Abstract
Objectives:
To assess the psychometric properties of the Peruvian version of the Rowland Universal Dementia Assessment Scale (RUDAS-PE) to discriminate controls from patients with mild cognitive impairment (MCI) and early dementia (ED) in a population with a mid-level education.
Methods:
A total of 133 patients from a memory clinic were administered the RUDAS-PE, INECO Frontal Screening, Addenbrooke’s Cognitive Examination, and Mini-Mental State Examination. Results were compared against a neuropsychological evaluation (gold standard). Validity measures, internal consistency, and concurrent validity were calculated.
Results:
Cronbach’s α was 0.68; Pearson’s ratio was 0.79 (P < .01). The area under the receiver–operating characteristics curve of the RUDAS to discriminate between ED and MCI was 89.0% (optimal cutoff at <21), whereas between MCI and controls, it was 99.0% (optimal cutoff at <24).
Conclusions:
The RUDAS-PE has acceptable psychometric properties performing well in its ability to discriminate controls from patients with MCI and ED.
Keywords: cognitive impairment, neuropsychological tests, dementia, neurocognitive disorders, Alzheimer’s disease, brief cognitive tests
Introduction
Due to a number of different factors, the number of people diagnosed with dementia in Latin America (LA) is projected to quadruple by 2050. 1 Therefore, strategies for early detection and intervention need to be prioritized. Access to obtaining a dementia diagnosis in LA poses severe constraints on the public health system, given that complete medical and cognitive assessments are conducted by specialists; rarely are they done by general practitioners. 2 The high level of cases gone undetected and misdiagnosed are proportional to the high number of doctors in LA who haven’t received proper training in diagnosing dementia, 3 particularly in the differential diagnosis of less common forms of dementia. 4 On the other hand, it’s been shown that doctors and nurses in a primary care setting can, if properly trained, obtain a dementia diagnosis with reasonable accuracy. Clinical data can be obtained during a routine consultation all within the time span of an average primary care office visit. 5 Likewise, community health workers, with only a few hours of training, can identify dementia with a positive predictive value of 66%. 6 Thus, it’s essential to promote programs that detect, in a timely manner, different stages of dementia as well as mild cognitive deterioration via the utilization of brief cognitive tests (BCTs). Moreover, BCTs should be adapted for and validated by each country within LA 7 and evaluated across different populations, including people who are illiterate and individuals with low levels of education. 1
Brief cognitive tests are tools frequently used in clinical practice as screening tests, as well as in follow-up and response to treatment. 8 The main characteristic of a BCT is its short administration time: no more than 5 minutes in a primary care consult and less than 10 minutes in a specialist consult. Additionally, it should be applicable to all persons (independent of education level, sociodemographic, ethnic, or cultural background), at all levels of health care, and in any setting. 2,8
The Mini-Mental State Examination (MMSE) is the most commonly used BCT 9 with multiple validations in LA. 10 –13 However, there are considerable limitations, including age, cultural and socioeducational level, lack of standardization, and lack of executive function evaluations. Similarly, the Montreal Cognitive Assessment requires cultural adaptations and different cutoff points depending on the education level. 14 –17 Several globally assessed BCTs have been validated in urban participants living in Lima, Peru including the Clock Drawing Test (CDT) as developed by Manos and Wu, 18,19 the Addenbrooke’s Cognitive Examination (ACE), 20 the Peruvian version of the Eurotest for dementia, 21 the INECO Frontal Screening (IFS) test, 22 and the Memory Alteration Test (M@T). 23,24
The Rowland Universal Dementia Assessment Scale (RUDAS) is a BCT developed and validated in Australia 25 showing acceptable psychometric properties and is not influenced by gender, years of education, or language, 26 giving similar results in communities with both mild and moderate stages of dementia. 27 In a meta-analysis, Canadian researchers 28 concluded that the RUDAS is a quick and effective BCT available free of charge and particularly useful in patients from different languages and cultures. In Spain 29 and Brazil, 9 the RUDAS proved to be superior to the MMSE in detecting dementia cases in populations with low levels of education.
This study set out to determine the ability of the RUDAS to discriminate between no cognitive impairment (no-CI), mild cognitive impairment (MCI), and early dementia (ED) in participants with a middle-level education in a memory clinic setting. Its psychometric properties were compared against the IFS, ACE, and MMSE.
Methods
Study Design
This is a diagnostic accuracy study designed to evaluate the performance of the RUDAS against a specialized neuropsychological evaluation—the gold standard.
Participants
The study took place in the outpatient clinics of neurology and rehabilitation medicine of the Instituto Peruano de Neurociencias in Lima between March 2017 and September 2018. Patients were aged 60 years or older, spoke Spanish, and completed a minimum of 6 years of formal education. We excluded patients whose cognitive function may have been affected through the use of certain drugs or through a particular medical condition, including those not of a neurodegenerative and/or vascular etiology such as history of addiction and substance abuse, depression, hypothyroidism, vitamin B12 deficiency, liver or chronic kidney disease, neuroinfections caused by HIV or syphilis, severe cranial trauma, and subdural hematoma. Furthermore, we excluded patients with any conditions that could affect his or her performance in the cognitive tests: hearing and visual deficiencies, motor sequelae of cerebrovascular disorder, or traumatic sequelae.
This study was conducted in accordance with the guidelines of the Council for International Organizations and Medical Sciences. A written informed consent was signed by all study participants or their primary caretakers in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committee of the Instituto de Medicina Tropical “Daniel Alcides Carrión” of the Universidad Nacional Mayor de San Marcos approval number CIEI-2018-020.
