Abstract
Background:
Sleep disturbances, gastrointestinal problems, and atypical heart rate are commonly observed in patients with autism spectrum disorder (ASD) and may relate to underlying function of the autonomic nervous system (ANS). The overall objective of the current study was to quantitatively characterize features of ANS function using symptom scales and available electronic health record (EHR) data in a clinically and genetically characterized pediatric cohort.
Methods:
We assessed features of ANS function via chart review of patient records adapted from items drawn from a clinical research questionnaire of autonomic symptoms. This procedure coded for the presence and/or absence of targeted symptoms and was completed in 3 groups of patients, including patients with a clinical neurodevelopmental diagnosis and identified genetic etiology (NPD, n=244), those with an ASD diagnosis with no known genetic cause (ASD, n=159), and age and sex matched controls (MC, n=213). Symptoms were assessed across four main categories: (1) Mood, Behavior, and Emotion; (2) Secretomotor, Sensory Integration; (3) Urinary, Gastrointestinal, and Digestion; and (4) Circulation, Thermoregulation, Circadian function, and Sleep/Wake cycles.
Results:
Chart review scores indicate an increased rate of autonomic symptoms across all four sections in our NPD group as compared to scores with ASD and/or MC. Additionally, we note several significant relationships between individual differences in autonomic symptoms and quantitative ASD traits.
Conclusion:
These results highlight EHR review as a potentially useful method for quantifying variance in symptoms adapted from a questionnaire or survey. Further, using this method indicates that autonomic features are more prevalent in children with genetic disorders conferring risk for ASD and other neurodevelopmental diagnoses.
Keywords: autonomic function, chart review, autism spectrum disorder, developmental brain dysfunction, individual differences
Graphical abstract
BACKGROUND
ASD is characterized by social communication impairments and repetitive behaviors. Although not a part of the core diagnostic criteria, sleep disturbances, gastrointestinal problems, and atypical cardiovascular functioning are commonly observed in ASD, and have been theoretically linked to underlying function of the autonomic nervous system (ANS) (Aldinger et al., 2015; Dell’Osso et al., 2022; Devnani & Hegde, 2015; Kotagal & Broomall, 2012; Kushki et al., 2014; Liu et al., 2006; Mayes, 2002; Neuhaus et al., 2014; Patriquin et al., 2013). Studies in those with ASD have reported differences in sleep-wake cycles, heart rate variability (HRV), skin conductance, and pupil response metrics including the pupillary light reflex (PLR) (Anderson & Colombo, 2009; Condy et al., 2017; Daluwatte et al., 2013; Devnani & Hegde, 2015; Fan et al., 2009; Kotagal & Broomall, 2012; Liu et al., 2006; Wang et al., 2016). These differences have been interpreted as reflecting altered discordant arousal states, potentially stemming from a dysregulated ANS. A growing body of research has put forth the hypothesis that dysregulated patterns of ANS function may underlie ASD traits including sensory disintegration, psychosocial impairments and repetitive behaviors (Bharath et al., 2019; Kushki et al., 2014; Patriquin et al., 2013; Quintana et al., 2012). Reported differences in measures of HRV and sinus arrythmia in ASD have been linked with cognitive and behavioral traits that are core clinical features of ASD (Pace et al., 2016; Patriquin et al., 2013) and differences in these measures can predict symptom severity (Condy et al., 2017). Sleep disturbances have been repeatedly observed in individuals with ASD (Baker et al., 2019; Dell’Osso et al., 2022; Devnani & Hegde, 2015; Kotagal & Broomall, 2012; Liu et al., 2006; Malow et al., 2006; Schreck et al., 2004). Finally, differences in the PLR as well as baseline pupil diameter have been reported in autism (Anderson & Colombo, 2009; Fan et al., 2009; Laeng et al., 2018), and significant relationships between individual differences in pupil response metrics and quantitative ASD traits (DiCriscio & Troiani, 2017, 2020). While the findings reported above tend to interpret results as directional, with increased or decreased autonomic activity reflecting hypo- or hyper-parasympathetic versys sympathetic activity, it is important to note that the directionality of the relationship between autonomuc function and ASD remains unclear (Kushki et al., 2014; Wang et al., 2016). Taken together, the findings above suggest that continued exploration of ANS function as a possible pathophysiological mechanism for ASD features is warranted.
Electronic health record (EHR) phenotyping refers to the use of data from existing digital charts that is captured as part of standard clinical care within health systems and offices that are supported by electronic records. Retrospective research using EHRs offer an unprecedented opportunity to explore information documented via patient encounters, demographics, diagnostic screening, progress notes, and assessment summaries. Within the past decade, a growing number of studies have utilized available EHR data in order to predict diagnostic outcomes, characterize clinically significant co-occurring conditions (i.e. sleep problems) and assess treatment response in ASD patients (Coleman et al., 2015; Lingren et al., 2016; Malow et al., 2022; Sharp et al., 2018; Singer et al., 2022). EHR phenotyping has also been transformative for genomic discovery across many diseases (Robinson et al., 2022; Sealock et al., 2021; Smoller, 2018; Zheutlin et al., 2019), including autism and other related neurodevelopmental disorders (Doshi-Velez et al., 2014; Lingren et al., 2016; Slaby et al., 2022). Within ASD, EHRs have been used to better understand disorder trajectories (Myers et al., 2019), health care utilization (Brooks et al., 2021), prediagnostic prediction (Rahman et al., 2020), and data driven strategies to understand comorbidities and phenotypic variance within ASD (Zhao et al., 2022). Our own biobank at Geisinger has also been used to better understand the prevalence of a broad spectrum of spectrum of neurodevelopmental/psychiatric disorders (NPD) stemming from copy number variants in a population-based genetic screening program (Martin et al., 2020).
We recently used a parent-report, clinical research questionnaire of autonomic symptoms (Ming et al., 2011) to demonstrate the increased presence of features of ANS function across multiple organ systems in a pediatric cohort characterized by clinical neurodevelopmental diagnoses (DiCriscio et al., 2022). We also identified significant relationships between individual differences in features of autonomic function and quantitative ASD traits. In this published work and in the current study, we opt to include comprehensive information on both clinical diagnoses as well as clinical genetic etiology of the patients in the study. Although the heterogeneity of genetic causes of ASD make it challenging to examine the role of the ANS in one specific etiology in a single hospital cohort, by including details of genetic diagnoses in these real-world patient samples, we hope to lay the groundwork for future phenotype-genotype discovery and meta-analyses.
The main purpose of the current study is to assess whether autonomic symptoms can be captured using a rubric based EHR chart review procedure. To build from the results reported in DiCriscio et al. (2022), we adapted the structure of the Pediatric Autonomic Symptom Scales (Ming et al., 2011; PASS) to develop a manual, autonomic chart review (ACR) procedure for use within the EHR to support the characterization of features of ANS function across NDP, patients with ASD w/o a genetic etiology, and age and sex matched controls. Our adapted chart review protocol coded for the presence and/or absence of autonomic symptoms that paralleled those assessed in the PASS. Based on previously published work, we predicted increased autonomic symptoms in NPD and significant relationships between quantitative ASD traits and autonomic chart review scores. Finally, in order to assess the internal validity of our manual chart review protocol, we compared autonomic symptoms assessed via the EHR and PASS scores in a subset of participants.
METHOD & PROCEDURE
Participants
A total of N=563 participants (ages 3-22 years), making up three groups, were identified from ongoing research protocols at Geisinger’s Autism and Developmental Medicine Institute (ADMI) in Lewisburg, Pennsylvania, which is focused on characterizing genetic influences on neurodevelopment and atypical neurodevelopmental conditions such as ASD. Patients were identified based on either having received an NPD diagnosis, including ASD, with a confirmed genetic etiology or having received an ASD diagnosis without an associated genetic cause for the disorder. All clinical diagnoses, including ASD, within our NPD and ASD cohorts are reported in Supplemental table 1. We utilized a data broker to identify controls that have no history of or a current diagnosis of a neurodevelopmental disorder, intellectual disability, or developmental delay, or a genetic diagnosis that would confer risk for the same. Groups were matched based on age, sex, length of their electronic health record and the frequency of access to the healthcare system (i.e. well-child visits). All participants consented to protocols approved by the institutional review board (IRB) at the authors’ home institution. Thus, the three groups included in the current research are as follows: patients with a genetic variant associated with atypical neurodevelopment and/or ASD, NPD (n=210), individuals that possess an ASD diagnosis with no known genetic cause, ASD (n=156), and matched controls (n=197). The most common genetic syndromes present in the NPD group include Trisomy 21 (n=26), 22q11.2 deletion (n=24), and 15q11.2-13.1 duplication (n=18); a complete list of all genetic syndromes in the NPD group is reported in Supplemental table 2.
After receiving a referral to Geisinger’s Autism & Developmental Medicine Institute (ADMI), patients undergo assessment by a multi-disciplinary team that includes neurodevelopmental pediatricians, clinical psychologists, behavioral specialists, and speech pathologists. All patients with qualifying clinical diagnoses are offered comprehensive clinical genetic testing and results follow-up with a genetic counselor. All clinical diagnoses, including ASD and any comorbidities, genetic test results and results from diagnostic assessments and clinical research measures are entered into the patient’s digital health record. Most patients (>85%) consent to a clinic-wide research protocol, which allows researchers to access the consented patient’s health record.
Parent Report Measures
The Social Responsiveness Scale – 2nd Edition (SRS-2).
