Abstract
Alzheimer’s disease (AD) is one of the main causes of dementia in senium and presenium. It is clinically characterized by memory impairment, deterioration of intellectual faculties, and loss of professional skills. The cerebellum is a critical part in the distributed neural circuits participating not only in motor function but also in autonomic, limbic, and cognitive behaviors. In present study, we aim to investigate the morphological changes in the Purkinje cells in different cerebellar regions in AD and to correlate them with the underlying AD pathology. Purkinje cells exhibit significant morphometric alterations in AD and prominently in the anterior lobe which is related to higher cognitive functions. The present study gives new insights into the cerebellar pathology in AD and confirms that Purkinje cells pathology is a key finding in AD brains and that AD is characterized by regional-specific atrophy picked in the anterior cerebellar lobe.
Keywords: Alzheimer’s disease, Purkinje cells, cerebellum, cerebellar cognitive syndrome, Golgi method, 3D neuronal reconstruction
Introduction
Alzheimer’s disease (AD) is one of the main causes of dementia in senium and presenium. It is clinically characterized by memory impairment, deterioration of intellectual faculties, and loss of professional skills. 1,2 Alzheimer disease brains are characterized by significant atrophy, being most obvious in the temporal and parietal lobes. 1 Light microscopy reveals deposition of senile plaques and neurofibrillary degeneration initially in the enthorhinal cortex, the hippocampus, and in the acoustic and visual cortices and in the frontal lobe and the cerebellum in the advanced stages. 1,3 -5 Dendritic and spinal pathology as well as loss of synapses are also key neuropathological features. 6
The cerebellum is a critical part in the distributed neural circuits participating not only in motor function but also in autonomic, limbic, and cognitive behaviors. Lesions of the motor cerebellum, mostly in lobules III to V in the anterior lobe and the secondary sensorimotor region in lobule VIII, result in dysmetria of movement; however, lesions of the cognitive and limbic cerebellum in the posterior lobe, represented in lobules VI, VIIA (including lobules VIIAf and VIIAt at the vermis, and crus I and crus II in the hemispheres) and VIIB, and possibly lobule IX, are followed by dysmetria in the realms of intellect and emotion 7,8 (Figure 1). Cerebellar functional topography has been demonstrated by tract tracing studies in nonhuman primates and in physiological and behavioral studies in rodents, cats, and monkeys. 7,9,10
Figure 1.
Schematic representation of anatomical regions of the human cerebellum. Amended from original which uploaded by Nretsand transferred to Commons., CC BY-SA 3.0.
Further studies revealed the existence of a mosaic of intrinsic connectivity networks that match the topographically precise cerebrocerebellar connections, while topographic organization of cerebellum is also evident in task-based functional magnetic resonance imaging in healthy controls 11 and in clinical neurology, neuropsychology, and neuropsychiatry studies in patients with cerebellar lesions. 8
The cerebellum has not been studied extensively in AD; however, recent studies have revealed certain changes in the cerebellum specific for AD. 12
As we have shown in a previous study of ours, the cerebellar cortex is characterized by a unique pattern of Alzheimer-type pathology, while there are only diffuse neuritic plaques and no neurofibrillary changes. 13 Furthermore, a loss of Purkinje cells and synaptic alterations in the mossy fibers, granule cell dendrites, parallel fibers, and Purkinje cell dendrites with substantial loss of dendritic spines and considerable decrease in number of granule and Golgi cells in the granule cell layer have been reported. 2,3,14
In the present study, we aim to investigate the morphological changes of the Purkinje cells in different cerebellar regions in AD and to correlate them with the underlying AD pathology.
Materials and Methods
The present study is based on the morphological analysis of the dendritic arborizations of the Purkinje cells from 3 different parts of the cerebellum from 10 patients withAD and from 10 healthy individuals who died accidentally and were used as normal controls (Table 1). Mean age for patients with AD was 72 years (standard deviation [SD]: 5.4) and for normal controls 73.2 (SD: 7.2). The first part was taken from lobule X (flocculonodular lobe), the second from lobule IV (anterior lobe), and the third one from lobule crus I (posterior lobe). All the brains have been offered by the Laboratory of Forensic medicine and Toxicology of the Aristotle University of Thessaloniki. A written informed consent was obtained from the relatives of the patients who died for each one of the brain, in which it was clearly defined that the tissue would be used for research purposes. The research was carried out with full respect to the appropriate legislation of the Greek Democracy (ν. 2472/1997, 2819/2000, 2915/2001, 3235/2004, 3471 /2006) as is clearly stated by the Committee for Research Deontology Principles of the Aristotle University of Thessaloniki. 15 The average autolysis time for all patients was 10 ± 3.5 hours. All the patients had a clinical diagnosis of AD by a neurologist and Mini-Mental State Examination scores below 12. Gross examination of the brains was performed by a neuropathologist who was blinded to the participant’s cognitive scores and medical history. Tissue blocks from multiple neocortical regions, hippocampus, entorhinal cortex, amygdala, visual cortex, auditory cortex, and cerebellum were embedded in paraffin. To assess Braak stage, sections at the range of 10 μm were stained with Gallyas method for neurofibrillary pathology, while for staining of neuritic plaques, the Bielchowsky and Methenamine Silver method were used. Neuritic plaque scores were determined by semiquantitative estimation of neocortical plaque density using the Consortium to Establish a Registry for Alzheimer's disease (CERAD) criteria. All the AD brains were on Braak stages V/VI, fulfilled the Khachaturian criteria for definite AD, and had indicative CERAD scores for AD. 16
Table 1.
