Abstract
Specific adhesion among like cells is a key determinant of the architecture of tissues. Homophilic (like binds like) adhesive interactions between cells are mediated by cadherins. These integral membrane glycoproteins have a crucial role in tissue morphogenesis during development and the maintenance of tissue integrity in adults. There is also an increasing recognition of a regulatory role for cadherins in a variety of cell functions, including cell migration. The recent identification of cadherin-11 expression in fibroblast-like synoviocytes (FLSs) has shed light on the mechanisms of synovial tissue organization and differentiation. Moreover, cadherin-11 expression in FLSs might also provide insight into pathways that determine the mesenchymal tissue response of the synovium to inflammation.
Introduction
The synovium is a highly organized tissue that resides between the joint cavity and the fibrous joint capsule. In healthy states, the predominant cell type is of mesenchymal origin and demonstrates fibroblast-like features. These cells condense and accumulate at the tissue–joint cavity interface to form a distinct structure called the synovial lining layer. Electron microscopy revealed extensive cell-to-cell contacts within the lining layer [1]. These adhesive cell interactions are probably critical for the organization as well as the structural and functional integrity of the synovial lining layer. Yet the molecular basis for these interactions is not known. The recent identification of cadherin-11 expression in fibroblast-like synoviocytes (FLSs) provides new insight into synovial tissue organization and morphogenesis.
Cadherins are integral membrane adhesion molecules that typically mediate calcium-dependent adhesion between cells of the same type within a tissue [2]. Cadherins are expressed in a tissue-restricted pattern and are well known for their role in cell recognition and cell sorting during development [3]. Some cadherins are named for the tissue in which they are found, such as the epithelial (E-) cadherin, neural (N-) cadherin and placental (P-) cadherin [4]. Importantly, each cadherin typically binds to another cadherin of the same type (E-E, P-P, N-N). Cadherins are composed of five extracellular domains that mediate binding to a cadherin molecule on an adjacent cell (Fig. 1). The molecular interactions with intracellular catenins at the cytoplasmic tail link the cadherin adhesive junction to the actin cytoskeleton and determine cadherin adhesiveness and cell shape (Fig. 1) [5]. Cadherins have also been implicated in contact inhibition of cell proliferation [6]. Compelling evidence indicates a regulatory role for cadherins in cell migration, cell invasion and in the malignant transformation of cancer cells [7,8].
Figure 1.
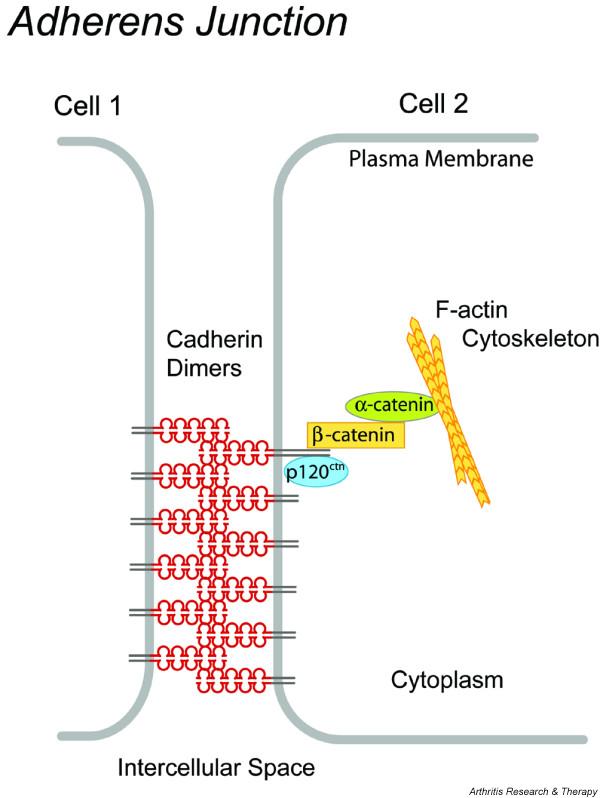
Schematic representation of the cadherin-11 adhesive junction. In the intercellular space, cadherin-11 extracellular domains interact with cadherin-11 extracellular domains of adjacent cells to mediate cell adhesion. Lateral clustering of cadherin molecules is required to form stable cell-to-cell contacts. The intracellular catenins bind to the cytoplasmic tail of cadherin-11. p120 catenin (p120ctn) binds the cadherin tail at the juxtamembrane domain, whereas β-catenin binds the distal domain, the β-catenin binding sequence. α-Catenin associates with β-catenin and is directly linked to the actin cytoskeleton.
