Abstract
Background:
Needle and syringe programs (NSPs) are effective at preventing HIV and hepatitis C virus (HCV) among people who inject drugs (PWID), yet global coverage is low, partly because governments lack data on the cost and cost-effectiveness of NSP in their countries to plan and fund their responses. We conducted a global systematic review of unit costs of NSP provision to inform estimation of cost drivers and extrapolated costs to other countries.
Methods:
We conducted a systematic review to extract data on the cost per syringe distributed and its cost drivers. We estimated the impact of country-level and program-level variables on the cost per syringe distributed using linear mixed-effects models. These models were used to predict unit costs of NSP provision, with the best performing model used to extrapolate the cost per syringe distributed for 137 countries. The total cost for a comprehensive NSP (200 syringes per PWID/year) was also estimated for 68 countries with PWID population size estimates.
Results:
We identified 55 estimates of the unit cost per syringe distributed from 14 countries. Unit costs were extrapolated for 137 countries, ranging from $0.08 to $20.77 (2020 USD) per syringe distributed. The total estimated spend for a high-coverage, comprehensive NSP across 68 countries with PWID size estimates is $5 035 902 000 for 10 887 500 PWID, 2.1-times higher than current spend.
Conclusion:
Our review identified cost estimates from high-income, upper-middle-income, and lower-middle-income countries. Regression models may be useful for estimating NSP costs in countries without data to inform HIV/HCV prevention programming and policy.
Keywords: costs, global cost extrapolation, harm reduction, needle and syringe programs, people who inject drugs, syringe exchange program
Introduction
Needle and syringe programs (NSPs) are community-based harm reduction programs for people who inject drugs (PWID) [1]. The World Health Organization (WHO) recommends that comprehensive NSPs provide clean needles and syringes, condoms, filters, sterile water, alcohol swabs, spoons, needle containers, acidifiers, tourniquets, bleach and disinfectants, first aid, HIV and STI testing and treatment, education, drug treatments, and when possible, legal and social services [2]. NSPs are delivered in a variety of ways, including fixed sites, mobile sites, outreach, vending machines, and pharmacies [2]. Globally, NSPs have been shown to reduce injection drug use, injection and sexual risk behaviors, prevent HIV, and mixed evidence regarding hepatitis C virus (HCV) transmission [1,3,4].
The WHO/United Nations Office on Drugs and Crime (UNODC)/Joint United Nations Programme on HIV/AIDS (UNAIDS) set a high-coverage target of 200 needles and syringes distributed per PWID per year to prevent HIV transmission [5,6]. Unfortunately, global coverage is low [6], and across countries, there is high variability in the implementation and coverage of NSPs. Although 92 countries have some form of NSP availability, the distribution of needles and syringes is generally low and insufficient to reach all PWID [6,7]. Globally, an estimated 33 syringes are distributed per PWID per year; however, there are large regional differences, with, for example, Australia distributing 396 syringes per PWID and 0 syringes distributed per PWID in the Pacific Islands [6]. Reasons for low coverage of NSPs are multifactorial, including stigma, lack of resources, misconceptions, limited political will, and legal challenges [8–14].
Although it has been shown that NSPs are cost-effective or cost-saving [15], there is a lack of data on the unit cost of NSP provision in many countries. Unit cost estimates are critical to support evidence-based resource mobilization to scale up NSPs, particularly in areas with low coverage. To help inform government policies and resource allocation related to the cost of a comprehensive NSP, we conducted a global systematic review of unit costs of NSP provision, which included the operations costs, and extrapolated the costs of a comprehensive NSP to countries with and without existing cost data.