Demographics of AD Patients and NC used in the Present Study.
| Brain | Age | Gender | Braak and Braak stage |
|---|---|---|---|
| AD1 | 67 | M | V/VI |
| AD2 | 65 | M | V/VI |
| AD3 | 72 | F | V/VI |
| AD4 | 72 | M | V/VI |
| AD5 | 69 | M | V/VI |
| AD6 | 76 | F | V/VI |
| AD7 | 73 | M | V/VI |
| AD8 | 79 | F | V/VI |
| AD9 | 81 | F | V/VI |
| AD10 | 66 | M | V/VI |
| NC1 | 59 | M | I/II |
| NC2 | 75 | M | 0 |
| NC3 | 86 | F | 0 |
| NC4 | 72 | M | 0 |
| NC5 | 75 | F | 0 |
| NC6 | 80 | F | 0 |
| NC7 | 69 | M | 0 |
| NC8 | 74 | M | 0 |
| NC9 | 77 | F | 0 |
| NC10 | 65 | F | 0 |
Abbreviation: NC, neocerebellum.
The brains after the excision from the skull were immediately immersed in a formaldehyde 10% fixing solution, where they remained for at least 25 days. Then, we excised small parts from the flocculonodular lobe, the anterior lobe, and the posterior lobe, which have been used for Golgi method. The specimens were immersed in a dilution of potassium dichromate (7 g of potassium dichromate and 1 mL of formaldehyde 37% in 300 mL of tap water) at a temperature of 18 C. They remained in that solution for 1 week, and then they were immersed in aqueous solution of 1% silver nitrate where they remained for 1 more week at a temperature of 15 C in a photoprotected environment. Afterward, the specimens were embedded in low-melting paraffin, cut with a slicing microtome at thick sections at the range of 120 μm, covered with entellan, and studied with a Carl Zeiss Axiostar plus light microscope (Zeiss International). Further adjacent specimens were used for Nissl methylene blue staining.
Cell Selection Criteria
For each one of the brains, 15 Purkinje cells were selected. Neurons examined for quantitative alterations met the criteria set forth by Jacobs et al that request uniform staining of neuronal processes, absence of precipitated debris, good contrast between cells and background, and relatively uniform tissue thickness. 17
For purposes of randomization, all the cells that met the selection criteria were randomly pooled, and every third neuron in the series was chosen.
Neuronal Tracing and Dendritic Quantification
For every one of the cells, we captured a 30-second video at a magnification of 400×, while the microscope table was moving at the standard velocity of 20 μm/s. The microscope stage was moving using a motorized XYZ microscope stage system (MLS203/MZS500-E-ThorLabs, ThorLabs, Inc.), with the movement on the Z-axis being controlled by the MZS500-E – Z-Axis Piezo Stage and Controller Kit, with the aim of the APC software provided by Thorlabs with a JogStep of 1 μm and a travel range of 250 μm. The videos were analyzed in digital image sequences of 200 serial pictures, which were ultimately imported in Neuromantic application to trace the cells, quantifying them along x-, y-, and z coordinates. 18 Each one of the selected cells was traced using the Neuromantic application. Neuronal tracing was carried out in the semi-automatic form by 2 different investigators, and the average of these measurements was used for statistical analysis. The neuronal tracing started with the cell soma and moved onto the basilar dendrites and the apical shaft. Dendritic trees were quantitatively evaluated in a centrifugal manner for apical dendrites and basal dendrites according to Uylings et al. 19 Dendrites arising from the cell soma are considered first-order segments, up to their first symmetrical bifurcation. Dendritic branches arising from first-order segments are considered second-order segments, in turn, up to their symmetrical bifurcation into third-order segments, and so on. When asymmetric branching is met during the neuronal tracing, the offspring dendritic branch, recognized by a qualitatively thinner diameter, is classified as a next order branch, whereas the parent dendrite would retain its order level past the branching point.
