Abstract
The principal limitations of the terms NAFLD and NASH are the reliance on exclusionary confounder terms and the use of potentially stigmatising language. This study set out to determine if content experts and patient advocates were in favor of a change in nomenclature and/or definition. A modified Delphi process was led by three large pan-national liver associations. The consensus was defined a priori as a supermajority (67%) vote. An independent committee of experts external to the nomenclature process made the final recommendation on the acronym and its diagnostic criteria. A total of 236 panelists from 56 countries participated in 4 online surveys and 2 hybrid meetings. Response rates across the 4 survey rounds were 87%, 83%, 83%, and 78%, respectively. Seventy-four percent of respondents felt that the current nomenclature was sufficiently flawed to consider a name change. The terms “nonalcoholic” and “fatty” were felt to be stigmatising by 61% and 66% of respondents, respectively. Steatotic liver disease was chosen as an overarching term to encompass the various aetiologies of steatosis. The term steatohepatitis was felt to be an important pathophysiological concept that should be retained. The name chosen to replace NAFLD was metabolic dysfunction–associated steatotic liver disease. There was consensus to change the definition to include the presence of at least 1 of 5 cardiometabolic risk factors. Those with no metabolic parameters and no known cause were deemed to have cryptogenic steatotic liver disease. A new category, outside pure metabolic dysfunction–associated steatotic liver disease, termed metabolic and alcohol related/associated liver disease (MetALD), was selected to describe those with metabolic dysfunction–associated steatotic liver disease, who consume greater amounts of alcohol per week (140–350 g/wk and 210–420 g/wk for females and males, respectively). The new nomenclature and diagnostic criteria are widely supported and nonstigmatising, and can improve awareness and patient identification.
INTRODUCTION
Unified global approaches to nomenclature and disease definition are critical for increasing disease awareness, driving policy change, identifying those at risk, and facilitating diagnosis and access to care. Language can create or exacerbate stigma, marginalise segments of the affected population, and, ultimately, contribute to health inequalities. It has been known for many years that being overweight or obese is associated with hepatic steatosis, hepatocyte injury, liver inflammation, and fibrosis. This was formally recognised by the term “nonalcoholic steatohepatitis” in 1980 by Jurgen Ludwig.1 Subsequently, the term nonalcoholic fatty liver disease (NAFLD) was used to describe the histological spectrum of steatosis to steatohepatitis with its subtypes NAFL and NASH. The histological classification was further expanded on by various scoring systems categorising steatosis, disease activity, and fibrosis.2–4 This framework has served as the anchor for our current understanding of the disease, data on the burden of the disease, and efforts to develop a treatment for the condition.
While the nomenclature is widely used, it has always been appreciated that the term “nonalcoholic” did not accurately capture what the aetiology of the disease was, and notably, the term “fatty” has been considered to be stigmatising by some. Furthermore, there are individuals with risk factors for NAFLD, such as type 2 diabetes, who consume more alcohol than the relatively strict thresholds used to define the nonalcoholic nature of the disease, which are not adequately recognised by existing nomenclature and are excluded from trials and consideration for treatments.5 Indeed, there is a recognition now that there are overlapping biological processes that may contribute to both NAFLD and alcohol-associated/related liver disease (ALD). All of these factors have led to growing dissatisfaction with the current nomenclature. This was summarised in a paper by Eslam et al6,7 in 2020 and led to the proposal to use the term metabolic dysfunction–associated fatty liver disease (MAFLD), which includes patients with a fatty liver regardless of the amount and pattern of alcohol intake under this terminology. While MAFLD was accepted by some, concerns were raised about the mixing of etiologies, continued use of the term “fatty” considered stigmatising by many, and restricting the population to those with 2 metabolic risk factors and allowance of more liberal alcohol use, thus impacting our understanding of natural history.8–10 One area of particular concern was the potential negative impact of changes in diagnostic criteria for the disease in terms of biomarker and therapeutic development.7,9,10
These concerns led to a multistakeholder effort under the auspices of the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) in collaboration with the Asociación Latinoamericana para el Estudio del Hígado (ALEH) with the engagement of academic professionals from around the world, including hepatologists, gastroenterologists, pediatricians, endocrinologists, hepatopathologists, and public health and obesity experts along with colleagues from industry, regulatory agencies, and patient advocacy organisations to resolve these concerns and develop a consensus on a change in nomenclature and the diagnostic criteria for the condition. This manuscript summarises the methodology, including a multistep Delphi process, the results of the process and provides the consensus recommendations endorsed by societies, patient advocacy groups, journals, and industry for adoption by all stakeholders.
METHODS
Panel generation and statement development
The panel for this Delphi study was generated through an iterative, inclusive process involving diverse liver organisations around the world (Table 1). The Steering Committee (n = 36) was composed of 2 co-chairs (Mary E. Rinella and Philip N Newsome), representing AASLD and EASL, respectively, and 34 other members nominated by their respective associations with a view to ensuring broad geographic representation.
TABLE 1.
Delphi panel characteristics (N = 225)
| N (%) | |
|---|---|
| Professional characteristics | |
| Primary sector of employment | |
| Civil society | 7 (3) |
| Private | 21 (9) |
| Public | 34 (15) |
| Academic | 158 (70) |
| Other | 4 (2) |
| Primary field/area of work | |
| Clinical research | 118 (54) |
| Health care provider | 61 (28) |
| Nonclinical research | 13 (6) |
| Patient/policy advocacy | 18 (9) |
| Other | 7 (4) |
| Primary area of specialty/expertisea (among health care providers, clinical and nonclinical researchers) | |
| Gastroenterology | 7 (4) |
| Endocrinology | 13 (7) |
| Hepatology | 151 (82) |
| Other | 13 (8) |
| Years working in the field post-training | |
| 0–12 | 53 (29) |
| 13–24 | 69 (37) |
| 25–36 | 51 (27) |
| 37–48 | 13 (7) |
| % of work in NAFLD-related clinical care, research, or both | |
| 0–25 | 26 (12) |
| 26–50 | 61 (27) |
| 51–75 | 68 (30) |
| 76–100 | 44 (19) |
| No. articles (co)authored on topic of NAFLD | |
| <6 | 32 (17) |
| 6–20 | 42 (22) |
| 21–50 | 39 (21) |
| >50 | 74 (40) |
| Liver organisation associated with (N invited) | |
| AASLD (72) | 60 (27) |
| ALEH (30) | 27 (12) |
| APASL, AMAGE, INASL, SAASL, TASL (41) | 29 (13) |
| EASL (70) | 66 (29) |
| GI and endocrinological societies (21) | 15 (7) |
| Pathology societies (4) | 3 (1) |
| Patient organisation (29) | 24 (11) |
| Personal characteristics | |
| Sex | |
| Woman | 88 (40) |
| Man | 135 (60) |
| Nonbinary or sex diverse | 0 |
| Prefer not to say | 0 |
| Country where born (N = 59)b | |
| High income | 163 (73) |
| Low and middle income | 61 (27) |
| Country where currently working (N = 54)b | |
| High income | 183 (82) |
| Low and middle income | 41 (18) |
Notes: Ns for different characteristics vary due to missing data; percentages may not sum to 100 due to rounding. With respect to the respondent’s area of expertise, 184 of 192 participating health care providers and researchers responded to the request to provide their area of expertise.
24 panelists indicated that, in their clinical practice or liver-focused research, they routinely care for or focus on liver disease patients who are under 18 years old. Note that numbers represent those who engaged in the process, rather than those who were invited to join the process, but did not respond.
N of total countries represented.
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALEH, Asociación Latinoamericana para el Estudio del Hígado (Latin American Association for the Study of the Liver); AMAGE, African Middle East Association of Gastroenterology; APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver; GI, gastrointestinal; INASL, Indian National Association for the Study of the Liver; SAASL, South Asian Association for the Study of the Liver; TASL, Taiwan Association for the Study of the Liver.
The consensus process used a modified Delphi method11–13 to incorporate input from the literature and a diverse group of content experts, practitioners, and patient advocates. The steering committee identified 5 areas deemed fundamental to the consideration of a revised nomenclature, namely: (1) What are issues with the current nomenclature, and can they be addressed? (2) What is the importance of steatohepatitis in disease definition and endpoints? (3) How should the role of alcohol be accounted for? (4) How might a name change impact disease awareness, clinical trials, and regulatory approval pathways? and (5) Can an alternative name reduce heterogeneity and allow for future advances? Between late 2021 and early 2022, the steering committee was divided into 6 working groups, each with a designated lead (Sven M. Francque, Mary E. Rinella, Philip N Newsome, Arun J. Sanyal, Vlad Ratziu, and Fasiha Kanwal), responsible for reviews of the literature to inform the development of draft statements for their assigned topic area: patient-centered perspective (Sven M. Francque); pros and cons of the current nomenclature (Mary E. Rinella); defining fatty liver disease in the setting of metabolic dysfunction (Philip N Newsome); disease heterogeneity (Arun J. Sanyal); histopathology (Vlad Ratziu); and how to manage the role of alcohol in dual aetiology (Fasiha Kanwal). The preliminary draft statements were compiled and shared with the larger steering committee for review, and the feedback was incorporated into a revised set of draft consensus statements (Supplemental Table S1, http://links.lww.com/HEP/H885).
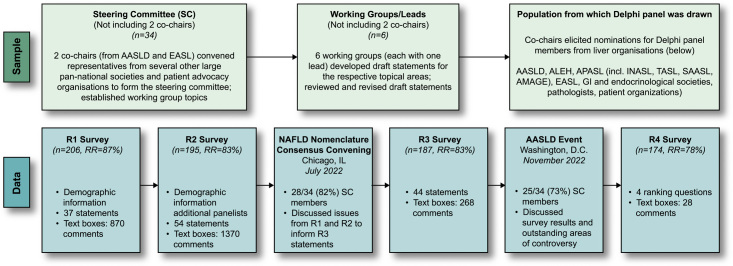
Pan-national societies were asked to nominate additional experts and other stakeholders including patient advocacy organisations to be invited (n = 267) to participate in the Delphi panel (Figure 1). Nominating societies were instructed to select individuals actively engaged in NAFLD research or clinical practice of patients with NAFLD. The consensus was defined a priori as a supermajority (67%) vote. To increase geographic diversity in the Delphi panel, an additional 30 experts were invited to participate in R2. The characteristics, including demographics, professional expertise, and geographic representation, of Delphi panel participants (n = 224) are summarised in Table 1 and Supplemental Table S2, http://links.lww.com/HEP/H885.
FIGURE 1.