Cadherins in tissue morphogenesis
Tissue formation during development requires adhesive cell interactions to gain tissue integrity and to organize cells into a structure that confers proper tissue and cell function. Classical cadherins and catenins, together with the cytoskeletal components, provide the molecular means for cell interactions that stably connect cells together. Cadherins also regulate cell movement that is required for morphogenic processes such as cell sorting, cell condensation, and cell rearrangement [3]. Importantly, these cadherin-mediated processes continue to be critical in later life to the maintenance of tissue integrity and architecture.
The process of cadherin-based cell-to-cell contact formation results in the assembly of a multiprotein junctional complex called the adherens junction (Fig. 1). Adherens junction formation requires specific structural properties of the cadherin molecule. Calcium binding to the cadherins provides the structural rigidity of the five extracellular domains that emanate from the plasma membrane and form stable molecular interactions with cadherins on adjacent cells (Fig. 1) [9]. Disruption of calcium binding has been shown to abolish adhesive function [10]. Atomic structures of cadherin domains have led to a model for the cadherin adhesive interaction in which the membrane-distal extracellular domains mediate the dimerization of cadherins on the same cell and their attachment to the membrane-distal domains of cadherins from adjacent cells. Critical molecular pockets accept amino acid residues from cadherins on adjacent cells, resulting in their binding [11]. More extensive lateral clustering of cadherin molecules at sites of cell-to-cell contact is also needed to establish stable intercellular adhesion [3]. At the cytoplasmic face, cadherins must form complexes with intracellular catenins and the actin cytoskeleton to gain adhesive activity [5]. β-Catenin binds at the distal domain (β-catenin binding sequence, CBS) and mediates the linkage of the cadherin-based junction to the actin cytoskeleton by binding α-catenin, which in turn directly associates with actin filaments (Fig. 1). Because tyrosine phosphorylation of β-catenin is correlated with decreased adhesive activity in response to certain stimuli, β-catenin also operates as a regulator of cadherin adhesiveness [12]. p120 catenin binds at the cytoplasmic juxtamembrane domain and has a key role in maintaining normal levels of cadherin in cells by regulating cadherin trafficking (Fig. 1) [13].
Besides the necessity to stably connect cells to one another, morphogenesis involves dynamic changes in the arrangement of cells within a tissue [3]. These changes require the constant reorganization of adhesive contacts. Cadherins influence this process in several ways. First, disengagement of cells that are connected to one another requires the release of cadherin-adhesive junctions so that the cells can move apart. Second, cadherin adhesive interactions may allow cell movement by directly generating the traction between cells for cell rearrangements to occur [14]. Third, cadherins by themselves may serve as a substrate for the migration of cells across other cells [14]. A compelling example has been provided for a direct role of a classical cadherin in cell migration on a cellular substrate. During Drosophila oogenesis, DE-cadherin (the Drosophila equivalent of vertebrate E-cadherin) is required for border cells to move on the surface of the germline cells. Notably, in this situation, DE-cadherin serves as the substrate that promotes the migration of cells on top of other cells [14].
