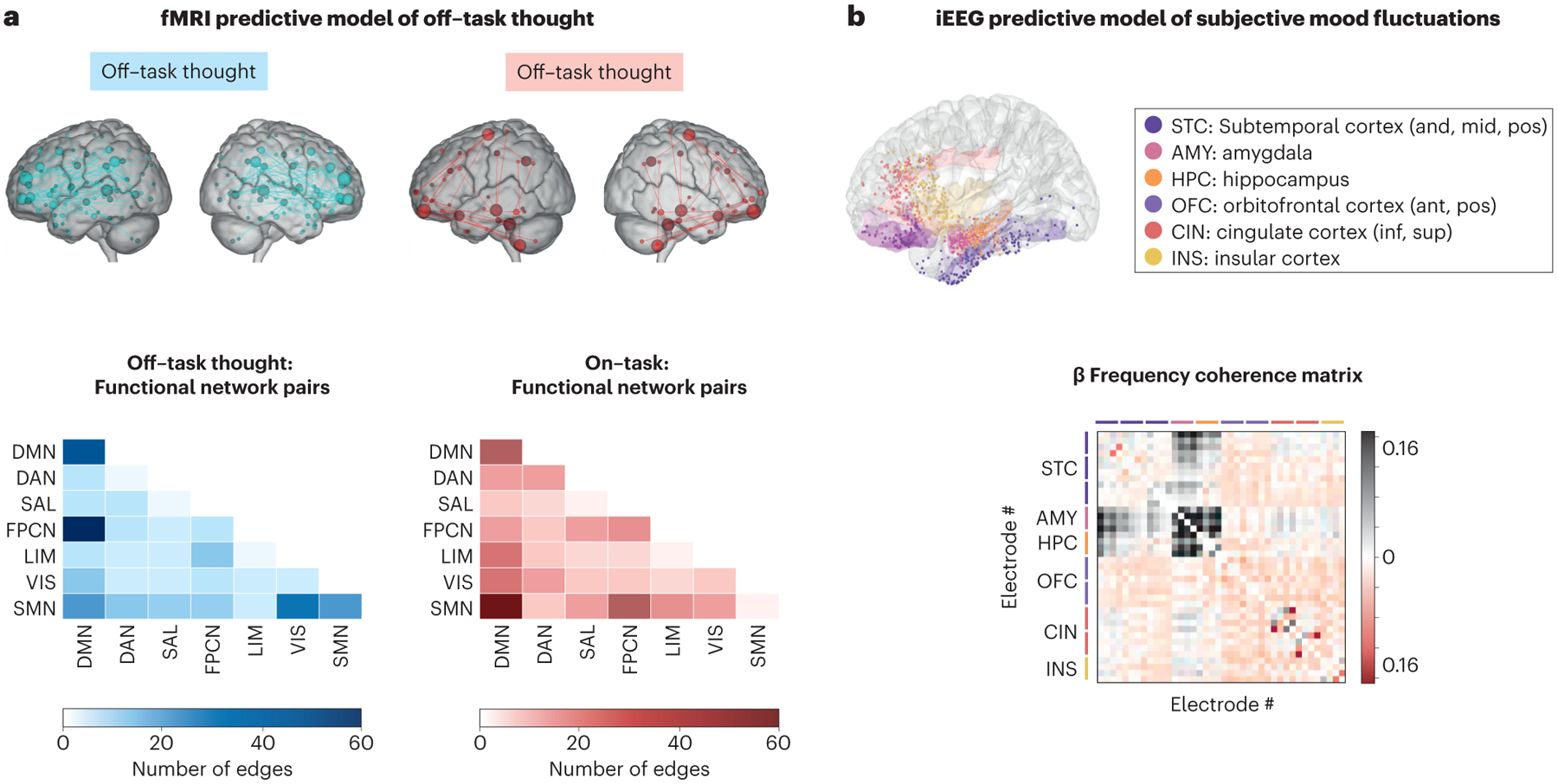
(a) (Top) Connectivity features of an fMRI-based functional network model that is predictive of intra-subject off-task thought and on-task attention. (Bottom) The number of edges (pairs of regions) between multiple intrinsic networks that contribute to the model (reproduced with modification from.92). (b) (Top) Implanted electrode locations and neuroanatomical regions involved in an iEEG-based functional network model that is predictive of subjective mood fluctuations. (Bottom) Connectivity features (beta-frequency coherence matrix) within a subnetwork involving strong amygdala-hippocampus coupling that is predictive of intra-subject mood ratings (reproduced with modification from157).
Most studies examining network dynamics and spontaneous thought have used fMRI, which has limited temporal resolution, indirectly measures brain activity, and is usually limited to short sessions. To overcome these limitations, one study combined experience-sampling with intracranial EEG (iEEG).157 Patients with semi-chronic, implanted electrodes (within limited cortical and subcortical regions) were asked to rate task-free fluctuations in subjective mood over several days. Based on inter-electrode temporal coherence, multiple intrinsic functional networks were identified involving the amygdala, hippocampus, and salience network regions (e.g. insula, cingulate) (Figure 3b). Self-report mood fluctuations were predicted from the variability of coherence in the beta (13–30 Hz) frequency range within a network involving amygdala-hippocampus coupling.157 This pattern may be indicative of interactions between the salience network (amygdala150) and DMN (hippocampus131). These iEEG findings provide strong neurophysiological evidence for the relevance of network dynamics to fluctuations in subjective experience, highlighting the role of synchronized oscillatory patterns that have been more broadly linked to cognition158 but have been rarely explored in relation to spontaneous and off-task thought. Extending the findings, the iEEG experience-sampling approach was recently applied in treatment-resistant depression for the purpose of identifying a deep brain stimulation target.93 This novel direction highlights how mapping large-scale network dynamics to spontaneous thought may provide clinical biomarkers to guide treatment.