Summary of the Delphi process. The top section depicts the iterative sampling approach employed to generate a large, diverse Delphi panel (267 experts invited and 225 participated across the 4 rounds). The 2 co-chairs, from AASLD and EASL, respectively, convened representatives from several other large pan-national societies and patient advocacy organisations to form the Steering Committee. This group identified 6 topics/working groups that led the development of a preliminary set of consensus statements, which were reviewed by the larger steering committee and subsequently revised. The co-chairs elicited nominations for Delphi panel members from a diverse group of liver organisations. The bottom section depicts the 4 survey rounds (R1–R4) of data collection from the full Delphi panel, which involved panelists’ indicating their level of agreement/disagreement (ie, consensus) with statements in each survey round, as well as the ability to provide comments in open-ended text boxes. Draft consensus statements were revised based on panelists’ comments for subsequent rounds. Two large expert convenings were held following R2 and R3 to permit group discussion of issues raised from the survey data collection components of the Delphi methodology. Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALEH, Asociación Latinoamericana para el Estudio del Hígado (Latin American Association for the Study of the Liver); AMAGE, African Middle East Association of Gastroenterology; APASL, Asian Pacific Association for the Study of the Liver; EASL, European Association for the Study of the Liver; GI, gastrointestinal; INASL,Indian National Association for the Study of the Liver; RR, response rate; SAASL, South Asian Association for the Study of the Liver; TASL, Taiwan Association for the Study of the Liver.
Data collection
The Delphi process was comprised of 6 components of online data collection (through the Qualtrics platform) and in-person discussions, including a first round (R1) survey (April 7 to May 9, 2022); a second round (R2) survey (June 15–27, 2022), plus additional panelists (8 September–16 October); a large-group nomenclature consensus meeting (Chicago, IL, USA, July 2022); a third round (R3) survey (October 17–27, 2022); a second convening (AASLD annual meeting, Washington, DC, November 2022) involving both steering committee and larger panel discussions; and a fourth round (R4) survey (December 2, 2022, to January 22, 2023) (Figure 1). Draft consensus statements contained predominantly 4-point Likert-type response categories related to agreement/disagreement (eg, agree/somewhat agree/somewhat disagree/disagree), support/opposition, 3-point responses (eg, increase, no change, and decrease), and so on. All statements included a “not qualified to respond” option to accommodate the diverse expertise represented in the panel. In addition, in line with established Delphi processes,11–13 text boxes appeared after panelists entered responses to each statement, so they could provide comments and suggest edits, if desired. These were reviewed and used to modify statements in subsequent survey rounds.
Analysis plan
The survey question and textbox data in the Delphi study required quantitative and qualitative analysis. For each survey question, responses were generated, and frequencies for all response categories were recoded to the 4-point response statements to dichotomous construction (eg, agree + somewhat agree vs. somewhat disagree + disagree) to determine whether the level of consensus with individual statements reached the minimum supermajority (ie, ≥67%) cutoff, which was agreed on a priori. For each statement, those selecting “not qualified to respond” were removed from the denominator to calculate statement frequencies from the relevant sample. The qualitative data collected from the text boxes were reviewed individually by the co-chairs and working group leaders, and then discussed in a series of meetings following each survey round to inform decisions regarding statement modification, deletion, and/or addition.
For the final decision on both acronym and definition, an external expert committee, comprising content experts from hepatology, endocrinology, paediatrics, and patient advocacy representatives, was created and led by two members of the Steering Committee (Vlad Ratziu and Arun J. Sanyal). The committee was established to represent diversity in terms of expertise and geography, with members chosen based on a prior substantial high-impact publication record in the field. It was composed of 21 members (including 15 who were not part of the Steering Committee) and included 4 endocrinologists and 5 pediatric hepatologists. The external committee discussed and recommended the final name and acronym from the top 3 choices that emerged from the final Delphi round. In addition, based on the output from the Delphi process up to this point, the external committee refined the definition, including metabolic parameters for both adult and pediatric diseases. The proposal from this external committee was discussed and approved by the broader NAFLD Nomenclature Steering Committee and then presented to the societies’ leadership (AASLD, EASL, and ALEH) for additional commentary and approval.
RESULTS
Delphi panel characteristics
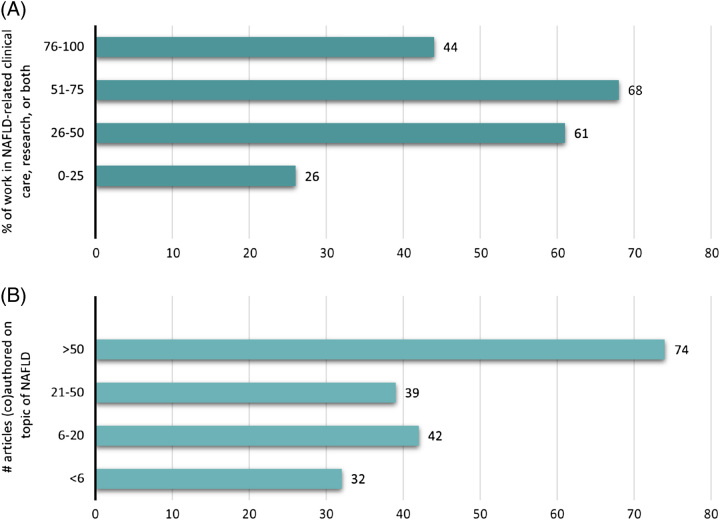
Invitation to participate on the Delphi panel included seven societies or organisation types, with 29% from EASL, 27% from AASLD, 13% from APASL, 12% from ALEH, 7% from other societies, and 11% from patient advocacy organisations. We collected descriptive information from all Delphi panel participants, including demographic and professional data (Table 1). The panel was geographically and demographically diverse; panelists from over 50 countries participated with regard to both country of birth (n = 59 countries) and country where currently working (n = 54 countries). (Supplemental Table S2, http://links.lww.com/HEP/H885) Among the panelists, 40% identified themselves as female and 60% as male. Seventy of the panelists were from the academic sector, with smaller proportions from the public (15%), private (9%), and civil society (3%) sectors. The 2 largest fields/areas of work were clinical research (54%) and clinical care (28%), with hepatology (82%) accounting for an overwhelming majority of the areas of specialisation. There was substantial NAFLD-related expertise among panelists with 76% indicating that they spend 26%–100% of their work time in NAFLD-related clinical care, research, or both, 61% reporting having authored ≥21, and 40% having >50 publications on the topic of NAFLD (Figure 2).
FIGURE 2.
NAFLD-related professional characteristics of Delphi panelists. (A) Data represent the number of respondents (x-axis) and percentage (y-axis) of time spent in NAFLD-related clinical care, research, or both. Similarly, (B) depicts the number of respondents (x-axis) and percentage (y-axis) that have (co)authored articles on the topic of NAFLD.
Response rates and panel participation
The R1 survey consisted of 37 statements within 3 domains: (1) nomenclature and distinctions among disease elements (eg, diagnostic criteria, prognosis, and treatment); (2) other factors possibly influencing consideration of additional or alternative terms; and (3) name/term preferences (Supplemental Table S1, http://links.lww.com/HEP/H885). Of 236 invited experts in R1, 206 participated and rated these statements [response rate (RR) = 87%). They also provided 870 comments that were reviewed and incorporated as additional statements and a new pediatric-focused domain into the second round of consensus statements, with a total of 54 statements. Of the 236 panelists invited for R2, 195 participated in R2 (overall participation, 195 + 30, RR = 83%), providing 1370 comments. Comments were organised thematically by their content and reviewed by the leads who then proposed modifications to statements if appropriate, eliminated statements if redundant or as suggested by comments, or carried the statements forward to the next round. To minimise survey fatigue, statements thought to be repetitive or ambiguous were removed from the following round. In addition, statements covering areas of high consensus were not carried forward to R3. Revised statements were shared with the full Steering Committee before proceeding with the next round. For example, in R3, statement revision resulted in 44 statements; there were 187 participants (of 226 invited, RR = 83%) who provided an additional 268 comments.
After R2, all Delphi panelists were invited to a 1.5 day in-person meeting with remote access provided for those unable to travel (n = 130, 61 in-person, 69 virtual in attendance). The nomenclature consensus conference was cohosted by AASLD and EASL in Chicago, IL, July 8 and 9, 2022, for an in-depth discussion of the extensive feedback generated from the first 2 rounds of data collection. This convening provided valuable guidance from a broader group that included the steering committee and the broader group of survey panelists to inform statement revision for the third round. The second in-person convening occurred at the annual AASLD conference on November 6, 2022, in Washington DC, with two fora for consideration and discussion of the third Delphi round—a closed meeting of the steering committee (n = 34 in attendance) followed by a large-group session open to all 2022 AASLD participants including all Delphi panelists. These discussions provided further clarity on the key elements to include in the final round of the nomenclature consensus process.
Based on this feedback, the R4 survey took panelists through a series of four questions that allowed them to select their first and second choices pertaining to terminology preference, whether the term metabolic should be included in the name, the preferred nomenclature (based on their prior choices), and whether or not diagnostic criteria should be revised. Of 224 invited panelists, 174 participated (RR = 78%) and provided 28 comments in a final open-ended textbox (Supplemental Figure S1, http://links.lww.com/HEP/H886).
