Abstract
Background:
We assessed implicit and explicit emotion in older patients with dementia using biosignals.
Methods:
Fifty patients with dementia and 34 healthy individuals watched 3 videos that aimed to elicit various emotional responses. Electroencephalogram and heart rate variability were recorded.
Results:
Patients with dementia experienced less fun and more fear than controls. The high frequency (HF) from the baseline in response to funny stimulation as well as HF from neutral to fear stimulation in the dementia group increased further than in the control group. The slow wave (SW)–fast wave (FW) ratio from neutral to funny stimulation in the control group increased further than in the dementia group. The SW-FW from neutral to fear stimulation was further decreased in the dementia group than in the control group.
Conclusions:
Although patients with dementia were more sensitive to implicit affect, they showed more enhanced imbalance between positive and negative affect in explicit affect assessment.
Keywords: dementia, electroencephalography, heart rate variability, affect, slow wave
Introduction
Emotion in Patients with Dementia
The ability of older people to accurately recognize emotion is crucial to maintaining interpersonal relationships, which can lessen withdrawal from social interactions and poor communication in patients with dementia. 1 -3 Several studies have suggested that older adults are emotionally more negative (measuring fear, anger, sadness, and disgust) than younger adults. 4,5 As a result of poor recognition of negative emotions, older adults have an attentional bias away from negative emotion stimuli and an attentional bias toward positive emotion stimuli, such as a happy face. 5 Interestingly, this age bias was not associated with explicit affect but with implicit affect. 6 Explicit affect can be defined as conscious affective experience that is assessed using questionnaires or scales. 7 Implicit affect is associated with the impulsive systems and automatic activation of cognitive representations of affective experiences. 8 The Implicit Positive and Negative Affect Test (IPANAT) 7 and implicit association test (IAT) 9 are different methods used to test implicit affect. However, these tests for implicit affect that also require cognitive ability are too complex to be applied to people with dementia. Recently, studies have attempted to assess implicit affect using psychophysiological responses such as electroencephalography (EEG) 10 and heart rate variability (HRV). 11
Slow Wave–Fast Wave Ratio of EEG in Response to Affective Stimulation
Electroencephalogram signals are conventionally classified into slow wave (SW) activity including δ (1-3 Hz) and θ (4-7 Hz) and fast wave (FW) activity including the β band (13-30 Hz). The SW within the frontal lobe is reported to be associated with emotion and motivation. 12,13 The θ activity of EEG within the anterior cingulate cortex is reported to predict depressive mood states in patients with major depressive disorder. 13 The θ activity of EEG within medial frontal cortex could be associated with reward response. 12 Moreover, several studies have suggested that the SW-FW ratio could be a useful tool to assess emotional–cognitive interactions. 14 The SW-FW ratio may represent a transient affect 15 and performance in emotion–cognitive experimental tasks. Increased SW-FW ratio is known to predict less fearful or fear-inhibited behaviors. 14,16 Fear-reducing situations increase frontal δ power and δ-β ratio within the frontal lobe. 16 The frontal lobe has provided a particular area of focus when investigating emotion recognition in old age. 17 -19 Although aging leads to widespread gradual atrophy of the whole brain, frontal and temporal lobes undergo substantial age-related changes. 18 In particular, earlier and more rapid brain volume loss is observed within frontal areas in old age. 17,19
Heart Rate Variability in Response to Affective Stimuli
In past decades, HRV has been used to represent autonomic nervous system responses to phobic stimulation. 20,21 Couyoumdjian et al 20 suggested that complex emotion problems were not associated with subjective emotional reaction but associated with physiological changes (decreased heart rate and increased HRV) in response to phobic stimulation. In varying spectral bands of HRV, high-frequency (HF) band and HF/low frequency (LF) band are known to be suitable indices to assess emotions such as joy and relaxation. 22 The HF band with a range between 0 and 0.04 Hz is considered to measure parasympathetic activity due to respiratory sinus arrhythmia. 22 The LF band with a range between 0.04 and 0.15 Hz is considered to measure sympathetic and parasympathetic activity on the heart. 22
Hypothesis
In the present study, we sought to assess implicit and explicit emotion in patients with dementia using simple methods with biosignals. We hypothesized that patients with dementia would show an imbalance of emotional expression in terms of implicit and explicit affective experiences, when compared to healthy older people.