3.3: Hippocampal Sharp-Wave Ripples and Replay
The field has likely focused extensively on large-scale brain networks because their function is readily detected with current human neuroscience methods. However, certain brain events that have been observed in nonhuman animals display properties that may be consistent with the occurrence of spontaneous or off-task thought. One neurophysiological event that is receiving growing attention is the so-called sharp-wave ripple (SWR).16,21,159 The SWR is an electrophysiological event, generated from highly synchronous neuronal activity, that lasts <150 ms and occurs spontaneously in the hippocampus within the MTL. Information from SWRs is transmitted widely to cortical and subcortical targets. Micro-scale local field potential signals are typically used to detect hippocampal SWRs, but recently, putative SWRs have been documented at coarser spatial scales in the human brain using iEEG160,161 and magnetoencephalography (MEG).162
There are several reasons to suspect that hippocampal SWRs have a mechanistic role in spontaneous thought. First, SWRs are observed selectively during “offline” periods such as wakeful rest (e.g. when animals are remaining still) when external constraints on thought are low.163 Second, SWRs coincide with extrahippocampal neuronal firing sequences that replay past experiences (reverse replay) or construct potential future scenarios (forward replay).164 Combinations of these sequences could support unconstrained semantic associations that are characteristic of spontaneous thought.21 Spontaneous memory reactivations have also been identified in hippocampus and cortex in human resting state fMRI165 and MEG.162 Third, hippocampal SWRs166,167 and replay events168 occur in concert with activation of the DMN, the large-scale network most frequently associated with spontaneous thought.169
Despite these theoretical considerations, a direct link between SWRs and spontaneous thought has not yet been firmly established. While it is possible that SWRs are directly involved in conscious experience, an alternative is that they support preconscious contemplations that influence subsequent experiential phenomena.163 As SWRs have largely been studied in animal models, it has been difficult to draw conclusions about relationships with mental experience. Notably, intracranial electrical stimulation of the hippocampus and nearby white matter tracts in neurosurgical patients often evokes spontaneous vivid experiences of past events133 However, it remains unknown whether such stimulation influences SWRs. A recent iEEG study showed that SWRs increased in frequency prior to spontaneous, conscious recall of previously viewed images,161 providing correlative evidence for involvement in mental experience.
Several authors have speculated that spontaneous hippocampal SWRs are involved in adaptive forms of thought, such as creative thinking and problem-solving.21,159 During SWRs, replay-related hippocampal and cortical sequences can generate new combination ‘strings’ of sequences.162 When conditions promote spontaneous and off-task thought (e.g. rest), the continuous stitching together of different sequence combinations provides a potential basis for abstract thinking, novel ideas, or sudden insights.159
On the flip side, SWRs and associated replay may lead to disruptive forms of thought in conditions affecting mental health. An MEG study showed that patients with schizophrenia, relative to control subjects, showed a reduction in spontaneous neural replay and elevated (potentially compensatory) SWR power.171 Such distortions in resting state brain activity, which may relate to DMN abnormalities in schizophrenia,107 could be associated with experiences such as disorganized or delusional thinking. Additionally, it has been hypothesized that disrupted SWRs and replay play a role in disrupted thought in depression and anxiety; sustained SWRs that drive forward and reverse replay, respectively, could be the bases of uncontrollable worrying and ruminative thoughts.172 Overall, SWRs may play key roles in both adaptive and maladaptive thought, and there is need to further characterize these events and their interplay with cortical networks during experience.
3.4: Ascending Neuromodulatory Systems.
Ascending neuromodulatory systems—such as the cholinergic, noradrenergic, and serotonergic systems—originate in brainstem or subcortical nuclei and send broad projections to cortical networks. These systems, and their complex interactions with one another, strongly influence the neural phenomena described here (DMN activity, large-scale network dynamics, hippocampal SWRs).173 Ongoing fluctuations within neuromodulatory systems regulate many aspects of behavior and cognition and have a vital role in mental health. Pharmacological agents that target these systems are regularly used in treatment of mental illness, yet little is known about the role that neuromodulators play in spontaneous and off-task thought. In this section, we discuss two systems where knowledge is beginning to accumulate: the noradrenergic and serotonergic systems. Other systems (e.g. cholinergic, dopaminergic) that have been theoretically implicated in spontaneous thought but require empirical study are beyond the scope of this review (see16 for an excellent overview).
3.4.1: Noradrenergic System.
The noradrenergic system originates in the locus coeruleus, projects diffusely to cortical regions, and has a major role in the regulation of global brain states to control vigilance and arousal. Tonic and phasic fluctuations in noradrenaline levels, respectively, refer to slow and fast changes that mediate separate functions.174 The size of the pupil is often used as a proxy for noradrenergic activity level, which is supported by neurophysiological and neurostimulation evidence.175,176 However, this relationship between pupil size and noradrenergic activity is somewhat variable over time.177 Other systems (e.g. cholinergic) are also associated with pupil size.176 As such, findings from studies examining pupil size in relation to spontaneous thought should be interpretated as preliminary regarding involvement of the noradrenergic system.
During off-task thought, tonic pupil size was found to be both elevated and reduced in different studies.120,178,179 These findings have been interpreted with reference to the inverted U-shaped relationship that noradrenergic activity has with task performance: too much (high arousal) or too little (low arousal) activity can impair behavior.174 One suggested possibility is that low levels of noradrenaline, associated with low arousal and increased SWRs,173 promote freely-moving and transitory thoughts (mind-wandering, according to the DFT14) while high levels promote disjointed thoughts with reduced awareness (that have been referred to as ‘mind blanking’).16,179 In contrast to tonic pupil size, phasic responses to stimuli have consistently been found to decrease during off-task thought120,180 (though see110). This may reflect disengagement of arousal responses to external stimuli related to perceptual decoupling. However, phasic pupil dilation, which occurs regularly during wakeful rest, could reflect engagement with inner thoughts. A recent iEEG study showed that spontaneous pupil dilations (resembling task-evoked, phasic dilations) were consistently preceded by neuronal population activations in the anterior insula within salience network.181 A possibility that could be explored is that coupling between the noradrenergic system and salience network may impose automatic constraints during worrying and ruminative thoughts.
Taken together, pupillometric studies tentatively suggest involvement of the noradrenergic system in spontaneous thought. These findings have important implications for the use of stimulant medications that are commonly used to treat mental illness (e.g. ADHD, depression) and include the noradrenergic system as a central target. Further research may clarify how pharmacological manipulation alters spontaneous and off-task thought to impact mental health.
3.4.2: Serotonergic System.