Outcome Measures
Rowland Universal Dementia Assessment Scale
The RUDAS is a simple tool that takes 10 minutes to administer and is comprised of 6 components exploring memory, body orientation, visuospatial praxis, motor praxis, judgment, and language. Like the MMSE, it has a maximum score of 30, where a lower score denotes poor cognitive performance. 25 Validation studies of the RUDAS in both Spain 29 and Brazil 9 reported similar area under the receiver–operating characteristic (ROC) curve as the MMSE for the detection of dementia in an outpatient sample population. Spain reported an ideal cutoff point for the RUDAS at <22 with a sensitivity of 94.3% and a specificity of 72.6%. 29 Meanwhile, in Brazil, the ideal cutoff point for the RUDAS was <23 with a sensitivity of 81.5% and a specificity of 76.1%. 9
RUDAS adaptation process
Direct translation and back-translation
To begin the process of adapting the RUDAS, we used the translated Spanish version validated by the Universidad de Compostela in Coruña, Spain. 30
Recommendations from experts
We analyzed word characterization in order to understand word frequency according to semantic categories. Identifying words of high, middle, and low usage in the urban Peruvian population allowed us to adjust the test accordingly. Additionally, experts in linguistics, geriatrics, psychiatry, licensed translators, and bilingual investigators assessed the adaptation; the final version was approved by consensus.
We performed 3 cultural modifications. The first modification was within the cognitive domain of memory (memory stores) where we substituted the word “tea” (an item seldom purchased in our region) for “coffee.” Within the cognitive domain of judgment, the following low-frequency words were substituted: “footpath” for “sidewalk” and “crosswalk” for “pedestrian crossing.”
This final version was subjected to further review by 3 experts in neuropsychology to determine the validity of the Peruvian version of the RUDAS (RUDAS-PE). Experts took into account content validity, criteria, and idea by means of a qualitative judgment. This assessment utilized a “grid” format questionnaire based on a 4-point Likert-type scale examining 4 categories (sufficiency, clarity, coherence, and relevance) for each of the 6 RUDAS components (Supplemental Material 1). The 4 ordered response level format is as follows:
Does not meet the criteria
Low level
Moderate level
High level
Pilot study (RUDAS in a healthy literate urban population)
The final version of the RUDAS-PE was applied to a group of 20 seniors (average age 78) with secondary level of schooling who regularly seek medical attention at the neurology and rehabilitation units of the Instituto Peruano de Neurociencias for neurological yet noncognitive complaints. Based on the BCTs validated for use in Lima (CDT, MMSE, and M@T) and the specialized neuropsychological evaluation, these patients were considered cognitively healthy. This study allowed us to verify the validity of the content and criteria.
INECO Frontal Screening
The IFS test evaluates executive functions taking ∼10 minutes to conduct. Its maximum score is 30 points (8 subtests): motor programming (3 points), conflicting instructions (3 points), motor inhibitory control (3 points), backward digital span (6 points), verbal working memory (2 points), spatial working memory (4 points), abstraction capacity (3 points), and verbal inhibitory control (6 points). A Peruvian version of the IFS was validated identifying a cutoff of <23.5 with a sensitivity 97.1% and a specificity of 97.9%. 22
Addenbrooke’s Cognitive Examination
The ACE is a BCT sensitive to ED. It evaluates 6 cognitive dominions, includes the MMSE, needs a high level of training for administration, and takes 25 to 30 minutes to conduct. 20 The maximum score attainable is 100: orientation (10 points), attention (8 points), memory (35 points), verbal fluency (14 points), language (28 points), and visuospatial abilities (5 points). A Peruvian version of the ACE was validated identifying a cutoff of <86 (sensitivity and specificity of 100%). 20
Gold standard for dementia
The neuropsychological evaluation consisted of a battery of tests adapted for use in the Peruvian population. The decision criteria to determine deficiency in the cognitive dominion evaluated were 2 standard deviations less than the average. Psychological testing included the Rey Auditory Verbal Learning Test, 31 the Logical Memory subtest of the Wechsler Memory Scale–Revised, 32 the Trail Making Test A and B, 33 the Rey-Osterrieth Complex Figure Test, 31 the Boston Naming Test, 34 the Wisconsin Card Sorting Test, 35 and the Letter-Number and Digit Span, subtests of the Wechsler Adult Intelligent Scale III. 32 Experts conducting the neuropsychological tests were blind to the BCT results.
Procedure
A convenience sampling was performed. The evaluation of CI was carried out in 3 successive phases: (1) screening—to detect cases with CI, (2) diagnostic nosology—to determine the specific disease causing the CI, and (3) final classification—to determine dementia severity. In the screening phase, we conducted a comprehensive clinical and neurological evaluation including anthropometric measurements and arterial pressure. We also administered the ACE, IFS, RUDAS, and MMSE to all participants. The Pfeffer Functional Activities Questionnaire (PFAQ) was used solely on caretakers. When a BCT was positive for CI, a different evaluator repeated it. Cases confirmed were designated as patients with cognitive impairment.
Patients with CI went onto a second phase including blood tests (hemogram, glucose, transaminase, urea, creatinine, vitamin B12, folic acid, free T3 and free T4, ultrasensitive thyroid-stimulating hormone, rapid plasma reagin test, and an enzyme-linked immunosorbent assay for HIV), brain imaging tests (computed tomography or magnetic resonance images), and Beck Depression Inventory II results to discard non-neurodegenerative causes of CI.
Finally, the severity of CI was classified based on a comprehensive neuropsychological evaluation and by applying the Diagnostic and Statistical Manual of Mental Disorders, fifth edition, criteria 36 of major and minor neurocognitive disorders—noted in our study as ED and MCI, respectively. Additionally, the Clinical Dementia Rating (CDR) 37 scale was used to stage the severity of dementia. In this study, only data from the following groups were entered into the analyses: CDR = 0 (no-CI), CDR = 0.5 (MCI), and CDR = 1 (ED). The CDR was applied to both study participants and caretakers. Inconclusive cases (with respect to classification) were resolved by consensus between neurologists, geriatricians, and neuropsychologists.