Data informing nomenclature considerations (R1–R4+)
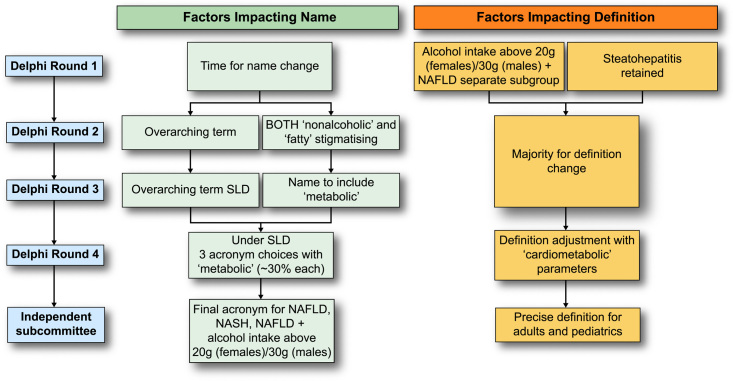
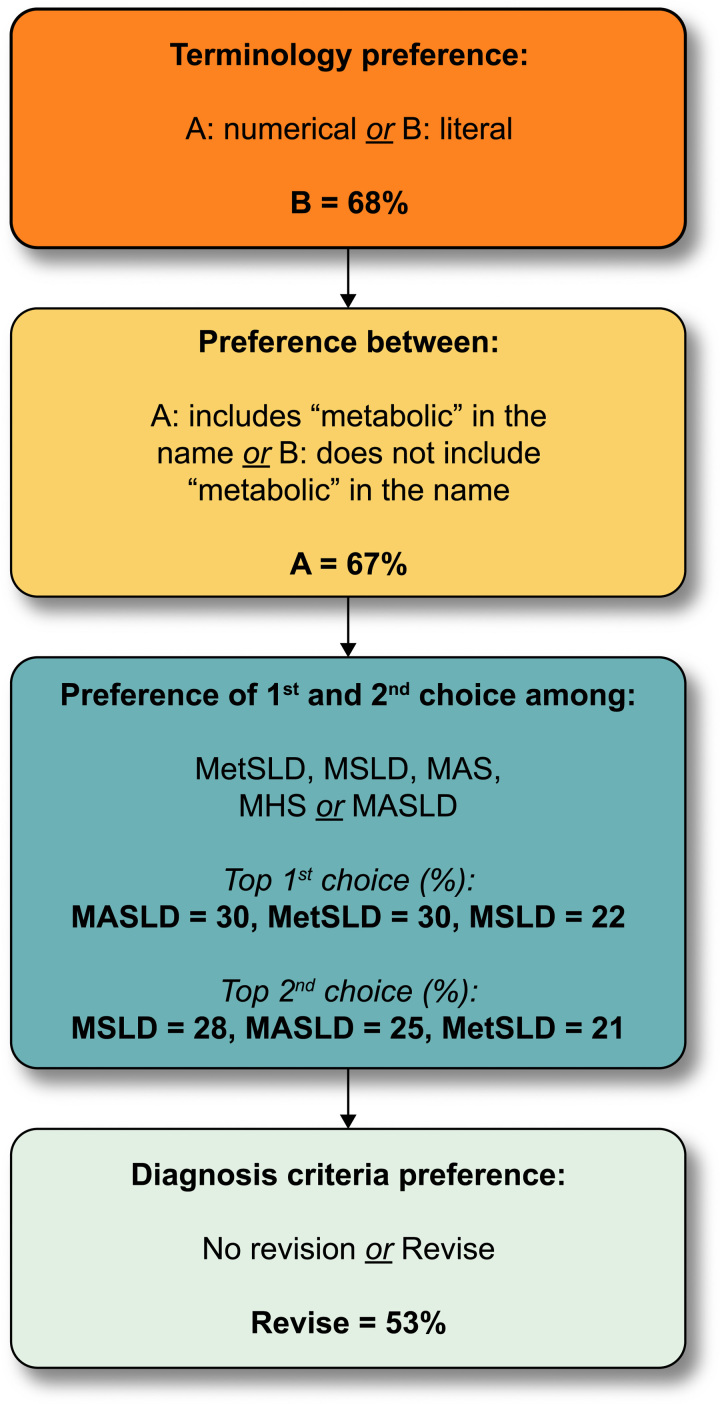
Supplemental Table S1, http://links.lww.com/HEP/H885, shows the evolution of survey statements across survey rounds 1–3 and the degree of agreement in each round. Statements were modified for clarity, changed, or removed based on the review of open-text comments, and output from face-to-face meetings. The main conclusions emerging from survey rounds are summarised in Figure 3. In the fourth Delphi round, only 4 questions were asked to clarify the remaining points of disagreement (Figure 4, Supplemental Figure S1, http://links.lww.com/HEP/H886).
FIGURE 3.
Overview of main findings by Delphi round. The conclusions reached at the end of each Delphi round are depicted here. Results are shown at each corresponding Delphi round with respect to name change and definition, depicted in light green and orange, respectively. An independent subcommittee that comprised expert endocrinologists, hepatologists, paediatricians, and patients chose between the top 3 acronyms emerging from the fourth Delphi round and outlined the specifics of the definition to include cardiometabolic parameters, as dictated by the fourth Delphi round. Abbreviation: SLD, steatotic liver disease.
FIGURE 4.
NAFLD nomenclature results: round 4 (summary). Delphi round 4 consisted of 4 questions. All panelists responded to all questions irrespective of their response to the preceding question. These are the aggregate results for respondents on each question. The first question addressed whether a literal term to replace NAFLD was preferred over a numerical subtype (eg, types 1–3) and 68% preferred the literal term. The second was whether or not the term “metabolic” should be included in the name, and 67% felt it should. The third presented a choice of acronyms that had emerged as the top 4 in Delphi R3 and the top 3 (nearly equal in preference) were advanced to the expert panel for a final decision as there was no clear majority. The last question was binary and simply asked if the definition of the NAFLD replacement term should be retained or refined to include a cardiometabolic qualifier. Abbreviations: MAS, metabolic dysfunction associated steatosis; MASLD, metabolic dysfunction associated steatotic liver disease; MetSLD, metabolic dysfunction associated steatotic liver disease; MHS, metabolic hepatic steatosis; MSLD, metabolic steatotic liver disease.
Desire for a name change and the role of stigma
During round 1, a supermajority of respondents (74%) felt that the current names NAFLD and NASH were sufficiently flawed to consider a name change (Supplemental Table S1, http://links.lww.com/HEP/H885) The terms “nonalcoholic” and “fatty” were deemed to be stigmatising by 61% and 66% of respondents, respectively. A nomenclature that describes the underlying cause of the disease was preferred by 89% of respondents. While there were concerns over the precise meaning of “metabolic” and to what extent this term was understood by clinicians, a supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%). Only a simple majority (56%) felt the terminology of “metabolic dysregulation” to be a clearly defined clinical entity although a supermajority (86%) felt that it highlighted a central aspect of disease pathophysiology.
Considerations regarding structure and composition of a new name
When given the choice of whether to select an “umbrella” term encompassing different disease subcategories, 78% of respondents preferred the idea of an overarching term to encompass the replacement term for NAFLD, ALD, and other conditions resulting in hepatic steatosis. Potential overarching terms were informed by survey rounds 2 and 3 and included fatty liver disease, steatotic liver disease (SLD), and lipogenic liver disease. Panelists were instructed to rank order their preferences, as first, second, and third choices. Fatty liver disease, SLD, and lipogenic liver disease garnered 46%, 48%, and 7% of first-choice selections, respectively. When considering the combination of first and second choice votes, SLD was chosen by 95% of respondents. Further, SC members preferred SLD due to the avoidance of potentially stigmatising language. Sixty-eight percent of the panelists preferred the use of a literal name (such as SLD) as opposed to using a numerical subtype (such as type 1 and type 2) as part of the new nomenclature. In round 4, 67% of respondents felt that the term “metabolic” should be included in the revised nomenclature for the alternative name for NAFLD, as a subtype under the overarching term of SLD chosen in R3 (Figure 5). Respondents were also asked whether a cardiometabolic risk factor (CMRF) should be added to the current definition and a simple majority was in favor of adding a cardiometabolic qualifier to the definition.
FIGURE 5.
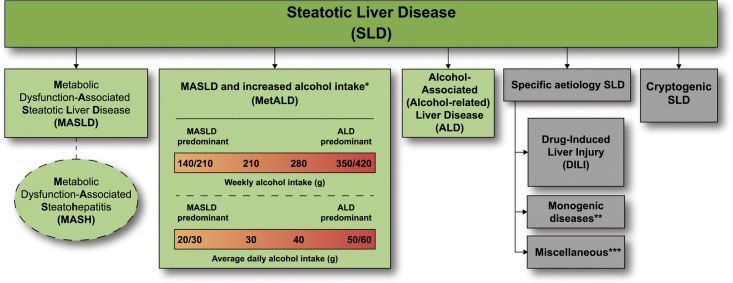
Steatotic liver disease (SLD) subclassification. This depicts the schema for SLD and its subcategories. SLD, diagnosed histologically or by imaging, has many potential aetiologies. Metabolic dysfunction associated steatotic liver disease (MASLD), defined as the presence of hepatic steatosis in conjunction with one cardiometabolic risk factor (CMRF) and no other discernible cause, ALD, and an overlap of the 2 (MetALD), comprises the most common causes of SLD. Persons with MASLD and steatohepatitis will be designated as metabolic dysfunction associated steatohepatitis (MASH). Within the MetALD group, there exists a continuum across which the contribution of MASLD and ALD will vary. To align with current literature, limits have been set accordingly for weekly and daily consumption, understanding that the impact of varying levels of alcohol intake varies between individuals. Other causes of SLD need to be considered separately, as is already done in clinical practice, given their distinct pathophysiology. Multiple aetiologies of steatosis can coexist. If there is uncertainty and the clinician strongly suspects metabolic dysfunction despite the absence of CMRF, this may be early MASLD and prompt additional testing (eg, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) and oral glucose tolerance tests). Those with no identifiable cause (cryptogenic SLD) may be recategorised in the future pending developments in our understanding of disease pathophysiology. Finally, the ability to provide an affirmative diagnosis allows for the coexistence of other forms of liver disease with MASLD, for example, MASLD + autoimmune hepatitis or viral hepatitis. *Weekly intake 140–30 g female, 210–420 g male (average daily 20–50 g female, 30–60 g male). **eg, Lysosomal acid lipase deficiency (LALD), Wilson disease, hypobetalipoproteinemia, inborn errors of metabolism. ***eg, HCV, malnutrition, celiac disease, human immunodeficiency virus (HIV).
Considerations for disease definition
Respondents were asked their opinion regarding the concept of steatohepatitis as an important entity, and 95% of respondents felt that the presence of steatohepatitis had prognostic implications and should remain an important distinction. In addition, given the role of “resolution of steatohepatitis” as one of the 2 European Medicines Agency (EMA) and US Food and Drug Administration (FDA) approvable endpoints, 93% felt that it should remain for both clinical practice and trial endpoints.14,15 The current definition of NAFLD excludes the consumption of >20 g/30 g of alcohol per day in females and males, respectively, with a more liberal approach to concomitant alcohol use proposed in the original MAFLD definition.5,7 To establish the permissibility of greater alcohol consumption, several questions were asked to better understand the impact of alcohol on the natural history of the disease and also how to characterise various levels of alcohol use in the definition. A supermajority felt that consumption of 30–60 g of alcohol daily in the setting of NAFLD alters the natural history of the disease (95%) and may alter the response to therapeutic interventions (90%). Furthermore, 90% felt that individuals with steatosis related to CMRFs who consume more than minimal alcohol (30–60 g daily) represented an important group that should be considered in a different disease category and studied independently.
Perceived impact of name and/or definition change on disease awareness, development of biomarkers, or clinical trials
When considering the potential impact of a change in name, definition, or both, 56% felt that a change in nomenclature would positively impact disease awareness. In assessing the impact of a change in name only on the interpretation of existing and emerging clinical trial results, 18%, 72%, and 11% (Supplemental Table S1, http://links.lww.com/HEP/H885; R3, Statement 27) felt that it would hinder, have no impact, and enhance, respectively. When a similar question was asked about the impact on regulatory approval of biomarkers if the name but not the definition changes, 12%, 63%, and 25% felt that it would accelerate, have no impact, or delay approval, respectively. In the event of both a name and definition change, 60% of respondents were concerned that this could hinder the interpretation of existing and emerging clinical trial results that used the currently accepted definition of NAFLD, whereas 20% felt that it would enhance, and 20% thought that there would be no impact. A simple majority (59%) felt that a change in disease definition would likely delay regulatory approval of biomarkers (R3–S24), whereas 63% felt that a change in name only would have no effect. Of note, these questions did not discuss the proposed change to the definition.