Dendritic Measures and Sholl Analysis
The parameters measured were total dendritic length (TDL), total number of dendritic segments and terminal branches, total dendritic area, and total dendritic volume. Furthermore, the tracing was quantitatively analyzed with Fiji and Simple Neurite Tracer plugin based on Sholl’s method of concentric spheres. 20 Concentric spheres were drawn, at intervals of 10 μm centered on the cell bodies, and dendritic intersections within each sphere were counted (Figures 2A and B).
Figure 2.

(A) Representative example of Sholl’s analysis technique. We used concentric circles starting from cell soma with an increasing radius of 5 μm. Yellow dots represent intersections, (B) schematic representation from the same analysis, where different colors represent different dendritic density.
Spine Counts
Spine counts were carried out at 500 pictures, which were taken with an AxioCam HR, at the standard magnification of 1000×, on an Axiostar Plus photomicroscope. Visible spines were counted on 3 segments of the dendritic field. The first segment, 20 to 30 μm in length, was located in a distance of 50 μm of cells soma, the second segment 20 to 30 μm in length in 150 μm from cells soma, and the third one 20 to 30 μm in 250 μm from cells soma.
Purkinje Cell Density
We also measured the linear density of the Purkinje cells in Nissl-stained specimens, on 20 images randomly selected for each cerebellar area for each brain at a standard magnification of 20×, with the help of cell counter plugin in Image J software.
Statistical Analysis
For the statistical analysis of the findings and the plotting graphs, we used Python in a Jupyter notebook, using standard scientific environments such as numpy, scipy, and matplotlib. 21 All the results were stored in csv files and presented for the statistical analysis as pandas data frames. A Student t test was used to determine whether significant differences existed across the independent parameters from Purkinje cells from normal controls and AD brains (significance was taken as P < .05). A Pearson correlation test was performed to investigate the relationship between morphological changes and plaques density. Furthermore, a Pearson correlation test was carried out in order to figure out whether there is any correlation between the autolysis time and the Purkinje cell dendritic complexity variables.
Results
Golgi Silver Staining
Golgi-stained tissue did not exhibit the autolytic changes described by Williams et al, 22 and Pearson’s correlation test did not show any link between the autolysis time and Purkinje cells complexity variables.
Dendritic Parameters
The Golgi impregnation technique revealed substantially lesser tertiary dendritic branches of the Purkinje cells in AD brains in comparison to normal controls and markedly lower density of dendritic arborization in all the 3 cerebellar regions that were examined in the present study, but predominantly in the neocerebellum. Total dendritic length was lesser by 25% in the archicerebellum (AC) and the paleocerebellum (PC) and by 40% in the neocerebellum (NC) in comparison to normal controls (Figure 3). The number of terminal branches was also lowered by 30% in both the AC and PC and by 44% in the NC, and dendritic area and dendritic volume were also lowered by 19% and 35%, respectively, in the AC, 25% and 38% in the PC, and 29% and 49% in the NC, compared to controls (Figures 4 –6).
Figure 3.
A, Purkinje cells from AD brains, Golgi method, magnification ×400 (B and C). Purkinje cells from NC, Golgi method, magnification ×100. Purkinje cells from AD brains showed significant lessening in dendritic density compared to NC. AD indicates Alzheimer’s disease; NC, neocerebellum.
Figure 4.
Total dendritic length, number of terminal branches, total dendritic area, and volume of the Purkinje cells from the flocculonodular lobe were all significantly lower in AD brains. AD indicates Alzheimer’s disease.
Figure 5.
Total dendritic length, number of terminal branches, total dendritic area. and volume of the Purkinje cells from the anterior lobe were all significantly lower in AD brains. AD indicates Alzheimer’s disease.
Figure 6.
Total dendritic length, number of terminal branches, total dendritic area, and volume of the Purkinje cells from the posterior lobe were all significantly lower in AD brains. AD indicates Alzheimer’s disease.