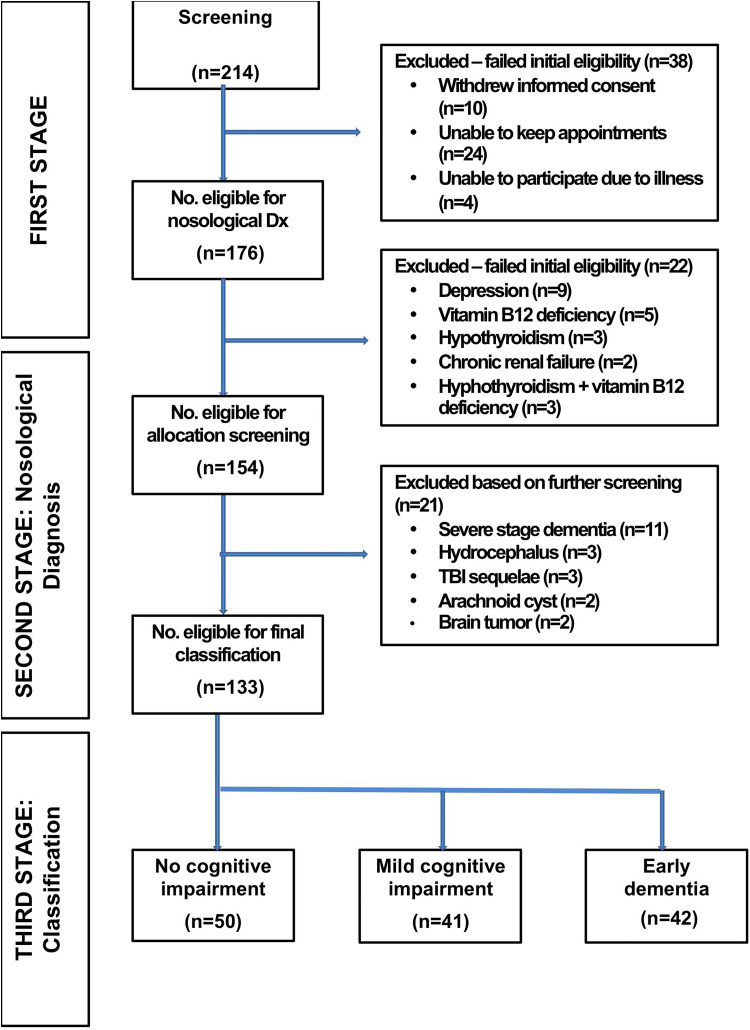
Individuals seeking medical attention for cognitive and subjective complaints with negative results on all BCTs formed part of the no-CI group. The RUDAS was used in the first phase of the study; evaluators were blinded to the specialized neuropsychological evaluation results. The RUDAS results weren’t used as part of the neuropsychological diagnostic battery to assign the study groups. Evaluators during the second and third phase included neurological and neuropsychological experts in dementia; the first phase included geriatric residents and neuropsychology and neuroscience postgraduate students supervised by neurology and medical rehabilitation experts in dementia. Figure 1 illustrates the process on how we created the 3 study groups: no-CI, MCI, and ED.
Figure 1.
Flowchart of participants.
Statistical Analysis
We performed descriptive statistics, comparing paired cognitive groups (ED/MCI, MCI/no-CI, and ED/no-CI) using t tests for discrete variables and chi-square for categorical variables. Additionally, we conducted a logistic regression (logit) for each study group pairs using a 2-variable model: final diagnostic as dependent variable and each BCT as independent variable. Furthermore, a bivariate analysis was conducted analyzing the influence of age, gender, and years of education on group status. Internal consistency was calculated using Cronbach’s α. Concurrent validity was measured with a Pearson’s correlation coefficient between scores on the RUDAS and the MMSE, ACE, IFS, and CDR. A postestimation analysis configuring the area under the curve (AUC) and ROC curve graphics was performed. Other metrics of discriminative validity (percentage of participants correctly classified) and diagnostic accuracy (sensitivity, specificity, positive predictive values, negative predictive values, and likelihood ratios) were calculated. In conjunction with ROC analysis, the Youden index 38 was used to derive optimal cutoff values. Finally, we compared AUCs using the Hanley and McNeil method. 39 The BCTs were evaluated with a 95% confidence interval using STATA software (version 12.0). The level of significance was set at .05.
Results
Participant Recruitment
A total of 214 individuals were initially screened, of whom 38 (17.8%) were excluded. Following nosological diagnosis, an additional 22 (12.5%) patients were excluded. Of the 154 participants determined eligible to complete the allocation screening, 21 (13.6%) were not included in the present analysis. The remaining 133 participants progressed to the next and final phase of classification. Of the 176 individuals who met the initial eligibility criteria, 133 were later enrolled (75.6% enrollment rate) and included in this analysis (Figure 1).
Of the 133 participants, 50 were determined to have had no-CI, in other words, intact condition, and therefore served as the control group (n = 50), while the remaining 83 were classified as either having MCI (n = 41) or ED (n = 42; Figure 1).
Participant Characteristics
Demographic and cognitive variables for the 3 diagnostic groups are shown in Table 1. There was a significant difference in age between the ED group and the other comparison groups, with the ED group being older than the patients with no-CI (P < .001) and with MCI (P < .001). Patients in the no-CI versus ED group differed in both age and years of education, while patients in the MCI versus ED group differed only in age; patients in the no-CI versus MCI group, however, differed neither in age nor in years of education. There was no significant difference among the 3 groups regarding gender.
Table 1.
Sociodemographic and Clinical Characteristics of Participants.
| No-CI, n = 50 | Mild Cognitive Impairment, n = 41 | Early Dementia, n = 42 | P Value (No-CI vs MCI) | P Value (MCI vs Early Dementia) | P Value (No-CI vs Early Dementia) | |
|---|---|---|---|---|---|---|
| Female % | 26 (52) | 19 (46.3) | 22 (52.4) | .591 | .582 | .971 |
| Age, yearsa | 68.74 (4.25) | 68.24 (3.68) | 72.43 (3.83) | .558 | .000b | .000b |
| Years of educationa | 12.72 (3.42) | 11.58 (2.56) | 10.64 (2.65) | .082 | .103 | .0019b |
| RUDAS scoresa | 26.28 (1.43) | 21.97 (1.27) | 18.55 (2.38) | .000b | .000b | .000b |
| IFS scorea | 26.52 (1.47) | 20.73 (1.70) | 14.55 (2.19) | .000b | .000b | .000b |
| ACE scoresa | 85.02 (5.64) | 70.02 (4.86) | 63.38 (4.51) | .000b | .000b | .000b |
| MMSE scorea | 25.62 (1.60) | 22.76 (2.35) | 18.67 (2.82) | .000b | .000b | .000b |
Abbreviations: ACE, Addenbrooke’s Cognitive Examination; CI, cognitive impairment; IFS, INECO Frontal Screening; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; RUDAS, Rowland Universal Dementia Assessment Scale.
aData is shown as arithmetic mean (standard deviation).
bP < 0.001.