Materials and methods
Systematic review
A systematic literature review of peer-reviewed and grey literature was conducted between January and October 2020, in consultation with a librarian with systematic review expertise. No restrictions for dates, language, or geographic location were used. The project was conducted following Cochrane guidelines and is reported using PRISMA guidelines [16,17]. The peer-reviewed literature was searched using 13 databases and the search strategy consisted of intervention-specific terms in English (e.g., “needle sharing,” “needle exchange programs,” or “NSEP”) and economic terms (e.g., “cost,” “economic,” or “funding”). For grey literature, 16 sources were selected a priori based on results from a previous global systematic review on the costs of medications for opioid use disorders. Keywords for the grey literature search included intervention-specific terms if searching an economic site and economic terms if the site was focused on harm-reduction, such as “cost AND (contains: needle OR syringe).” Search strategies varied based on the type of source as there was substantial variation in how documents could be searched (see Supplement Table 1). Unit cost estimates of cost per syringe distributed were extracted and standardized to 2020 USD$. More on the systematic review process, data extraction, and cost standardization of extracted estimates is detailed in the supplement.
Statistical analyses
Descriptive statistics
With the outcome measure of cost per syringe distributed being continuous, this variable was first natural log-transformed and assessed for normality; no outliers were identified. Next, we described country and programmatic variables with frequencies for categorical covariates and means, standard deviations, medians, interquartile ranges (IQRs), and minimum and maximum values for continuous covariates. Payer perspective was also obtained as either societal, provider, or not reported. Countries were categorized using the World Bank Gross National Income (GNI) at country level to examine the characteristics by income level. Bivariate analyses were conducted using an ANOVA for the categorical covariates and a Pearson's correlation coefficient for continuous covariates by country GNI level.
Regression model development
To extrapolate the NSP unit costs for countries with no data, we first developed multivariable linear regression models using data from countries with complete cost data.
Covariates for the unit cost extrapolation models were selected based on economic theory and our literature review and comprised of both country and program-level variables. The country-level covariates included the 2020 GDP per capita, the WHO Health Systems Ranking Index (HSRI), the number of needles and syringes distributed per PWID per year within a country, and the number of NSPs within a country. We hypothesized that higher 2020 GDP per capita was associated with higher NSP unit costs. The WHO HSRI was used to represent overall health system performance [18], and we hypothesized that the higher the country's health system ranking, the higher the expected unit cost. We further hypothesized that the higher number of needles and syringes distributed per PWID per year at the country level [6] would be associated with lower NSP costs because of increased program efficiency (economies of scale). Lastly, the number of NSPs within a country was examined [6], with the hypothesis being that the more NSPs that are in a country the lower the cost per syringe distributed due to program efficiency.
The program-level variables included program age, number of components offered, and inclusion of ancillary services. Variables were selected due to their potential as important cost drivers of the overall program. The age of the program was dichotomized as less than 6 months versus at least 6 months of operations and we hypothesized that a program with less than 6 months of operation would be more expensive than older programs. The 6-month operation window was used as this is the time period used by the GHCC Unit Cost Study Repository [19]. The number of additional WHO-recommended program components (services) variable was a count of how many services were included at the NSP, regardless of whether they were costed. We hypothesized that programs with more components were more expensive. Finally, we hypothesized that the costing of ancillary services would be associated with higher unit costs. More on the details of all country-level and program-level model covariates can be found in the supplement.
We estimated: , where: was the natural log cost per syringe distributed and the independent variables were the country-level and program-level characteristics. The country-level covariates included the 2020 GDP per capita (GDPpc), the country HSRI (HSRI), the number of needles and syringes distributed per PWID per year , and the number of NSPs in the country (NSPs). The program-level covariates were program age (Age), the number of components offered (Components), and if ancillary services (Ancillary) were costed. We created models with different combinations of the above components, resulting in 19 models, with two of the models examining random effects for the country-level intercept, and the remaining 17 models assuming fixed effects. Multiple models were developed to determine and assess the drivers of the unit cost per syringe distributed for NSPs from the extracted data. The top four models, assessed by having the lowest Akaike information criterion (AIC) and highest R2 adjusted, were examined for cost prediction. Detailed specifications of all models are in Supplement Table 3 and the four best-performing models are in Table 2.
Table 2.
