Abstract
Lesional induced pluripotent stem cell-derived endothelial cells can resemble pathological vascular phenotypes of port-wine birthmark (PWB). Our data demonstrate that multiple pathways, including Hippo and Wnt, NFκB, TNF, MAPK and cholesterol metabolism, are dysregulated. These data suggest new therapeutics can be developed to target such dysregulated pathways in the treatment of PWB.
Dear Editor, Port-wine birthmark (PWB) is a congenital vascular malformation with an estimated prevalence of 0.1–0.3% per live births.1 PWB lesions typically show proliferation of endothelial cells (ECs) and smooth muscle cells (SMCs), replication of basement membranes, disruption of vascular barriers and progressive dilatation of the vasculature.2–4 Pathologically, PWB ECs are differentiation-defective ECs.2,5 In this study, we generated PWB patient-derived induced pluripotent stem cells (iPSCs), differentiated them into ECs and characterized the vascular phenotypes.
One surgically excised nodular PWB lesion from one patient (a White man aged 46 years) and one age- and sex-matched deidentified surgically discarded normal skin tissue sample were collected. The donor had very large lesions, with nodular and hypertrophic PWB on his back, chest, arm and hand; he had received multiple rounds of pulsed dye laser treatments prior to surgical procedures. Skin biopsies were collected for the outgrowth of human dermal fibroblasts. iPSC generation was performed with a CytoTune-iPS Sendai Reprogramming Kit (Thermo Fisher Scientific, Waltham, MA, USA). iPSC colonies were selected, isolated and propagated. iPSC induction to mesoderm/mesenchymal stem cells (MSCs), then differentiation into ECs were followed a previously published method,6 with tailored modifications. RNA sequencing was performed on iPSCs, MSCs, and ECs. STAR (https://github.com/alexdobin/STAR), FeatureCounts (http://bioconductor.org/packages/Rsubread) and edgeR (https://bioconductor.org/packages/edgeR) were used for data analysis. For functional characterization of the intersecting gene sets, we used ToppCluster (https://toppcluster.cchmc.org), an extended version of the ToppFun application of the ToppGene Suite.7
For in vitro capillary-like structure (CLS) formation assay, ECs (n = 4 for each cell model, 4.5 × 104 in 200 µL) was added into wells precoated with GeltrexTM (Thermo Fisher Scientific). After CLS formation, cells were fixed and images were acquired. We implanted iPSC-derived control and PWB ECs and MSCs (2 × 106 cells with a ratio of EC: MSC of 2 : 3) with Geltrex into the subcutaneous layer of the skin of severe combined immunodeficiency (SCID) mice using a previously described protocol.8 Male SCID mice [Nude NIH-III; Charles River Laboratories (Wilmington, MA, USA)] were used. The animals were euthanased on day 10 postinjection. Plug-ins were removed, fixed, embedded and sectioned.
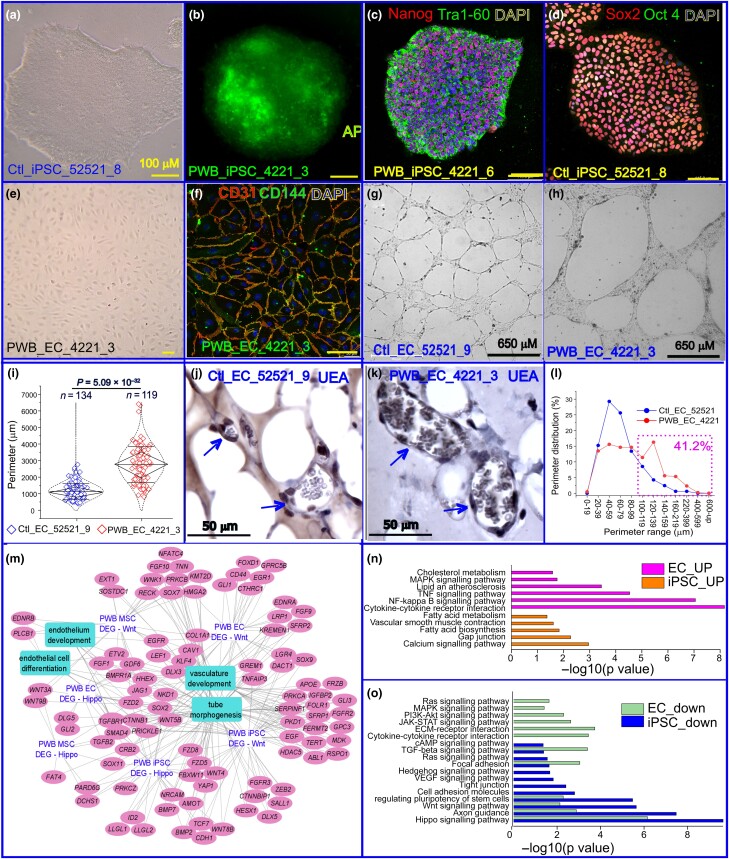
Approximately 12–14 days after the delivery of Yamanaka factors into human dermal fibroblasts, iPSC colonization was observed (Figure 1a). iPSC colonies were confirmed using alkaline phosphatase live staining (Figure 1b). Two iPSC lines from a human PWB lesion (#4221_3; #4221_6) and three lines from normal skin (#52521_4; #52521_8; #52521_9) were successfully expanded and maintained for > 50 passages. These cells were further verified to express the iPSC biomarkers Tra1-60, Nanog, Oct4 and Sox2 (Figure 1c, d). We next differentiated these iPSCs into MSCs then ECs. Fully differentiated monolayer ECs from PWB iPSCs were verified using immunofluorescence staining with antibodies to the specific EC biomarkers CD31 and CD144 (Figure 1e, f).