Paediatric perspective
There was a high degree of consensus among the Paediatric panelists when considering statements/questions pertaining to the Paediatric population. Only paediatricians answered the paediatric-specific questions, and the main themes addressed the role of stigma, the use of the term “metabolic,” and the histological definition of the disease. In children and adolescents, 60% felt that the use of the term “nonalcoholic” was stigmatising for parents and/or Paediatric patients, with 55% finding this to be the case with the term “fatty.” When asked if the current definition of NASH is less useful in children and adolescents due to a lower frequency of hepatocyte ballooning, 95% agreed that a reassessment of the definitions of steatohepatitis in the paediatric setting would be beneficial. In considering the incorporation of the term “metabolic” into the nomenclature, 90% estimated that this term may be confusing in the paediatric context since inborn errors of metabolism are referred to as “metabolic liver disease.”
Proposed new nomenclature for NAFLD, NASH, and NAFLD with increased alcohol consumption
When considering different subcategories under the overarching term of SLD, 67% of respondents preferred the NAFLD replacement term to include the word “metabolic.” The top 3 acronyms, metabolic dysfunction–associated steatotic liver disease (MASLD), MetSLD or metabolic steatotic liver disease (MSLD), were 30%, 30%, and 22%, respectively (Figure 4). In total, 75% of respondents of the external expert committee chose MASLD as the replacement term for NAFLD and 88% metabolic dysfunction–associated steatohepatitis (MASH) as the replacement term for NASH. The acronym MetALD was chosen by 28% and metabolic and alcohol related/associated liver disease (MAASLD) by 33% to represent a separate group of patients with MASLD that consumes 140–350 g/wk for females and 210–420 g/wk for males. MetALD was chosen to avoid the possible confusion or perception associated with the acronym AASLD within MAASLD that may link the acronym to a specific professional association. Within MetALD, there is a continuum where, conceptually, the condition can be seen to be MASLD or ALD predominant. This may vary over time within a given individual.
Proposed modifications to current definition
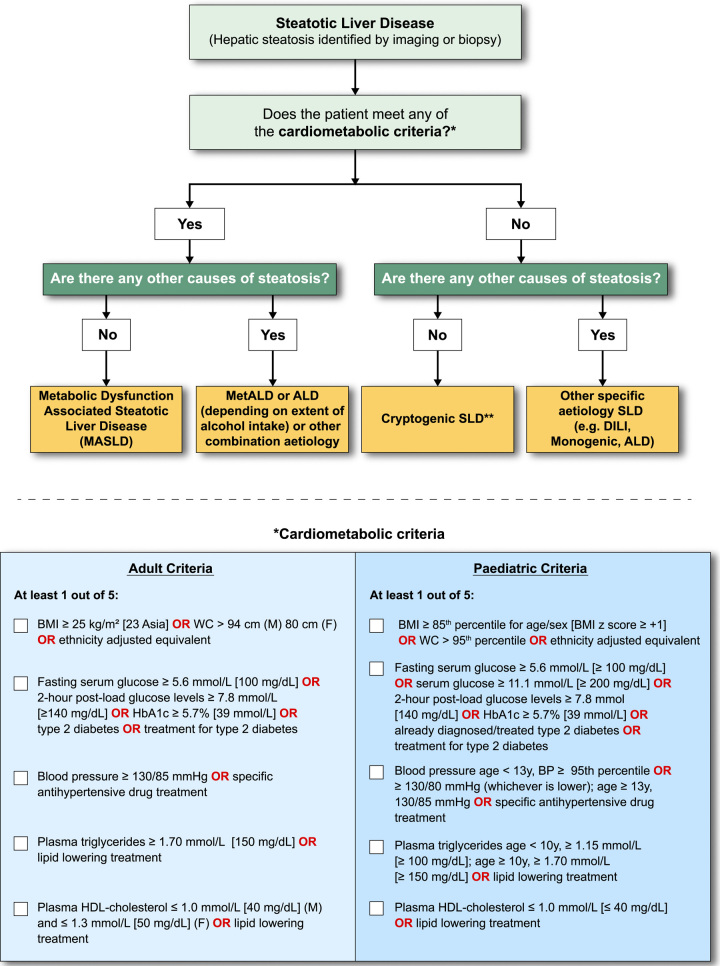
The strong epidemiological and pathogenic link between NAFLD, metabolic dysfunction, and insulin resistance informed a view in the external expert committee that the diagnosis be based on affirmative rather than exclusionary criteria, such as nonalcoholic. There was near universal agreement that the criteria be defined sufficiently broadly to identify both individuals with obesity and CMRFs in the context of regional/ethnic differences. Simple, readily available, and easily measurable parameters were also deemed necessary for this set of criteria to be broadly applied in clinical practice and in various clinical settings. Finally, the diagnostic criteria were selected to align with CMRFs believed to be associated with insulin resistance, and already well established and validated in the context of cardiovascular disease.16 It was agreed that patients with steatosis and any one of the cardiometabolic criteria outlined in Figure 6 would be considered to have MASLD. Of note, making a diagnosis of MASLD does not imply that other causes of SLD do not need to be considered, which is particularly relevant in children where it is imperative to exclude other causes of hepatic steatosis before applying the MASLD diagnostic criteria to ensure that dual pathology is not missed.17 Additionally, recognising that most patients with ALD have CMRFs, the distinction between MetALD and ALD with CMRFs, would be made on the basis of quantity of alcohol intake (Figure 6).
FIGURE 6.
MASLD diagnostic criteria. In the presence of hepatic steatosis, the finding of any CMRF would confer a diagnosis of MASLD if there are no other causes of hepatic steatosis. If additional drivers of steatosis are identified, then this is consistent with a combination aetiology. In the case of alcohol, this is termed MetALD or ALD, depending on extent of alcohol intake. In the absence of overt cardiometabolic criteria, other aetiologies must be excluded, and if none is identified, this is termed cryptogenic SLD although, depending on clinical judgment, it could also be deemed to be possible MASLD and, thus, would benefit from periodic reassessment on a case-by-case basis. In the setting of advanced fibrosis/cirrhosis, steatosis may be absent, requiring clinical judgment based on CMRFs and absence of other aetiologies. Abbreviations: ALD, alcohol-associated/related liver disease; BMI, body mass index; BP, blood pressure; CMRF, cardiometabolic risk factors; DILI, drug-induced liver disease; MetALD, metabolic dysfunction and alcohol associated steatotic liver disease; SLD, steatotic liver disease; WC, waist circumference.
Switching from a definition based on the exclusion of any other liver disease (ie, NAFLD) to a definition based on specific, primarily CMRFs (ie, MASLD) has potential limitations. First, the key metabolic dysfunction underlying MASLD is insulin resistance, and the selected metabolic risk factors do not equally predict insulin resistance, as, for example, diastolic blood pressure and HDL-C are only weakly associated with insulin resistance.18 Second, insulin resistance and steatosis may be present in the absence of any CMRFs, especially in younger adults in the primary care setting. Thus, patients with steatosis without overt CMRFs or other discernible causes are labeled as cryptogenic. If there is uncertainty and the clinician strongly suspects metabolic dysfunction despite the absence of CMRF, then the term possible MASLD can be considered pending additional testing (e.g., Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) and oral glucose tolerance tests) although this should be left to the discretion of the clinical team. Such cases and also cryptogenic cases that subsequently manifest CMRF can be rebadged as MASLD.
Role of alcohol in disease definitions
With respect to alcohol intake, the overwhelming consensus was to continue to limit alcohol intake (as previously limited for NAFLD) in the context of steatosis. The purpose of this process was to focus on NAFLD, not alcohol-associated/related liver disease, but it was seen as relevant to comment on situations where there was overlap. We, therefore, created a separate category outside of pure MASLD, namely, metabolic dysfunction and alcohol associated/related liver disease (MetALD), with alcohol intake greater than that allowed for NAFLD/MASLD. Within the group of patients with MetALD, there may be individuals where MASLD is the perceived dominant driver and others where ALD is the perceived dominant driver, and indeed, this may change over time (Figure 5).
DISCUSSION
Identification of a new name and definition for the condition formerly known as NAFLD has been a challenging process given the broad range of global stakeholders. It is imperative that any new proposal is sufficiently better than the existing nomenclature, and it enhances awareness, understanding of the disease, without negatively impacting drug/biomarker development. This robust, representative, patient-centric Delphi process systematically addressed all the issues and views over the past years and, through consensus, has arrived at both a new name and a refined definition. By inclusion of patient advocacy groups throughout the entire process, the new nomenclature strives to accelerate disease awareness while minimising stigma associated with the use of terms perceived as stigmatising by some patients and their caregivers.
Several important findings emerged from the nomenclature consensus process; there was clear support for a name change, the use of an overarching term that could accommodate the evolution of disease understanding, and the use of a metabolic descriptor in the new nomenclature. Both the overarching term of SLD and the more specific MASLD provide an affirmative nonstigmatising description of the condition rather than a diagnosis of exclusion. This is also seen in the definition, which requires the presence of at least 1 CMRF in addition to hepatic steatosis. The proposed nomenclature is not intended to be static but rather allows the flexibility for refinement as new evidence emerges about underlying pathophysiology and risk factors.
A key consideration is the preservation of existing data on natural history, biomarkers, and clinical trials as part of these changes. To address the impact of the refined definition, an analysis of the LITMUS consortium European was performed, which demonstrated that 98% of the existing registry cohort of patients with NAFLD would fulfill the new criteria for MASLD.19 Conceptually patients with the previous definition (NAFLD) can now be seen to be completely covered by the categories of MASLD and possible MASLD. The introduction of a separate MetALD subcategory where metabolic and alcohol-associated risk factors coexist sits outside MASLD/NAFLD and is an opportunity to generate new knowledge for this common group of patients. In addition, maintenance of the term, and clinical definition, of steatohepatitis ensures retention and validity of prior data from clinical trials and biomarker discovery studies of patients with NASH to be generalisable to individuals classified as MASLD or MASH under the new nomenclature, without impeding the efficiency of research.