The serotonergic system originates in the raphe nuclei and, like the noradrenergic system, projects diffusely to most cortical regions. This system acts on a diverse set of receptor subtypes, enabling precise neuromodulation that depends on the target neuronal population. In psychiatric practice, the serotonergic system has long been a target of pharmacotherapies that aim to improve mood (e.g. selective serotonin reuptake inhibitors). Also acting upon the serotonergic system are psychedelic agents—including psilocybin, lysergic acid diethylamide (LSD), and dimethyltryptamine—that can have profound, short-term effects on subjective experience as well as potentially longer-lasting therapeutic effects for a variety of mental illnesses.182 There has been a recent resurgence of scientific and clinical interest in psychedelics. Because psychedelics impact spontaneous thought in an acute and unique manner, we focus here on insights from these agents as a lens into the role of the serotonergic system.
Although psychedelic agents do not act exclusively on the serotonergic system, their agonist action upon the serotonin receptor subclass 5HT2A has a key role in generating experiential effects.183 The psychedelic experience, which may last between ~30 minutes and 12 hours, involves vivid alterations in perception and feelings that take on many variations but often involve hyperassociative and discontinuous thought, visual imagery/hallucination, intense emotion, and an altered or diminished sense of self (“ego dissolution”).184,185 Given these features, the psychedelic state was recently incorporated into the DFT as a form of spontaneous thought with lower deliberate and automatic constraints than mind-wandering, creative thinking, and dreaming.c This characterization situates the psychedelic state as the most unconstrained and least ruminative known version of thought; thus, understanding the role of the serotonergic system in this state provides a potential unique window into neural basis of spontaneous thought.
How psychedelic action at the 5HT2A receptor leads to subjective effects remains unclear and an active area of investigation.186 Recently, resting state neuroimaging has been used to examine the acute effects of psilocybin and LSD. Though multiple neural metrics have been examined and are difficult to compare across studies, one consistent finding is that that LSD reduces functional connectivity between the thalamus and sensory cortical regions,187,188 a phenomenon that may be associated with perceptual decoupling.153 Psilocybin and LSD have also been shown to decrease functional connectivity within the DMN,183,185,189 which may be linked to subjective experiences of creative insights.190 Given the role of the DMN in self-referential thought, this effect has also been attributed to ego dissolution. However, decreased functional connectivity within the salience network191 and increased coupling between regions rich in 5HT2A receptors192 have also been associated with individual differences in psilocybin-induced ego dissolution. Critically, these resting state studies did not examine intra-individual variability in spontaneous experiences within the psychedelic state. Future studies involving psychedelics, neuroimaging, and experience-sampling may shed light on the mechanistic role of the serotonergic system in functions such as enhancing the spontaneity of thought, reducing rumination, and exerting long-term benefits to mental health.
4.0: Conclusions and Future Directions
In this review, we have highlighted some of the diverse approaches and findings emerging from the quickly growing body of research on the neuroscience of spontaneous and off-task thought. We explored novel findings that build on the well-studied role of the DMN and large-scale network dynamics. We further discussed the potential roles of less-studied phenomena: hippocampal SWRs, memory replay, and neuromodulatory activity in noradrenergic and serotonergic systems. We surveyed how this knowledge may advance our understanding of mental health and lead to clinical applications. In this final section, we spotlight a few methodological directions that may clarify mechanisms, lead to reliable biomarkers, and facilitate development of personalized therapies toward the goals of better understanding and improving mental health.
Studying spontaneous and off-task thoughts and their neural mechanisms presents some unique challenges. Most standard experimental paradigms rely on external perceptual or task-based manipulations that cannot typically be used to elicit and precisely measure these thoughts (though see82–84 for examples of how such manipulations can indirectly influence involuntary thoughts). The introduction of experience-sampling as a novel technique has led to important advances, and additional new approaches could yield further improvements in our ability to study thought dynamics. Recently, paradigms involving free-flowing descriptions of thoughts have been applied to characterize the dynamic trajectories of conceptual associations.136,193–195 Moreover, the way that thoughts change over time can be altered in conditions affecting mental health. Semantic network analyses revealed that individuals high in rumination frequently became ‘stuck’ on negative, self-referential topics.136 The recent integration of descriptive experience-sampling,196 free association,80 and think aloud paradigms81 with fMRI highlight the potential of these approaches to offer further insights into altered neural and thought dynamics affecting mental health.
Innovations are also needed to improve efficiency of discovering brain-experience relationships. Online experience-sampling neuroimaging studies treat experience and brain activity, respectively, as independent and dependent variables. In an alternative, brain-triggered experience-sampling paradigm, neural activity is analyzed in real time, and experience-sampling moments are initiated when a pre-specified event of interest (e.g. SWR, DMN activation) occurs. Compared to the standard approach of sampling experience at random intervals, this approach could enhance efficiency because sampling occurs selectively when a pre-hypothesized phenomenological experience is arising.69 In one application, real-time analysis detected a pre-specified EEG pattern that was predictive of dream contents.197 It was also suggested that real-time analyses could shed light on whether spontaneous memory reactivations are consciously experienced.198 In clinical applications employing experience-sampling to identify neuromodulation targets, brain-triggered experience-sampling could reduce the time that a patient needs to be monitored (10 days of iEEG recording in a recent case report93).
Finally, it is critical to note that research on the neural basis of spontaneous and off-task thought has almost exclusively focused on groups of individuals. Brain mechanisms giving rise to self-labelled experiences may vary across and even within an individual across contexts. Thus, personally-derived neural markers may allow researchers to test, rather than assume, generalizability across individuals and instances. This issue is critical in the context of mental illness, where thought phenomenology can deviate substantially from the typical pattern. Recent findings illustrate that idiographic, rather than group-level, fMRI predictive models are needed to predict affective valence when spontaneous thoughts have high self-relevance. Moreover, dense, individual-level, online experience-sampling with fMRI recently revealed individual-specific neural patterns predictive of spontaneous sensory imagery.87 Building on these findings, future studies may derive personalized biomarkers of spontaneous and off-task thought that could aid in guiding brain-based therapies (e.g. neurostimulation, neurofeedback).
These future directions highlight just a few ways in which research on spontaneous and off-task thought may be leveraged to guide assessments and treatments for mental health. These directions also highlight that the field is still in its infancy, and as such there remains enormous potential for research and clinical practice.
Acknowledgements
This work was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers R21MH127384 (to AK), R21MH129630 (to AK and SWG) and R01MH125414 (JAH). JWYK was supported by the Natural Sciences and Engineering Research Council Discovery Grant.