Figure 1.
Generation and functional characterization of port-wine birthmark (PWB)-derived induced pluripotent stem cells (iPSCs) and their differentiated endothelial cells (ECs). (a) Typical morphology of a control iPSC colony, ctl_iPSC_52521_8. (b) Alkaline phosphatase (AP) staining of the PWB iPSC 4221_3 colony. (c) PWB_iPSC_4221_6 colony-expressing stem cell biomarkers Nanog and Tra1-60 (image overlay). (d) Stem cell biomarkers Sox2 and Oct4 (image overlay) were used to stain control iPSCs. (e) Fully differentiated monolayer ECs from PWB iPSCs were observed on day 8 during differential induction. (f) PWB iPSC-derived ECs expressed membrane biomarkers CD31 (red) and CD144 (green; image overlay). Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). (a–f) Yellow scale bar = 100 µm. (g) Control EC_52521_9 and (h) PWB EC_4221_3 formed capillary-like structures (CLS) on GeltrexTM. (i) PWB EC_4221_3 showed impaired CLS in vitro with larger perimeters (P = 5.09 × 10–32) than the control EC_52521_9. Whiskers indicate mean (SD), diamond boxes indicate the interquartile range, and dotted curves indicate the data distribution. Mann–Whitney U test was used. (j, k) Formation of perfused human vasculature 10 days after intradermal xenograft of the (j) control and (k) PWB ECs with corresponding mesoderm/mesenchymal stem cells (MSCs) into severe combined immunodeficiency mice. Arrows indicate perfused blood vessels in xenografts comprising human ECs, confirmed by immunohistochemistry by an antihuman Ulex europaeus agglutinin 1 (UEA1) antibody. (l) Perimeter distribution of xenografted vasculature formed by PWB ECs vs. control ECs. Pink dashed rectangle indicates the total percentage (41.2%) of perfused vessels formed by PWB iPSC-derived ECs vs. 16.5% of perfused vessels formed by control iPSC-derived ECs with perimeters > 100 µm. (m) Functional interaction network of Hippo- and Wnt-related differentially expressed genes (DEGs) in PWB vasculature showing significant enrichment (false discovery rate < 0.05) for tube morphogenesis, endothelium and vasculature development, and EC differentiation. (n, o) KEGG enrichment analysis showing the top significantly (n) upregulated and (o) downregulated pathways related to vascular differentiation and development in PWB iPSCs and ECs. cAMP, cyclic adenosine monophosphate; ECM, extracellular matrix; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor κB; PI3K, phosphoinositide 3-kinase; STAT, signal transducer and activator of transcription; TGF, transforming growth factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
Next, we performed CLS formation on Geltrex in vitro. CLS formed by PWB EC_4221 in Geltrex in vitro had larger perimeters and greater branch thickness than those formed by normal EC_52521 (Figure 1g–i). In a Geltrex plug-in assay, perfused PWB or normal vasculatures were formed in dermal implants 10 days after xenografting, which comprised human ECs recognized by an antihuman Ulex europaeus agglutinin 1 (UEA1) antibody (Figure 1j, k). Vasculature formed by PWB ECs had larger perimeters and higher densities than that formed by normal ECs; size distribution showed that the total percentage of perfused large vessels (perimeters > 100 µm) formed by PWB iPSC-derived ECs was significantly higher compared with that of those formed by control iPSC-derived ECs (41.2% vs. 16.5%; Figure 1 l), consistent with previously published data from patient lesions and a xenografted animal model.2,8 Finally, we determined differentially expressed genes (DEGs) in PWB iPSCs, MSCs and ECs vs. their control counterparts. Functional enrichment networks showed that DEGs in Hippo and Wnt pathways confer significant functions in vasculature development and EC differentiation (Figure 1 m). Other significantly dysregulated pathways in PWB iPSCs and ECs included regulation of stem cell pluripotency; calcium signalling and gap junctions; fatty acid biosynthesis; vascular SMC contraction; tumour necrosis factor (TNF); nuclear factor κB (NFκB); mitogen-activated protein kinase (MAPK); cholesterol metabolism; transforming growth factor β; axon guidance; and focal adhesion (Figure 1n, o). More detailed data can be found at https://www.biorxiv.org/content/10.1101/2023.07.02.547408v2.