Multivariable linear regression model results for the four best performing models.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| Predictors | Estimates | CI | p | Estimates | CI | p | Estimates | CI | p | Estimates | CI | p |
| Intercept | −1.41 | −3.37–0.55 | 0.154 | −0.79 | −3.29–1.71 | 0.527 | −2.53 | −4.47 – −0.60 | 0.011 | −2.73 | −4.81 – −0.66 | 0.011 |
| Log of GDP per Capita (2020) | −0.21 | −0.48–0.06 | 0.127 | −0.25 | −0.54–0.04 | 0.090 | −0.13 | −0.42–0.16 | 0.373 | −0.15 | −0.43–0.14 | 0.314 |
| Health Systems Ranking Index (HSRI) | 4.69 | 2.10–7.28 | 0.001 | 4.77 | 2.16–7.39 | 0.001 | 3.55 | 0.96–6.15 | 0.008 | 3.69 | 1.03–6.36 | 0.008 |
| Log Country-Level Number of Syringes Distributed per PWID | −1.11 | −2.21 – −0.02 | 0.046 | −1.17 | −2.28 – −0.06 | 0.039 | ||||||
| Program Less than 6 months old (Yes/Not Reported) | 0.55 | 0.16–0.95 | 0.006 | 0.52 | 0.11–0.92 | 0.013 | 0.59 | 0.18–1.01 | 0.006 | 0.61 | 0.20–1.02 | 0.004 |
| Number of Program Components | −0.11 | −0.33–0.11 | 0.319 | −0.09 | −0.32–0.14 | 0.444 | −0.09 | −0.34–0.16 | 0.469 | −0.11 | −0.36–0.14 | 0.392 |
| Ancillary Services were Costed (Yes) | 0.78 | 0.42–1.14 | <0.001 | 0.83 | 0.44–1.22 | <0.001 | 0.83 | 0.46–1.21 | <0.001 | 0.83 | 0.45–1.20 | <0.001 |
| Log Number of NSPs in Country | −0.23 | −0.80–0.34 | 0.422 | |||||||||
| Log Program-Level Number of Syringes Distributed (Regional Mean for Missing Values) | −0.03 | −0.30–0.23 | 0.795 | |||||||||
| Log Program-Level Number of Syringes Distributed (Global Mean for Missing Values) | 0.01 | −0.25–0.27 | 0.934 | |||||||||
| Observations | 55 | 55 | 55 | 55 | ||||||||
| R2/R2 adjusted | 0.529/0.471 | 0.536/0.467 | 0.489/0.425 | 0.488/0.424 | ||||||||
| AIC | 102.784 | 104.022 | 107.323 | 107.393 | ||||||||
Statistical significance indicated by bold values.
Cost prediction using the full dataset
Each model's robustness was tested by sequentially predicting the NSP cost outcome for every country with available cost data. For countries with more than one estimate, the most common response was used for the categorical program-level characteristics. Within individual country program-level characteristics that accounted for the number of components, a continuous variable, were used to calculate the median number of components. The predicted cost estimates were compared with the observed cost estimates for each country. To evaluate the performance of each model, we assessed the mean absolute error, the mean absolute relative percentage error, and the median absolute relative percentage error between the predicted cost estimate and the extracted cost estimates for all 14 countries where we had estimates from the systematic review. We also assessed whether the observed cost estimate fell within the 95% prediction interval of the model estimate and if the model overestimated or underestimated the cost estimate per country.
Cost prediction using a sequential leave one country out process
The predictive performance of the models was then assessed by sequentially removing each country's cost data and using the adapted model to predict the excluded country's NSP unit cost. For the models that used a random intercept, we assigned a country-specific random intercept using a nearest neighbor match on either the WHO HSRI or the country's GDP, depending on the model. The match was made on the smallest absolute value between the country's GDP or HSRI with the next closest country. The predicted cost estimates for the excluded countries were compared against the observed cost estimates and the models were evaluated using the same methods as described in the cost prediction with full dataset. The final best prediction model was selected based on the highest number of true observations within the model's prediction interval, and the lowest mean absolute relative percentage error. The absolute relative percentage error was calculated for each country by subtracting the extracted cost estimate from the predicted estimate, then dividing this difference by the extracted cost estimate, and taking the absolute value. The mean of the absolute relative percentage error was calculated by taking the mean of the absolute relative percentage error for all of the countries.