The SRS-2 (Constantino & Frazier, 2013; Constantino & Gruber, 2012) is a 65-item rating scale widely used to identify social impairment and persons at risk for ASD from 2.5 years to adulthood. The SRS offers the advantage of being sensitive to subclinical ASD behaviors in the general population. Each item such as, “avoids eye contact, or has unusual eye contact” and “would rather be alone than with others” is measured on a 4-point (0-3) scale: 0= not true, 1= sometimes true, 2= often true, and 3= almost always true. SRS scores for NPD (n= 72 of 210) and ASD (n= 48 of 156) available within patients’ charts are reported in Table 1, as well as demographic information for all three groups.
Table 1.
Demographics and SRS in NPD with genetic syndromes and individuals with ASD with no known genetic etiology.
| NPD (N=210) | ASD (N=156) | HC (N=197) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex Ratio | 132 males, 78 females | 127 males, 29 females | 124 males, 73 females | ||||||
| (σ) | Min | Max | (σ) | Min | Max | (σ) | Min | Max | |
|
| |||||||||
| Age (in years) | 12.07 (5.31) | 1 | 22 | 9.65 (4.47) | 3 | 22 | 11.89 (5.09) | 3 | 22 |
|
| |||||||||
| SRS Total T-Scores | 72.88 (11.92) | 40 | 98 | 79.21 (10.07) | 56 | 96 | |||
|
| |||||||||
| n= 9 Sub-clinical (T-score ≤ 59) | n= 2 Sub-clinical (T-score ≤ 59) | ||||||||
| n= 10 Mild (T-score 60 -65) | n= 2 Mild (T-score 60 -65) | ||||||||
| n= 24 Moderate (T-score 66 -75) | n= 14 Moderate (T-score 66 -75) | ||||||||
| n= 29 Severe (T-score ≥ 76) | n= 30 Severe (T-score ≥ 76) | ||||||||
| SRS Raw Scores | n= 72 | n= 48 | |||||||
|
| |||||||||
| Total Raw Score | 91.00 (29.55) | 16 | 154 | 111.92 (25.58) | 58 | 164 | |||
| SCI | 75.06 (23.09) | 15 | 122 | 92.15 (19.19) | 47 | 130 | |||
| RBRI | 15.93 (7.64) | 0 | 32 | 19.72 (8.25) | 4 | 34 | |||
| Social Awareness | 12.10 (3.68) | 3 | 20 | 14.73 (3.58) | 7 | 22 | |||
| Social Cognition | 18.25 (6.40) | 2 | 30 | 22.40 (4.91) | 11 | 34 | |||
| Social Communication | 30.49 (10.57) | 5 | 50 | 37.77 (8.65) | 16 | 53 | |||
| Social Motivation | 14.22 (6.05) | 2 | 27 | 17.19 (6.31) | 0 | 28 | |||
Pediatric Autonomic Symptom Scales (PASS).
The PASS is a parent/caregiver reported questionnaire designed to assess the severity of autonomic function in children (Ming et al., 2011). It includes 80, close-ended questions from 4 sections grouped by the affected organ systems – Section I: Mood, Behavior, and Emotion; Section II: Secretomotor/Sensory Integration: Section III: Urinary/Gastrointestinal Systems; and Section IV: Circulation, Thermoregulation, Sleeping Patterns, and Breathing. The first type of question is based on the presence or absence of a symptom, such as “Have you noticed that your child seems to have difficulty seeing after coming out of a dark room?” If the answer represented atypical autonomic function, it was scored as “1” whereas absence of the symptom or appropriate function was scored “0”. The second type of question assessed severity or frequency of the symptom, such as “Does your child urinate frequently, such as more than 10 times daily?” or “Does your child typically skip having a bowel movement for 2 days or more?”. Item responses are totaled, resulting in a Total Autonomic score as well as four subscale scores for each of the sections outlined above. The PASS questionnaire was primarily used to inform the development of our autonomic manual chart review procedure. We also had PASS survey scores from n=32 (a subset of patients previously reported in (DiCriscio et al., 2022)) that were used to compare the internal consistency and reliability of our autonomic chart review protocol as compared to autonomic symptoms assessed via the PASS.
Adaptation of PASS for EHR and manual chart review procedures
A list of search terms and smart phrases based on PASS items was used to develop a manual, autonomic chart review (ACR) rubric for determination of the presence/absence of symptoms within patients’ charts. Patients’ electronic health records were searched, resulting in 48 symptom fields assessed within each patient’s record. See Figure 1 for examples of PASS items and corresponding search terms and/or smart phrases that were used in our adapted ACR procedure. Following a format similar to the PASS, symptoms were categorized in four sections, I. Mood, Behavior and Emotion, II. Secretomotor/Sensory Integration, III. Urinary/Gastrointestinal System and IV. Circulation, Thermoregulation, Sleeping Patterns and Breathing. In some cases, more than one PASS item corresponds to a single symptom field within our chart review rubric. For example, items 1 and 2 within section I. Mood, Behavior, and Emotion (“Is your child easily distractible?”; “Does your child have any problems focusing on what they are doing at home or in school?”), are both associated with symptoms of “Inattentiveness” in chart review. For a complete list of PASS items and corresponding ACR symptoms, please see Supplemental Table 3.
Figure 1.
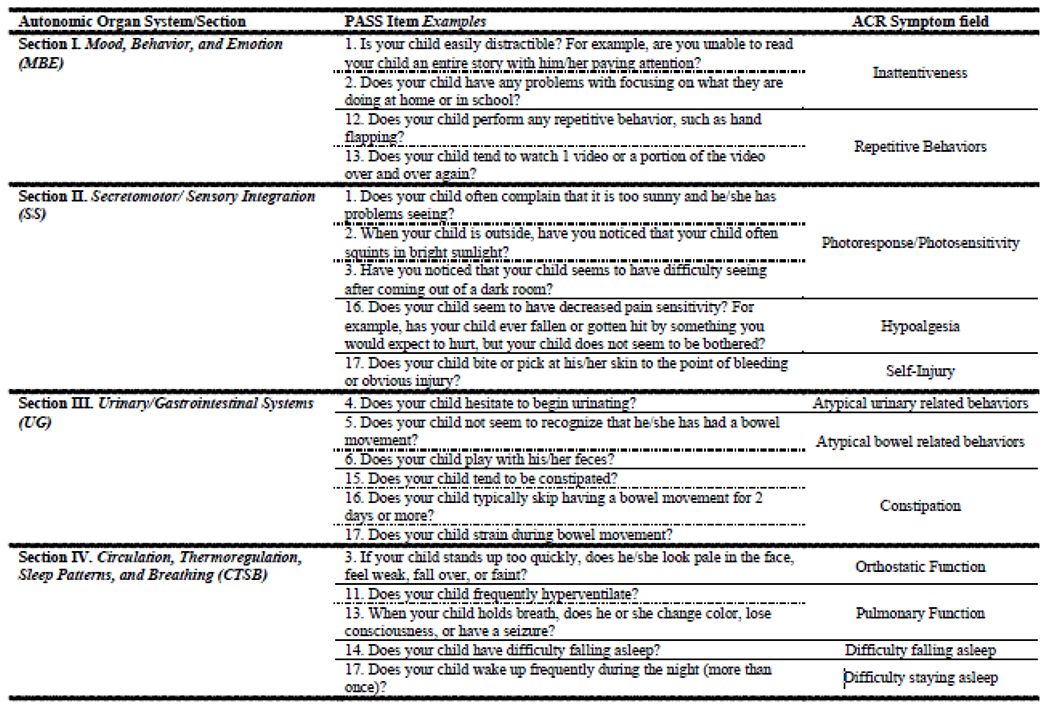
Our autonomic chart review (ACR) procedure was adapted based on the item structure and subsections within the Pediatric Autonomic Symptoms Scale (PASS; (Ming et al., 2011)). Items within the each of the four sections (I. Mood, Behavior, and Emotion; II. Secretomotor;/Sensory Integration; III. Urinary/ Gastrointestinal Systems; and IV. Circulation, Thermoregulation, Sleep Patterns, and Breathing) were used to established search terms and relevant smart phrases for manual chart review related to autonomic symptoms. Symptoms captured were summed, resulting in a ACR Total score as well as subsection scores that paralleled those PASS sections outlined above.
Patients’ charts were binary scored based on the presence or absence of symptoms where presence of a symptom was scored as ‘1’ and absence of a symptom received a ‘0’. ACR scoring mirrored the process of PASS scoring, to produce a total score of autonomic symptom load for each study group as well as four subscale scores based on the four categories of autonomic symptoms included in the PASS, I. Mood, Behavior, and Emotion, II. Secretomotor/Sensory Integration, III. Urinary/Gastrointestinal Systems, and IV. Circulation, Thermoregulation, Sleeping Patterns, and Breathing. To ensure validity of our chart review scores, we used inter-rater reliability checks and randomly selected 30% of the charts reviewed to be evaluated. Charts were found to be no less than 85% similar between assessors.
Analysis
ACR scores (total and subsection scores) were summarized for each group and used to determine the estimated prevalence of each autonomic symptom category in the study sample. Total and subsection scores were entered into a multivariate analysis of variance (MANOVA) to assess differences in ACR scores between NPD, ASD, and Controls (i.e. to assess a main effect of group). Appropriate follow-up tests were used to investigate pairwise comparisons between groups. All p-values were corrected for multiple comparisons via Benjamini-Hochberg procedures. The relationships between quantitative ASD traits and the presence of autonomic features were examined by performing partial correlations, controlling for age and sex, between autonomic chart review scores (total and subsection scores) and SRS raw scores. As in our previous work, raw scores were used in order to maximize phenotypic variability across the included sample. We used subsamples within the NPD (n=72) and ASD (n=48) groups with completed SRS assessments for this analysis available within their electronic records. As mentioned above, p-values were corrected for multiple comparisons.