A major morphogenic transition mediated by cadherins is the process of cell condensation (Fig. 2). During this process, loosely organized cells condense and form intimate contacts along their surfaces. Cell condensation is a key feature of cadherin function and has important implications for the accumulation of cells at distinct areas of the tissue. For example, in the early mouse embryo, rapid activation of E-cadherin function forces loosely adherent blastomeres to form an epithelium, in which cells condense in an ordered fashion [15]. Cadherins are also instrumental in the process of tissue extension. This is brought about by the directed migration of cells and involves cellular rearrangements in which cells remain in close contact while crawling over each other to expand the tissue (Fig. 2) [14]. This cadherin-driven migratory activity determines many developmental processes, including limb or neural tube formation [3]. In later life, cellular rearrangement as a method of tissue extension has been proposed as a mechanism for tumor progression in which tumor cells rearrange so as to extend the tumor mass into host tissues.
Figure 2.
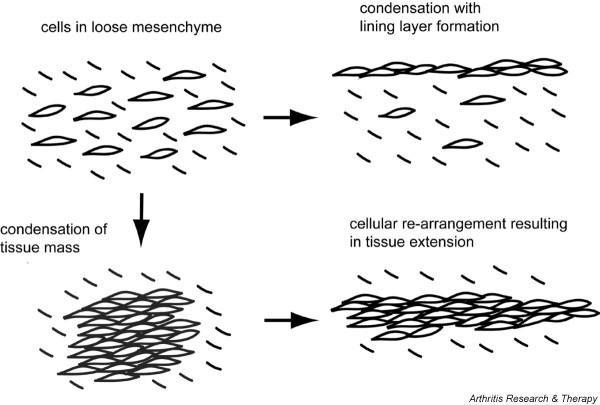
Cadherin-dependent morphogenic processes. Condensation is mediated by cadherins, the intracellular catenins, and the actin cytoskeleton and results in the regional accumulation of like cells. The condensed accumulation of fibroblast-like synoviocytes is responsible for the morphogenesis of the synovial lining layer. Cellular rearrangement is another morphogenic process that involves cell movement and the reorganization of cellular adhesive contacts within the tissue. Compelling evidence indicates that cadherin participates in this process by providing a molecular stratum for cells to crawl over each other, thereby extending the tissue.
Cadherin-11 mediates FLS cell-to-cell adhesion
Given the fact that essentially every solid tissue expresses a cadherin, Valencia and colleagues hypothesized that there exists a synovial cadherin and that this cadherin might have a function in synovial tissue organization [16]. To identify a cadherin species expressed in synoviocytes, they applied a reverse transcription polymerase chain reaction approach using degenerate oligonucleotides corresponding to conserved sequences among human cadherin cytoplasmic tails. This approach revealed the expression of cadherin-11 in cultured FLSs [16]. Indirect immunohistochemistry of frozen synovial tissue sections derived from RA patients revealed specific staining of cadherin-11 in the synovial lining layer. Cadherin-11 reactivity was also seen on a small subset of cells in the sublining. Analysis of osteoarthritic or normal synovium revealed a similar staining pattern. Flow cytometric analysis of freshly dispersed synovial cells indicated cadherin-11 expression predominantly on FLSs but not on cells of hematopoietic origin, including macrophage-like synoviocytes. Functional studies confirmed homophilic adhesive activity of cadherin-11 in FLSs. Morphogenic activity of the synovial cadherin was demonstrated with the use of stably transfected L-cells. On the expression of cadherin-11, L-cells became connected to one another, then condensed and formed aggregates. At higher cell density, the cells formed extensive and intimate contacts along their surfaces, ultimately leading to the formation of a continuous sheet of cells. In contrast, control L-cells transfected with empty vector were loosely organized and the assembly of cells into a tissue-like structure was not observed (Fig. 3). Moreover, cellular organization assays in vitro demonstrated that cadherin-11 expression confers upon L-cells the ability to become organized into a lining-layer-like structure (Fig. 4). These results in vitro support the notion that cadherin-11 in vivo mediates cell-to-cell adhesion and confers upon FLSs the capacity to organize into the synovial architecture.
Figure 3.