Methods
Participants
Since the implementation of long-term care insurance systems in Korea in 2008, an increasing number of senior citizens with dementia reside within nursing homes. 23 From March 2017 to June 2017, 62 elderly patients (defined as 65 years or older) who were residents at a nursing home in Incheon, South Korea, and 38 elderly people who were visiting Incheon-Seogu welfare assembly center as outpatients were asked to participate in the study. Of 100 elderly people, 3 were excluded because they could not understand the process and goals of the study. In total, 97 people agreed to participate. Thirteen people were excluded owing to a history of psychiatric treatment, including major depressive and anxiety disorders. Fifty patients with dementia and 34 healthy controls were recruited. All participants were assessed using the Mini-Mental State Examination (Korean version; K-MMSE), the clinical rating scale for dementia (CDR), and the Korean version of the positive and negative affect schedule (K-PANAS). Criteria for inclusion were participants ≥65 years of age. The exclusion criteria were (1) participants with history of, or a current episode of, another psychiatric disease; (2) participants with substance abuse or dependence problems (except drinking alcohol without an intent to become intoxicated in a social setting); and (3) participants with a history of neurological or medical diseases. All participants were categorized based on K-MMSE and CDR scores; less than 24 in K-MMSE and greater than 0.5 in CDR for dementia, and greater than 25 in K-MMSE for healthy control participants. Final diagnoses were confirmed with data and clinical consensus from 2 clinical doctors (S.H.L. and H.G.). The Incheon Eun-Hye Hospital Institutional Review Board approved this study. All participants provided informed, written consent. The contents of the informed consent were written by 2 doctors (S.H.L and H.G.) to be read easily and comprehended by patients with dementia.
Mini-Mental State Examination Korean Version
The MMSE, a brief mental status assessment tool, has been validated for use in many countries. 24 The K-MMSE is a Korean version of MMSE that showed similar validity with the original MMSE. 25
Clinical Rating Scale for Dementia
The CDR is a structured interview protocol that focused on 6 areas: memory, orientation, judgment, problem-solving, community affairs, home and habit, and personal care. Scores in each area are combined to obtain a total score ranging from 0 to 3. 26
Korean Version of the Positive and Negative Affect Schedule
The PANAS is one of the most widely used affect assessment tools and was developed to assess positive affect from 10 items and negative affect from 10 items. Each item is rated from 1 (not at all) to 5 (very much). 27 The Korean version of PANAS (K-PANAS) was developed and verified with Cronbach α = .84. 28
Heart Rate Variability Recording
During the trials, the HRV of each patient was also monitored using a Bluetooth heart rate sensor wrapped around the patient’s chest (Polar Electro Inc., Bethpage, NY, USA).
Electroencephalogram Recording
All participants were asked to watch videos that represented funny, fear, or neutral emotions, respectively. Each video was presented for 1 minute. After each video was finished, the degrees of emotion in all participants were assessed using the 5-point Likert- visual analogue scale for 20 seconds. The order of videos was as follows: neutral (1 minute), funny (1 minute), neutral (1 minute), and fear (1 minute). Funny videos contained a comic reindeer dancing and babies smiling. The fear video consisted of death scenes from the fire location and Korean War. The neutral video consisted of a visual noise scene and a screen-tuning scene. To verify the characteristics of each video, we asked 20 control participants to rate the videos. The agreement rates for each video were as follows: 19 (95%) people in the video for funny and 18 (90%) people in the video for fear. To check for levels of distress, 5 people with dementia watched the videos including neutral, funny, and fearful scenes before confirming the use of the videos for this study. The range of MMSE scores of people with dementia was from 12 to 17. All of the participants were able to watch all of the videos. While watching the videos during the study, participants could stop watching at any time if they felt distress in response to the videos. However, there was no one who stopped watching the videos during the study.
The EEG data were acquired with a single channel-sensing headset with a ThinkGear-AM module, at 10 μV/mm sensitivity, which outputs 12-bit Raw Brainwaves (3-100 Hz) with a sampling rate of 512 Hz (NeuroSky, Silicon Valley, California). Electroencephalogram acquisition was obtained from the mono electrode site of the forehead above the eye (FP1) in the dominant hemisphere. Electroencephalogram activity was recorded continuously during the entire video trial (total 4 minutes; Figure 1). The electrode included the chipset for analog filtering with a band-pass filter (0.5-30 Hz) and notch filter to eliminate electrical noise at 50 Hz. Other noise artifacts caused by eye blinks, ocular movement, and facial muscle movement were also eliminated automatically using proprietary algorithms supplied by NeuroSky. 29 Since other noise artifacts have a higher frequency than EEG signals, these can be filtered out using a low-pass filter with a 30 to 40 Hz cutoff frequency. 30 The analogue signal is then converted to a digital format in the headset circuit board and transmitted via Bluetooth to the NeuroView date acquisition software. Based on previous studies of the correlation between SW-FW ratio and emotional change, 14,15,18 we analyzed only the changes in the SW-FW ratio in response to all 3 videos.