Finally, a subset of n=32 NPD with existing parent-report versions of the PASS were selected for further analysis to compare ACR scores with the results from PASS scoring. Internal reliability of ACR and PASS scores were assessed using Cronbach’s alpha. Comparisons of Cronbach’s alpha values were performed to assess differences in reliability between PASS and corresponding ACR sections. Pearson’s correlations were also performed to assess the significance of associations between section and total scores of PASS and ACR symptom scores.
RESULTS
Group differences in autonomic symptoms captured via chart review
Prior to the reporting of results from our formal analysis, we first summarize the distribution of ANS symptoms assessed via ACR across NPD, ASD, and Controls. See Table 2. Next, multivariate analysis of variance (MANOVA) procedures were used to assess the differences in autonomic symptoms between NPD, ASD and Control groups. Results from mutltivariate tests indicate that there was a statistically significant difference in ACR scores based on group membership (F(2,510)=117.12, p<0.0001). Groups differed in ACR Total as well as subsection scores (p’s<0.0001). See Table 3 for results from follow-up tests and specific between-subjects effects. Overall, significantly more autonomic symptoms were noted in NPD as compared to ASD and Controls based on ACR Total as well as all subsection scores.
Table 2.
Summary of autonomic features captured as part of ACR. Cells represent summed totals of documented symptoms for each group and proportions of each group (%) in which symptoms were documented.
| NPD | ASD | HC | |||||
|---|---|---|---|---|---|---|---|
| n=210 | n=156 | n=197 | |||||
|
| |||||||
| Symptom | TOTAL | % of n | TOTAL | % of n | TOTAL | % of n | |
| Section I. Mood, Behavior, and Emotion (MBE) | Inattentiveness | 160 | 76.19% | 124 | 79.49% | 19 | 9.64% |
| Hyperactivity or Impulsivity | 170 | 80.95% | 134 | 85.90% | 22 | 11.17% | |
| Mood | 92 | 43.81% | 90 | 57.69% | 8 | 4.06% | |
| Tantrums or Aggression | 149 | 70.95% | 132 | 84.62% | 16 | 8.12% | |
| Anxiety, Unusual fears or phobias | 107 | 50.95% | 79 | 50.64% | 28 | 14.21% | |
| OCD | 80 | 38.10% | 72 | 46.15% | 1 | 0.51% | |
| Repetitive behaviors | 90 | 42.86% | 132 | 84.62% | 1 | 0.51% | |
| Social impairment | 103 | 49.05% | 145 | 92.95% | 4 | 2.03% | |
|
| |||||||
| Total | 951 | 56.61% | 908 | 72.76% | 99 | 6.28% | |
|
| |||||||
| Section II. Secretomotor/Sensory Integration (SS) | Photoresponse/Photosensitivity | 5 | 2.38% | 2 | 1.28% | 1 | 0.51% |
| Hyperactive Secretomotor | 48 | 22.86% | 14 | 8.97% | 1 | 0.51% | |
| Hypoactive Secretomotor | 1 | 0.48% | 0 | 0.00% | 2 | 1.02% | |
| Dermatologic Symptoms | 94 | 44.76% | 48 | 30.77% | 54 | 27.41% | |
| Hypoalgesia | 11 | 5.24% | 10 | 6.41% | 1 | 0.51% | |
| Self-injury | 71 | 33.81% | 61 | 39.1% | 3 | 1.52% | |
| Sensory Processing | 114 | 54.29% | 83 | 53.21% | 4 | 2.03% | |
| Vestibular Symptoms | 22 | 10.48% | 3 | 1.92% | 1 | 0.51% | |
|
| |||||||
| Total | 366 | 21.79% | 221 | 17.71% | 67 | 4.25% | |
|
| |||||||
| Section III. Urinary/Gastrointestinal Systems (UG) | Diapering/Toilet Training | 157 | 74.76% | 129 | 82.69% | 41 | 20.81% |
| Frequent urination | 8 | 3.81% | 5 | 3.21% | 2 | 1.02% | |
| Atypical urinary related behaviors | 17 | 8.10% | 7 | 4.49% | 4 | 2.03% | |
| Atypical bowel related behaviors | 28 | 13.33% | 24 | 15.38% | 3 | 1.52% | |
| Constipation | 126 | 60.00% | 61 | 39.10% | 48 | 24.37% | |
| Diarrhea or loose stool | 45 | 21.43% | 17 | 10.90% | 7 | 3.55% | |
| Reflux | 83 | 39.52% | 26 | 16.67% | 38 | 19.29% | |
| Bloating/Distension | 31 | 14.76% | 6 | 3.85% | 1 | 0.51% | |
| Vomit/Nausea | 60 | 28.57% | 20 | 12.82% | 5 | 2.54% | |
| Growth/Weight | 49 | 23.33% | 27 | 17.31% | 3 | 1.52% | |
| Abnormal Feeding | 99 | 47.14% | 28 | 17.95% | 3 | 1.52% | |
| Swallowing/Chewing | 50 | 23.81% | 15 | 9.62% | 3 | 1.52% | |
| Sucking | 40 | 19.05% | 11 | 7.05% | 3 | 1.52% | |
|
| |||||||
| Total | 793 | 29.05% | 376 | 18.54% | 161 | 6.29% | |
|
| |||||||
| Section IV. Circulation, Thermoregulatio n, Sleep Patterns, and Breathing (CTSB) | Pulmonary Function | 57 | 27.14% | 27 | 17.31% | 50 | 25.38% |
| Fatigue | 42 | 20.00% | 13 | 8.33% | 5 | 2.54% | |
| Syncope/Pre-syncope or fainting | 8 | 3.81% | 1 | 0.64% | 4 | 2.03% | |
| Apnea | 76 | 36.19% | 45 | 28.85% | 29 | 14.72% | |
| Orthostatic function | 1 | 0.48% | 0 | 0.00% | 1 | 0.51% | |
| Sinus Arrhythmia | 9 | 4.29% | 4 | 2.56% | 3 | 1.52% | |
| Tachycardia | 55 | 26.19% | 10 | 6.41% | 3 | 1.52% | |
| Motion sickness | 3 | 1.43% | 2 | 1.28% | 9 | 4.57% | |
| Skin/Complexion | 22 | 10.48% | 5 | 3.21% | 4 | 2.03% | |
| Edema | 8 | 3.81% | 0 | 0.00% | 0 | 0.00% | |
| Abnormal body temperature | 4 | 1.90% | 0 | 0.00% | 0 | 0.00% | |
| Circulation | 5 | 2.38% | 0 | 0.00% | 0 | 0.00% | |
| Difficulty falling asleep | 100 | 47.62% | 86 | 55.13% | 21 | 10.66% | |
| Difficulty staying asleep | 102 | 48.57% | 71 | 45.51% | 13 | 6.60% | |
| Difficulty staying awake or waking | 39 | 18.57% | 9 | 5.77% | 4 | 2.03% | |
| Parasomnia | 36 | 17.14% | 12 | 7.69% | 11 | 5.58% | |
|
| |||||||
| Total | 567 | 16.88% | 285 | 11.42% | 157 | 4.98% | |
Table 3.
Autonomic symptom scores based on EHR chart review data in NPD with genetic syndromes, individuals with ASD, and healthy controls.
| NPD | ASD | HC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| (σ) | Min | Max | (σ) | Min | Max | (σ) | Min | MaX | p-value | |
| ACR Total | 12.75(5.53) | 0 | 28 | 11.47 (4.52) | 3 | 31 | 2.46 (2.83) | 0 | 16 |
ap=0.007* bp<0.0001** cp<0.0001** |
|
| ||||||||||
| Section I. Mood, Behavior, & Emotion (MBE) | 4.53 (2.12) | 0 | 8 | 5.82 (1.73) | 1 | 8 | 0.50 (1.16) | 0 | 6 |
ap<0.0001** bp<0.0001** cp<0.0001** |
| Section II. Secretomotor/Sensory Integration (SS) | 1.74 (1.31) | 0 | 6 | 1.42 (1.00) | 0 | 5 | 0.34 (0.55) | 0 | 3 |
ap=0.003* bp<0.0001** cp<0.0001** |
| Section III. Urinary/Gastrointestinal Systems (UG) | 3.78 (2.45) | 0 | 11 | 2.41 (1.95) | 0 | 10 | 0.82 (1.11) | 0 | 6 |
ap<0.0001** bp<0.0001** cp<0.0001** |
| Section IV. Circulation, Thermoregulatio n, Sleep Patterns, and Breathing (CTSB) | 2.70 (2.02) | 0 | 9 | 1.83 (1.62) | 0 | 10 | 0.80 (1.19) | 0 | 7 |
ap<0.0001** bp<0.0001** bp<0.0001** |
Results from a multivariate analysis of variance (ANOVA) indicated a significant effect of Group on Autonomic symptom Total and sub-section scores (p’s<0.0001). Results from follow-up tests on between groups comparisons are reported in the table above.
p<0.01;
p<0.001
Linear relationship between ANS symptom scores and ASD traits
We also assessed the relationship between individual differences in ACR scores and quantitative ASD traits across our NPD and ASD groups (i.e. n=120 from across the two groups). Partial correlation procedures, controlling for age and sex, were used to assess the relationships between ACR total and subsection chart review scores and SRS raw scores. ACR Total scores were significantly related to SRS Total raw score (p<0.005) as well as SRS SCI, Social Cognition, and Social Communication subscales (p’s<0.01). ACR Section I. Mood, Behavior, and Emotion scores were found to be significantly related to SRS Total raw scores (p<0.001) as well as subscale scores (p’s<0.001). Significant relationships were also noted between ACR Sections II. Secretomotor/Sensory Integration and IV. Circulation, Thermoregulation, Sleeping Patterns and Breathing and SRS Social Cognition subscale scores (p’s<0.045). Complete results from partial correlations are reported in Table 4. Figure 2 illustrates relationships between autonomic chart review scores and SRS Social Cognition subscale scores. These results highlight the association between individual differences in atypical autonomic symptoms, captured via chart review, and quantitative ASD traits across NPD with a genetic etiology associated with atypical neurodevelopment, including those with a clinical diagnosis of ASD. Results from partial correlations between ACR and SRS scores for NPD and ASD groups separately can be found in Supplemental tables 4 and 5.