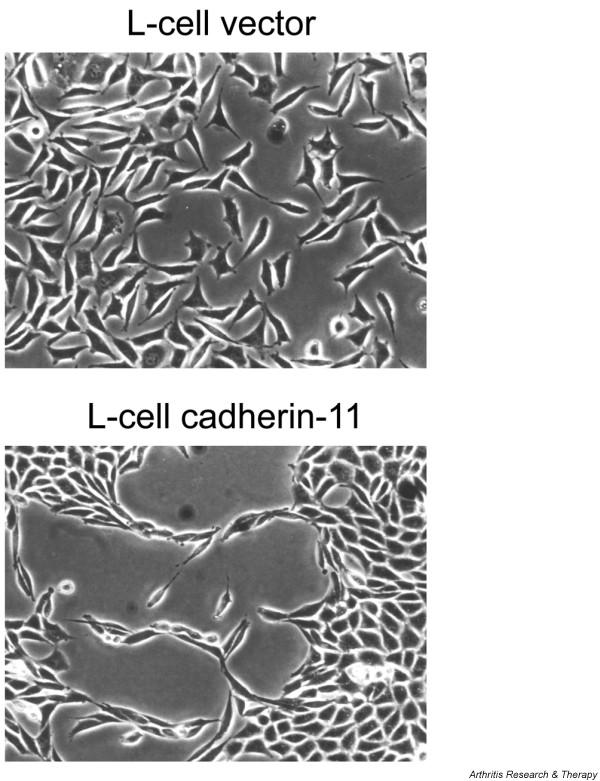
Cadherin-11 mediates cell condensation. Cadherin-11-transfected L-cells or vector control L-cells were seeded in equal numbers. After 4 days in culture, cadherin-11-expressing L-cells formed extensive contacts along their surfaces and condensed at higher cell density to form a continuous sheet of cells. In contrast, vector control L-cells were loosely organized and did not form a tissue-like structure. (Reproduced from The Journal of Experimental Medicine 2004, 200:1677 by permission of The Rockefeller University Press [16].)
Figure 4.
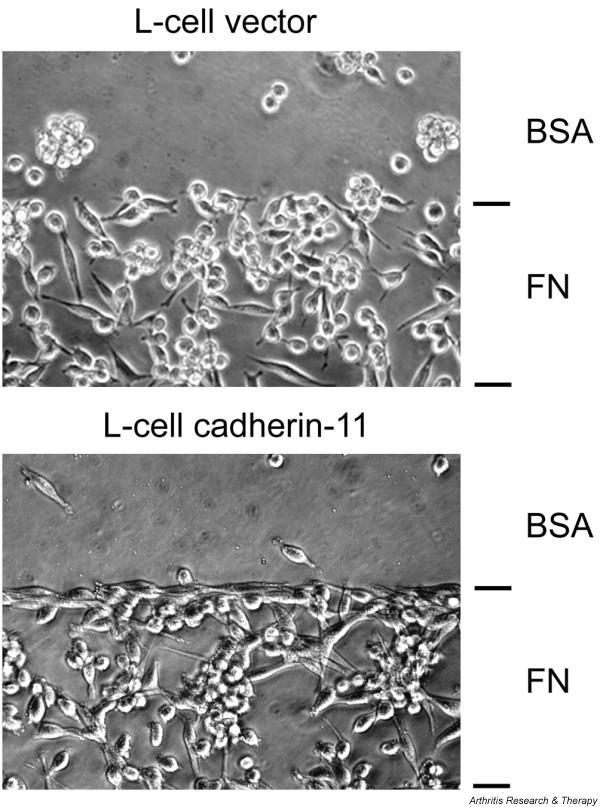
Cadherin-11 mediates lining layer-like formation. Discrete regions of tissue culture dishes were coated with fibronectin (FN), followed by blocking of the entire dish with bovine serum albumin (BSA). L-cells were seeded at equal numbers and cultured under serum-free conditions. After 2 days in culture, cadherin-11-expressing L-cells condensed and accumulated at the FN–BSA interphase to form a lining layer-like structure. In contrast, vector control L-cells were randomly distributed at the FN-coated area and did not form a lining layer. (Reproduced from The Journal of Experimental Medicine 2004, 200:1678 by permission of The Rockefeller University Press [16].)