Dendritic Spines
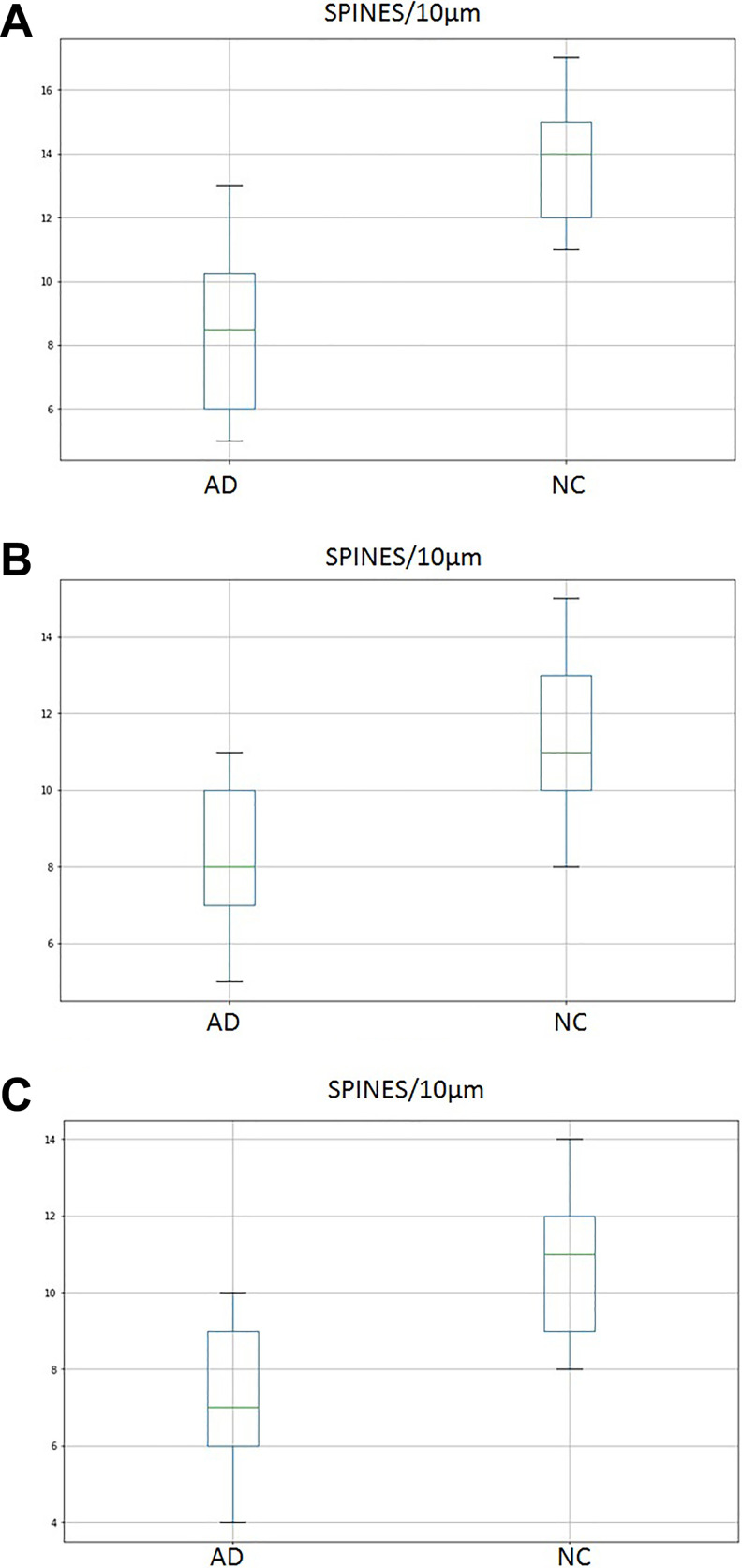
Compared to normal controls, the density of the dendritic spines of the Purkinje cells of AD was 27% lesser in the AC, 28% in the PC, and 38% in the NC (Figure 7A-C, Figure 8A and B).
Figure 7.
Density of dendritic spines was significantly lower in the flocculonodular lobe (A), the anterior lobe (B) and the posterior lobe (C) of the cerebellum in AD. AD indicates Alzheimer’s disease.
Figure 8.

Representative segment of a tertiary dendritic branch from a Purkinje cell from AD (A), and NC (B), showing a significant difference in dendritic spine density. Golgi method, ×1000. AD indicates Alzheimer’s disease; NC, neocerebellum.
Sholl’s Analysis
We used Sholl analysis to measure the number of intersections as a function of distance from the soma. Concentric circles analysis of the dendritic arborization of the Purkinje cells demonstrated marked lessening in the density of dendritic arborization in AD brains in all the cerebellar areas (Figures 9A1, A2, 9B1, B2, and 9C1, C2).
Figure 9.
Sholl’s analysis revealed significant lessening in the dendritic density of the Purkinje cells from the flocculonodular lobe (A), the anterior (B) and the posterior lobes (C) of the cerebellum in AD. AD indicates Alzheimer’s disease.
The peak of the dendritic density of the Purkinje cells occurred between 150 and 200 at 300 μm from the cell soma for AD brains and above 300 μm for the normal controls.