Extrapolation of cost of a comprehensive needle and syringe program globally
The best prediction model was used to predict unit cost estimates for a comprehensive NSP for countries both with and without an observed cost estimate. On the basis of the requirements of our best prediction model, we generated predictions for countries with estimates for the number of needles and syringes distributed per PWID per year, country GDP, and WHO HSRI [6]. For the program-level characteristics, the predetermined characteristics selected were a program that was older than 6 months, inclusion of four program components, and that had costed ancillary services. The cost estimates of a comprehensive NSP for countries for which we do and do not have observed cost data were extrapolated with this information.
Estimation of total spending per country for current vs. comprehensive needle and syringe programs
For countries with PWID population size estimates, we estimated total annual current NSP spend and the annual cost of achieving high-coverage NSPs (200 syringes distributed per PWID per year) with a comprehensive NSP. We estimated current spend by multiplying the unit cost per syringe distributed of current programs (using regional averages for program-level components) by the country-level estimates for the number of syringes distributed per PWID [6] and the total number of PWID [20]. The unit cost per syringe distributed was inclusive of all costs related to the NSP operation. To estimate comprehensive high-coverage program costs, we multiplied the extrapolated comprehensive unit cost estimates by 200 (high-coverage target) multiplied by the estimated number of PWID in that country [20]. The increase needed in spending was calculated by subtracting the current spend estimate from the comprehensive high-coverage cost estimate and dividing the difference by the current spend estimate. For all analyses, R version 3.6.3 was used [21].
Results
Systematic review results
Systematic review results are presented in the PRISMA flow diagram (Fig. 1). The full-text review identified 44 studies eligible for data extraction, which included unit cost estimates for 31 countries (Supplement Table 2). Of these, 14 countries had at least one cost per syringe distributed estimate, resulting in 55 individual estimates (Supplement Figure 1a and Supplement Table 3). Details on the systematic review results are provided in the supplement.
Fig. 1.
Eligible study identification, screening, and inclusion process.
Descriptive statistics for the cost per syringe distributed estimates by country GNI group are displayed in Table 1. The majority of the unit cost estimates were from high-income countries (78%), particularly the USA and Australia, with the remaining cost estimates originating from upper-middle-income countries (13%) and lower-middle-income countries (9%). We did not find published cost estimates from low-income countries. The majority of the costing studies (91%) used a provider prospective. Most programs did not report any additional WHO-recommended program components (60%) other than distributing clean needles and syringes; however, most included the cost of ancillary services (66%), and a large percentage were in operation less than 6 months or did not report program age (71%). The median cost per syringe distributed across all studies was 2020 USD$0.81 (IQR: $0.49--$1.19). Median unit cost was higher among high-income countries ($0.86; IQR: $0.55--$1.29), compared with upper-middle ($0.49; IQR: $0.22--$0.82) and lower-middle-income countries ($0.36; IQR: $0.18--$0.64).
Table 1.
Characteristics of studies that provided primary data on needle and syringe program costs.