Cadherin-11 in synovial tissue architecture
The normal synovial lining layer is a condensed accumulation of cells one to four cells thick that resides between the fluid-filled joint cavity and a more loosely packed stroma [17] (Fig. 2). In contrast to the highly organized epithelia, the synovial lining lacks tight junctions, desmosomes and a discrete basement membrane [1]. Rather, it is composed of a compacted network of cells within a lattice of extracellular matrix. This combination of condensed cells and matrix components form a functional barrier between the synovial fluid compartment and the synovial sublining region. Mechanisms contributing to the structural integrity of the synovial lining are beginning to emerge. Although electron microscopy demonstrates synovial lining discontinuity with evidence of significant intercellular matrix space, it also shows the formation of cell-to-cell contacts with communicating cellular processes [1]. The recent identification of cadherin-11 expression by FLSs provides further insight into mechanisms of synovial lining formation and structural organization and integrity [16]. The homophilic adhesion properties of cadherin-11 probably provide a molecular basis for specifying the FLS-to-FLS adhesion that is crucial for the structural integrity of the synovial lining. Indeed, we have recently found that cadherin-11-deficient mice have an attenuated synovial lining (unpublished). In addition, heterophilic adhesion molecule receptor–ligand pairs including α4β1-integrin–CD106 (VCAM-1) are expressed by FLSs and synovial macrophages, providing a means for mediating cellular interactions within the synovial lining layer [18].
In the context of inflammatory arthritis, the synovium undergoes profound changes in cellular content and physiology. In particular, rheumatoid synovitis is characterized by a distinct mesenchymal reaction that yields the formation of a condensed mass of cells (pannus) that encroaches over and invades the cartilage from the periphery of the joint [19]. The predominant cell type found in pannus exhibits fibroblast-like features and is presumably derived from synovial FLSs. Unlike other portions of the hyperplastic synovium, no lining cell layer can be distinguished in the pannus. Instead, it is a continuous mass of cells that is attached to and extends onto the articular cartilage. Cadherin-11 expression on FLSs might be important in the formation and behavior of pannus tissue. Given the role of cadherins in other tissues in mediating cell condensation and tissue extension (Fig. 2), the synovial cadherin is probably involved in the process of cell condensation during pannus formation and might provide a molecular means for pannus invasion in which FLSs crawl over each other to extend the tissue onto the cartilage surface and become invasive.
Cadherin-11 as a regulator of cell behavior beyond cell-to-cell adhesion
Accumulating evidence indicates that classical cadherins control a wide array of cellular functions [6]. In this regard, E-cadherin in epithelial tissues has been the most studied. The significance of E-cadherin for epithelial cell function is suggested by the fact that malignant transformation frequently coincides with the loss of E-cadherin function [20]. Experiments with tumor cell lines and transgenic mouse models have now established that the loss of E-cadherin function is causally involved in the development of carcinomas. Remarkably, reconstitution of functional E-cadherin by transfection in poorly differentiated carcinoma cell lines suppresses their invasive phenotype. Maintenance of E-cadherin expression during tumor development in a transgenic mouse model of pancreatic β-cell tumorigenesis resulted in the arrest of tumor progression at the non-invasive stage, whereas the expression of a dominant-negative E-cadherin yielded invasiveness and early metastasis [7]. The mechanisms by which E-cadherin mediates its tumor suppressor function are being elucidated. Studies now indicate that E-cadherin is not simply effective by physically joining cells, thereby preventing them from breaking away from the tumor mass and becoming invasive. E-cadherin actively regulates cell functions by interfering with intracellular signaling circuits. β-Catenin, which links cadherins to the cytoskeleton, has a central function in these signaling circuits. Thus, besides being crucial for cadherin-mediated cell-to-cell adhesion, β-catenin also binds to and activates the TCF/LEF-1 transcription factor, a key element in the Wnt signaling pathway [5,21]. Alterations in the expression or function of E-cadherin alter the cytosolic pool of the β-catenin pool and thereby influence the TCF/LEF-1 transcriptional program of tumor cells. In addition to p120 catenin (p120ctn) binding to the cadherin cytoplasmic tail at the juxtamembrane domain, p120ctn regulates Rho-family GTPases [22]. The small GTPases RhoA, Rac1, and Cdc42 are well known for their roles in controlling cytoskeletal organization and cell motility [23]. Importantly, only cytosolic p120ctn influences small GTPase activity, whereas cadherin-bound p120ctn does not. Furthermore, p120ctn is able to translocate to the nucleus and bind to Kaiso, a newly discovered member of the POZ/ZF family of transcription factors [24]. Thus, both β-catenin and p120ctn, proteins that bind the cadherin cytoplasmic tail, may translocate to the nucleus and directly influence the transcriptional program of cells.