Footnotes
Code Availability Statement
The code that was used to generate Figure 1 is available at https://github.com/DynamicBrainMind/NatMentHealth2023.
Competing Interest Statement
The authors declare no competing interests.
Data Availability Statement
The dataset that was generated for Figure 1 is available at https://github.com/DynamicBrainMind/NatMentHealth2023.
References
- 1.Kane MJ et al. For Whom the Mind Wanders, and When: An Experience-Sampling Study of Working Memory and Executive Control in Daily Life. Psychol Sci 18, 614–621 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Killingsworth MA & Gilbert DT A Wandering Mind Is an Unhappy Mind. Science 330, 932–932 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Klinger E & Cox WM Dimensions of Thought Flow in Everyday Life. Imagination, Cognition and Personality 7, 105–128 (1987). [Google Scholar]
- 4.Kane MJ et al. For Whom the Mind Wanders, and When, Varies Across Laboratory and Daily-Life Settings. Psychol Sci 28, 1271–1289 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seli P et al. How pervasive is mind wandering, really?,. Consciousness and Cognition 66, 74–78 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Baird B et al. Inspired by Distraction: Mind Wandering Facilitates Creative Incubation. Psychol Sci 23, 1117–1122 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Baird B, Smallwood J & Schooler JW Back to the future: autobiographical planning and the functionality of mind-wandering. Conscious Cogn 20, 1604–1611 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Smallwood J & Andrews-Hanna J Not all minds that wander are lost: the importance of a balanced perspective on the mind-wandering state. Frontiers in Psychology 4, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer KM, Lieberman A, Sever AC & Joiner T Prevalence rates of anxiety, depressive, and eating pathology symptoms between the pre- and peri-COVID-19 eras: A meta-analysis. Journal of Affective Disorders 298, 364–372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhdanava M et al. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J Clin Psychiatry 82, 20m13699 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Bozhilova NS, Michelini G, Kuntsi J & Asherson P Mind wandering perspective on attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev 92, 464–476 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaieb L, Hoppe C & Fell J Mind wandering and depression: A status report. Neurosci Biobehav Rev 133, 104505 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Marchetti I, Koster EHW, Klinger E & Alloy LB Spontaneous Thought and Vulnerability to Mood Disorders: The Dark Side of the Wandering Mind. Clinical Psychological Science 4, 835–857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christoff K, Irving ZC, Fox KCR, Spreng RN & Andrews-Hanna JR Mind-wandering as spontaneous thought: a dynamic framework. Nature Reviews Neuroscience 17, 718–731 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Kucyi A Just a thought: How mind-wandering is represented in dynamic brain connectivity. NeuroImage 180, 505–514 (2018). [DOI] [PubMed] [Google Scholar]
- 16.O’Callaghan C, Walpola IC & Shine JM Neuromodulation of the mind-wandering brain state: the interaction between neuromodulatory tone, sharp wave-ripples and spontaneous thought. Philos Trans R Soc Lond B Biol Sci 376, 20190699 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smallwood J et al. The neural correlates of ongoing conscious thought. iScience 24, 102132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittner M, Hawkins GE, Boekel W & Forstmann BU A Neural Model of Mind Wandering. Trends in Cognitive Sciences 20, 570–578 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Girn M, Mills C, Roseman L, Carhart-Harris RL & Christoff K Updating the dynamic framework of thought: Creativity and psychedelics. Neuroimage 213, 116726 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Kam JWY, Mittner M & Knight RT Mind-wandering: mechanistic insights from lesion, tDCS, and iEEG. Trends Cogn Sci 26, 268–282 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mildner JN & Tamir DI Spontaneous Thought as an Unconstrained Memory Process. Trends in Neurosciences 42, 763–777 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Fox KCR, Andrews-Hanna JR & Christoff K The neurobiology of self-generated thought from cells to systems: Integrating evidence from lesion studies, human intracranial electrophysiology, neurochemistry, and neuroendocrinology. Neuroscience 335, 134–150 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Fox KCR & Christoff K The Oxford Handbook of Spontaneous Thought: Mind-wandering, Creativity, and Dreaming. (Oxford University Press, 2018). [Google Scholar]
- 24.DuPre E & Spreng RN Rumination Is a Sticky Form of Spontaneous Thought. in The Oxford Handbook of Spontaneous Thought (eds. Fox KCR & Christoff K) (Oxford University Press, 2018). [Google Scholar]
- 25.Seli P et al. Mind-Wandering as a Natural Kind: A Family-Resemblances View. Trends in Cognitive Sciences 22, 479–490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzinger T Why Is Mind-Wandering Interesting for Philosophers? in The Oxford Handbook of Spontaneous Thought (eds. Fox KCR & Christoff K) (Oxford University Press, 2018). [Google Scholar]
- 27.Maillet D & Schacter DL When the mind wanders: Distinguishing stimulus-dependent from stimulus-independent thoughts during incidental encoding in young and older adults. Psychol Aging 31, 370–379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babo-Rebelo M, Richter CG & Tallon-Baudry C Neural Responses to Heartbeats in the Default Network Encode the Self in Spontaneous Thoughts. J Neurosci 36, 7829–7840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avitan L & Stringer C Not so spontaneous: Multi-dimensional representations of behaviors and context in sensory areas. Neuron 110, 3064–3075 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Smallwood J Distinguishing how from why the mind wanders: a process-occurrence framework for self-generated mental activity. Psychol Bull 139, 519–535 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Mills C, Raffaelli Q, Irving ZC, Stan D & Christoff K Is an off-task mind a freely-moving mind? Examining the relationship between different dimensions of thought. Conscious Cogn 58, 20–33 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Mills C, Porter AR, Andrews-Hanna JR, Christoff K & Colby A How task-unrelated and freely moving thought relate to affect: Evidence for dissociable patterns in everyday life. Emotion 21, 1029–1040 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Turnbull A et al. Reductions in task positive neural systems occur with the passage of time and are associated with changes in ongoing thought. Sci Rep 10, 9912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christoff K et al. Mind-Wandering as a Scientific Concept: Cutting through the Definitional Haze. Trends Cogn Sci 22, 957–959 (2018). [DOI] [PubMed] [Google Scholar]
- 35.McVay JC & Kane MJ Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008). Psychol Bull 136, 188–197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mace JH Involuntary autobiographical memory chains: implications for autobiographical memory organization. Front Psychiatry 5, 183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McVay JC & Kane MJ Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. J Exp Psychol Learn Mem Cogn 35, 196–204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stawarczyk D, Majerus S, Maj M, Van der Linden M & D’Argembeau A Mind-wandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychologica 136, 370–381 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Smallwood JM, Baracaia SF, Lowe M & Obonsawin M Task unrelated thought whilst encoding information. Conscious Cogn 12, 452–484 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Smallwood J, Fishman DJ & Schooler JW Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance. Psychonomic Bulletin & Review 14, 230–236 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Smallwood J Mind-wandering While Reading: Attentional Decoupling, Mindless Reading and the Cascade Model of Inattention. Language and Linguistics Compass 5, 63–77 (2011). [Google Scholar]