| Descriptive statistics for cost per syringe distributed by country GNI level | ||||
| Overall (n = 55) | High income (n = 43) | Upper-middle income (n = 7) | Lower-middle income (n = 5) | |
| n (%) | n (%) | n (%) | n (%) | |
| Countries | 14 | 4 | 5 | 5 |
| Number of intervention componentsa | ||||
| 0 | 33 (60.0%) | 25 (58.1%) | 5 (71.4%) | 3 (60.0%) |
| 1 | 18 (32.7%) | 14 (32.6%) | 2 (28.6%) | 2 (40.0%) |
| 2 | 3 (5.5%) | 3 (7.0%) | 0 (0.0%) | 0 (0.0%) |
| 3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 4 | 1 (1.8%) | 1 (2.3%) | 0 (0.0%) | 0 (0.0%) |
| Ancillary services costed | ||||
| Yes | 36 (65.5%) | 27 (62.8%) | 7 (100.0%) | 3 (60.0%) |
| No/NR | 19 (34.6%) | 16 (37.2%) | 0 (0.0%) | 2 (40.0%) |
| Program less than 6 months old | ||||
| Yes/NR | 39 (70.9%) | 34 (79.1%) | 2 (28.6%) | 3 (60.0%) |
| No | 16 (29.1%) | 9 (20.9%) | 5 (71.4%) | 2 (40.0%) |
| Payer prospective | ||||
| Societal | 2 (3.6%) | 1 (2.3%) | 1 (14.3%) | 0 (0.0%) |
| Provider | 50 (90.9%) | 41 (95.4%) | 6 (85.7%) | 3 (60.0%) |
| Not Reported | 3 (5.5%) | 1 (2.3%) | 0 (0.0%) | 2 (40.0%) |
| Cost per syringe distributed (inflated 2020 USD) | ||||
| Mean (SD) | 1.01 (0.96) | 1.14 (1.03) | 0.61 (0.45) | 0.49 (0.41) |
| Median (IQR: Q1-Q3) | 0.81 (0.49–1.19) | 0.86 (0.55–1.29) | 0.49 (0.22–0.82) | 0.36 (0.18–0.64) |
| Range | 0.13–5.69 | 0.13–5.69 | 0.18–1.44 | 0.14–1.13 |
Intervention components refers to additional WHO-recommended program services that were included in treatment regardless if they were costed.
Assessment of needle and syringe program cost drivers
The four regression models that best fit the data and best predicted the unit cost per syringe distributed estimates were assessed and are presented in Table 2(results for all 19 models are in Supplement Table 4). The best performing four models all used fixed effects. Models 1 and 2 included the country-level estimates of the log number of syringes distributed per PWID [6] that came from another systematic review, while Models 3 and 4 did not include this country-level variable and instead included the program-level estimate of the log number of syringes distributed, which was extracted from the systematic review. Model 3 used a regional mean for data with a missing value for this variable, whereas Model 4 used a global mean if there were missing values for this variable. Model 2 also included a country-level variable for the number of NSPs in the country [6]. Among the four models, Model 1 had the lowest AIC and the highest R2 adjusted, suggesting that it was the best fit model for the data. All four models were used for prediction with the full dataset and in the leave-one-out process to examine which of the models were best at predicting the unit cost per syringe distributed.
All of the models showed high theoretical validity as the direction of associations for the covariates aligning with a priori expectations. In all of the four highlighted models, the WHO HSRI was found to be significantly and positively correlated to the log cost per syringe distributed. The country-level variable for the log number of syringes per PWID in each country [6] was significant and negatively correlated in both models it was included, Models 1 and 2. The program-level variables of whether a program was less than 6 months old (yes/not reported) and if ancillary services were costed (yes) were both significantly and positively associated with the outcome of unit cost per syringe distributed in all of the models. The country-level variable log of the 2020 GDP and the program-level variable of the number of intervention components offered were not statistically significant predictors in any of the models. For Model 2, the country-level variable of the number of NSPs in the country was not statistically significantly associated with the unit cost per syringe distributed. For Model 3, the log of the program-level number of syringes distributed, using a regional mean for missing data, was not statistically significant. Lastly, for Model 4, there was no statistically significant association between the log of the number of syringes distributed at the program-level, with a global mean used for missing data, and the unit cost per syringe distributed.
Cost prediction using the full dataset
Models 1--4 all performed well at predicting the unit costs using the full dataset, with all 14 of the observed mean country unit costs falling within the 95% prediction intervals for the estimates from these models (Table 3). Models 1 and 2 had the lowest mean absolute error (0.25 and 0.26, respectively) and the lowest mean relative percentage error (37.3 and 37.1%, respectively). Model 1 also had the lowest median relative percentage error (22.1%), with higher error in other models (ranging from 24.9 to 27.4%).
Table 3.