Purkinje Cell Density
Purkinje cells’ density was significantly lower in all 3 cerebellar areas in the AD brains in comparison to NC, but the posterior lobe was more severely affected. The flocculonodular lobe showed a density of 5.29 (SD 0.72) in AD compared to 8.94 (SD 0.21) in controls (P < .001), the anterior lobe 6.05 (SD 0.59) and 9.5 (SD 0.49) in AD and controls, respectively (P < .001), and the posterior lobe 5.94 (SD 0.74) and 10.4 (SD 0.47) in AD and controls (P < .001; Figure 10A-C).
Figure 10.
Purkinje cell density in the cerebellar area studies in the present study, in AD and controls. Error bars indicate standard deviation. AD indicates Alzheimer’s disease.
Neurofibrillary Tangles and Plaque Density
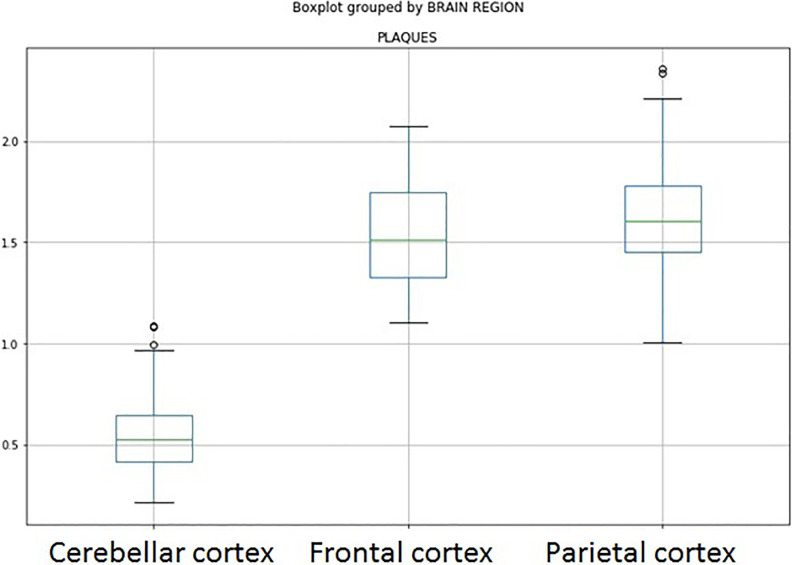
Gallyas technique revealed neurofibrillary degeneration in 30% of the Purkinje cells. Two types of plaque-like deposits were demonstrated in AD cerebellar cortex. Diffuse plaques in the molecular layer and compact amyloid cored plaques in the Purkinje and granular cell layers. Diffuse plaques in the molecular layer of cerebellar cortex were not detected in normal aged controls. Typical neuritic plaques were not seen in the cerebellar cortex of AD brains. The plaques found in the cerebellum were detected in far smaller proportion than senile plaques in the prefrontal and parietal cortices of the same cases (Figure 11). No association between plaque density and morphological changes has been noticed in AD brains.
Figure 11.
The density of senile plaques was significantly lower in the cerebellar cortex in comparison to frontal and temporal lobes in AD, as revealed by Silver staining methods. AD indicates Alzheimer’s disease.
Discussion
Cerebellum was thought to be spared by AD; however, recent studies showed a number of pathological changes. The cerebellar cortex is characterized by a unique pattern of Alzheimer-type pathology, with diffuse neuritic plaques, very rare sparse neuritic plaques, 23 and only minor neurofibrillary changes. 3,13
The network theory of neurodegeneration builds upon the Hebbian notion that neurons that are functionally and anatomically connected may also degenerate and die together. Interconnected neural networks in the nonhuman primates and human brain were identified with physiological techniques and functional neuroimaging studies. 24
Prusiner proposed that neurodegeneration occurs within interconnected networks as a result of self-propagation/prion-like spread of neurotoxic agents along neural pathways linking distributed nodes into functional modules. 25 Guo and colleagues mapped the patterns of atrophy in AD and frontotemporal dementia, showing that in the first condition there is regional atrophy in the cerebellum involving crus I and II bilaterally, while in frontotemporal dementia the peak atrophied region was in lobule VI, predominantly on the left Guo et al. 26 They compared the degree of atrophy between cerebrum and cerebellum within the same connectivity networks, and they concluded that the cerebellum undergoes focal atrophy in concert with interconnected cerebral nodes within the same functional module. 12
Previous neuropathological and morphological studies have shown loss of distal dendritic segments, decrease in the total number of dendritic spines, ubiquitin-immunoreactive dystrophic neurites, and microglial proliferation of the Purkinje cells. 4,13,14,27,28,29
In the present study, we found significantly lower density of Purkinje cells and substantial morphological changes in AD brains, including a lower dendritic density, and lower numbers of dendritic branches and dendritic spines, in the flocculonodular lobe, the anterior, and the posterior lobes. These findings confirm the involvement of the cerebellum and more specifically the Purkinje cells in AD neuropathology and gives insights into the difference of the morphological changes in different parts of the cerebellum. Although the changes in the Purkinje cells density and the morphological alterations of the Purkinje cells from all the areas examined in the present study were statistically significant, crus I, which corresponds to the Neocerebellum, or the posterior lobe, have been predominantly affected. This is in keeping with the finding of Guo et al that in AD there is regional atrophy in the cerebellum involving crus I and II. 26