- 42.Galéra C et al. Mind wandering and driving: responsibility case-control study. BMJ 345, e8105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robison MK, Gath KI & Unsworth N The neurotic wandering mind: An individual differences investigation of neuroticism, mind-wandering, and executive control. Q J Exp Psychol (Hove) 70, 649–663 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Seli P, Risko EF, Purdon C & Smilek D Intrusive thoughts: linking spontaneous mind wandering and OCD symptomatology. Psychological Research 2, 392–398 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Smallwood J, Fitzgerald A, Miles LK & Phillips LH Shifting moods, wandering minds: negative moods lead the mind to wander. Emotion 9, 271–276 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Engert V, Smallwood J & Singer T Mind your thoughts: associations between self-generated thoughts and stress-induced and baseline levels of cortisol and alpha-amylase. Biol Psychol 103, 283–291 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Cárdenas-Egúsquiza AL & Berntsen D Sleeping poorly is robustly associated with a tendency to engage in spontaneous waking thought. Conscious Cogn 105, 103401 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann F, Banzhaf C, Kanske P, Bermpohl F & Singer T Where the depressed mind wanders: Self-generated thought patterns as assessed through experience sampling as a state marker of depression. J Affect Disord 198, 127–134 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Smith AC, Brosowsky NP, Caron EE, Seli P & Smilek D Examining the relation between mind wandering and unhealthy eating behaviours. Personality and Individual Differences 200, 111908 (2023). [Google Scholar]
- 50.Seli P, Smallwood J, Cheyne JA & Smilek D On the relation of mind wandering and ADHD symptomatology. Psychon Bull Rev 22, 629–636 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Vatansever D, Bozhilova NS, Asherson P & Smallwood J The devil is in the detail: exploring the intrinsic neural mechanisms that link attention-deficit/hyperactivity disorder symptomatology to ongoing cognition. Psychol Med 49, 1185–1194 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Fox KCR et al. Affective neuroscience of self-generated thought. Ann N Y Acad Sci (2018) doi: 10.1111/nyas.13740. [DOI] [PubMed] [Google Scholar]
- 53.Franklin MS et al. The silver lining of a mind in the clouds: interesting musings are associated with positive mood while mind-wandering. Front Psychol 4, 583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welz A, Reinhard I, Alpers GW & Kuehner C Happy Thoughts: Mind Wandering Affects Mood in Daily Life. Mindfulness 9, 332–343 (2018). [Google Scholar]
- 55.Smallwood J & O’Connor RC Imprisoned by the past: unhappy moods lead to a retrospective bias to mind wandering. Cogn Emot 25, 1481–1490 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Ruby FJM, Smallwood J, Engen H & Singer T How Self-Generated Thought Shapes Mood—The Relation between Mind-Wandering and Mood Depends on the Socio-Temporal Content of Thoughts. PLOS ONE 8, e77554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb CA et al. Spontaneous thought characteristics are differentially related to heightened negative affect versus blunted positive affect in adolescents: An experience sampling study. JCPP Advances n/a, e12110 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stawarczyk D Phenomenological properties of mind-wandering and daydreaming: A historical overview and functional correlates. in The Oxford Handbook of Spontaneous Thought (eds. Fox KCR & Christoff K) (Oxford University Press, 2018). [Google Scholar]
- 59.Mildner JN & Tamir DI The people around you are inside your head: Social context shapes spontaneous thought. J Exp Psychol Gen 150, 2375–2386 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Ji JL, Holmes EA, MacLeod C & Murphy FC Spontaneous cognition in dysphoria: reduced positive bias in imagining the future. Psychol Res 83, 817–831 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tulving E Multiple memory systems and consciousness. Hum Neurobiol 6, 67–80 (1987). [PubMed] [Google Scholar]
- 62.Gable SL, Hopper EA & Schooler JW When the Muses Strike: Creative Ideas of Physicists and Writers Routinely Occur During Mind Wandering. Psychol Sci 30, 396–404 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Thiemann RF, Mills C & Kam JWY Differential relationships between thought dimensions and momentary affect in daily life. Psychological Research (2022) doi: 10.1007/s00426-022-01766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turnbull A et al. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nature Communications 10, 3816 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ho NSP et al. Facing up to the wandering mind: Patterns of off-task laboratory thought are associated with stronger neural recruitment of right fusiform cortex while processing facial stimuli. Neuroimage 214, 116765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gusnard DA, Raichle ME & Raichle ME Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2, 685–694 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Ingvar DH ‘Hyperfrontal’ distribution of the cerebral grey matter flow in resting wakefulness; on the functional anatomy of the conscious state. Acta Neurol Scand 60, 12–25 (1979). [DOI] [PubMed] [Google Scholar]
- 68.Andreasen NC et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152, 1576–1585 (1995). [DOI] [PubMed] [Google Scholar]
- 69.Gonzalez-Castillo J, Kam JWY, Hoy CW & Bandettini PA How to Interpret Resting-State fMRI: Ask Your Participants. J Neurosci 41, 1130–1141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGuire PK, Paulesu E, Frackowiak RS & Frith CD Brain activity during stimulus independent thought. Neuroreport 7, 2095–2099 (1996). [PubMed] [Google Scholar]
- 71.Binder JR et al. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11, 80–95 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Mason MF et al. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science 315, 393–395 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christoff K, Gordon AM, Smallwood J, Smith R & Schooler JW Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. PNAS 106, 8719–8724 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lehmann D, Henggeler B, Koukkou M & Michel CM Source localization of brain electric field frequency bands during conscious, spontaneous, visual imagery and abstract thought. Cognitive Brain Research 1, 203–210 (1993). [DOI] [PubMed] [Google Scholar]