Scores on the RUDAS-PE, IFS, ACE, and MMSE differed significantly between all 3 groups, as expected: Patients with no-CI scored higher than those with MCI (P < .001), and patients with MCI scored higher than patients with ED (P < .001).
Diagnostic Accuracy
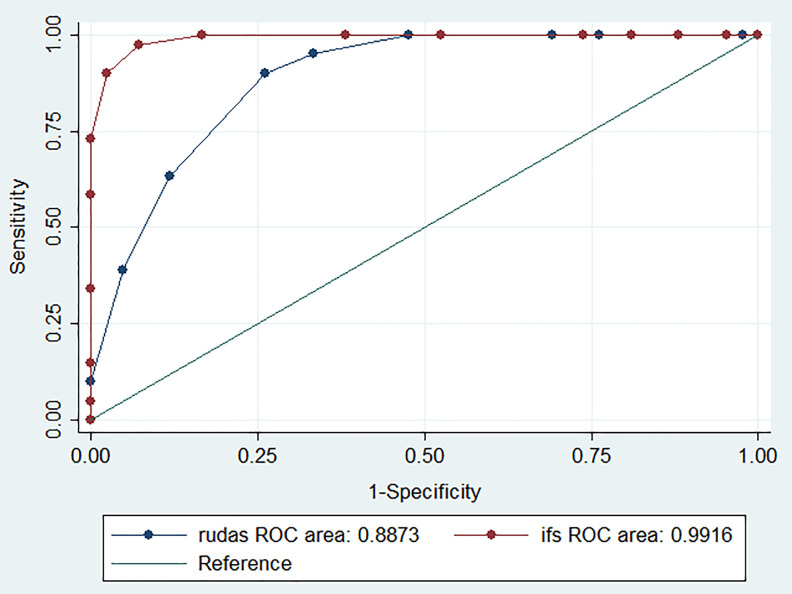
For each BCT, ROCs and AUCs were calculated comparing the 3 groups of participants: (1) no-CI versus MCI (n = 91), (2) no-CI versus ED (n = 92), and (3) MCI versus ED (n = 83). Figure 2 depicts the ROC curve for RUDAS-PE, IFS, and ACE for the comparison between no-CI and MCI. The RUDAS-PE AUC (0.99, 95% confidence interval: 0.97-1.00) was similar to the IFS (0.99, 95% confidence interval: 0.98-1.00; Table 2). The RUDAS-PE was slightly better than the ACE (0.97, 95% confidence interval: 0.94-1.00); however, there was no statistically significant difference between the 2 groups (P value = .313).
Figure 2.
Receiver–operating characteristic (ROC) curve for the Peruvian version of the Rowland Universal Dementia Assessment Scale (RUDAS-PE), the INECO Frontal Screening (IFS), and the Addenbrooke’s Cognitive Examination (ACE) in 133 patients for discrimination between no cognitive impairment (CI) and mild cognitive impairment (MCI).
Table 2.
Cutoff Points and Diagnostic Performance.
| Discrimination Between Patients With No-CI and Patients With MCI | Discrimination Between Patients With MCI and Patients With Early Dementia | |||||||
|---|---|---|---|---|---|---|---|---|
| RUDAS | IFS | ACE | MMSE | RUDAS | IFS | ACE | MMSE | |
| Optimal cutoff point | <24 | <24 | <85 | <25 | <21 | <19 | <67 | <19 |
| Sensitivity, % | 96.00 | 94.00 | 54.00 | 78.00 | 90.24 | 90.24 | 82.93 | 95.12 |
| Specificity, % | 90.24 | 95.12 | 97.56 | 73.17 | 73.81 | 97.62 | 69.05 | 74.62 |
| Youden Index | 0.86 | 0.89 | 0.52 | 0.51 | 0.66 | 0.88 | 0.52 | 0.43 |
| Correctly classified, % | 93.41 | 94.51 | 73.63 | 75.82 | 81.93 | 93.98 | 75.9 | 71.08 |
| Likelihood ratio + | 9.84 | 19.27 | 22.14 | 2.91 | 3.45 | 37.9 | 2.68 | 1.82 |
| Likelihood ratio− | 0.04 | 0.06 | 0.47 | 0.3 | 0.13 | 0.01 | 0.25 | 0.1 |
| AUC (95% CI) | 0.99 (0.97-1.00) |
0.99 (0.98-1.00) |
0.97 (0.94-1.00) |
0.84 (0.77-1.00) |
0.89 (0.82-1.00) |
0.99 (0.98-1.00) |
0.83 (0.75-1.00) |
0.86 (0.78-1.00) |
Abbreviations: ACE, Addenbrooke’s Cognitive Examination; AUC, area under the curve; CI, confidence interval; CI, cognitive impairment; ED, early dementia; IFS, INECO Frontal Screening; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; RUDAS, Rowland Universal Dementia Assessment Scale.
The ability of the RUDAS-PE to correctly discriminate between MCI and ED showed an AUC of 0.89 (95% confidence interval: 0.82-0.96), somewhat less than the IFS (AUC = 0.99 [95% confidence interval: 0.98-1.00], P < .05; Figure 3 and Table 2). The RUDAS-PE performed slightly higher than the ACE (AUC = 0.83 [95% confidence interval: 0.75-0.92], P < .03) and MMSE (AUC = 0.86 [95% confidence interval: 0.78-0.94], P < .04; Table 2).
Figure 3.
Receiver–operating characteristic (ROC) curve for the Peruvian version of the Rowland Universal Dementia Assessment Scale (RUDAS-PE) and the INECO Frontal Screening (IFS) in 133 patients for discrimination between mild cognitive impairment (MCI) and early dementia (ED).