Predictive performance of the best four performing multivariable linear models.
| Model results (Number of countries = 14) | ||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Random effects? | No | No | No | No | ||||
| Full Dataset | Exclude Country | Full Dataset | Exclude Country | Full Dataset | Exclude Country | Full Dataset | Exclude Country | |
| Number of observations within prediction interval | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 13 |
| Mean absolute error | 0.25 | 0.30 | 0.26 | 0.36 | 0.36 | 0.49 | 0.37 | 0.48 |
| Mean relative percentage error | 37.30% | 49.10% | 37.14% | 51.47% | 44.61% | 62.37% | 45.99% | 60.56% |
| Median relative percentage error | 22.05% | 36.30% | 25.32% | 34.42% | 27.38% | 51.06% | 24.89% | 50.21% |
| Number of overestimating predictions | 8 | 9 | 8 | 9 | 9 | 9 | 8 | 8 |
| Number of underestimating predictions | 6 | 5 | 6 | 5 | 5 | 5 | 6 | 6 |
| AIC | 102.78 | 104.02 | 106.22 | 106.37 | ||||
Cost prediction using a sequential leave one country out process
Using the leave one out process, three models (1, 2, and 3) correctly predicted all 14 of the observed country unit costs within the 95% prediction intervals, with Model 4 predicting 13 of the 14. As in the full dataset prediction, Model 1 had the lowest mean absolute error (0.30) and the lowest mean relative percentage error (49.1%). As Model 1 predicted all countries and had the lowest mean absolute and relative error, it was selected as the best fitting model to use for the cost extrapolation of a comprehensive NSP globally.
Extrapolation of cost of a comprehensive needle and syringe program globally
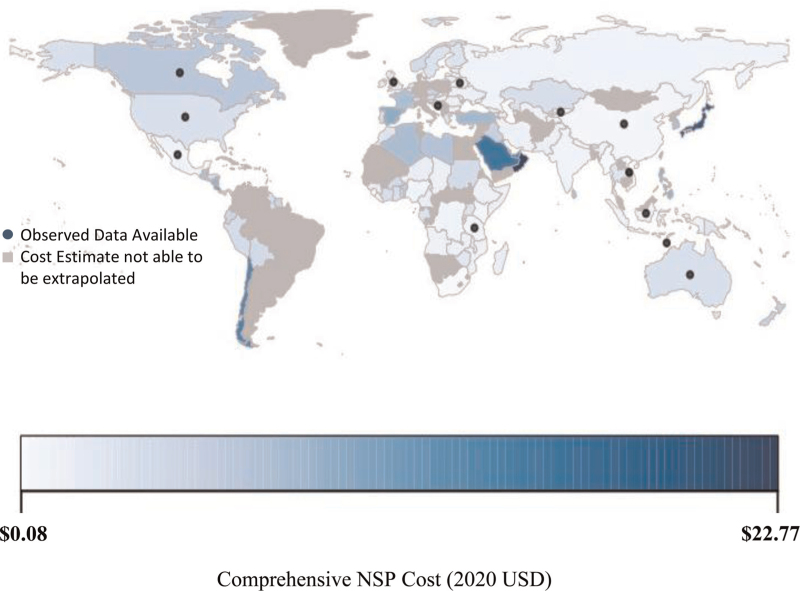
Using the best performing prediction model (Model 1), we extrapolated unit cost per syringe distributed in 2020 USD$ for a comprehensive program in 137 countries (countries with estimates for the number of needles and syringes distributed per PWID [6], a model covariate). These unit cost extrapolations ranged from $0.08 to $20.77 (2020 USD) (Fig. 2), with all extrapolated costs by country in Supplement Table 5.
Fig. 2.
Predicted unit cost of a comprehensive needle and syringe program per syringe distributed (2020 USD) for 137 countries.