Table 4.
Results from partial correlation (age and sex) between Autonomic chart review (ACR) scores and SRS in n=72 NPD and n=48 ASD (i.e. Total N= 120). All p-values are adjusted for multiple comparisons by the Benjamini-Hochberg method.
| ACR Total |
ACR I-MBE |
ACR II-SS |
ACR III-UG |
ACR IV-CTSB |
|
|---|---|---|---|---|---|
| SRS Raw Scores | |||||
| Total |
0.292
p= 0.005* |
0.471
p< 0.001* |
0.107 p= 0.408 |
0.028 p= 0.890 |
0.089 p= 0.511 |
| Social Communication and Interaction |
0.303
p= 0.004* |
0.468
p= 0.001* |
0.111 p= 0.408 |
0.022 p= 0.919 |
0.125 p= 0.364 |
| Restricted Interests & Repetitive Behavior | 0.199 p= 0.071 |
0.390
p< 0.001* |
0.064 p= 0.642 |
0.035 p= 0.888 |
−0.029 p= 0.890 |
| Social Awareness | 0.193 p= 0.079 |
0.401
p< 0.001* |
0.064 p= 0.642 |
−0.012 p= 0.971 |
−0.007 p= 0.971 |
| Social Cognition |
0.448
p< 0.001* |
0.461
p< 0.001* |
0.227
p= 0.039* |
0.210 p= 0.056 |
0.220
p= 0.045* |
| Social Communication |
0.259
p= 0.014* |
0.432
p< 0.001* |
0.084 p= 0.536 |
−0.008 p= 0.971 |
0.108 p= 0.408 |
| Social Motivation | 0.113 p= 0.408 |
0.288
p< 0.001* |
0.003 p= 0.971 |
−0.105 p= 0.408 |
0.063 p= 0.642 |
Figure 2.
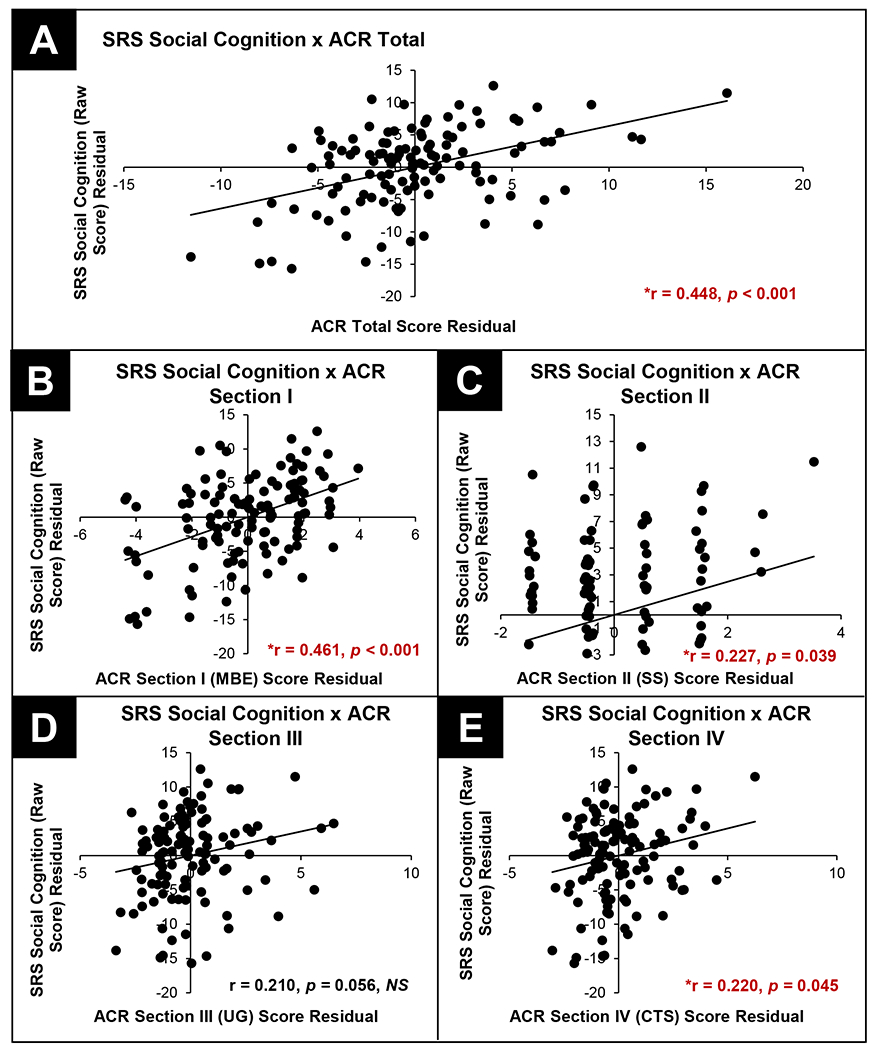
Results from Pearson, pairwise comparisons between SRS Total (raw scores) and ACR Total (Panel A) and subscale scores (Panels B – E).
Validation of ACR procedure versus PASS in subset of NPD
As a critical next step, we assessed the performance of our ACR procedure versus the PASS as a method for capturing autonomic symptoms in neurodevelopmental populations. In order to determine the reliability of chart review, we assessed the internal consistency and the relationship between the PASS and our ACR protocol based on subsection scores. It is important to note that the subset of participants included in this analysis did not demonstrate different chart review scores from the larger NPD cohort, nor did this subset differ in demographic characteristics (i.e. age, sex). Complete results from this analysis can be found in Supplemental table 6. Using chart review scores in a subset of n=32 NPD with available PASS scores (DiCriscio et al., 2022), we first established Cronbach’s alpha (α) as an index of internal reliability for our ACR and PASS measures. α’s for ACR and PASS subsection scores were compared in order to investigate potential differences in internal consistency between the two measures. Comparisons based on dependent (i.e. the same) group indicated no significant differences in α, as a measure of internal consistency and reliability, for chart review and PASS subsection scores, with the exception of ACR Section II. Secretomotor/Sensory Integration (p=0.001). See Table 5 for complete results from this analysis. Using Pearson, pairwise comparisons we also assessed the relationship between ACR and PASS scores and note significant direct relationships between ACR and PASS Total scores (p<0.0001) as well as each of the four corresponding subsections (p’s<0.04). See Table 6 for complete results. Thus, in addition to significant linear relationships between autonomic symptoms assessed via chart review and quantitative ASD traits, we are also able to demonstrate the successful adaptation of a parent-report measure as a chart review procedure for use within the EHR.
Table 5.
Cronbach’s alpha (α) for PASS and ACR in n=32 NPD. Comparisons based on dependent (i.e. the same) group.
| PASS (α) | ACR (α) | χ2(p-value) | |
|---|---|---|---|
| ACR Total | 0.785 | 0.789 | 0.005, p=0.962, NS |
|
| |||
|
Section I.
Mood, Behavior, & Emotion (MBE) |
0.524 | 0.708 | 3.686, p=0.055, NS |
|
Section II.
Secretomotor/Sensory Integration (SS) |
0.782 | 0.323 | 10.509, p=0.001** |
|
Section III.
Urinary/Gastrointestinal Systems (UG) |
0.703 | 0.658 | 0.228, p=0.633, NS |
|
Section IV.
Circulation, Thermoregulation, Sleep Patterns, and Breathing (CTSB) |
0.767 | 0.604 | 2.674, p=0.102, NS |
p<0.01;
p<0.001
Table 6.