The Delphi process utilised a supermajority threshold of ≥67% with 2 exceptions, the consideration of stigma, and a binary question to retain or revise the current definition. While recognising that perceptions of stigma differ widely,20,21 especially across different languages and cultures, in this study, it became clear that substantial proportions of the respondents deemed terms such as “fatty” stigmatising, hence its exclusion as part of any new name. Although healthcare professionals may contend that patients have not reported this previously, this likely reflects in part a failure to ask the question in the first place and the power imbalance in the doctor-patient relationship. Moreover, a recent large study indicated that some healthcare professionals and patients considered the terms fatty and nonalcoholic to be pejorative and stigmatising.21 The use of medical terminology such as steatosis may at one level be seen as overmedicalising the lexicon, yet it affords patients the opportunity to disclose their condition to friends and colleagues without having to face prejudice and stigma that can be inherent to the word “fatty.”21,22 Efforts to increase disease awareness have had modest success, possibly impacted by the perception that care providers deem the term “fatty liver” as describing an indolent condition. With therapeutics on the horison, there is renewed energy to identify “at-risk” patients, which, in conjunction with new terminology, may bolster awareness and a sense of importance.
The overarching term of SLD encompasses the spectrum of causes of hepatic steatosis, thus allowing precise classification once a specific aetiology has been identified. The new names also allow for further characterisation of fibrotic severity, for example, MASH with stage 3 fibrosis. Disease staging and severity are not altered by this process although it is anticipated that, in the near to medium term, disease staging will be achieved using noninvasive tests, which can be incorporated into further clarifications of disease stage. Thus, the current consensus process does not deviate from prior case definitions for steatohepatitis and disease stages.23 The diagnosis of MASLD/MASH with advanced fibrosis or cirrhosis, when steatosis may not be present, will be based on existing agreed criteria for NASH cirrhosis.23 This also applies to patients with MetALD and ALD with significant fibrosis who may not have steatosis yet have SLD as part of the overarching nomenclature, reflecting the mechanism of injury.
The proposed nomenclature also improves on the prior “nonalcoholic” label and appropriately assigns a metabolic basis for this liver disease, which was long recognised as “the hepatic manifestation of the metabolic syndrome.”5 This important conceptual change has several practical consequences. First, when addressing patients, it allows for a coherent and straightforward explanation of the disease as it is intuitively easier to understand in the context of its underlying cardiometabolic abnormalities linked to insulin resistance and its association with the patient’s other conditions, rather than in the framework of a diagnosis of exclusion. This also helps to communicate to the patient the main therapeutic actions to be taken both from a liver and a holistic perspective. Second, we believe that using this classification will enhance disease awareness since the alignment of the diagnostic criteria for MASLD with widely recognised phenotypic traits in diabetes and cardiovascular medicine will make it easier for the larger community of health care providers to identify individuals with this condition. There is a strong convergence between the metabolic set of criteria that we propose for diagnosing MASLD and those proposed by Eslam et al24 for MAFLD. However, the current consensus approach decided to prioritise robust and easily accessible clinical criteria and biological measurements, and as such, these criteria do not include direct measurements of insulin resistance (such as fasting insulin or Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)) because of their complexity, cost, and variability between laboratories. However, in patients with hepatic steatosis in the absence of overt CMRFs, secondary testing for insulin resistance may be useful to identify those with possible MASLD. It is important to understand that the set of diagnostic criteria for MASLD is not intended to diagnose “metabolic syndrome” or predict the occurrence of cardiovascular outcomes. The CMRFs are intended to identify patients likely to have insulin resistance as the main cause of hepatic steatosis. There was consideration of providing differential weighting for the CMRFs, such as type 2 diabetes, although the literature is conflicting in that regard with some indicating that no parameter is better than another at identifying hepatic steatosis.25
This process focuses on the nomenclature and definition of NAFLD rather than a determination of what constitutes hepatic steatosis or assessment of disease severity. There is extensive literature on the confirmation of hepatic steatosis,26,27 which we did not seek to interrogate; often, this is a pragmatic determination in clinical practice, which is where this guidance starts. Moreover, this nomenclature process, in line with published guidance,21,28,29 is not advocating for the routine use of tests to confirm hepatic steatosis although, in reality, most, if not all, patients will usually have imaging at some point. Finally, we recognise it is the evaluation of fibrosis either as part of screening strategies or individual clinical decisions, which is relevant for most clinical settings.30 That remains unchanged after this process, other than the name (eg, MASLD with advanced fibrosis).
Contrary to the initial proposal by Eslam and colleagues, the Delphi process revealed that most experts consider that MetALD patients should be classified in a category distinct from MASLD, mainly because of the added pathogenic value of alcohol consumption and consequential prognostic implications. The condition MetALD provides an opportunity to better define the natural history of such patients and the development of biomarkers and therapies that are currently lacking for this group of patients.31 ALD is a distinct liver disease (of which steatosis is one of the features) and, thus, is categorised under the SLD umbrella. This should raise awareness of alcohol as a driver of steatosis and highlight the impact of excessive alcohol consumption (ie, >50–60 g daily in females and males, respectively) irrespective of their association with metabolic dysfunction. Studies have shown that, even in excessive drinkers, obesity increases the prevalence of cirrhosis, and glycaemic dysregulation increases fibrosis severity.32,33 Patterns of alcohol use must also be taken into consideration as bingeing (even within the total weekly “allowable limit” for MASLD) can be detrimental. We also recognise that objective tools are not available or sufficiently validated to determine the relative contribution of MASLD and ALD in patients with MetALD, and hence, we rely on self-reported alcohol intake, which can be inaccurate. In that regard, this is a conceptual construct and might be better seen as a disease spectrum with differing of modifiable disease drivers (CMRFs and alcohol). This is also relevant for the distinction between patients with MetALD and those drinking more heavily, which are termed as having ALD. Also, the category of ALD without metabolic factors is relatively rare among patients with significant fibrosis, but it was felt to represent part of the spectrum.
In addition to defining a distinct category for patients with MASLD and greater alcohol consumption (MetALD), the proposed nomenclature allows, by introducing the umbrella term of SLD, for diagnostic subgroups of SLD to be identified, namely, those that are drug-related and others. The latter encompasses the many “secondary” causes of NAFLD, most of which are rare diseases, including monogenic diseases.31 This is particularly relevant in children, in whom rare genetic metabolism defects can cause steatosis and must be considered.17 Patients with steatosis without overt CMRFs or other discernible causes are labeled as cryptogenic although, depending on clinical judgment, they could also be deemed to have possible MASLD and would benefit from periodic reassessment on a case-by-case basis. Of note, genetic variants influencing the prevalence and/or severity of MASLD such as PNPLA3, TM6SF2, and HSD17B13, and other genetic risk variants that are common in the general population were not considered a distinct nosological entity. This was because these variants are disease modifiers for both MASLD and ALD rather than causative factors, in contrast to rare variants responsible for monogenic diseases. The change in nomenclature in favor of a positive diagnosis based on the presence of CMRFs will also allow for a rational reclassification of most cases of the condition formerly known as “lean NASH” into the regular MASLD category, as long as the currently defined metabolic risk factors are present. The “cryptogenic” category will, as mentioned, also accommodate the rare specific causes of SLD unrelated to metabolic dysfunction, alcohol consumption, drug intake, or other causes34 while waiting for precise identification of the causal agent by future research.
Despite the many strengths of this rigorous process, we acknowledge limitations. The individual statements changed between R1 and R3 (Supplemental Table S1, http://links.lww.com/HEP/H885), and there was variation in levels of agreement for individual statements although this reflects their evolution, as important issues arose, which we needed to consider regarding the NAFLD nomenclature. Furthermore, the lack of uniform agreement on many topics reflects the diversity of opinions involved in the process. A priori, we chose a threshold of 67% (supermajority) to define consensus, which meant that some opinions, although held by a simple majority (>50% but <67%), did not influence the final decisions, with the exception of stigma and the decision to alter the disease definition. Nonetheless, we are confident that statements supported by a supermajority were addressed and incorporated.
In conclusion, we believe that this process, which has multistakeholder endorsement, provides a strong platform from which we can increase disease awareness, reduce stigma, and accelerate drug and biomarker development for the benefit of patients with MASLD, MASH, and MetALD.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the governing boards of the 3 lead societies, AASLD, ALEH, and EASL, and specifically society leadership, Norah Terrault (AASLD President 2023), Laurie DeLeve (AASLD President 2022), W. Ray Kim (AASLD President-elect), Graciela E. Castro Narro (ALEH President), Thomas Berg (EASL Secretary General 2021–2023), and Aleksander Krag (EASL Secretary General 2023–2025). The authors thank Matthew D’Uva from AASLD for his tireless work and support of this project and recognise the contributions of all of the members of the NAFLD nomenclature consensus group who provided feedback, analysis, and recommendations over multiple Delphi rounds (Table 2). The authors acknowledge the assistance of the EU-IMI2–funded LITMUS (Liver Investigation: Testing Marker Utility in Steatohepatitis) consortium for the analysis of data derived from the European NAFLD Registry. Finally, the authors and the methodology team thank Delfina Boudou and Trenton White (ISGlobal, Spain).
TABLE 2.