Estimation of total spending per country for current vs. comprehensive needle and syringe programs
Among the 137 countries where the cost of a comprehensive NSP was extrapolated, 68 countries had estimates for the number of PWID within the country [20]. Estimated current spend and the estimated high-coverage, comprehensive spend by country can be found in Supplement Table 6. The total global estimated current spend on NSPs for these 68 countries is $1 603 501 830 (2020 USD). For high-coverage, comprehensive NSPs among these 68 countries, the estimated global spend is $5 035 902 000 (2020 USD), which is 2.1-times higher than the estimated current spend. This would provide comprehensive NSPs to 10 887 500 PWID in these 68 countries. Some countries are already spending the amount they would need and other countries need to spend an additional 128-times the current spend is to reach the high-coverage, comprehensive NSP targets (Supplement Figure 2).
Discussion
Our systematic review identified 55 NSP unit cost estimates from 14 middle and high-income countries. Higher unit costs were associated with countries with higher HSRI and fewer syringes distributed, and with newer programs, which confirmed our hypothesis. The number of intervention components included was not seen to affect the unit cost, possibly because the majority of programs did not include any additional WHO-recommended intervention components. Using our best performing model, the cost per syringe distributed of a comprehensive NSP was extrapolated to 137 countries. We find that current spend on NSP among 68 countries examined needs to increase by 2.1-times the current spend to achieve the WHO/UNODC/UNAIDS 2020 target goals of 200 syringes distributed per PWID. Reaching the high-coverage targets for NSPs can reduce the burden of HIV and HCV infection among PWID [22] and has been found cost-effective in several settings [15,23,24].
There were limitations to this study. Our review included a disproportionate number of estimates from the USA and Australia, and the search was conducted using terms in English. The overrepresentation of high-income countries could lead to inflated estimates for the extrapolated cost of high-income countries and wider uncertainty for extrapolated cost estimates for low- and middle-income countries. No low-income country estimates were identified, and in the absence of any available data for these settings, our estimates provide a reasonable approach. However, there is a need for resources to support cost data collection in low-income countries to confirm our estimates and inform policymakers on the funding needed to implement harm-reduction programs in these countries.
There are also limitations surrounding the representativeness of the data regarding the number of countries with published data and subsequent generalizability. There were only 14 countries with unit costs estimates, and many countries with known PWID populations that lack cost data. This limits the generalizability for all NSP cost estimates. In addition, among the 14 countries with data, eight countries had only one cost estimate and five countries had two only cost estimates. The lack of several cost estimates for these countries is a limitation for within-country generalizability. Given the paucity of estimates from each country, we lack certainty about how representative the estimates are to these countries. Predictions from the model are as good as the available data can now provide; however, we believe our model provides useful estimates now that may be validated or improved as new data become available.
Lastly, a number of potential covariates were not included in our model due to a lack of data reporting or the high frequency of missing values. These included, for example, whether the NSP was in a rural or urban area, or hours of NSP operation. The variable regarding funding support for the programs was not used as a covariate as it had considerable missing data, and for those without missing data a large portion listed combinations of government and private funding; thus, it was not possible to disentangle these funding types. Despite these limitations, the results were robust to alternative specifications of the model.
Conclusion
This is the first systematic literature review of NSP unit costs and extrapolation of findings to estimate unit costs for a comprehensive NSP for countries with no NSP cost data. Findings can be used to inform NSP policies and resource allocation among other harm reduction strategies to improve health of PWID and prevent transmission of HIV and HCV. More studies are required in low-income countries. Limitations notwithstanding, increased funding for comprehensive NSPs is urgently required globally to achieve WHO/UNODC/UNAIDS high-coverage goals.
Acknowledgements
N.K., A.W., P.V., J.A.K., D.P.W. conceptualized the study and designed the research. C.M., A.V., A.W., C.L.C., K.M.H. designed the systematic review with C.M., A.V., M.H., A.W. conducting the systematic review. J.A.K., C.M., A.W. performed data management and quality control. J.A.K. conducted data analysis, with input from N.K.M., A.W., J.A.C, and A.V. Article development was led by J.A.K. with assistance from N.K.M., A.W., R.S.G., M.L.Z, H.A.P., J.G.Z., P.V., J.A.C., and F.T.P. All authors have read, reviewed, contributed to, and approved the final article.