Distal dendritic segments and dendritic spines, which are the most plastic components of the dendritic arborisation, 30 were prominently affected in Purkinje cells from AD brains. These changes may indicate an impairment of mechanisms underlying neuroplasticity in the cerebellar cortex AD. Indeed, soluble Aβ peptide and Aβ oligomers unsettle neuroplasticity imbalance, resulting in an impairment of synaptic stabilization 31,32 and loss of dendritic spines and synapses in AD brains. Hyperphosphorylation of tau protein may also cause deleterious effects of neuroplasticity and may underlie its role in the etiology of AD 33,34
The molecular layer of the cerebellar cortex is characterized by a unique pattern of pathology in AD, which includes deposition of spare of diffuse plaques and the absence of typical senile plaques, and only minor neurofibrillary changes. 3,13
Subsequently, other mechanisms, such as oxidative damage, vascular pathology, blood–brain barrier abnormalities, may also participate in the neuronal degeneration in AD. 13,27 Prusiner’s suggestion is that self-propagation/prion-like spread of neurotoxic agents along neural pathways linking distributed nodes into functional modules could also explain the pattern of neurodegeneration in the cerebellum in AD.
There was no neuritic plaques’ deposition in the cerebellar cortex in AD, and only few diffuse plaques demonstrated. Compared to cerebral Aβ plaques in AD, cerebellar Aβ plaques could be considered to possibly represent an earlier form of plaque evolution or even an attenuated stage in the process of plaque maturation.
It is important to emphasize that the cerebellar cortex is affected in AD despite the fact that neurofibrillary degeneration and typical senile plaques deposition are not prominent. Cerebellar cortex offers a valuable background for the further study of AD pathogenesis is and future treatment strategies.
The loss of Purkinje cells and the morphological changes seen in thick sections of Golgi-stained material leads to a substantial decrease in the synaptic area and synaptic contacts of the Purkinje cells, and this could contribute to the changes in equilibrium, limb coordination, and cognitive decline that are clinically demonstrable in persons with AD. 35 Furthermore a cerebellar cognitive affective syndrome should be additionally considered to the cognitive profile of AD.
Discussion on Selection of Methods
Although Golgi method is capricious and unpredictable, it has been used for more than 100 years for the study of the neuronal morphology and continues to provide a unique view of the neuronal cell. With Golgi method, the cell soma, as well as the dendrites, are clearly stained in brown and black and can be followed through to their entire length. Gallyas staining has been used for the depiction of neurofibrillary tangles, while it shows almost the same sensitivity as the Thioflavin S staining and is more sensitive in neurofibrillary tangles (NFT) demonstration than PHF-1 or NFT immunostaining. 36
Senile plaques are best seen with silver stains and immunocytochemistry for amyloid-β protein. Furthermore, for most practical and diagnostic reasons, they are divided into the “diffuse” or “preamyloid” plaques and the “classical” or “neuritic” plaques. Diffuse plaques stain with Bielschowsky and Methenamine Silver staining. Wisniewski et al, in a comparative study of 4 staining methods on the detection of neuritic plaques, demonstrated that Bielschowsky’s method revealed the same number of neuritic plaques in comparison to immunostaining with monoclonal antibody (4G8, IgG2b) to β-amyloid. 37 According to Yamaguchi et al, Bielschowsky’s method is more effective than b protein immunostaining in the visualization of the diffuse types of plaques. 38 Furthermore, Methenamine Silver staining is more specific to the amyloid of the primitive and typical types of senile plaques than Periodic Acid Modified Methenamine stain stain, results similar to that of the b protein immunostain 39,40 and is the most sensitive method for detecting the presence of the diffuse type of senile plaques. 41
For neuronal tracing, we chose Neuromantic because it is a stand-alone freeware application; it is designed to provide a simple and intuitive interface for the exploration of serial image stacks and the reconstruction of dendritic trees and can be used to translate, scale, and move through the images of stacks using the mouse via a simple click-and-drag interface. Furthermore, the morphology may be easily modified by deleting segments/branches or changing connectivity to correct possible tracing errors. 18