Results for the comparison of no-CI versus ED showed the RUDAS-PE, IFS, and ACE as each having an AUC of 1.00; the MMSE showed an AUC of 0.98 (95% confidence interval: 0.95-1.00).
The cutoff score with the highest Youden index was <25 for the RUDAS-PE where sensitivity was 96% and specificity 90%; this is 2 points higher than the recommended standard cutoff score. 40
A standard cutoff score of <23 for the MMSE in our population had a very high sensitivity (96%) and very low specificity (37%), indicating a fairly high rate of false positives. 41 We therefore selected our cutoff point at <25, also 2 points higher than the standard.
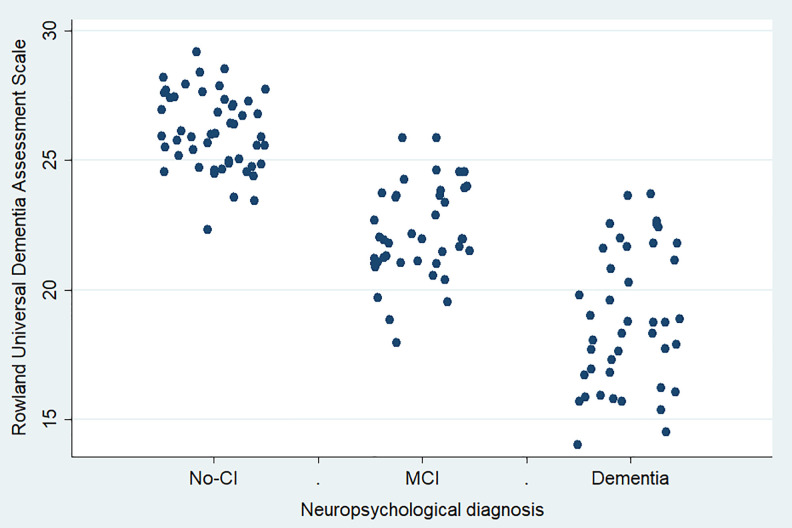
Additional comparisons listing sensitivity, specificity, Youden index, and likelihood ratios are reported in Table 2. The RUDAS scores showed a differential distribution according to the comparison group, behaving as a trend as shown in Figure 4.
Figure 4.
The Peruvian version of the Rowland Universal Dementia Assessment Scale (RUDAS-PE) scores distribution according to their neuropsychological diagnosis (n = 133).
Correlations
Bivariate analysis proved the RUDAS-PE to be a significant predictor of cognitive status, according to correlation with the CDR (RUDAS-PE odds ratio 0.65 [95% confidence interval: 0.32-0.81], P < .001), as well as functional status, according to correlation with the PFAQ (RUDAS-PE Odds Ratio 0.53 [95% confidence interval: 0.12-0.67], P < .001). There was no correlation with respect to age, gender, or years of education. A strong positive correlation between the RUDAS-PE and other BCTs (r = 0.79 [95% confidence interval: 0.412-0.829], P < .01) was identified. The correlation between cognitive domains in RUDAS-PE versus MMSE = 0.63 (95% confidence interval: 0.408-0.727, P < .001); in RUDAS-PE versus IFS = 0.83 (95% confidence interval: 0.525-0.928, P < .001); and in RUDAS-PE versus ACE = 0.78 (95% confidence interval: 0.415-0.817, P < .001).
Internal Consistency
Cronbach’s α for the the RUDAS-PE was .68.
Discussion
The RUDAS has already been directly translated into other languages and validated in a number of different countries as a screening test for dementia. 9,29,30,42,43 We aimed to evaluate the test accuracy for an adaptation of the Spanish version of the RUDAS as applied to an urban population with a middle-level education in Peru. The sample consisted of 133 participants whose ages ranged from 59 to 86 years and approximately 50% of whom were female and had a median of 11 years of education. We presumed that, like the original version of the RUDAS, the RUDAS-PE would outperform the MMSE in diagnostic accuracy. We also presumed that it would have at least an equal if not better diagnostic accuracy compared to other BCTs, such as the IFS and ACE. The diagnostic accuracy of the RUDAS-PE and other BCTs was assessed by comparison against the clinical reference standard—the neuropsychological evaluation.
We can speculate that the RUDAS performed better than the (longer) ACE for probably either of 2 reasons. First of all, this was the first study that compared RUDAS versus ACE and to discriminate between no-CI and MCI. And second, perhaps because the ACE includes a grading system of 100 points dedicating more items in the memory and language dominions, it doesn’t include areas of visuospatial orientation, motor praxis, and judgment in which the RUDAS does, allowing it to detect cognitive changes in other areas sooner and not just problems of memory and language that the ACE identifies. However, the weight system applied to the cognitive domains of the ACE and RUDAS is different. And as such, while the ACE focuses on memory evaluation (35%), verbal fluency (14%), and language (28%), the RUDAS gives more weight to verbal fluency (26.7% vs 14% ACE), body orientation (16.7% vs 0% ACE), and visuospatial praxis (10% vs 5% ACE), allowing it to detect different types of dementia syndromes.
Interestingly, the IFS performed better than the RUDAS in discriminating between MCI and ED, thereby lending support to previously published research indicating that discriminating between patients with no-CI and ED is much easier than discriminating between MCI and ED. 40,44 While originally designed to quickly assess executive functioning, the IFS has proved to be useful in detecting dementias of both cortical (behavioral variant frontotemporal dementia [bvFTD] and AD) 22,45 and subcortical type (mixed dementia, semantic dementia and dementia with Lewy bodies, multiple sclerosis, and vascular dementia). 46 –48 This may, in part, explain as to why this occurred.
In our study, we identified a cutoff score of <24 to make the distinction between patients who have no-CI and those with MCI, 1 point higher than the standard cutoff score. 40 In our study, the standard cutoff score of <23 for the RUDAS showed a high sensitivity 100% and a low specificity of 61%, indicating a false-positive rate of 39%.