Recent studies identified the expression of cadherin-11 on cancer cells [8,25]. Strikingly, cadherin-11 expression was associated with enhanced tumor cell motility and invasiveness, thus showing an effect opposite to that of E-cadherin. Furthermore, transfection of cadherin-11 into cells in vitro resulted in increased, rather than decreased, motility and invasiveness [8]. The basis for the functional difference between the effects of E-cadherin and cadherin-11 is not clear. However, these data suggest that cadherin-11 expression confers upon cells a fundamental change in cellular behavior. Therefore, cadherin-11 expression on FLSs might have a determining role for FLS behavior and differentiation with implications for the synovial lining layer as well as the organization and behavior of pannus tissue in RA.
Conclusion
Cadherins have emerged as the predominant group of cellular adhesion molecules involved in morphogenesis, determining tissue integrity and architecture, and regulating cell differentiation [3]. The identification of cadherin-11 expressed on FLSs provides the opportunity to unravel the mechanisms of synovial tissue morphogenesis and differentiation. Indeed, transfection of cadherin-11 confers upon cells the ability to become organized into a tissue-like structure that resembles the synovial lining layer. Given the expression of cadherin-11 in FLSs, elucidating its regulatory role on FLS behavior will represent a major advance in our understanding of synovial biology, providing new insights into processes that control the synovial mesenchymal response to inflammatory reactions. Ultimately, this new path of studies might reveal novel therapeutic targets for intervention in the destructive process of rheumatoid arthritis.
Abbreviations
E-cadherin = epithelial cadherin; FLS = fibroblast-like synoviocyte; p120ctn = p120 catenin.
Competing interests
The author(s) declare that they have no competing interests.
Acknowledgments
Acknowledgements
We thank members of the Brenner laboratory for useful discussions and reading of the manuscript. HPK is supported by the Arthritis Foundation. We thank Steve Moskowitz for artistic assistance.
References
- Barland P, Novikoff AB, Hamerman D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/S0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Proteins associated with the cytoplasmic surface of adhesion molecules. Neuron. 1993;11:551–564. doi: 10.1016/0896-6273(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–560. doi: 10.1016/S0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell–cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TP, Johnson MH. From egg to epithelium. Annu Rev Cell Biol. 1988;4:459–485. doi: 10.1146/annurev.cb.04.110188.002331. [DOI] [PubMed] [Google Scholar]
- Valencia X, Higgins JMG, Kiener HP, Lee DM, Podrebarac TA, Dascher CC, Watts GFM, Mizoguchi E, Simmons B, Patel DD, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200:1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor CW. The microscopic structure of normal human synovial tissue. Arthritis Rheum. 1960;3:140. doi: 10.1002/art.1780030205. [DOI] [PubMed] [Google Scholar]
- Morales-Ducret J, Wayner E, Elices MJ, Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992;149:1424–1431. [PubMed] [Google Scholar]
- Henderson B, Edwards JCW. Structural and microscopic changes. In: Henderson B, Edwards JCW, editor. The Synovial Lining in Health and Disease. London: Chapman & Hall; 1987. pp. 233–283. [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Seidensticker MJ, Behrens J. Biochemical interactions in the wnt pathway. Biochim Biophys Acta. 2000;1495:168–182. doi: 10.1016/S0167-4889(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. The catenin p120ctn interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947–952. [PubMed] [Google Scholar]