NAFLD nomenclature consensus group
| Recipient first name | Recipient last name | Country |
|---|---|---|
| Manal | Abdelmalek | United States |
| Leon | Adams | Australia |
| Veeral | Ajmera | United States |
| Mamun | Al Mahtab | Bangladesh |
| William | Alazawi | United Kingdom |
| Maryam | Alkhatry | United Arab Emirates |
| Naim | Alkhouri | United States |
| Alina | Allen | United States |
| Michael | Allison | United Kingdom |
| Khalid | Alswat | Saudi Arabia |
| Michele | Alves-Bezerra | Spain |
| Quentin | Anstee | United Kingdom |
| Juan Pablo | Arab | Canada |
| Matthew J. | Armstrong | United Kingdom |
| Marco | Arrese | Chile |
| Diego | Arufe | Argentina |
| Pablo | Aschner | Colombia |
| Amon | Asgharpour | United States |
| Gyorgy | Baffy | United States |
| Maya | Balakrishnan | United States |
| Meena | Bansal | United States |
| Pierre | Bedossa | United States |
| Cynthia | Behling | United States |
| Renata | Belfort | United States |
| Carlos | Benítez | Chile |
| Thomas | Berg | Germany |
| Annalisa | Berzigotti | Switzerland |
| Michael | Betel | United States |
| Ulrich | Beuers | Netherlands |
| Cristiana | Bianco | Italy |
| Jerome | Boursier | France |
| Clifford | Brass | United States |
| Carol L. | Brosgart | United States |
| Elizabeth Matthews | Brunt | United States |
| Elisabetta | Bugianesi | Italy |
| Maria | Buti | Spain |
| Christopher | Byrne | United Kingdom |
| Steve | Caldwell | United States |
| Rotonya | Carr | United States |
| Teresa | Casanovas | Spain |
| Marlene | Castellanos-Fernández | Cuba |
| Laurent | Castera | France |
| Graciela | Castro Narro | México |
| Cyrielle | Caussy | France |
| Eira | Cerda | México |
| Naga | Chalasani | United States |
| Wah Kheong | Chan | Malaysia |
| Phunchai | Charatcharoenwitthaya | Thailand |
| Michael | Charlton | United States |
| Amanda | Cheung | United States |
| Daniela | Chiodi | Argentina |
| Ray | Chung | United States |
| David | Cohen | United States |
| Kathleen | Corey | United States |
| Helena | Cortez-Pinto | Portugal |
| Helma P. | Cotrim | Brazil |
| Javier | Crespo | Spain |
| Deborah | Crosby | United States |
| Donna | Cryer | United States |
| Kenneth | Cusi | United States |
| Yock Young | Dan | Singapore |
| Anuradha | Dassanayake | Sri Lanka |
| Nicholas | Davidson | United States |
| Robert | De Knegt | Netherlands |
| Victor | De Ledinghen | France |
| Münevver | Demir | Germany |
| Moutaz | Derbala | Qatar |
| Sebastian | Diaz | Colombia |
| Anna Mae | Diehl | United States |
| Bruce | Dimmig | United States |
| Melisa | Dirchwolf | Argentina |
| Ajay | Duseja | India |
| Karel | Dvorak | Prague |
| Mattias | Ekstedt | Sweden |
| Reda | El Wakil | Egypt |
| Mohammed | El-Kassas | Egypt |
| Wayne | Eskridge | United States |
| Jian-Gao | Fan | China |
| Geoffrey | Farrell | Australia |
| María Lucía | Ferraz | Brazil |
| Yasser | Fouad | Egypt |
| Sven | Francque | Belgium |
| Dave | Frank | United States |
| Scott | Friedman | United States |
| Angie | Fry Carpenter | United States |
| Michael | Fuchs | United States |
| Rino | Gani | Indonesia |
| Amalia | Gastaldelli | Italy |
| Anja | Geerts | Belgium |
| Andreas | Geier | Germany |
| Marcos | Girala | Paraguay |
| George | Goh | Singapore |
| Nicolas | Goossens | Switzerland |
| Cheryl | Grainger | United States |
| Isabel | Graupera | Spain |
| Cynthia | Guy | United States |
| Hannes | Hagström | Sweden |
| Stephen | Harrison | United States |
| Zachary | Henry | United States |
| Bela | Hunyady | Hungary |
| Alan | Hutchison | United States |
| Scott | Isaacs | United States |
| Jidong | Jia | China |
| François | Jornayvaz | Switzerland |
| Fasiha | Kanwal | United States |
| Cynthia | Kemp | United States |
| Denise | Kile | United States |
| Won | Kim | South Korea |
| Seung Up | Kim | South Korea |
| George | KK Lau | China |
| Samuel | Klein | United States |
| David | Kleiner | United States |
| Rohit | Kohli | United States |
| Bart | Koot | Netherlands |
| Yannoula | Koulla | Cyprus |
| Marcelo | Kugelmas | United States |
| Joel | Lavine | United States |
| Jeffrey | Lazarus | Spain |
| Mariana | Lazo | United States |
| Hye Won | Lee | South Korea |
| Nathalie | Leite | Brazil |
| Han-Chieh | Lin | Taiwan |
| Michelle | Long | United States |
| Rohit | Loomba | United States |
| Susan | Love Hawfield | United States |
| Adelina | Lozano | Peru |
| Panu | Luukkonen | Finland |
| Paula | Macedo | Portugal |
| Dina | Mansour | United Kingdom |
| Christos | Mantzoros | United States |
| Giulio | Marchesini | Italy |
| Sebastián | Marciano | Argentina |
| Claudia P. | Marques Souza de Oliveira | Brazil |
| Kim | Martinez | United States |
| Lyudmila Vladimirova | Mateva | Bulgaria |
| Jose M | Mato | Spain |
| Alexis | McCary | United States |
| Jeff | McIntyre | United States |
| Luca | Miele | Italy |
| Ivana | Mikolasevic | Croatia |
| Veronica | Miller | United States |
| Pam | Miller | United States |
| Maria “Terri” | Milton | United States |
| Milan | Mishkovikj | North Macedonia |
| Robert | Mitchell-Thain | United Kingdom |
| Rosalba | Moreno | United States |
| Timothy | Morgan | United States |
| Cynthia | Moylan | United States |
| Atsushi | Nakajima | Japan |
| Jean Charles | Nault | France |
| Phillip | Newsome | United Kingdom |
| Suzanne | Norris | Ireland |
| Mazen | Noureddin | United States |
| Claudia P. | Oliveira | Brazil |
| Masao | Omata | Japan |
| Arlin | Ong | Philippines |
| Martín | Padilla | Perú |
| Raluca | Pais | France |
| Arturo | Panduro | Mexico |
| Manas K | Panigrahi | India |
| George | Papatheodoridis | Greece |
| Edison | Parise | Brazil |
| Sonali | Paul | United States |
| Diana | Payawal | Philippines |
| Serena | Pelusi | Italy |
| Marlene | Pérez | Brazil |
| Juanita | Perez Escobar | Mexico |
| Gianluca | Perseghin | Italy |
| Mario | Pessoa | Brazil |
| Salvatore | Petta | Italy |
| Massimo | Pinzani | United Kingdom |
| Monica | Platon Lupsor | Romania |
| Atoosa | Rabiee | United States |
| Vlad | Ratziu | France |
| Mario R. | Alvares-da-Silva | Brazil |
| Mary | Rinella | United States |
| Michael | Roden | Germany |
| Stefano | Romeo | Sweden |
| Manuel | Romero Gomez | Spain |
| Yaron | Rotman | United States |
| Ian | Rowe | United Kingdom |
| Riina | Salupere | Estonia |
| Arun | Sanyal | United States |
| Shiv Kumar | Sarin | India |
| Sanjaya K. | Satapathy | United States |
| Jörn M. | Schattenberg | Germany |
| Wendy | Schaufert | Canada |
| Bernd | Schnabl | United States |
| Jeff | Schwimmer | United States |
| Lynn | Seim | United States |
| Lawrence | Serfaty | France |
| David | Shapiro | United States |
| Marcelo | Silva | Argentina |
| Ashwani K. | Singal | United States |
| Shivaramn Prasad | Singh | India |
| Lubomir | Skladany | Slovakia |
| Silvia | Sookoian | Argentina |
| Norbert | Stefan | Germany |
| Jonathan | Stine | United States |
| Shikha | Sundaram | United States |
| C. Wendy | Spearman | South Africa |
| Gianluca | Svegliati-Baroni | Italy |
| Gyonzgi | Szabo | United States |
| Frank | Tacke | Germany |
| Tawesak | Tanwandee | Thailand |
| Giovanni | Targher | Italy |
| Brent | Tetri | United States |
| Maja | Thiele | Denmark |
| Dina | Tiniakos | Greece |
| Baron | Tisthammer | United States |
| Aldo | Torre Delgadillo | Mexico |
| Diane | Tovar | United States |
| Michael | Trauner | Austria |
| Emmanuel | Tsochatzis | United Kingdom |
| Luca | Valenti | Italy |
| Laurens | Van Kleef | Netherlands |
| Saskia | Van Mil | Netherlands |
| Lisa | VanWagner | United States |
| Adriana | Varon Puerta | Colombia |
| Jose Antonio | Velarde Ruiz Velasco | Mexico |
| Mette | Vesterhus | Norway |
| Eduardo | Vilar-Gomez | United States |
| Anthony | Villiotti | United States |
| Miriam | Vos | United States |
| Kymberly | Watt | United States |
| Julia | Wattacheril | United States |
| Fonda | Wilkins | United States |
| José | Willemse | Netherlands |
| Vincent | Wong | China |
| Stavra | Xanthakos | United States |
| Yusuf | Yilmaz | Turkey |
| Lorna | Younger | United States |
| Zobair | Younossi | United States |
| Amany | Zekry | United Kingdom |
| Shira | Zelber-Sagi | Israel |
Footnotes
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALD, alcohol-associated/related liver disease; ALEH, Asociación Latinoamericana para el Estudio del Hígado (Latin American Association for the Study of the Liver); AMAGE, African Middle East Association of Gastroenterology; APASL, Asian Pacific Association for the Study of the Liver; BMI, body mass index; BP, blood pressure; CMRF, cardiometabolic risk factor; DILI, drug-induced liver disease; EASL, European Association for the Study of the Liver; GI, gastrointestinal; INASL, Indian National Association for the Study of the Liver; LALD, lysosomal acid lipase deficiency; MAFLD, metabolic dysfunction-associated fatty liver disease; MAS, metabolic dysfunction associated steatosis; MAASLD, metabolic dysfunction and alcohol associated steatotic liver disease; MASH, metabolic dysfunction -associated steatohepatitis; MASLD, metabolic dysfunction associated steatotic liver disease; MetALD, metabolic dysfunction and alcohol associated steatotic liver disease; MetSLD, metabolic dysfunction associated steatotic liver disease; MHS, metabolic hepatic steatosis; MSLD, metabolic steatotic liver disease; RR, response rate; SAASL, South Asian Association for the Study of the Liver; SLD, steatotic liver disease; TASL, Taiwan Association for the Study of the Liver; WC, waist circumference.
The list of additional non-author contributors for the NAFLD Nomenclature consensus group can be found in the Supplemental Materials, www.hepjournal.com
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepjournal.com.
This article is being copublished by Hepatology, Journal of Hepatology, and Annals of Hepatology. Minor differences in style may appear in each publication, but the article is substantially the same in each journal.
Contributor Information
Mary E. Rinella, Email: mrinella@bsd.uchicago.edu.