Based on the results of the study, the RUDAS-PE is positively correlated with the IFS, ACE, and MMSE with a Pearson r value of .79, P < .01, showing good agreement. A strong correlation indicates that the RUDAS-PE can be a good alternative for dementia screening.
While it’s true that age was significantly greater in the ED group in comparison to the no-CI and MCI groups, it didn’t affect the results; it’s merely a reflection of what happens in the real world, as there is an age-dependent relation to cognitive function and dementia. Additionally, the bivariate analysis didn’t show any influence of age on the RUDAS. Similarly, the ED group was significantly less in comparison to the no-CI group, in terms of years of education; however, the bivariate analysis showed that the RUDAS wasn’t influenced by years of education. Our calculations show that age, gender, and years of education didn’t act as confounders on the diagnosis of CI. The RUDAS-PE score, therefore, can be interpreted without adjustment for age, gender, and years of education for most cases in patients with a middle-level education. Internal reliability of the RUDAS-PE as measured by Cronbach α coefficient was .68, where .70 is generally considered acceptable. The majority of studies seem to cite a reliability score at or above .70 as “adequate” based on earlier research by Nunnally. 49 However, there are various interpretations that report an ideal αof being between .70 and .90, with .60 considered as “acceptable.” Our results are in line with previous studies reporting similar results ranging from .54 to .80. 9,26,27,41,50
We speculate that the somewhat low value of Cronbach’s α coefficient may shed some light as to the reason for the subpar performance of the RUDAS-PE in its ability to discriminate between patients with MCI and ED. We suspect that an increase in sample size (n > 30) for the piloting study and then assessing the results may have improved our ability to identify areas that should’ve been addressed on the questionnaire prior to going “live” with the survey. 51,52 We also suspect that by having restructured the questionnaire and/or deleted an item in the instrument might have resulted in an increased Cronbach’s α and consequently an increased RUDAS-PE diagnostic performance in discriminating between this subgroup.
Another strength is that the IFS, ACE, and MMSE have already been validated in Peru. 22,23 This made for a more direct comparison as defined by the similar population and type of setting involved. The majority of BCTs in Peru focus specifically on assessing episodic memory, 4,23 which tends to be impaired during the very early stages of Alzheimer’s disease (AD) but less frequently in other subtypes of dementia. Even more, clear evidence exists that cognitive vascular disorder and vascular dementia occur more frequently in low- and middle-income countries (LMICs). Wherefore, a cognitive evaluation test such as the RUDAS is ideal for our community in Peru.
One limitation in our study is the low number of persons used in the piloting study, which we have already addressed above. Another limitation is that our findings lack generalizability as a result of having been conducted in a memory clinic; in fact, our facility serves as the national reference center for all of Peru. As such, clinicians who are experts in conducting BCTs better identified the participants enrolled in our study and those used as controls were individuals who sought out medical treatment for complaints dealing with memory (eg, feeling abnormally confused or forgetful). Thus, this particular setting and population may not be an adequate reflection of the community at large.
Also, contrary to previously published studies, we report an AUC of 1.00 for the no-CI versus ED group on the RUDAS, ACE, and IFS. As such, this exceptional classification accuracy may actually be considered a serious limitation to our study. It’s possible that we may have included patients whose level of CI was more severe than what was able to be detected by a CDR of 0.5 or 1. However, the final classification of severity was not solely based on a CDR score but in conjunction with a diagnosis reached by a consensus of neurologists, geriatricians, and neuropsychologists.
Another limitation is not obtaining biological evidence of the disease either in vivo by biomarkers or by postmortem examination. A new set of diagnostic criteria for AD relying on biomarkers has been proposed by the workgroup led by Dubois et al 53 together with the National Institute on Aging (NIA) at National Institutes of Health and the Alzheimer’s Association (AA), known as the NIA-AA criteria. 54 It proposed evidence of elevated levels of hallmark AD biomarkers—amyloid and tau—in both cerebrospinal fluid (CSF) and cerebral imaging by positron emission tomography (PET) scan for a definitive diagnosis. In April 2018, the NIA-AA recommendations were determined to be more of a “research framework” and not as diagnostic criteria, emphasizing its applicability in clinical research only (longitudinal cohort studies and randomized, placebo-controlled clinical trials) and advising against everyday clinical practice. 55
Admittedly, PET scans may help in understanding the overall structural and functional changes occurring in the brain of a patient with AD. Yet the high cost of subsequent scans may not be suitable in LMICs. Although the neuropsychological evaluation takes longer (four 45-minute sessions), it’s less expensive and provides a reliable and accurate diagnosis with minimum risk; it’s also less invasive than obtaining a CSF sample. Moreover, although the use of biomarkers and imaging modalities offer some intriguing research possibilities, they lack the ability to classify the level of cognitive and functional impairment that the patient displays.
Overall, the performance of the RUDAS-PE when compared to the gold standard is noteworthy. The neuropsychological evaluation—in accordance with and strict adherence to the Instituto Peruano de Neurociencias guidelines—requires four 45-minute sessions, whereas the RUDAS takes only 10 minutes. Our study results have important implications on the clinical use of the RUDAS-PE in the primary care setting where it has proven itself as a viable alternative to other commonly used BCTs for the detection of dementia, particularly in the elderly population with a mid-level education living in Lima-Peru whose consultation time tends to be limited to 10 minutes.