- 75.Weinstein Y, De Lima HJ & van der Zee T Are you mind-wandering, or is your mind on task? The effect of probe framing on mind-wandering reports. Psychon Bull Rev 25, 754–760 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Jordão M, Ferreira-Santos F, Pinho MS & St Jacques PL Meta-analysis of aging effects in mind wandering: Methodological and sociodemographic factors. Psychol Aging 34, 531–544 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Martinon LM, Smallwood J, McGann D, Hamilton C & Riby LM The disentanglement of the neural and experiential complexity of self-generated thoughts: A users guide to combining experience sampling with neuroimaging data. Neuroimage 192, 15–25 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Ellamil M et al. Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage 136, 186–196 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Hasenkamp W, Wilson-Mendenhall CD, Duncan E & Barsalou LW Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 59, 750–760 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Kim B, Andrews-Hanna JR, Han J, Lee E & Woo C-W When self comes to a wandering mind: Brain representations and dynamics of self-generated concepts in spontaneous thought. Sci Adv 8, eabn8616 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H-X et al. Neural representations of self-generated thought during think-aloud fMRI. Neuroimage 265, 119775 (2022). [DOI] [PubMed] [Google Scholar]
- 82.Berntsen D, Staugaard SR & Sørensen LMT Why am I remembering this now? Predicting the occurrence of involuntary (spontaneous) episodic memories. Journal of Experimental Psychology: General 142, 426–444 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Hall SA, Brodar KE, LaBar KS, Berntsen D & Rubin DC Neural responses to emotional involuntary memories in posttraumatic stress disorder: Differences in timing and activity. Neuroimage Clin 19, 793–804 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schlagman S & Kvavilashvili L Involuntary autobiographical memories in and outside the laboratory: how different are they from voluntary autobiographical memories? Mem Cognit 36, 920–932 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Tusche A, Smallwood J, Bernhardt BC & Singer T Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. Neuroimage 97, 107–116 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Sormaz M et al. Default mode network can support the level of detail in experience during active task states. PNAS 115, 9318–9323 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hung S-M & Hsieh P-J Mind wandering in sensory cortices. Neuroimage: Reports 2, 100073 (2022). [Google Scholar]
- 88.Kam JWY et al. Distinct electrophysiological signatures of task-unrelated and dynamic thoughts. Proc Natl Acad Sci U S A 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andrillon T, Burns A, Mackay T, Windt J & Tsuchiya N Predicting lapses of attention with sleep-like slow waves. Nat Commun 12, 3657 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mortaheb S et al. Mind blanking is a distinct mental state linked to a recurrent brain profile of globally positive connectivity during ongoing mentation. Proc Natl Acad Sci U S A 119, e2200511119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bozhilova N, Kuntsi J, Rubia K, Asherson P & Michelini G Event-related brain dynamics during mind wandering in attention-deficit/hyperactivity disorder: An experience-sampling approach. Neuroimage Clin 35, 103068 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kucyi A et al. Prediction of stimulus-independent and task-unrelated thought from functional brain networks. Nature Communications 12, 1793 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scangos KW et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med (2021) doi: 10.1038/s41591-021-01480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomescu MI et al. Spontaneous thought and microstate activity modulation by social imitation. NeuroImage 249, 118878 (2022). [DOI] [PubMed] [Google Scholar]
- 95.Shulman GL et al. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci 9, 648–663 (1997). [DOI] [PubMed] [Google Scholar]
- 96.Raichle ME et al. A default mode of brain function. Proc Natl Acad Sci U S A 98, 676–682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greicius MD, Krasnow B, Reiss AL & Menon V Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100, 253–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andrews-Hanna JR, Smallwood J & Spreng RN The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316, 29–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andrews-Hanna JR, Reidler JS, Huang C & Buckner RL Evidence for the Default Network’s Role in Spontaneous Cognition. Journal of Neurophysiology 104, 322–335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fox MD et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102, 9673–9678 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dixon ML et al. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage 147, 632–649 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Kucyi A et al. Electrophysiological dynamics of antagonistic brain networks reflect attentional fluctuations. Nat Commun 11, 325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buckner RL, Andrews-Hanna JR & Schacter DL The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Stawarczyk D, Majerus S, Maquet P & D’Argembeau A Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One 6, e16997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kucyi A, Salomons TV & Davis KD Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. PNAS 110, 18692–18697 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sonuga-Barke EJS & Castellanos FX Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 31, 977–986 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Whitfield-Gabrieli S & Ford JM Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8, 49–76 (2012). [DOI] [PubMed] [Google Scholar]
- 108.Beaty RE, Benedek M, Silvia PJ & Schacter DL Creative Cognition and Brain Network Dynamics. Trends Cogn Sci 20, 87–95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kucyi A, Esterman M, Riley CS & Valera EM Spontaneous default network activity reflects behavioral variability independent of mind-wandering. PNAS 113, 13899–13904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Groot JM et al. Probing the neural signature of mind wandering with simultaneous fMRI-EEG and pupillometry. NeuroImage 224, 117412 (2021). [DOI] [PubMed] [Google Scholar]
- 111.Murphy C et al. Distant from input: Evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. Neuroimage 171, 393–401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Konishi M, McLaren DG, Engen H & Smallwood J Shaped by the Past: The Default Mode Network Supports Cognition that Is Independent of Immediate Perceptual Input. PLoS One 10, e0132209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gratton C et al. Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation. Neuron 98, 439–452.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harmelech T & Malach R Neurocognitive biases and the patterns of spontaneous correlations in the human cortex. Trends Cogn Sci 17, 606–615 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Kucyi A & Davis KD Dynamic functional connectivity of the default mode network tracks daydreaming. NeuroImage 100, 471–480 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Godwin CA et al. Functional connectivity within and between intrinsic brain networks correlates with trait mind wandering. Neuropsychologia 103, 140–153 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Zhang J et al. What have we really learned from functional connectivity in clinical populations? Neuroimage 242, 118466 (2021). [DOI] [PubMed] [Google Scholar]
- 118.Liu X & Duyn JH Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A 110, 4392–4397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karapanagiotidis T et al. The psychological correlates of distinct neural states occurring during wakeful rest. Sci Rep 10, 21121 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mittner M et al. When the Brain Takes a Break: A Model-Based Analysis of Mind Wandering. J. Neurosci 34, 16286–16295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hutchison RM et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Callaghan C, Shine JM, Hodges JR, Andrews-Hanna JR & Irish M Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proc Natl Acad Sci U S A 116, 3316–3321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kucyi A Just a thought: How mind-wandering is represented in dynamic brain connectivity. Neuroimage 180, 505–514 (2018). [DOI] [PubMed] [Google Scholar]