Lesional iPSC-derived ECs can resemble pathological vascular phenotypes of PWB. Our data have demonstrated that multiple pathways, including Hippo and Wnt, NFκB, TNF, MAPK and cholesterol metabolism, are dysregulated. These data suggest new therapeutics may be developed for targeting such dysregulated pathways for the treatment of PWB.
Supplementary Material
Acknowledgements
we are very thankful for the support and assistance of the Instrumentation Resource Facility at University of South Carolina School of Medicine. We greatly appreciate Dr Joyce Bischoff at Boston Children’s Hospital, Harvard University, for providing us with the in vivo xenograft assay protocol. We are also grateful to Arieleus Taine from the Spring Valley High School, Columbia, SC, who participated and performed the RNA sequencing data analysis of the mesenchymal stem cells.
Contributor Information
Vi Nguyen, Department of Cell Biology and Anatomy, School of Medicine.
Chao Gao, Department of Cell Biology and Anatomy, School of Medicine.
Jacob Kravitz, Department of Cell Biology and Anatomy, School of Medicine.
Camilla F Wenceslau, Department of Cell Biology and Anatomy, School of Medicine; Department of Biomedical Engineering, College of Engineering and Computing, University of South Carolina, Columbia, South Carolina 29208, USA.
Yunguan Wang, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio 45229, USA.
Anil G Jegga, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio 45229, USA; Division of Biomedical Informatics, Cincinnati Children Hospital Medical Center, Cincinnati, Ohio 45229, USA.
Wenbin Tan, Department of Cell Biology and Anatomy, School of Medicine; Department of Biomedical Engineering, College of Engineering and Computing, University of South Carolina, Columbia, South Carolina 29208, USA.
Funding sources
this work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R01AR073172 and 1R21AR083066 to W.T.) and Department of Defense (HT9425-23-10008 to W.T.).
Data availability
the processed.bam RNA sequencing files have been deposited into the National Institutes of Health Sequence Read Archive (BioProject ID: PRJNA997591). The transcriptome data (raw and pseudo counts for each sample) have been deposited into the National Center for Biotechnology Information’s Gene Expression Omnibus (accession number: GSE240770). A full version of preprint can be found at https://www.biorxiv.org/content/10.1101/2023.07.02.547408v2.
Ethics statement
the study was approved by the Institutional Review Board at Prisma Health Midlands (#1853132). Experimental procedures for in vivo xenograft assay in severe combined immunodeficiency (SCID) mice were approved by the University of South Carolina Institutional Animal Care and Use Committee.
References
- 1. Lever WF, Schaumburg-Lever G. Histopathology of the Skin, 7th edn. Philadelphia, PA: J.B. Lippincott; , 1990. [Google Scholar]
- 2. Tan W, Wang J, Zhou F et al. Coexistence of EphB1 and EphrinB2 in port wine stain endothelial progenitor cells contributes to clinicopathological vasculature dilatation. Br J Dermatol 2017; 177:1601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan W, Zakka LR, Gao L et al. Pathological alterations involve the entire skin physiological milieu in infantile and early childhood port wine stain. Br J Dermatol 2016; 177:293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan W, Chernova M, Gao L et al. Sustained activation of c-Jun N-terminal and extracellular signal-regulated kinases in port-wine stain blood vessels. J Am Acad Dermatol 2014; 71:964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams J et al. Embryonic stem cell-like population in hypertrophic port-wine stain. JOVA 2021; 2:e006. [Google Scholar]
- 6. Ikuno T, Masumoto H, Yamamizu K et al. Efficient and robust differentiation of endothelial cells from human induced pluripotent stem cells via lineage control with VEGF and cyclic AMP. PLOS ONE 2017; 12:e0173271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 2009; 37:W305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang L, Bichsel C, Norris AL et al. Endothelial GNAQ p.R183Q increases ANGPT2 (angiopoietin-2) and drives formation of enlarged blood vessels. Arterioscler Thromb Vasc Biol 2022; 42:e27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
the processed.bam RNA sequencing files have been deposited into the National Institutes of Health Sequence Read Archive (BioProject ID: PRJNA997591). The transcriptome data (raw and pseudo counts for each sample) have been deposited into the National Center for Biotechnology Information’s Gene Expression Omnibus (accession number: GSE240770). A full version of preprint can be found at https://www.biorxiv.org/content/10.1101/2023.07.02.547408v2.