Collaborators: Veeral Ajmeral, William Alazawi, Maryam Alkhatry, Naim Alkhouri, Alina Allen, Michael Allison, Khalid Alswat, Mario R. Alvares-da-Silva, Michele Alves-Bezerra, Matthew J. Armstrong, Diego Arufe, Pablo Aschner, Gyorgy Baffy, Meena Bansal, Pierre Bedossa, Renata Belfort, Thomas Berg, Annalisa Berzigotti, Michael Betel, Cristiana Bianco, Clifford Brass, Carol L. Brosgart, Elizabeth Matthews Brunt, Maria Buti, Steve Caldwell, Rotonya Carr, Teresa Casanovas, Laurent Castera, Cyrielle Caussy, Eira Cerda, Naga Chalasani, Wah Kheong Chan, Phunchai Charatcharoenwitthaya, Michael Charlton, Amanda Cheung, Daniela Chiodi, Ray Chung, David Cohen, Kathleen Corey, Helma P. Cotrim, Javier Crespo, Anuradha Dassanayake, Nicholas Davidson, Robert De Knegt, Victor De Ledinghen, Münevver Demir, Sebastian Diaz, Anna Mae Diehl, Bruce Dimmig, Melisa Dirchwolf, Ajay Duseja, Karel Dvorak, Mattias Ekstedt, Reda El Wakil, María Lucía Ferraz, Scott Friedman, Michael Fuchs, Amalia Gastaldelli, Anja Geerts, Andreas Geier, Marcos Girala, George Goh, Nicolas Goossens, Isabel Graupera, Hannes Hagström, Zachary Henry, Bela Hunyady, Alan Hutchison, Scott Isaacs, François Jornayvaz, Cynthia Kemp, Denise Kile, Won Kim, David Kleiner, Rohit Kohli, Marcelo Kugelmas, Joel Lavine, Mariana Lazo, Nathalie Leite, Adelina Lozano, Panu Luukkonen, Paula Macedo, Dina Mansour, Christos Mantzoros, Giulio Marchesini, Sebastián Marciano, Kim Martinez, Lyudmila Vladimirova Mateva, Jose M. Mato, Alexis McCary, Luca Miele, Ivana Mikolasevic, Veronica Miller, Rosalba Moreno, Cynthia Moylan, Atsushi Nakajima, Jean Charles Nault, Suzanne Norris, Mazen Noureddin, C.P. Oliveira, Arlin Ong, Martín Padilla, Raluca Pais, Arturo Panduro, Manas K. Panigrahi, George Papatheodoridis, Serena Pelusi, Marlene Pérez, Juanita Perez Escobar, Gianluca Perseghin, Mario Pessoa, Salvatore Petta, Massimo Pinzani, Monica Platon Lupsor, Atoosa Rabiee, Stefano Romeo, Yaron Rotman, Ian Rowe, Riina Salupere, Sanjaya Satapathy, Jörn M. Schattenberg, Wendy Schaufert, Bernd Schnabl, Lynn Seim, Lawrence Serfaty, David Shapiro, Ashwani K. Singal, Lubomir Skladany, Norbert Stefan, Jonathan Stine, Shikha Sundaram, Gianluca Svegliati-Baroni, Gyonzgi Szabo, Frank Tacke, Tawesak Tanwandee, Giovanni Targher, Norah Terrault, Brent Tetri, Maja Thiele, Baron Tisthammer, Aldo Torre Delgadillo, Michael Trauner, Emmanuel Tsochatzis, Laurens Van Kleef, Saskia Van Mil, Lisa VanWagner, Jose Antonio Velarde Ruiz Velasco, Mette Vesterhus, Eduardo Vilar-Gomez, Kymberly Watt, Julia Wattacheril, Fonda Wilkins, José Willemse, Amany Zekry, and Shira Zelber-Sagi
ENDORSING ORGANISATIONS
American Association of Clinical Endocrinology, American College of Gastroenterology, American Gastroenterological Association, American Liver Foundation, American Society for Gastrointestinal Endoscopy, American Society for Preventive Cardiology, Asian Pan-Pacific Sociy for Pediatric Gastroenterology, Hepatology, and Nutrition, Asociación Chilena de Hepatología ACHHEP, Asociación Colombiana de Hepatología, Asociación de Especialistas en Gastroenterología y Endoscopia Digestiva de Costa Rica, Asociación Española para el Estudio del Hígado, Asociación Guatemalteca de Gastroenterología, Hepatología y Endoscopía Gastrointestinal, Asociación Mexicana de Hepatología, Asociación Peruana para el Estudio del Hígado, Asociación Puertorriqueña de Gastroenterología, Associação Portuguesa Para o Estudo do Fígado, Association Française pour l’Etude du Foie, Association of Black Gastroenterologists and Hepatologists, Association of Pakistani-Descent Gastroenterologists of North America (APGNA), Associazione Italiana per lo Studio del Fegato, Azerbaijan Gastroenterologists and Hepatologists Association, Banner Liver Support Group, Belgian Week of Gastroenterology, British Association for the Study of the Liver, British Liver Trust, British Society of Gastroenterology, Canadian Association for the Study of the Liver, Croatian Society of Gastroenterology, Danish Society for Gastroenterology and Hepatology, Deutsche Gesellschaft für Gastroenterolgie, Verdauungs- und Stoffwechselkrankheiten, European Liver Patient Association, European Society for Paediatric Gastroenterology, Hepatology, and Nutrition, Facebook Cirrhosis Support Group, Fatty Liver Foundation, Fatty Liver Study Foundation in Middle East and North Africa, Federation of the International Societies for Paediatric Gastroenterology, Hepatology, and Nutrition, Global Liver Institute, Hannover Medical School, Hellenic Association for the Study of the Liver, Indian National Association for Study of the Liver, Japan Society of Hepatology, Japan Study Group of NAFLD, Korean NAFLD Study Group, Latin American Society of Pediatric Gastroenterology, Hepatology, and Nutrition, Leverforeningen Danmark, Liver Patients International, Mexican Association of Gastroenterology, North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition, Norwegian Gastroenterology Society, Österreichische Gesellschaft für Gastroenterologie und Hepatologie, Pakistan Society of Hepatology, Polish Association for the Study of Liver, Romanian Society of Gastroenterology and Hepatology, Sociedad Argentina de Hepatología, Sociedad Cubana de Gastroenterología, Sociedad de Gastroenterología del Uruguay, Sociedad Dominicana de Gastroenterologia, Sociedad Española de Patología Digestiva, Sociedad Paraguaya de Diabetologia, Sociedad Venezolana de Gastroenterologia, Sociedade Brasileira de Hepatologia, Society of Gastroenterology and Hepatology of the Republic of Moldova, Society of Liver Disease in Africa, South Asian Association for Study of the Liver, Swedish Society of Gastroenterology, Swiss Association for the Study of the Liver, The Liver Forum, Turkish Association for the Study of the Liver, Ukrainian Association for the Study of Liver Diseases, United European Gastroenterology, Zambia Association of Gastroenetrology and Nutrition.
CONFLICTS OF INTEREST
Manal F. Abdelmalek consults, advises, and received grants from Bristol Myers Squibb, Hanmi, Intercept, Inventiva, and Madrigal. She consults and advises 89Bio, Merck, NGM Bio, Novo Nordisk, Sonic Incytes, and Theratechnologies. She is on the speakers’ bureau for the Chronic Liver Disease Foundation, Clinical Care Options, Fishawack, Medscape, and Terra Firma. She received grants from Allergan, Boehringer Ingelheim, Celgene, Durect, Enanta, Enyo, Galmed, Genentech, Gilead, Novo Nordisk, Poxel, Target NASH, and Viking. Quentin M. Anstee, on behalf of Newcastle University, consults for Alimentiv, Akero, AstraZeneca, Axcella, 89Bio, Boehringer Ingelheim, Bristol Myers Squibb, Galmed, GENFIT, Genentech, Gilead, GSK, Hanmi, HistoIndex, Intercept, Inventiva, Ionis, IQVIA, Janssen, Madrigal, Medpace, Merck, NGM Bio, Novartis, Novo Nordisk, PathAI, Pfizer, Pharmanest, Prosciento, Poxel, RTI, Resolution Therapeutics, Ridgeline Therapeutics, Roche, Shionogi, and Terns. He is on the speakers’ bureau for Fishawack, Integritas Communications, Kenes, Novo Nordisk, Madrigal, Medscape, and Springer Healthcare. He received grants from AstraZeneca, Boehringer Ingelheim, and Intercept. He holds intellectual property rights with Elsevier, Ltd. Ramon Bataller is on the speakers’ bureau for Abbvie and Gilead. Ulrich Beuers consults for CSL Behring. He is on the speakers’ bureau for Abacus and Zambon. Elisabetta Bugianesi advises Boehringer Ingelheim, MSD, and Novo Nordisk. Helena Cortez-Pinto consults and received grants from Novo Nordisk and Roche. She received grants from Eisai, Gilead, GMP-Orphan, and Intercept. Kenneth Cusi Consults for Aligos, Arrowhead, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Covance, Lilly, Madrigal, Myovant, Novo Nordisk, Prosciento, Sagimet, and Siemens. He received grants from Echosens, Inventiva, LabCorp, Nordic Biosciences, and Target NASH. Sven M. Francque consults and received grants from Astellas, Falk, GENFIT, Gilead, Glympse Bio, Janssen, Inventiva, Merck, Pfizer, and Roche. He consults for AbbVie, Actelion, Aelin Therapeutics, Allergan, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Coherus, CSL Behring, Echosens, Eisai, ENYO, Galapagos, Galmed, Genetech, Intercept, Julius Clinical, Madrigal, Medimmune, NGM Bio, Novartis, Novo Nordisk, and Promethera. Samer Gawrieh consults for Pfizer and TransMedics. He received grants from LiverIncytes, Viking, and Zydus. Manuel Romero-Gómez advises and received grants from Novo Nordisk and Siemens. He advises AbbVie, Alpha-sigma, Allergan, AstraZeneca, Axcella, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Intercept, Inventia, Kaleido, MSD, Pfizer, Prosciento, Rubió, Shionogi, Sobi, and Zydus. He received grants from Echosens and Theratechnologies. Cynthia D. Guy consults for 89Bio, CymaBay, HistoIndex, Madrigal, and NGM. Stephen Harrison consults, advises, is involved with trials, received grants, and owns stock in Akero, Galectin, GENFIT, Hepion, and NGM Bio. He consults, advises, is involved with trials, and received grants from Axcella, Gilead, Intercept, Madrigal, and Poxel. He consults, advises, received grants, and owns stock in NorthSea Therapeutics. He consults, advises, and is involved with trials for Terns. He consults, advises, and received grants from HighTide, Novartis, Novo Nordisk, and Sagimet. He consults, advises, and owns stock in HistoIndex, Metacrine, and Sonic Incytes. He consults, received grants, and owns stock in Cirius. He consults, is involved with trials, and received grants from ENYO and Viking. He is involved with trials and received grants from Genentech. He consults and is involved with trials for Ionis. He consults and received grants from CiVi, CymaBay, Galmed, and Pfizer. He consults and owns stock in Hepta Bio. He consults and advises for Altimmune, Echosens North America, Foresite Labs, and Medpace. He advises and owns stock in ChronWell. He consults for AgomAb, Alentis, Aligos Therapeutics, Alimentiv, Blade, Bluejay, Boston Pharmaceuticals, Boxer Capital, Can-Fite BioPharma, the Chronic Liver Disease Foundation (CLDF), CohBar, Corcept, Fibronostics, Fortress Biotech, Galecto, Gelesis, GSK, GNS Healthcare, GRI Bio, Hepagene, Indalo, Inipharm, Innovate Biopharmaceuticals, Kowa Research Institute, Merck, MGGM, NeuroBo, Nutrasource, Perspectum, Piper Sandler, Prometic (now Liminal BioSciences), Ridgeline