Supplemental Material
Supplemental Material, Supplemental_1._Expert_Judgment_Questionnaire_Template_EN for Validation of the RUDAS in Patients With a Middle-Level Education in Lima, Peru by Nilton Custodio, Rosa Montesinos, David Lira, Eder Herrera-Perez, Kristy Chavez, Gustavo Hernandez-Córdova, José Cuenca, Carlos Gamboa and Tatiana Metcalf in American Journal of Alzheimer's Disease & Other Dementias
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tatiana Metcalf  https://orcid.org/0000-0002-1517-6725
https://orcid.org/0000-0002-1517-6725
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: epidemiological evidence and implications for public policy. Front Aging Neurosci. 2017;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parra M, Baez S, Allegri R. et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology. 2018;90(5):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gleichgerrcht E, Flichtentrei D, Manes F. How much do physicians in Latin America know about behavioral variant frontotemporal dementia? J Mol Neurosci. 2011;45(3):609–617. [DOI] [PubMed] [Google Scholar]
- 4. Custodio N, Becerra-Becerra Y, Cruzado L. et al. Nivel de conocimientos sobre demencia frontotemporal en una muestra de médicos que evalúa regularmente a pacientes con demencia en Lima-Perú. Revista Chilena de Neuro-Psiquiatria. 2018;56(2):77–88. [Google Scholar]
- 5. Prince MJ, Acosta D, Ferri CP. et al. A brief dementia screener suitable for use by non-specialists in resource poor settings—the cross-cultural derivation and validation of the brief Community Screening Instrument for Dementia. Int J Geriatr Psych. 2011;26(9):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramos-Cerqueira AT, Torres AR, Crepaldi AL. et al. Identification of dementia cases in the community: a Brazilian experience. J Am Geriatr Soc. 2005;53(10):1738–1742. [DOI] [PubMed] [Google Scholar]
- 7. Dua T, Barbui C, Clark N. et al. Evidence-based guidelines for mental, neurological, and substance use disorders in low-and middle-income countries: summary of WHO recommendations. PLoS Med. 2011;8(11):e1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carnero-Pardo C. Should the Mini-Mental State Examination be retired? Neurología. 2014;29(8):473–481. [DOI] [PubMed] [Google Scholar]
- 9. de Araujo NB, Nielsen TR, Engedal K, Barca ML, Coutinho ES, Laks J. Diagnosing dementia in lower educated older persons: validation of Brazilian Portuguese version of the Rowland Universal Dementia Assessment Scale (RUDAS). Rev Bras Psiquiatr. 2018;40:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franco-Marina F, Garcia-Gonzalez JJ, Wagner-Echeagaray F. et al. The Mini-Mental State Examination revisited: ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int Psychogeriatr. 2010;22(1):72–81. [DOI] [PubMed] [Google Scholar]
- 11. Ostrosky-Solís F, López-Arango G, Ardila A. Sensitivity and specificity of the Mini-Mental State Examination in a Spanish-speaking population. Appl Neuropsychol. 2000;7(1):25–31. [DOI] [PubMed] [Google Scholar]
- 12. Reyes S, de Beaman SR, Beaman PE. et al. Validation of a modified version of the Mini-Mental State Examination (MMSE) in Spanish. Aging Neuropsychol Cognition. 2004;11(1):1–11. [Google Scholar]
- 13. Rosselli D, Ardila A, Pradilla-Ardila G. et al. El examen mental abreviado (Mini-Mental State Examination) como prueba de tamizaje para el diagnóstico de demencia: estudio poblacional colombiano. Rev Neurologia. 2000;30(5):428–432. [PubMed] [Google Scholar]
- 14. Delgado C, Araneda A, Behrens MI. Validación del instrumento Montreal Cognitive Assessment en español en adultos mayores de 60 años. Neurología. 2017;32(3):1–10. [DOI] [PubMed] [Google Scholar]
- 15. Gil L, Ruiz de Sánchez C, Gil F, Romero SJ, Pretelt Burgos F. Validation of the Montreal Cognitive Assessment (MoCA) in Spanish as a screening tool for mild cognitive impairment and mild dementia in patients over 65 years old in Bogotá, Colombia. Int J Geriatr Psych. 2015;30(6):655–662. [DOI] [PubMed] [Google Scholar]
- 16. Pedraza OL, Salazar AM, Sierram FA. et al. Confiabilidad, validez de criterio y discriminante del Montreal Cognitive Assessment (MoCA) test, en un grupo de adultos de Bogotá. Acta Médica Colombiana. 2016;41(4):221–228. [Google Scholar]
- 17. Pereira-Manrique F, Reyes MF. Confiabilidad y validez del test Montreal Cognitive Assessment (MoCA) en población mayor de Bogotá, Colombia. Revista Neuropsicol Neuropsiquiatría y Neuroci. 2013;13(2):39–61. [Google Scholar]
- 18. Manos PJ, Wu R. The ten-point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. Int J Psychiat Med. 1994;24(3):229–244. [DOI] [PubMed] [Google Scholar]
- 19. Custodio N, García A, Montesinos R, Lira D, Bendezú L. Validación de la prueba de dibujo del reloj-versión de Manos como prueba de cribado para detector demencia en una población adulta mayor de Lima, Perú. Revista Peruana de Med Experiment y Salud Publica. 2011;28(1):29–34. [DOI] [PubMed] [Google Scholar]
- 20. Custodio N, Lira D, Montesinos R, Gleichgerrcht E, Manes F. Utilidad del Addenbrookes’s Cognitive Examination versión en español en pacientes peruanos con enfermedad de Alzheimer y demencia frontotemporal. Vertex Revista Argentina de Psiquiatria. 2012;23(103):165–172. [PubMed] [Google Scholar]
- 21. Oscanoa TJ, Cieza E, Parod JF, Paredes N. Evaluación de la prueba de la moneda peruana en el tamizaje de trastorno cognitivo en adultos mayores. Revista Peruana de Medicina Expe y Salud Publica. 2016;33(1):67–73. [PubMed] [Google Scholar]
- 22. Custodio N, Herrera-Perez E, Lira D. et al. Evaluation of the INECO Frontal Screening and the Frontal Assessment Battery in Peruvian patients with Alzheimer’s disease and behavioral variant frontotemporal dementia. eNeurologicalSci. 2016;5:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Custodio N, Lira D, Herrera-Perez E. et al. The Memory Alteration Test discriminates between cognitively healthy status, mild cognitive impairment and Alzheimer’s disease. Dementia Geriatric Cognitive Disorders Extra. 2014;4(2):314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Custodio N, Lira D, Herrera E. et al. Memory Alteration Test to detect amnestic mild cognitive impairment and early Alzheimer’s dementia in population with low educational level. Front Aging Neurosci. 2017;9:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Storey JE, Rowland JT, Conforti DA, Dickson HG. The Rowland Universal Dementia Assessment Scale (RUDAS): a multicultural cognitive assessment scale. Int Psychogeriatr. 2004;16(1):13–31. [DOI] [PubMed] [Google Scholar]