Python scientific libraries were selected and used for the statistical analysis for the reason that it is completely free, light-weight, easy to learn, super-efficient, highly scalable, always updated, and the richness of Python is an invaluable asset when it comes to building complex analysis pipelines with mixed statistics. 21
Conclusions
The cerebellar cortex provides an excellent background for the study of the neuropathology and pathophysiology in AD. The present study gives new insights into neuropathology of the cerebellar cortex in AD and confirms the findings of previous studies on the involvement of the cerebellum in AD. Other mechanisms, such as oxidative damage, vascular pathology, and blood–brain barrier abnormalities may also participate in the neuronal degeneration in AD. Furthermore, self-propagation/prion-like spread of neurotoxic agents along neural pathways linking distributed nodes into functional modules could also explain the pattern of neurodegeneration in the cerebellum in AD. It is also important to emphasize that morphological changes in the Purkinje cells is one of the pathological features of AD, and compared to cerebral Aβ plaques in AD, cerebellar Aβ plaques could be considered to possibly represent an earlier form of plaque evolution or even an attenuated stage in the process of plaque maturation. Last but not the least, a cerebellar cognitive affective syndrome to the cognitive profile of AD should also be considered.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ioannis Mavroudis  https://orcid.org/0000-0001-9844-4010
https://orcid.org/0000-0001-9844-4010
References
- 1. Baloyannis SJ. Neuropathology of Dementia. In: Baloyannis SJ, ed. Thessaloniki; 1993. Greece: 1st Department of Neurology, Aristotelian University of Thessaloniki; 1993:2–5. [Google Scholar]
- 2. Perry RJ, Hodges JR. The relationship between functional and neuropsychological performance in early Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2000;14(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3. Wegiel J, Wisniewski HM, Dziewiatkowski J, et al. Cerebellar atrophy in Alzheimer’s disease—clinicopathological correlations. Brain Res. 1999;818(1):41–50. [DOI] [PubMed] [Google Scholar]
- 4. Larner AJ. The cerebellum in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1997;8(4):204–209. [DOI] [PubMed] [Google Scholar]
- 5. Padurariu M, Ciobica A, Mavroudis I, Fotiou D, Baloyannis S. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr Danub. 2012;24(2):152–158. [PubMed] [Google Scholar]
- 6. Mavroudis IA, Fotiou DF, Manani MG, et al. Dendritic pathology and spinal loss in the visual cortex in Alzheimer’s disease: a Golgi study in pathology. Int J Neurosci. 2011;121(7):347–354. [DOI] [PubMed] [Google Scholar]
- 7. Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48(11):1178–1187. [DOI] [PubMed] [Google Scholar]
- 8. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 11): 561–579. [DOI] [PubMed] [Google Scholar]
- 9. Schmahmann JD. The cerebellum and cognition. International Review of Neurobiology. In: Schmahmann JD, ed. Vol. 41. San Diego, CA: Academic Press; 1997:31–60. [DOI] [PubMed] [Google Scholar]
- 10. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. [DOI] [PubMed] [Google Scholar]
- 11. Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmahmann JD. Cerebellum in Alzheimer’s disease and frontotemporal dementia: not a silent bystander. Brain. 2016;139(Pt 5):1314–1318. [DOI] [PubMed] [Google Scholar]
- 13. Mavroudis IA, Fotiou DF, Adipepe LF, et al. Morphological changes of the human purkinje cells and deposition of neuritic plaques and neurofibrillary tangles on the cerebellar cortex of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2010;25(7):585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukutani Y, Cairns NJ, Rossor MN, Lantos PL. Purkinje cell loss and astrocytosis in the cerebellum in familial and sporadic Alzheimer’s disease. Neurosci Lett. 1996;214(1):33–36. [DOI] [PubMed] [Google Scholar]
- 15. Research Committee. Research Deontology Principles, 2nd ed. Thessaloniki, Greece: Aristotle University of Thessaloniki; 2010:23–24. [Google Scholar]
- 16. Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42(11):1097–1104. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi Study. J Comp Neurol. 1997;386(4):661–680. [PubMed] [Google Scholar]