Pearson’s correlations between PASS and ACR scores in n=32 NPD
| R value (p value) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| PASS Total | PASS I-MBE | PASS II-SS | PASS III-UG | PASS IV-CTSB | ACR Total | ACR I-MBE | ACR II-SS | ACR III-UG | ACR IV-CTSB | |
| PASS Total | 1.00 - |
. | ||||||||
| PASS I-MBE | 0.85** p<0.0001 | 1.00 - |
||||||||
| PASS II-SS | 0.84** p<0.0001 | 0.72** p<0.0001 | 1.00 - |
|||||||
| PASS III-UG | 0.60** p<0.0001 | 0.25 p=0.17 | 0.37 * p=0.04 | 1.00 - |
||||||
| PASS IV-CTSB | 0.64** p<0.0001 | 0.36* p=0.04 | 0.31 p=0.09 | 0.33 p=0.06 | 1.00 - |
|||||
| ACR Total | 0.64** p<0.0001 | 0.49** p<0.001 | 0.41 * p=0.02 | 0.60** p<0.0001 | 0.45 ** p=0.01 | 1.00 - |
||||
| ACR I-MBE | 0.62** p<0.0001 | 0.72** p<0.0001 | 0.47 ** p=0.01 | 0.28 p=0.12 | 0.26 p=0.15 | 0.59** p<0.0001 | 1.00 - |
|||
| ACR II-SS | 0.45** p=0.01 | 0.36* p=0.04 | 0.36 * p=0.04 | 0.37* p=0.04 | 0.25 p=0.17 | 0.73** p<0.0001 | 0.42 * p=0.02 | 1.00 - |
||
| ACR III-UG | 0.36* p=0.04 | 0.15 p=0.41 | 0.18 p=0.32 | 0.58** p<0.0001 | 0.27 p=0.13 | 0.76** p<0.0001 | 0.12 p=0.50 | 0.45 ** p=0.01 | 1.00 - |
|
| ACR IV-CTSB | 0.37* p=0.04 | 0.15 p=0.42 | 0.18 * p=0.03 | 0.42* p=0.02 | 0.46 ** p=0.01 | 0.74** p<0.0001 | 0.15 p=0.41 | 0.38 * p=0.03 | 0.49 ** p<0.001 | 1.00 - |
p<0.01;
p<0.001
DISCUSSION
The current investigation aimed to characterize autonomic features across children with and without neurodevelopmental diagnoses, specifically those with an NPD-related genetic etiology, using a novel chart review procedure adapted from a clinical research tool. The vast majority of research on autonomic function and physiologic indices of arousal in the context of ASD have focused on objective measures of sensory indices of arousal/autonomic function, such as heart rate variability (Pace et al., 2016; Patriquin et al., 2013; Wang et al., 2016), sleep (Malow et al., 2006; Schreck et al., 2004; Singer et al., 2022), and pupil measurements (Anderson & Colombo, 2009; Daluwatte et al., 2013; DiCriscio & Troiani, 2020). We and others have reported results in ASD using parent-report on symptom level questionnaires (DiCriscio et al., 2022; Ming et al., 2011). This growing body of research has been instrumental in shaping the landscape of research related to hypotheses of autonomic function as a biological correlate and/or underlying causal mechanism of the development of atypical neurodevelopmental traits. However, studies that capture direct measurements collected in experimental laboratories can be challenging to scale to meaningful sample sizes in real-world clinical samples. Thus, a novel component of the current investigation is the adaptation of a clinical research questionnaire of autonomic symptoms (DiCriscio et al., 2022; Ming et al., 2011) into a manual chart review protocol implemented across a large sample of clinically characterized patients within an integrated health care system. Using our ACR procedure, we report significantly more symptoms of autonomic function in NPD as compared to ASD and age and sex matched controls. These results align with previous studies reporting more symptoms related to atypical autonomic function in clinically and genetically characterized cohorts (DiCriscio et al., 2022) and expands upon previous studies of atypical autonomic processes (i.e. disruptions in sleep-wake cycles and disturbances to gastrointestinal function) within NPD-related genetic disorders (Brunetti-Pierri et al., 2008; DiCriscio et al., 2022; Kamara et al., 2021; Shayota & Elsea, 2019).
We used a broad sampling approach that aimed to explore the relationship between features of autonomic function and clinical ASD traits, while also using a heterogenous, genetically characterized cohort. We find significant relationships between autonomic features and neurodevelopmental trait dimensions, specifically symptoms associated with Social Cognition as assessed by the SRS, across individuals from NPD and ASD groups. While previous studies have linked autonomic processes such as HRV with core clinical features of ASD and other neurodevelopmental diagnoses such as psychosocial impairments and emotion regulation (Aldinger et al., 2015; Appelhans & Luecken, 2006; Bernstein et al., 1997; Bunford et al., 2017; Patriquin et al., 2013, 2019), results reported here align with previously published work that has found significant linear relationships between individual differences in autonomic features, assessed using parent-report on the PASS, and quantitative ASD traits (DiCriscio et al., 2022), as well as research that has noted differences in physiologic indices of autonomic function or arousal states associated with the ASD phenotype (Anderson & Colombo, 2009; Baker et al., 2019; Bast et al., 2021; Condy et al., 2017; DiCriscio & Troiani, 2020). The results presented here as well as our previously published research in NPD groups underscores the dimensional measurement of individual differences in autonomic features as a potential transdiagnostic trait domain associated with underlying neurobiological drivers of atypical neurodevelopment.
Of particular importance are reported results related to the adaptation of a clinical research tool, the PASS, for data collection and manual chart review of clinical data within the EHR. In a subset of individuals from our NPD group, we report no significant differences in measures of internal consistency in our chart review protocol as compared to the PASS, indicating this chart review method is measuring the same general construct as the parental report of these symptoms. We assessed the relationship between PASS scores and our autonomic chart review scores and reported significant relationships between PASS and autonomic chart review total scores across all four subscales. Taken together, these results demonstrate the successful adaptation of a parent-report, clinical research questionnaire for use at scale within the EHR. In section II. Secretomotor/Sensory Integration, we did identify differences in measures of internal consistency, with a lower Cronbach’s a from our chart review procedure as compared to the use of the PASS. Items within this section may differ in the way in which they are reported on a parent-report questionnaire versus identified and documented within a patient’s chart, which relies on parent-report in the context of a patient encounter and/or a provider’s assessment of atypical sensory features. In other words, the consistency at which a parent reports various sensory processing features (i.e. secretomotor symptoms, aversions to certain textures or sounds) may differ when directly prompted via questionnaire requiring a Yes/No response as compared to historically describing symptoms or suspected “problems” to a provider during a healthcare visit. Additionally, the way in which a patient provider documents a symptom or behavior related to sensory processing may vastly differ across providers and encounters, especially if sensory symptoms are being directly measured or assessed. Research in sensory integration/sensory processing in ASD has broadly reported differences in the manner in which individuals with ASD ‘take-in’ the world around them; however, results have varied in regards to subtyping groups based on variability in patterns of hypo versus hyper-responsivity (Tomchek et al., 2015) and depending on symptom measures used. The items within the Secretomotor/Sensory Integration section of the PASS may be limited in scope in capturing various patterns of sensory processing across multiple levels.
Previous studies have reported on the successful adaptation of clinical phenotyping tools and assessments for use with electronic health data to characterize patient groups based on diagnostic criteria of complex phenotypes including ASD, identify patients who may benefit from targeted intervention, or to characterize treatment response in patients receiving treatment (Coleman et al., 2015; Lingren et al., 2016; Malow et al., 2022; Singer et al., 2022). The use of available clinical data within the context of the EHR, in conjunction with diagnostic and individualized genomic data, represents a unique opportunity to identify meaningful neurodevelopmental subgroups or subtypes based on co-occurring conditions. Previous research in ASD has investigated specific autonomic processes such as gastrointestinal problems and sleep disturbances in conjunction with core diagnostic features of ASD as a means to stratifying subgroups (Unwin et al., 2013). Although we have demonstrated the potential expanded use of EHR data for phenotyping of complex neurodevelopmental disorders and clinically meaningful co-occurring conditions, it was beyond the scope of the current study to stratify our patient cohorts based on clinical diagnoses, genetic etiologies, and phenotypic traits including autonomic symptoms. Additional research is necessary in order to continue to validate the adaptation of clinical research questionnaires of complex neurobiological processes such as ANS function. As a part of future studies, it will also be necessary to also document and acknowledge other factors such as medication and prescription information (i.e. stimulants) and/or other clinically relevant comorbitidies that may disrupt or affect autonomic processes. Data related to medication and prescription documentation, clinically relevant comorbidities, and the frequency of reported symptoms should be captured and incorporated into future analysis as potential confounds and/or covariates. It is important to acknowledge that things such medications (i.e. stimulants and other psychoactive medications) may impact features that are included as autonomic symptoms in the current investigation such as sleep-wake cycles, cardiovascular functioning, and sensory processing (Cheng et al., 2020; Koolhaas et al., 2011; Taylor et al., 2021). Similarly, dietary needs and nutritional habits may cause symptoms related to constipation or other acute gastrointestinal distress that would not be characterized as a feature of autonomic function. Critical next steps for this research include (i) the continued dimensional assessment of features of autonomic function as neurobiological drivers of atypical neurodevelopment including ASD; (ii) multisite studies that focus on the expanded use and validation of chart review algorithms developed based on diagnostic criteria (e.g. DSM-V) and clinical assessment tools; and (iii) explore specific autonomic processes that may differentiate meaningful sub-phenotypes based on clinical expression as well as genetic etiology.