- 26. Rowland JT, Basic D, Storey JE, Conforti DA. The Rowland Universal Dementia Assessment Scale (RUDAS) and the Folstein MMSE in a multicultural cohort of elderly persons. Int Psychogeriatr. 2006;18(1):111–120. [DOI] [PubMed] [Google Scholar]
- 27. Basic D, Khoo A, Conforti D. et al. Rowland Universal Dementia Assessment Scale, Mini-Mental State Examination and general practitioner assessment of cognition in a multicultural cohort of community-dwelling older persons with early dementia. Aust Psychol. 2009;44(1):40–53. [Google Scholar]
- 28. Naqvi RM, Haider S, Tomlinson G, Alibhai S. Cognitive assessments in multicultural populations using the Rowland Universal Dementia Assessment scale: a systematic review and meta-analysis. Can Med Assoc J. 2015;187(5):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mateos-Álvarez R, Ramos-Ríos R, López-Moríñigo JD. Comparative analysis between the MMSE and the RUDAS for dementia screening in low educated people in a Spanish psychogeriatric clinic. Europe J Psychiatry. 2017;31(3):119–126. [Google Scholar]
- 30. Ramos-Ríos R, Mateos-Álvarez R, López-Moríñigo JD. Cribado de demencia en una población con un bajo nivel de instrucción. Validación de la versión española del RUDAS (Rowland Universal Dementia Assessment Scale) en una muestra asistencial. Psicogeriatría. 2009;1(2):89–99. [Google Scholar]
- 31. Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique (Les problems). Arch Psychologie. 1941;28:286–340. [Google Scholar]
- 32. Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 33. Partington JE, Leiter RG. Partington’s pathways test. Psycho Serv Center Bullet. 1949;1:9–20. [Google Scholar]
- 34. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 35. Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12(4):313–324. [DOI] [PubMed] [Google Scholar]
- 36. Asociación Americana de Psiquiatría. Manual Diagnóstico y Estadístico De Los Trastornos Mentales, Quinta Edición (DSM-V). Madrid, Spain: Panamericana; 2014. [Google Scholar]
- 37. Hughes CP, Berg L, Danzige WL, Coben LA, Martin LR. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 38. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 39. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. [DOI] [PubMed] [Google Scholar]
- 40. Basic D, Rowland JT, Conforti DA. et al. The validity of the Rowland Universal Dementia Assessment Scale (RUDAS) in a multicultural cohort of community-dwelling older persons with early dementia. Alz Dis Assoc Dis. 2009;23(2):124–129. [DOI] [PubMed] [Google Scholar]
- 41. Pedraza OL, Sanchez E, Plata SJ. et al. Punctuaciones del MoCA y el MMSE en pacientes con deterioro cognitivo leve y demencia en una clinica de memoria en Bogota. Acta Neurologica Colombiana. 2014;30(1):22–30. [Google Scholar]
- 42. Chaaya MT, Phung TKT, El Asmar K. et al. Validation of the Arabic Rowland Universal Dementia Assessment Scale (A-RUDAS) in elderly with mild and moderate dementia. Aging Ment Health. 2016;20(8):880–887. [DOI] [PubMed] [Google Scholar]
- 43. Iype T, Ajitha BK, Antony P, Ajeeth NB, Job S, Shaji KS. Usefulness of the Rowland Universal Dementia Assessment Scale in South India. J Neurol Neurosurg Psychiatry. 2006;77(4):513–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Albert MS, DeKosky ST, Dickson D. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dementia. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gleichgerrcht E, Roca M, Manes F, Torralva T. Comparing the clinical usefulness of the Institute of Cognitive Neurology (INECO) Frontal Screening (IFS) and the Frontal Assessment Battery (FAB) in frontotemporal dementia. J Clin Exp Neuropsyc. 2011;33(9):997–1004. [DOI] [PubMed] [Google Scholar]
- 46. Ihnen Jory J, Antivilo Bruna A, Muños-Neira C, Slachevsky Chonchol A. Chilean version of the INECO Frontal Screening (IFS-Ch): psychometric properties and diagnostic accuracy. Dementia Neuropsychol. 2013;7(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruno D, Torralva T, Marenco V. et al. Utility of INECO Frontal Screening (IFS) in the detection of executive dysfunction in patients with relapsing-remitting multiple sclerosis (RRMS). Neurol Sci. 2015;36(11):2035–2041. [DOI] [PubMed] [Google Scholar]
- 48. Custodio N, Montesinos R, Lira D. et al. Evolution of short cognitive test performance in stroke patients with vascular cognitive impairment and vascular dementia: baseline evaluation and followup. Dementia Neuropsychol. 2017;11(4):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nunnally JC. Psychometric theory. 2nd ed. New York, NY: McGraw-Hill; 1978. [Google Scholar]
- 50. Salary S, Shaeir M, Asghari-Moghadam MA. Assessing the validity and reliability of Rowland’s Universal Dementia Assessment Scale (RUDAS) in patients with dementia. Zahedan J Res Med Sci. 2014;16(9):72–74. [Google Scholar]
- 51. In J. Introduction of a pilot study. Korean J Anesthesiol. 2017;70(6):601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14(17):1933–1940. [DOI] [PubMed] [Google Scholar]
- 53. Dubois B, Feldman HH, Jacova C. et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. [DOI] [PubMed] [Google Scholar]
- 54. McKhann GM, Knopman DS, Chertkow H. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dementia. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jack C, Jr, Bennet DA, Blennow K. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer´s disease. Alzheimers Dementia. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplemental_1._Expert_Judgment_Questionnaire_Template_EN for Validation of the RUDAS in Patients With a Middle-Level Education in Lima, Peru by Nilton Custodio, Rosa Montesinos, David Lira, Eder Herrera-Perez, Kristy Chavez, Gustavo Hernandez-Córdova, José Cuenca, Carlos Gamboa and Tatiana Metcalf in American Journal of Alzheimer's Disease & Other Dementias