Therapeutics, Silverback, and Zafgen (now Larimar). He advises Arrowhead BVF Partners, Humana, and Pathai. He received grants from Bristol Myers Squibb, Conatus, Immuron, and Second Genome. Samuel Klein advises Alnylam, Altimmune, and Merck. Kris V. Kowdley advises, is on the speakers’ bureau, and received grants from Gilead and Intercept. He advises, received grants, and owns stock in Inipharm. He advises and received grants from 89bio, CymaBay, GENFIT, Ipsen, Madrigal, Mirum, NGM Bio, Pfizer, Pliant, and Zeds. He advises Enact, HighTide, and Protagonist. He is on the speakers’ bureau for AbbVie. He received grants from Boston Pharmaceuticals, Corcept, GSK, Hanmi, Janssen, Novo Nordisk, Terns, and Viking. Jeffrey V. Lazarus consults for Novavax. He received grants from AbbVie, Gilead, MSD, and Roche Diagnostics. Rohit Loomba consults and received grants from Arrowhead, AstraZeneca, Bristol Myers Squibb, Galmed, Gilead, Intercept, Inventiva, Ionis, Janssen, Lilly, Madrigal, Merck, NGM Bio, Novo Nordisk, Pfizer, and Terns. He consults and owns stock in 89Bio and Sagimet. Consults for Altimmune, Anylam, Amgen, CohBar, Glympse Bio, HighTide, Inipharm, Metacrine, Novartis, Regeneron, Theratechnologies, and Viking. He received grants from Boehringer Ingelheim, Galectin Therapeutics, Hanmi, and Sonic Incytes. He cofounded and owns stock in LipoNexus. Phillip N. Newsome consults, advises, is on the speakers’ bureau, and received grants from Novo Nordisk. He consults and advises Boehringer Ingelheim, Bristol Myers Squibb, Gilead, GSK, Intercept, Madrigal, Pfizer, Poxel, and Sun Pharma. He is on the speakers’ bureau for AiCME. Elizabeth E. Powell advises and received grants from Novo Nordisk. Vlad Ratziu consults and received grants from Intercept. He consults for Boehringer Ingelheim, Eny, Madrigal, NorthSea, Novo Nordisk, E. Poxel, and Sagimet. He received grants from Gilead. Mary E. Rinella consults for Boehringer Ingelheim, CytoDyn, GSK, Novo Nordisk, HistoIndex, Intercept, Madrigal, NGM Bio, and Sonic Incytes. Michael Roden consults and received grants from Boehringer Ingelheim and Novo Nordisk. He consults for Lilly. He is on the speakers’ bureau for AstraZeneca. Arun J. Sanyal consults and advises Avant Santé and AstraZeneca. He consults and received grants from Akero, Bristol Myers Squibb, Intercept, Lilly, Madrigal, and Novo Nordisk. He consults and owns stock in Rivus. He consults for AGED Diagnostics, Albireo, Alnylam, Altimmune, Boehringer Ingelhiem, 89Bio, Echosense, Genentech, Gilead, GSK, HistoIndex, Malinckrodt, Merck, NGM Bio, Novartis, PathAI, Pfizer, Poxel, Regeneron, Salix, Siemens, Surrozen, Takeda, Terns, and Zydus. He owns stock in Durect, Exhalenz, GENFIT, Indalo, Inversago, and Tiziana. He received royalties from Elsevier and Wolters Kluwer. Marcelo Silva consults, advises, and received grants from Zydus. He received grants from Inventiva and MSD. Dina Tiniakos consults for Clinnovate Health, ICON, Ionis, Inventiva, Merck, and Verily. Luca Valenti consults and received grants from Gilead. He consults for AstraZeneca, Boehringer Ingelheim, MSD, Novo Nordisk, Pfizer, and Resalis Therapeutics. Miriam B. Vos consults and advises Thiogenesis. She consults and received grants from Target Real World Evidence. She consults and owns stock in Intercept. She consults for Albireo, Boehringer Ingelheim, Lilly, Novo Nordisk, and Takeda. She received grants from Bristol Myers Squibb, Quest, and Sonic Incytes. Vincent Wai-Sun Wong consults and received grants from Gilead. He consults for AbbVie, Boehringer Ingelheim, Echosens, Intercept, Inventiva, Novo Nordisk, Pfizer, Sagimet, and TARGET PharmaSolutions. He owns stock in Illuminatio Medical Technology. Yusuf Yilmaz consults for Zydus. He advises Novo Nordisk. He is on the speakers’ bureau for Echosens. Zobair Younossi consults for Bristol Myers Squibb, Gilead, Intercept, Madrigal, Merck, Novartis, Novo Nordisk, Quest, Siemens, and Terns. The remaining authors have no conflicts to report.
AUTHOR CONTRIBUTIONS
Mary E. Rinella, Jeffrey V. Lazarus, Vlad Ratziu, Sven Franque, Arun J. Sanyal, Fasiha Kanwal, Diana Romero, and Philip N. Newsome contributed to study conception and design, data acquisition, and interpretation of the data. All authors (Mary E. Rinella, Jeffrey V. Lazarus, Vlad Ratziu, Sven M. Francque, Arun J. Sanyal, Fasiha Kanwal, Diana Romero, Manal F. Abdelmalek, Quentin M. Anstee, Juan Pablo Arab, Marco Arrese, Ramon Bataller, Ulrich Beuers, Jerome Boursier, Elisabetta Bugianesi, Christopher Byrne, Graciela E. Castro Narro, Abhijit Chowdhury, Helena Cortez-Pinto, Donna R. Cryer, Kenneth Cusi, Mohamed El-Kassas, Samuel Klein, Wayne Eskridge, Jian-gao Fan, Samer Gawrieh, Cynthia D. Guy, Stephen A. Harrison, Seung Up Kim, Bart G. Koot, Marko Korenjak, Kris V. Kowdley, Florence Lacaille, Rohit Loomba, Robert Mitchell-Thain, Timothy R. Morgan, Elisabeth E. Powell, Michael Roden, Manuel Romero-Gómez, Marcelo Silva, Shivaram Prasad Singh, Silvia C. Sookoian, C. Wendy Spearman, Dina Tiniakos, Luca Valenti, Miriam B. Vos, Vincent Wai-Sun Wong, Stavra Xanthakos, Yusuf Yilmaz, Zobair Younossi, Ansley Hobbs, Marcela Villota-Rivas, and Philip N. Newsome) contributed to drafting and critical revision of the manuscript. All authors, as well as the NAFLD nomenclature consensus group named in Table 2, reviewed and approved the final version of the manuscript.
REFERENCES
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 2.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM. Nonalcoholic steatohepatitis (NASH): further expansion of this clinical entity? Liver. 1999;19:263–4. [DOI] [PubMed] [Google Scholar]
- 4.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–9. [DOI] [PubMed] [Google Scholar]
- 5.Matteoni C, Younossi Z, Gramlich T, Boparai N, Liu Y, Mccullough A. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014 e1. [DOI] [PubMed] [Google Scholar]
- 7.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, Sarin SK, Wong VWS, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2021;73:1194–98. [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V, Rinella M, Beuers U, Loomba R, Anstee QM, Harrison S, et al. The times they are a-changin’ (for NAFLD as well). J Hepatol. 2020;73:1307–9. [DOI] [PubMed] [Google Scholar]
- 11.Beiderbeck D, Frevel N, von der Gracht HA, Schmidt SL, Schweitzer VM. Preparing, conducting, and analyzing Delphi surveys: cross-disciplinary practices, new directions, and advancements. MethodsX. 2021;8:101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevelyan EG, Turner WA, Robinson N. Developing an acupuncture protocol for treating phantom limb pain: a Delphi consensus study. Acupunct Med. 2015;33:42–50. [DOI] [PubMed] [Google Scholar]
- 13.L.H.a.T. M. The Delphi Method: Techniques and Applications. Portland State University; 2002. [Google Scholar]
- 14.Rinella ME, Tacke F, Sanyal AJ, Anstee QM. Report on the AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD. Hepatology. 2019;70:1424–36. [DOI] [PubMed] [Google Scholar]
- 15.Loomba R, Ratziu V, Harrison SA, Loomba R, McFarlane SC, Tamaki N, et al. Expert panel review to compare FDA and EMA Guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology. 2022;162:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 17.European Society for Paediatric Gastroenterology, H. Diagnosis of fatty liver in children should occur in parallel to investigation for other causes of liver disease. Lancet Gastroenterol Hepatol. 2023. doi: 10.1016/S2468-1253(23)00100-0 [DOI] [PubMed] [Google Scholar]
- 18.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–94. [DOI] [PubMed] [Google Scholar]
- 19.Hardy T, Wonders K, Younes R, Aithal GP, Aller R, Allison M, et al. The European NAFLD Registry: a real-world longitudinal cohort study of nonalcoholic fatty liver disease. Contemp Clin Trials. 2020;98:106175. [DOI] [PubMed] [Google Scholar]
- 20.Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116. [DOI] [PubMed] [Google Scholar]
- 21.Zobair M, Younossi YY, Jian-Gao F, Wai-Sun Wong V, El-Kassas M, Zelber-Sagi S, et al. Lazarus and the Global NASH Council, Stigma in NAFLD and NASH: a global survey of patients and providers. Hepatology. 2023:21–24. [Google Scholar]
- 22.Carrieri P, Mourad A, Marcellin F, Trylesinski A, Calleja JL, Protopopescu C, et al. Knowledge of liver fibrosis stage among adults with NAFLD/NASH improves adherence to lifestyle changes. Liver Int. 2022;42:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui MS, Harrison SA, Abdelmalek MF, Anstee QM, Bedossa P, Castera L, et al. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology. 2018;67:2001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eslam M, George J. Reply to: correspondence regarding “A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement”: bringing evidence to the NAFLD-MAFLD debate. J Hepatol. 2020;73:1575. [DOI] [PubMed] [Google Scholar]
- 25.Wong VWS, Chu WCW, Wong GLH, Chan RSM, Chim AML, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–15. [DOI] [PubMed] [Google Scholar]
- 26.Petroff D, Blank V, Newsome PN, Shalimar, Voican CS, Thiele M, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185–98. [DOI] [PubMed] [Google Scholar]
- 27.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 28.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berzigotti A, Tsochatzis E, Boursier J, Castera L, Cazzagon N, Friedrich-Rust M, et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J Hepatol. 2021;75:659–89. [DOI] [PubMed] [Google Scholar]
- 30.Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association Between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–25 e12. [DOI] [PubMed] [Google Scholar]
- 31.Moreno C, Sheron N, Tiniakos D, Lackner C, Mathurin P. “Dual aetiology fatty liver disease”: a recently proposed term associated with potential pitfalls. J Hepatol. 2021;74:979–82. [DOI] [PubMed] [Google Scholar]
- 32.Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput J. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. [DOI] [PubMed] [Google Scholar]
- 33.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–8. [DOI] [PubMed] [Google Scholar]
- 34.Liebe R, Esposito I, Bock HH, vom Dahl S, Stindt J, Baumann U, et al. Diagnosis and management of secondary causes of steatohepatitis. J Hepatol. 2021;74:1455–71. [DOI] [PubMed] [Google Scholar]