It is important to note that, in the current investigation, we adapted our reported chart review procedure from one measure of autonomic symptoms, the PASS. Our methods and reported results represent a novel extension of previously published work using the PASS in a neurodevelopmental patient cohort (DiCriscio et al., 2022). Research has demonstrated the utility of the PASS as well as the the Composite Autonomic Scoring Scale (CASS) (Low, 1993) and the Compositite Autonomic Symptom Scales (COMPASS; and abbreviated COMPASS-31) (Sletten et al., 2012; Suarez et al., 1999) and, from which the PASS was adapted, as ANS-specific scales for quantifying autonomic features that may be distinct in neurodevelopmental conditions including ASD (Lawson et al., 2020; Ming et al., 2011). While our adapted ACR and results are novel and distinct from other research related to autonomic function, it is important to recognize that other studies have implemented and reported findings from additional measures related to autonomic processes (Keith et al., 2019; Morlino et al., 2019; Zinn & Jason, 2021). However, a majority of these studies are based in adult samples or have focused on one symptom domain (Damian et al., 2012; Morlino et al., 2019; Sletten et al., 2012). Within neurodevelopmental populations, research has not used ANS-specific surveys, but relied on measures of anxiety, sensory processing, or sleep-wake cycles that were interpreted to reflect autonomic processes (Keith et al., 2019; Lawson et al., 2020; Wiggs & Stores, 1996, 2004). While our adaptation of a research measure for the scalable collection of clinical data related to ANS processes is distinct as compared to these previous studies, additional research is necessary, not only to validate the ACR protocol as mentioned above but also investigate the reliability and validity of ANS-specific scales such as the PASS as compared to other quantitative measures of ANS related symptoms in pediatric cohorts.
There are additional limitations in the current investigation that should be addressed as a part of future studies. We included a broad age range across all groups. While our previously published work also included a similar age range, autonomic symptoms as assessed via the PASS did not differ when we explored possible age related differences (DiCriscio et al., 2022). We have included a summary of symptoms captured via ACR across each age group (2-5 years; 6-10 years; 11+ years) within our current cohort (see Supplementary Table 7). Thus, our current work expands upon previously published studies and improves the generalization of findings across pediatric patients rather than isolated age groups; however, it is important to acknowledge that the PASS, from which our ACR protocol was adapted, was originally created to be used in a much younger and much narrower age range. Continued research, in much larger samples, is necessary in order to assess age-related changes in autonomic features and whether a critical period of autonomic function as it relates to atypical neurodevelopment can be identified. Next, additional data collection and more rigorous testing of our novel ACR procedures as well as the implementation of advanced analytic methods (i.e. principal components analysis) are necessary in order confirm the validity and reliability of manual chart review and the use of EHR data as a clinical phenotyping tool for underlying neurobiological processes associated with neurodevelopmental psychopathology. To our knowledge, while other studies have assessed the link between questionnaires related to features of autonomic function and direct measures of autonomic activity or clinical diagnoses resulting from atypical autonomic function (Ke et al., 2017; Ruška et al., 2018; Schultz et al., 2019; Strand et al., 2016; Zinn & Jason, 2021), there is no evidence linking PASS scores, or ANS symptom scores summarized using health record data, with objective or direct measures of autonomic function. However, there has been work linking other autonomic questionnaires, including the COMPASS-31, from which the PASS was adapted, to clinical symptoms (Cortez et al., 2015; Costa et al., 2023; Takri et al., 2023; Treister et al., 2015) as well as direct sensory measures (Bitirgen et al., 2021; D’Amato et al., 2020; Georges et al., 2022; Greco et al., 2017; Huang et al., 2023; Kang et al., 2016). Nevertheless, future work should assess the relationship between PASS symptom scores and direct measurements of arousal in children. It’s also important to acknowledge the sex ratios of male:female across our three groups. The distribution of males and females across each of our NPD, ASD, and control groups parallels currently accepted estimates of ASD in males versus females (Burrows et al., 2022; Loomes et al., 2017; Zeidan et al., 2022); however, additional data collection and larger, sex-matched samples (e.g. 1:1) in future studies will promote a more targeted approach for the investigation of sex-related differences in autonomic processes.
Finally, we wish to acknowledge that we did not explore differences in autonomic features based on genetic etiology. Our NPD cohort was heterogenous and included CNVs, single-gene disorders, as well as likely or uncertain pathogenic variants. Smaller sample sizes in our more highly represented genetic subgroups (i.e. 1q21.1 duplication syndrome) did not provide sufficient power to support the identification of group differences or correlations between autonomic symptoms and meaningful differences in ASD traits. Clinical genetic testing and research in clinical genomics has identified several pathogenic variants and single-gene disorders associated with increased risk for the development ASD, other neurodevelopmental and psychiatric conditions, and clinically significant comorbidities including medical conditions linked with ANS function (i.e. sleep disturbances, hearing and vision impairments, ataxias). Continued research on trait dimensions that may be considered transdiagnostic across multiple genetic etiologies, including those with a current uncertain or emerging pathogenicity, is critical to promote the identification and characterization of complex neurodevelopmental phenotypes. Thus, we would like to emphasize that a future direction for this area of research is the continued characterization of the genetic contribution to autonomic function in atypical neurodevelopment; however, it was beyond the scope of the current study to include specific subgroup analyses.
IMPLICATIONS
The work presented here presents the use of a manual chart review procedure, adapted from a clinical research tool, as a novel and scalable method for the assessment of autonomic features associated with atypical neurodevelopment. Our methods and broad sampling approach underscores autonomic symptoms as a potential transdiagnostic trait dimension found in neurodevelopmental and psychiatric disorders, including ASD. Our reported results extend previous studies of autonomic features in ASD using parent-report symptom scales (DiCriscio et al., 2022; Ming et al., 2011) and align with experimental studies that have reported differences in direct measures of autonomic processes (Anderson & Colombo, 2009; Condy et al., 2017; Daluwatte et al., 2013; Goodwin et al., 2006; Kushki et al., 2014; Pace et al., 2016; Patriquin et al., 2013, 2013, 2019). The current study provides a foundation for future studies regarding the relationship across ANS function, core clinical features of ASD and atypical neurodevelopment, and genetic differences.
Supplementary Material
HIGHLIGHTS.
Atypical autonomic processes have been described in autism spectrum disorder (ASD).
Health record data can be used to quantify features of autonomic function.
More symptoms of atypical autonomic function are reported in atypical neurodevelopment.
Autonomic symptoms are associated with quantitative ASD traits.
ACKNOWLEDGEMENTS
We would like to extend our gratitude to the patients and families who make this research possible. The authors are grateful to Dr. Christa Martin, PhD, FACMG, Chief Scientific Officer of Geisinger Research, Professor and Director of Geisinger ADMI. This research was funded by a National Institutes of Health (NIH) R01 Administrative Supplement (NIMH 3R01 107431-04S1) awarded to Drs. Troiani and DiCriscio as a part of a parent NIMH R01 (PI: C.L. Martin; NIMH 3R01 MH107431-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests:
The authors declare that they have no competing interests.
Author Credit Statement
Antoinette S. DiCriscio: Conceptualization, Methodology, Formal Analysis, Investigation, Data Curation, Writing, Visualization, Supervision, Project Administration; Donielle Beiler: Resources, Data Curation; Jaclyn Smtih: Data Curation; Patrick Asdell: Data Curation; Shannon Dickey: Data Curation; Marina DiStefano: Formal Analysis, Data Curation; Vanessa Troiani: Conceptualization, Methodology, Investigation, Writing, Supervision, Project Administration, Funding Acquisition
REFERENCES
- Aldinger KA, Lane CJ, Veenstra-VanderWeele J, & Levitt P (2015). Patterns of risk for multiple co-occurring medical conditions replicate across distinct cohorts of children with autism spectrum disorder. Autism Research, 8(6), 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, & Colombo J (2009). Larger Tonic Pupil Size in Young Children With Autism Spectrum Disorder. Developmental Psychobiology, 51(2), 207–211. 10.1002/dev.20352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229. [Google Scholar]
- Baker EK, Richdale AL, Hazi A, & Prendergast LA (2019). Assessing a hyperarousal hypothesis of insomnia in adults with autism spectrum disorder. Autism Research, 12(6), 897–910. [DOI] [PubMed] [Google Scholar]
- Bast N, Boxhoorn S, Supér H, Heifer B, Polzer L, Klein C, Cholemkery H, & Freitag CM (2021). Atypical arousal regulation in children with autism but not with attention-deficit/hyperactivity disorder as indicated by pupillometric measures of locus coeruleus activity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Massie ED, Thuras PD, Perwien AR, Borchardt CM, & Crosby RD (1997). Somatic symptoms in anxious-depressed school refusers. Journal of the American Academy of Child & Adolescent Psychiatry, 36(5), 661–668. [DOI] [PubMed] [Google Scholar]