- 18. Myatt DR, Hadlington T, Ascoli GA, Nasuto SJ. Neuromantic—from semi-manual to semi-automatic reconstruction of neuron morphology. Front Neuroinform. 2012;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uylings HBM, Van Eden CG, Parnavelas JG, Kalsbeek A. The prenatal and postnatal development of rat cerebral cortex. In: Kolb B, Tees RC, eds. The cerebral cortex of the rat. Cambridge, England: MIT Press; 1990;35–76. [Google Scholar]
- 20. Sholl DA. The organization of the visual cortex in the cat. J Physiol. 1955; 89(Pt 1):33–46. [PMC free article] [PubMed] [Google Scholar]
- 21. Millman KJ, Aivazis M. Python for scientists and engineers, Computing in Science & Engineering. 2011;13(1):9–12 [Google Scholar]
- 22. Williams RS, Ferrante RJ, Caviness VS, Jr. The Golgi rapid method in clinical neuropathology: morphological consequences of suboptimal fixation. J Neuropath Exp Neurol. 1978;37(1):13–33. [DOI] [PubMed] [Google Scholar]
- 23. Catafau AM, Bullich S, Seibyl JP, et al. Cerebellar Amyloid-β Plaques: How Frequent Are They, and Do They Influence 18F-Florbetaben SUV Ratios? J Nucl Med. 2016;57(11):1740–1745. [DOI] [PubMed] [Google Scholar]
- 24. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prusiner SB. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet. 2013;47:601–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo CC, Tan R, Hodges JR, Hu X, Saber S, Hornberger M. Network-selective vulnerability of the human cerebellum to Alzheimer’s disease and frontotemporal dementia. Brain. 2016;139(Pt 5):1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baloyannis SJ, Manolidis SL, Manolidis LS. Synaptic alterations in the vestibulocerebellar system in Alzheimer’s disease—a Golgi and electron microscope study. Acta Otolaryngol. 2000;120(2):247–250. [DOI] [PubMed] [Google Scholar]
- 28. Dickson DW, Wertkin A, Mattiace LA, et al. Ubiquitin immunoelectron microscopy of dystrophic neurites in cerebellar senile plaques of Alzheimer’s disease. Acta Neuropathol. 1990;79(5):486–493. [DOI] [PubMed] [Google Scholar]
- 29. Yamamoto T, Hirano A. Hirano bodies in the perikaryon of the Purkinje cell in a case of Alzheimer’s disease. Acta Neuropathol (Berl). 1985;67(1-2):167–169. [DOI] [PubMed] [Google Scholar]
- 30. Michmizos D, Koutsouraki E, Asprodini E, Baloyannis S. Synaptic plasticity: a unifying model to address some persisting questions. Int J Neurosci. 2011;121(6):289–304. [DOI] [PubMed] [Google Scholar]
- 31. Wang HW, Pasternak JF, Kuo H, et al. Soluble oligomers of ab-amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002; 924(2):133–140. [DOI] [PubMed] [Google Scholar]
- 32. Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8(15):3213–3217. [DOI] [PubMed] [Google Scholar]
- 33. Maccioni RB, Cambiazo V. Role of microtubule associated proteins in the control of microtubule assembly. Physiol Rev. 1995;23:1–27. [DOI] [PubMed] [Google Scholar]
- 34. Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging. 1995;16(3):355–362. [DOI] [PubMed] [Google Scholar]
- 35. Franssen EH, Souren LE, Torossian CL, Reisberg B. Equilibrium and limb coordination in mild cognitive impairment and mild Alzheimer’s disease. J Am Geriatr Soc. 1999;47(4):463–469. [DOI] [PubMed] [Google Scholar]
- 36. Sun A, Nguyen XV, Bing G. Comparative analysis of an improved thioflavin-S stain, gallyas silver stain, and immunohistochemistry for neurofibrillary tangle demonstration on the same sections. J Histochem Cytochem. 2002;50(4):463–472. [DOI] [PubMed] [Google Scholar]
- 37. Wisniewski HM, Wen GY, Kim KS. Comparison of four staining methods on the detection of neuritic plaques. Acta Neuropathol. 1989;78(1):22–27. [DOI] [PubMed] [Google Scholar]
- 38. Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Harigaya Y. Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol. 1988;77(2):113–119. [DOI] [PubMed] [Google Scholar]
- 39. Haga C, Ikeda K, Iwabuchi K, Akiyama H, Kondoh H, Kosaka K. Methenamine-silver staining: a simple and sensitive staining method for senile plaques and neurofibrillary tangles. Biotech Histochem. 1994;69(5):295–300. [DOI] [PubMed] [Google Scholar]
- 40. Yamaguchi H, Haga C, Hirai S, Nakazato Y, Kosaka K. Methenamine silver staining integrates amyloid-related components of senile plaques in the Alzheimer brains as clearly as b protein immunostaining. Ann Rep Col Care Techol Gumma Univ. 1989;10:121–130. [Google Scholar]
- 41. Kamiya S, Yamagami T, Fujii S, Yamano S, Umeda M, Sugiyama M. Senile plaques in the Beagle brain. Bull Nippon Vet Anim Sci. 1995;44:1–4. [Google Scholar]