- 124.Martin CG, He BJ & Chang C State-related neural influences on fMRI connectivity estimation. Neuroimage 244, 118590 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R & Buckner RL Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Axelrod V, Rees G & Bar M The default network and the combination of cognitive processes that mediate self-generated thought. Nat Hum Behav 1, 896–910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kernbach JM et al. Subspecialization within default mode nodes characterized in 10,000 UK Biobank participants. Proc Natl Acad Sci U S A 115, 12295–12300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Andrews-Hanna JR & Grilli MD Mapping the imaginative mind: Charting new paths forward. Curr Dir Psychol Sci 30, 82–89 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Braga RM & Buckner RL Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron 95, 457–471.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.DiNicola LM, Braga RM & Buckner RL Parallel distributed networks dissociate episodic and social functions within the individual. J Neurophysiol 123, 1144–1179 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buckner RL & DiNicola LM The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci 20, 593–608 (2019). [DOI] [PubMed] [Google Scholar]
- 132.Ranganath C & Ritchey M Two cortical systems for memory-guided behaviour. Nat Rev Neurosci 13, 713–726 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Konu D et al. A role for the ventromedial prefrontal cortex in self-generated episodic social cognition. Neuroimage 218, 116977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ho NSP et al. Individual variation in patterns of task focused, and detailed, thought are uniquely associated within the architecture of the medial temporal lobe. Neuroimage 202, 116045 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Smallwood J & Schooler JW The science of mind wandering: empirically navigating the stream of consciousness. Annu Rev Psychol 66, 487–518 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Andrews-Hanna JR et al. The conceptual building blocks of everyday thought: Tracking the emergence and dynamics of ruminative and nonruminative thinking. J Exp Psychol Gen 151, 628–642 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kajimura S, Kochiyama T, Abe N & Nomura M Challenge to Unity: Relationship Between Hemispheric Asymmetry of the Default Mode Network and Mind Wandering. Cereb Cortex 29, 2061–2071 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Bertossi E, Peccenini L, Solmi A, Avenanti A & Ciaramelli E Transcranial direct current stimulation of the medial prefrontal cortex dampens mind-wandering in men. Sci Rep 7, 16962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Filmer HL, Marcus LH & Dux PE Stimulating task unrelated thoughts: tDCS of prefrontal and parietal cortices leads to polarity specific increases in mind wandering. Neuropsychologia 151, 107723 (2021). [DOI] [PubMed] [Google Scholar]
- 140.Mowlem FD et al. Validation of the Mind Excessively Wandering Scale and the Relationship of Mind Wandering to Impairment in Adult ADHD. J Atten Disord 23, 624–634 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bertossi E & Ciaramelli E Ventromedial prefrontal damage reduces mind-wandering and biases its temporal focus. Soc Cogn Affect Neurosci 11, 1783–1791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McCormick C, Rosenthal CR, Miller TD & Maguire EA Mind-Wandering in People with Hippocampal Damage. J Neurosci 38, 2745–2754 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chou T, Hooley JM & Camprodon JA Transcranial direct current stimulation of default mode network parietal nodes decreases negative mind-wandering about the past. Cognit Ther Res 44, 10–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dixon ML et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci U S A 115, E1598–E1607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smallwood J, Brown K, Baird B & Schooler JW Cooperation between the default mode network and the frontal–parietal network in the production of an internal train of thought. Brain Research 1428, 60–70 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Axelrod V, Rees G, Lavidor M & Bar M Increasing propensity to mind-wander with transcranial direct current stimulation. Proceedings of the National Academy of Sciences 112, 3314–3319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boayue NM et al. The interplay between executive control, behavioural variability and mind wandering: Insights from a high-definition transcranial direct-current stimulation study. European Journal of Neuroscience 53, 1498–1516 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Uddin LQ Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16, 55–61 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Zerbi V et al. Rapid Reconfiguration of the Functional Connectome after Chemogenetic Locus Coeruleus Activation. Neuron 103, 702–718.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Seeley WW et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Webb CA et al. Mind-Wandering in Adolescents Predicts Worse Affect and Is Linked to Aberrant Default Mode Network-Salience Network Connectivity. J Am Acad Child Adolesc Psychiatry 60, 377–387 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lydon-Staley DM et al. Repetitive negative thinking in daily life and functional connectivity among default mode, fronto-parietal, and salience networks. Transl Psychiatry 9, 234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schooler JW et al. Meta-awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences 15, 319–326 (2011). [DOI] [PubMed] [Google Scholar]
- 154.Zhang M et al. Perceptual coupling and decoupling of the default mode network during mind-wandering and reading. Elife 11, e74011 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Woo C-W, Chang LJ, Lindquist MA & Wager TD Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 20, 365–377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shen X et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature Protocols 12, 506–518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kirkby LA et al. An Amygdala-Hippocampus Subnetwork that Encodes Variation in Human Mood. Cell 175, 1688–1700.e14 (2018). [DOI] [PubMed] [Google Scholar]
- 158.Varela F, Lachaux JP, Rodriguez E & Martinerie J The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2, 229–239 (2001). [DOI] [PubMed] [Google Scholar]