- Bharath R, Moodithaya SS, Bhat SU, Mirajkar AM, & Shetty SB (2019). Comparison of Physiological and Biochemical Autonomic Indices in Children with and without Autism Spectrum Disorders. Medicina, 55(7), 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitirgen G, Turkmen K, Zengin N, & Malik RA (2021). Altered pupillary light responses are associated with the severity of autonomic symptoms in patients with Fabry disease. Scientific Reports, 11(1), 8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JD, Bronskill SE, Fu L, Saxena FE, Arneja J, Pinzaru VB, Anagnostou E, Nylen K, McLaughlin J, & Tu K (2021). Identifying children and youth with autism spectrum disorder in electronic medical records: Examining health system utilization and comorbidities. Autism Research, 14(2), 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Berg JS, Scaglia F, Belmont J, Bacino CA, Sahoo T, Lalani SR, Graham B, Lee B, & Shinawi M (2008). Recurrent reciprocal 1q21. 1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nature Genetics, 40(12), 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunford N, Evans SW, Zoccola PM, Owens JS, Flory K, & Spiel CF (2017). Correspondence between heart rate variability and emotion dysregulation in children, including children with ADHD. Journal of Abnormal Child Psychology, 45(7), 1325–1337. [DOI] [PubMed] [Google Scholar]
- Burrows CA, Grzadzinski RL, Donovan K, Stallworthy IC, Rutsohn J, John TS, Marrus N, Parish-Morris J, MacIntyre L, & Hampton J (2022). A data-driven approach in an unbiased sample reveals equivalent sex ratio of autism spectrum disorder–associated impairment in early childhood. Biological Psychiatry, 92(8), 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y-C, Huang Y-C, & Huang W-L (2020). Heart rate variability in individuals with autism spectrum disorders: A meta-analysis. Neuroscience & Biobehavioral Reviews, 118, 463–471. [DOI] [PubMed] [Google Scholar]
- Coleman KJ, Lutsky MA, Yau V, Qian Y, Pomichowski ME, Crawford PM, Lynch FL, Madden JM, Owen-Smith A, Pearson JA, Pearson KA, Rusinak D, Quinn VP, & Croen LA (2015). Validation of Autism Spectrum Disorder Diagnoses in Large Healthcare Systems with Electronic Medical Records. Journal of Autism and Developmental Disorders, 45(7), 1989–1996. 10.1007/s10803-015-2358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condy EE, Scarpa A, & Friedman BH (2017). Respiratory sinus arrhythmia predicts restricted repetitive behavior severity. Journal of Autism and Developmental Disorders, 47(9), 2795–2804. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Frazier TW (2013). Commentary: The observed association between autistic severity measured by the Social Responsiveness Scale (SRS) and general psychopathology – a response to. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54(6), 695–697. 10.1111/jcpp.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social Responsiveness Scale: SRS-2 Software Kit. Western Psychological Services. [Google Scholar]
- Cortez MM, Nagi Reddy SK, Goodman B, Carter JL, & Wingerchuk DM (2015). Autonomic symptom burden is associated with MS-related fatigue and quality of life. Multiple Sclerosis and Related Disorders, 4(3), 258–263. 10.1016/j.msard.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Costa T, Taylor A, Black F, Hill S, McAllister-Williams RH, Gallagher P, & Watson S (2023). Autonomic dysregulation, cognition and fatigue in people with depression and in active and healthy controls: Observational cohort study. BJPsych Open, 9(4), e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daluwatte C, Miles JH, Christ SE, Beversdorf DQ, Takahashi TN, & Yao G (2013). Atypical pupillary light reflex and heart rate variability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43(8), 1910–1925. 10.1007/s10803-012-1741-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato C, Greco C, Lombardo G, Frattina V, Campo M, Cefalo CMA, Izzo V, Lauro D, & Spallone V (2020). The diagnostic usefulness of the combined COMPASS 31 questionnaire and electrochemical skin conductance for diabetic cardiovascular autonomic neuropathy and diabetic polyneuropathy. Journal of the Peripheral Nervous System, 25(1), 44–53. 10.1111/jns.12366 [DOI] [PubMed] [Google Scholar]
- Damian A, Adler CH, Hentz JG, Shill HA, Caviness JN, Sabbagh MN, Evidente VG, Beach TG, & Driver-Dunckley E (2012). Autonomic function, as self-reported on the SCOPA-autonomic questionnaire, is normal in essential tremor but not in Parkinson’s disease. Parkinsonism & Related Disorders, 18(10), 1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Osso L, Massoni L, Battaglini S, Cremone IM, Carmassi C, & Carpita B. (2022). Biological correlates of altered circadian rhythms, autonomic functions and sleep problems in autism spectrum disorder. Annals of General Psychiatry, 21(1), 13. 10.1186/s12991-022-00390-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devnani PA, & Hegde AU (2015). Autism and sleep disorders. Journal of Pediatric Neurosciences, 10(4), 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCriscio AS, & Troiani V (2017). Pupil adaptation corresponds to quantitative measures of autism traits in children. Scientific Reports, 7(1), 6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCriscio AS, & Troiani V (2020). Resting and functional pupil response metrics indicate features of reward sensitivity and Autism Spectrum Disorder in children. MedRxiv, 2020.01.24.20018648. 10.1101/2020.01.24.20018648 [DOI] [PubMed] [Google Scholar]
- DiCriscio AS, Wain KE, Smith J, Beiler D, Walsh LK, Holdren K, & Troiani V (2022). Higher scores on autonomic symptom scales in pediatric patients with neurodevelopmental disorders of known genetic etiology. Brain and Behavior, e2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi-Velez F, Ge Y, & Kohane I (2014). Comorbidity clusters in autism spectrum disorders: An electronic health record time-series analysis. Pediatrics, 133(1), e54–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Miles JH, Takahashi N, & Yao G (2009). Abnormal transient pupillary light reflex in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(11), 1499–1508. [DOI] [PubMed] [Google Scholar]
- Georges C, Lloret-Perez S, Ory-Magne F, Fabbri M, Foubert-Samier A, Meissner WG, Rascol O, & Pavy-Le Traon A (2022). Alterations in electrochemical skin conductance as a marker of autonomic dysfunction in multiple system atrophy. Parkinsonism & Related Disorders, 103, 56–59. 10.1016/j.parkreldis.2022.08.026 [DOI] [PubMed] [Google Scholar]
- Goodwin MS, Groden J, Velicer WF, Lipsitt LP, Baron MG, Hofmann SG, & Groden G (2006). Cardiovascular arousal in individuals with autism. Focus on Autism and Other Developmental Disabilities, 21(2), 100–123. [Google Scholar]
- Greco C, Di Gennaro F, D’Amato C, Morganti R, Corradini D, Sun A, Longo S, Lauro D, Pierangeli G, Cortelli P, & Spallone V (2017). Validation of the Composite Autonomic Symptom Score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabetic Medicine, 34(6), 834–838. 10.1111/dme.13310 [DOI] [PubMed] [Google Scholar]
- Huang Y-C, Huang C-C, Lai Y-R, Lien C-Y, Cheng B-C, Kung C-T, Chiang Y-F, & Lu C-H (2023). Assessing the Feasibility of Using Electrochemical Skin Conductance as a Substitute for the Quantitative Sudomotor Axon Reflex Test in the Composite Autonomic Scoring Scale and Its Correlation with Composite Autonomic Symptom Scale 31 in Parkinson’s Disease. Journal of Clinical Medicine, 12(4), Article 4. 10.3390/jcm12041517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamara D, De Boeck P, Lecavalier L, Neuhaus E, & Beauchaine TP (2021). Characterizing Sleep Problems in 16p11. 2 Deletion and Duplication. Journal of Autism and Developmental Disorders, 1–14. [DOI] [PubMed] [Google Scholar]
- Kang JH, Kim JK, Hong SH, Lee CH, & Choi BY (2016). Heart Rate Variability for Quantification of Autonomic Dysfunction in Fibromyalgia. Annals of Rehabilitation Medicine, 40(2), 301–309. 10.5535/arm.2016.40.2.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J-Q, Shao S-M, Zheng Y-Y, Fu F-W, Zheng G-Q, & Liu C-F (2017). Sympathetic skin response and heart rate variability in predicting autonomic disorders in patients with Parkinson disease. Medicine, 96(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith JM, Jamieson JP, & Bennetto L (2019). The importance of adolescent self-report in autism spectrum disorder: Integration of questionnaire and autonomic measures. Journal of Abnormal Child Psychology, 47(4), 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, & Palanza P (2011). Stress revisited: A critical evaluation of the stress concept. Neuroscience & Biobehavioral Reviews, 35(5), 1291–1301. [DOI] [PubMed] [Google Scholar]
- Kotagal S, & Broomall E (2012). Sleep in children with autism spectrum disorder. Pediatric Neurology, 47(4), 242–251. [DOI] [PubMed] [Google Scholar]
- Kushki A, Brian J, Dupuis A, & Anagnostou E (2014). Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism, 5(1), 39. 10.1186/2040-2392-5-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng B, F\a erevaag FS, Tanggaard S, & von Tetzchner S (2018). Pupillary Responses to Illusions of Brightness in Autism Spectrum Disorder. I-Perception, 9(3), 2041669518771716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LP, Richdale AL, Haschek A, Flower RL, Vartuli J, Arnold SR, &Trollor JN. (2020). Cross-sectional and longitudinal predictors of quality of life in autistic individuals from adolescence to adulthood: The role of mental health and sleep quality. Autism, 24(4), 954–967. [DOI] [PubMed] [Google Scholar]
- Lingren T, Chen P, Bochenek J, Doshi-Velez F, Manning-Courtney P, Bickel J, Welchons LW, Reinhold J, Bing N, Ni Y, Barbaresi W, Mentch F, Basford M, Denny J, Vazquez L, Perry C, Namjou B, Qiu H, Connolly J, … Savova G (2016). Electronic Health Record Based Algorithm to Identify Patients with Autism Spectrum Disorder. PLOS ONE, 11(7), e0159621. 10.1371/journal.pone.0159621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hubbard JA, Fabes RA, & Adam JB (2006). Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development, 37(2), 179–191. [DOI] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56(6), 466–474. [DOI] [PubMed] [Google Scholar]