This project was funded by NIAID/NIDA R01AI147490. N.M. was additionally supported by the San Diego Center for AIDS Research (CFAR) P30AI036214.
N.M. has received unrestricted research grants paid to her university from Gilead unrelated to this work.
The views expressed in this article do not necessarily represent the decisions, policy, or views of UNAIDS.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Jordan A. Killion and Christopher Magana are joint first authors.
Adriane Wynn and Natasha K. Martin are joint senior authors.
Supplemental digital content is available for this article.
References
- 1. The Centers for Disease Control and Prevention (CDC). Syringe Services Programs (SSPs) FAQs. 2019. https://www.cdc.gov/ssp/syringe-services-programs-faq.html. [Accessed August 2023] [Google Scholar]
- 2. World Health Organization (WHO). Guide to starting and managing needle and syringe programmes. WHO, Department of HIV/AIDS; 2007. [Google Scholar]
- 3.Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol 2014; 43:235–248. [DOI] [PubMed] [Google Scholar]
- 4.Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2018; 113:545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users. WHO/UNODC/UNAIDS; 2012. [Google Scholar]
- 6.Larney S, Peacock A, Leung J, Colledge S, Hickman M, Vickerman P, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health 2017; 5:e1208–e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harm Reduction International. The Global State of Harm Reduction, 2022. 8th ed. London: Harm Reduction International, 2022. [Google Scholar]
- 8.Kulesza M, Teachman BA, Werntz AJ, Gasser ML, Lindgren KP. Correlates of public support toward federal funding for harm reduction strategies. Subst Abuse Treat Prev Policy 2015; 10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Des Jarlais DC. Harm reduction in the USA: the research perspective and an archive to David Purchase. Harm Reduct J 2017; 14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyshka E, Anderson-Baron J, Karekezi K, Belle-Isle L, Elliott R, Pauly B, et al. Harm reduction in name, but not substance: a comparative analysis of current Canadian provincial and territorial policy frameworks. Harm Reduct J 2017; 14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo AD, Schmich L, Higgs P, Fischer A. Qualitative evaluation of a peer-based needle syringe programme in Vietnam. Int J Drug Policy 2009; 20:179–182. [DOI] [PubMed] [Google Scholar]
- 12.White B, Haber PS, Day CA. Community attitudes towards harm reduction services and a newly established needle and syringe automatic dispensing machine in an inner-city area of Sydney, Australia. Int J Drug Policy 2016; 27:121–126. [DOI] [PubMed] [Google Scholar]
- 13.Roe G. Harm reduction as paradigm: Is better than bad good enough? The origins of harm reduction. Crit Public Health 2005; 15:243–250. [Google Scholar]
- 14. UNAIDS. In danger: UNAIDS Global AIDS Update 2022. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2022. [Google Scholar]
- 15.Wilson DP, Donald B, Shattock AJ, Wilson D, Fraser-Hurt N. The cost-effectiveness of harm reduction. Int J Drug Policy 2015; 26: (Suppl 1): S5–11. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. 2000: health systems: improving performance. World Health Organization (WHO); 2000. [Google Scholar]
- 19. Reference case for estimating the costs of Global Health Services and Interventions. Global Health Cost Consortium; 2017. [Google Scholar]
- 20.Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 22.Abdul-Quader AS, Feelemyer J, Modi S, Stein ES, Briceno A, Semaan S, et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav 2013; 17:2878–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon JA, Anderson J, Kerr CC, Thein HH, Zhang L, Iversen J, et al. Estimating the cost-effectiveness of needle-syringe programs in Australia. AIDS 2012; 26:2201–2210. [DOI] [PubMed] [Google Scholar]
- 24.Guinness L, Vickerman P, Quayyum Z, Foss A, Watts C, Rodericks A, et al. The cost-effectiveness of consistent and early intervention of harm reduction for injecting drug users in Bangladesh. Addiction 2010; 105:319–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.