- 159.Aru J, Drüke M, Pikamäe J & Larkum ME Mental navigation and the neural mechanisms of insight. Trends Neurosci S0166–2236(22)00232–6 (2022) doi: 10.1016/j.tins.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 160.Axmacher N, Elger CE & Fell J Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131, 1806–1817 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Norman Y et al. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science 365, eaax1030 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Liu Y, Dolan RJ, Kurth-Nelson Z & Behrens TEJ Human Replay Spontaneously Reorganizes Experience. Cell 178, 640–652.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Buzsáki G Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carr MF, Jadhav SP & Frank LM Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci 14, 147–153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tambini A & Davachi L Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proc Natl Acad Sci U S A 110, 19591–19596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Norman Y, Raccah O, Liu S, Parvizi J & Malach R Hippocampal ripples and their coordinated dialogue with the default mode network during recent and remote recollection. Neuron 109, 2767–2780.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kaplan R et al. Hippocampal Sharp-Wave Ripples Influence Selective Activation of the Default Mode Network. Curr Biol 26, 686–691 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Higgins C et al. Replay bursts in humans coincide with activation of the default mode and parietal alpha networks. Neuron 109, 882–893.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kaefer K, Stella F, McNaughton BL & Battaglia FP Replay, the default mode network and the cascaded memory systems model. Nat Rev Neurosci 23, 628–640 (2022). [DOI] [PubMed] [Google Scholar]
- 170.Deeb W et al. Fornix-Region Deep Brain Stimulation-Induced Memory Flashbacks in Alzheimer’s Disease. N Engl J Med 381, 783–785 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nour MM, Liu Y, Arumuham A, Kurth-Nelson Z & Dolan RJ Impaired neural replay of inferred relationships in schizophrenia. Cell 184, 4315–4328.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heller AS & Bagot RC Is Hippocampal Replay a Mechanism for Anxiety and Depression? JAMA Psychiatry 77, 431–432 (2020). [DOI] [PubMed] [Google Scholar]
- 173.McGinley MJ et al. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 87, 1143–1161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aston-Jones G & Cohen JD An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28, 403–450 (2005). [DOI] [PubMed] [Google Scholar]
- 175.Joshi S, Li Y, Kalwani RM & Gold JI Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 89, 221–234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reimer J et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun 7, 13289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Megemont M, McBurney-Lin J & Yang H Pupil diameter is not an accurate real-time readout of locus coeruleus activity. Elife 11, e70510 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Smallwood J et al. Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS One 6, e18298 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Unsworth N & Robison MK Tracking arousal state and mind wandering with pupillometry. Cogn Affect Behav Neurosci 18, 638–664 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Konishi M, Brown K, Battaglini L & Smallwood J When attention wanders: Pupillometric signatures of fluctuations in external attention. Cognition 168, 16–26 (2017). [DOI] [PubMed] [Google Scholar]
- 181.Kucyi A & Parvizi J Pupillary Dynamics Link Spontaneous and Task-Evoked Activations Recorded Directly from Human Insula. J. Neurosci 40, 6207–6218 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schindler EAD & D’Souza DC The therapeutic potential of psychedelics. Science 378, 1051–1053 (2022). [DOI] [PubMed] [Google Scholar]
- 183.Madsen MK et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44, 1328–1334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Spitzer M et al. Increased activation of indirect semantic associations under psilocybin. Biol Psychiatry 39, 1055–1057 (1996). [DOI] [PubMed] [Google Scholar]
- 185.Carhart-Harris RL et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109, 2138–2143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kwan AC, Olson DE, Preller KH & Roth BL The neural basis of psychedelic action. Nat Neurosci 25, 1407–1419 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Preller KH et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife 7, e35082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Müller F et al. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatrica Scandinavica 136, 648–657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Müller F, Dolder PC, Schmidt A, Liechti ME & Borgwardt S Altered network hub connectivity after acute LSD administration. NeuroImage: Clinical 18, 694–701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mason NL et al. Spontaneous and deliberate creative cognition during and after psilocybin exposure. Transl Psychiatry 11, 209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lebedev AV et al. Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Human Brain Mapping 36, 3137–3153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tagliazucchi E et al. Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Current Biology 26, 1043–1050 (2016). [DOI] [PubMed] [Google Scholar]
- 193.Raffaelli Q et al. The think aloud paradigm reveals differences in the content, dynamics and conceptual scope of resting state thought in trait brooding. Sci Rep 11, 19362 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bellana B, Mahabal A & Honey CJ Narrative thinking lingers in spontaneous thought. Nat Commun 13, 4585 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li H-X et al. Exploring self-generated thoughts in a resting state with natural language processing. Behav Res Methods 54, 1725–1743 (2022). [DOI] [PubMed] [Google Scholar]
- 196.Kühn S, Fernyhough C, Alderson-Day B & Hurlburt RT Inner experience in the scanner: can high fidelity apprehensions of inner experience be integrated with fMRI? Frontiers in Psychology 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Siclari F et al. The neural correlates of dreaming. Nat Neurosci 20, 872–878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tambini A & Davachi L Awake Reactivation of Prior Experiences Consolidates Memories and Biases Cognition. Trends Cogn Sci 23, 876–890 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Van den Driessche C et al. Attentional Lapses in Attention-Deficit/Hyperactivity Disorder: Blank Rather Than Wandering Thoughts. Psychol Sci 28, 1375–1386 (2017). [DOI] [PubMed] [Google Scholar]
- 200.Watts FN, MacLeod AK & Morris L Associations between phenomenal and objective aspects of concentration problems in depressed patients. Br J Psychol 79 (Pt 2), 241–250 (1988). [DOI] [PubMed] [Google Scholar]
- 201.Kawagoe T, Onoda K & Yamaguchi S The neural correlates of ‘mind blanking’: When the mind goes away. Hum Brain Mapp 40, 4934–4940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fell J What is mind blanking: A conceptual clarification. Eur J Neurosci 56, 4837–4842 (2022). [DOI] [PubMed] [Google Scholar]
- 203.Borchers S, Himmelbach M, Logothetis N & Karnath H-O Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nat Rev Neurosci 13, 63–70 (2011). [DOI] [PubMed] [Google Scholar]
- 204.Holtzheimer PE & Mayberg HS Deep Brain Stimulation for Psychiatric Disorders. Annu Rev Neurosci 34, 289–307 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Curot J et al. Memory scrutinized through electrical brain stimulation: A review of 80 years of experiential phenomena. Neuroscience & Biobehavioral Reviews 78, 161–177 (2017). [DOI] [PubMed] [Google Scholar]
- 206.Fox KCR et al. Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain. Nat Hum Behav 4, 1039–1052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Parvizi J et al. Altered sense of self during seizures in the posteromedial cortex. Proc Natl Acad Sci U S A 118, e2100522118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shofty B et al. The default network is causally linked to creative thinking. Mol Psychiatry 1–7 (2022) doi: 10.1038/s41380-021-01403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset that was generated for Figure 1 is available at https://github.com/DynamicBrainMind/NatMentHealth2023.