- Low PA (1993). Composite Autonomic Scoring Scale for Laboratory Quantification of Generalized Autonomic Failure. Mayo Clinic Proceedings, 68(8), 748–752. 10.1016/S0025-6196(12)60631-4 [DOI] [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, & Stone WL (2006). Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep, 29(12), 1563–1571. [DOI] [PubMed] [Google Scholar]
- Malow BA, Veatch OJ, Niu X, Fitzpatrick KA, Hucks D, Maxwell-Horn A, & Davis LK (2022). A practical approach to identifying autistic adults within the electronic health record. Autism Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CL, Wain KE, Oetjens MT, Tolwinski K, Palen E, Hare-Harris A, Habegger L, Maxwell EK, Reid JG, Walsh LK, Myers SM, & Ledbetter DH (2020). Identification of Neuropsychiatric Copy Number Variants in a Health Care System Population. JAMA Psychiatry, 10.1001/jamapsychiatry.2020.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC (2002). A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology and Teratology, 24(3), 385–395. 10.1016/S0892-0362(02)00200-3 [DOI] [PubMed] [Google Scholar]
- Ming X, Bain JM, Smith D, Brimacombe M, von-Simson GG, & Axelrod FB (2011). Assessing autonomic dysfunction symptoms in children: A pilot study. Journal of Child Neurology, 0883073810381921. [DOI] [PubMed] [Google Scholar]
- Morlino S, Dordoni C, Sperduti I, Clark CJ, Piedimonte C, Fontana A, Colombi M, Grammatico P, Copetti M, & Castori M (2019). Italian validation of the functional difficulties questionnaire (FDQ-9) and its correlation with major determinants of quality of life in adults with hypermobile Ehlers–Danlos syndrome/hypermobility spectrum disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 180(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Myers SM, Voigt RG, Colligan RC, Weaver AL, Storlie CB, Stoeckel RE, Port JD, & Katusic SK (2019). Autism spectrum disorder: Incidence and time trends over two decades in a population-based birth cohort. Journal of Autism and Developmental Disorders, 49(4), 1455–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E, Bernier R, & Beauchaine TP (2014). Brief report: Social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders, 44(3), 730–737. [DOI] [PubMed] [Google Scholar]
- Pace M, Dumortier L, Favre-Juvin A, Guinot M, & Bricout V-A (2016). Heart rate variability during sleep in children with autism spectrum disorders. Physiology & Behavior, 167, 309. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Hartwig EM, Friedman BH, Porges SW, & Scarpa A (2019). Autonomic response in autism spectrum disorder: Relationship to social and cognitive functioning. Biological Psychology, 145, 185–197. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, & Porges SW (2013). Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology, 55(2), 101–112. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, Outhred T, Hickie IB, & Kemp AH (2012). Heart rate variability is associated with emotion recognition: Direct evidence for a relationship between the autonomic nervous system and social cognition. International Journal of Psychophysiology, 86(2), 168–172. [DOI] [PubMed] [Google Scholar]
- Rahman R, Kodesh A, Levine SZ, Sandin S, Reichenberg A, & Schlessinger A (2020). Identification of newborns at risk for autism using electronic medical records and machine learning. European Psychiatry: The Journal of the Association of European Psychiatrists, 63(1), e22. 10.1192/j.eurpsy.2020.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JR, Carroll RJ, Bastarache L, Chen Q, Pirruccello J, Mou Z, Wei W-Q, Connolly J, Mentch F, & Crane PK (2022). Quantifying the phenome-wide disease burden of obesity using electronic health records and genomics. Obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruška B, Pavičić T, Pavlović I, Junaković A, Adamec I, Crnošija L, Krbot Skorić M, & Habek M (2018). Performance of the COMPASS-31 questionnaire with regard to autonomic nervous system testing results and medication use: A prospective study in a real-life setting. Neurological Sciences, 39(12), 2079–2084. 10.1007/si0072-018-3542-8 [DOI] [PubMed] [Google Scholar]
- Schreck KA, Mulick JA, & Smith AF (2004). Sleep problems as possible predictors of intensified symptoms of autism. Research in Developmental Disabilities, 25(1), 57–66. [DOI] [PubMed] [Google Scholar]
- Schultz KR, Katz BZ, Bockian NR, & Jason LA (2019). Associations between autonomic and orthostatic self-report and physician ratings of orthostatic intolerance in youth. Clinical Therapeutics, 41(4), 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealock JM, Lee YH, Moscati A, Venkatesh S, Voloudakis G, Straub P, Singh K, Feng Y-CA, Ge T, Roussos P, Smoller JW, Chen G, & Davis LK (2021). Use of the PsycheMERGE Network to Investigate the Association Between Depression Polygenic Scores and White Blood Cell Count. JAMA Psychiatry, 78(12), 1–10. 10.1001/jamapsychiatry.2021.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp WG, Postorino V, McCracken CE, Berry RC, Criado KK, Burrell TL, & Scahill L (2018). Dietary intake, nutrient status, and growth parameters in children with autism spectrum disorder and severe food selectivity: An electronic medical record review. Journal of the Academy of Nutrition and Dietetics, 118(10), 1943–1950. [DOI] [PubMed] [Google Scholar]
- Shayota BJ, & Elsea SH (2019). Behavior and Sleep Disturbance in Smith-Magenis Syndrome. Current Opinion in Psychiatry, 32(2), 73–78. 10.1097/YCO.0000000000000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer EV, Niarchou M, Maxwell-Horn A, Hucks D, Johnston R, Sutcliffe JS, Davis LK, & Malow BA (2022). Characterizing sleep disorders in an autism-specific collection of electronic health records. Sleep Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby I, Hain HS, Abrams D, Mentch FD, Glessner JT, Sleiman P, & Hakonarson H (2022). An electronic health record (EHR) phenotype algorithm to identify patients with attention deficit hyperactivity disorders (ADHD) and psychiatric comorbidities. Journal of Neurodevelopmental Disorders, 14(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten DM, Suarez GA, Low PA, Mandrekar J, & Singer W (2012). COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clinic Proceedings, 87, 1196–1201. http://www.sciencedirect.com/science/article/pii/S0025619612010385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW (2018). The use of electronic health records for psychiatric phenotyping and genomics. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 177(7), 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand EB, Lillestøl K, Jason LA, Tveito K, Diep LM, Valla SS, Sunnquist M, Helland IB, Herder I, & Dammen T (2016). Comparing the DePaul Symptom Questionnaire with physician assessments: A preliminary study. Fatigue: Biomedicine, Health & Behavior, 4(1), 52–62. 10.1080/21641846.2015.1126026 [DOI] [Google Scholar]
- Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O’brien PC, & Low PA (1999). The autonomic symptom profile a new instrument to assess autonomic symptoms. Neurology, 52(3), 523–523. [DOI] [PubMed] [Google Scholar]
- Takri T, Mathew RR, Sivadasan A, Raju K, Karuppusami R, & Mariappan R (2023). The Utility of COMPASS-31 Questionnaire to Predict Autonomic Dysfunction in Patients With Cervical/Upper Thoracic Compressive Myelopathy. Journal of Neurosurgical Anesthesiology, 35(2), 243. 10.1097/ANA.0000000000000824 [DOI] [PubMed] [Google Scholar]
- Taylor EC, Livingston LA, Callan MJ, Ashwin C, & Shah P (2021). Autonomic dysfunction in autism: The roles of anxiety, depression, and stress. Autism, 25(3), 744–752. [DOI] [PubMed] [Google Scholar]
- Tomchek SD, Little LM, & Dunn W (2015). Sensory pattern contributions to developmental performance in children with autism spectrum disorder. The American Journal of Occupational Therapy, 69(5), 6905185040p1-6905185040p10. [DOI] [PubMed] [Google Scholar]
- Treister R, O’Neil K, Downs HM, & Oaklander AL (2015). Validation of the Composite Autonomic Symptom Scale-31 (COMPASS-31) in patients with and without Small-fiber Polyneuropathy. European Journal of Neurology : The Official Journal of the European Federation of Neurological Societies, 22(7), 1124–1130. 10.1111/ene.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin L, Maybery M, Wray J, & Whitehouse A (2013). A “Bottom-Up” Approach to Aetiological Research in Autism Spectrum Disorders. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hensley MK, Tasman A, Sears L, Casanova MF, & Sokhadze EM (2016). Heart Rate Variability and Skin Conductance During Repetitive TMS Course in Children with Autism. Applied Psychophysiology and Biofeedback, 41(1), 47–60. 10.1007/si0484-015-9311-z [DOI] [PubMed] [Google Scholar]
- Wiggs L, & Stores G (1996). Severe sleep disturbance and daytime challenging behaviour in children with severe learning disabilities. Journal of Intellectual Disability Research, 40(6), 518–528. [DOI] [PubMed] [Google Scholar]
- Wiggs L, & Stores G (2004). Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine and Child Neurology, 46(6), 372–380. [DOI] [PubMed] [Google Scholar]
- Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, Yusuf A, Shih A, & Elsabbagh M (2022). Global prevalence of autism: A systematic review update. Autism Research, 15(5), 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Havrilla J, Peng J, Drye M, Fecher M, Guthrie W, Tunc B, Schultz R, Wang K, & Zhou Y (2022). Development of a phenotype ontology for autism spectrum disorder by natural language processing on electronic health records. Journal of Neurodevelopmental Disorders, 14(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheutlin AB, Dennis J, Karlsson Linnér R, Moscati A, Restrepo N, Straub P, Ruderfer D, Castro VM, Chen C-Y, Ge T, Huckins LM, Charney A, Kirchner HL, Stahl EA, Chabris CF, Davis LK, & Smoller JW (2019). Penetrance and Pleiotropy of Polygenic Risk Scores for Schizophrenia in 106,160 Patients Across Four Health Care Systems. The American Journal of Psychiatry, 176(10), 846–855. 10.1176/appi.ajp.2019.18091085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn MA, & Jason LA (2021). Cortical autonomic network connectivity predicts symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). International Journal of Psychophysiology, 170, 89–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