Figure 1.
Representative still images from each of the trial videos.
Statistics
The difference in demographic data, emotional responses, and EEG at baseline between severe dementia, mid dementia, and healthy controls was analyzed using the independent Student t test. The changes in emotional response and EEG between emotional stages were assessed with a mixed analysis of variance. All statistical analyses were performed using IBM SPSS statistics 24.0 (IBM Corp, Armonk, New York).
Results
Participants
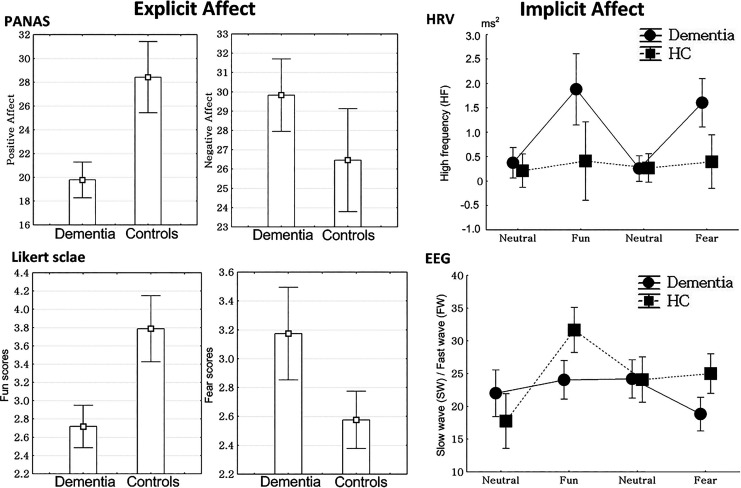
There were no significant differences in age, sex, and handedness between patients with dementia and healthy controls. However, there were significant differences in K-MMSE (t = 14.5, P < .01) and CDR scores (t = 12.3, P < .01) between the 2 groups. The patients with dementia had a mean K-MMSE score of 12.8 ± 4.4 and mean CDR score of 1.6 ± 0.6. In addition, patients with dementia showed lower scores for positive affect (t = 5.7, P < .01) but higher scores for negative affect (t = 2.2, P < .01) in PANAS scales, when compared to the results from the control group (Table 1; Figure 2).
Table 1.
Comparison of Demographic Characteristics Between Patients With Dementia and The Control Group.
| Dementia Group, n = 50 | Control Group, n = 34 | Statistics | |
|---|---|---|---|
| Age | 71.5 ± 4.3 years | 71.1 ± 5.5 years | t = 0.30, P = .76 |
| Sex, male/female | 6/44 | 5/29 | χ2 = 0.13, P = .72 |
| Education, years | 10.0 ± 3.7 | 10.1 ± 3.7 | t = 0.06, P = .94 |
| Handedness (right/left) | 44/6 | 31/3 | χ2 = 0.21, P = .64 |
| K-MMSE | 12.8 ± 4.4 | 24.7 ± 1.2 | t = 14.5, P < .01 |
| CDR | 1.6 ± 0.6 | 0.04 ± 0.14 | t = 12.3, P < .01 |
| PANAS | |||
| PA | 19.8 ± 5.0 | 28.4 ± 8.4 | t = 5.7, P < .01 |
| NA | 29.8 ± 6.3 | 26.5 ± 7.5 | t = 2.2, P < .01 |
Abbreviations: CDR, clinical dementia rating scale; K-MMSE, Mini-Mental State Examination Korean version; PANAS, Positive and Negative Affect Schedule Scale.
Figure 2.
Explicit and implicit affect in patients with dementia and the control group. HC controls indicates healthy control old age-group; HRV, heart rate variability; PANAS, the positive (PA) and negative affect (NA) schedule scale.
Comparison of Emotional Reactivity in Emotional Stimulation
The control group (3.8 ± 1.0) experienced more emotions related to fun in response to stimulation with funny content, compared those in the dementia group (2.7 ± 0.8; t = 5.3, P < .01). In addition, the control group (2.6 ± 0.7) experienced less fear emotions in response to fear stimulation, compared to those in the dementia group (3.2 ± 1.1; t = 2.9, P < .01; Figure 2).
Changes in HRV
There were significant differences in the pattern of HF changes between the dementia group and healthy controls (F = 5.23, P < .01). The HF from baseline in response to funny stimulation (F = 4.04, P = .04) as well as HF from neutral to fear stimulation (F = 5.94, P = .02) in the patients with dementia was increased, compared to the responses in the control group (Figure 2).
Changes in EEG
There were significant differences in SW-FW changes between the dementia group and healthy controls (F = 5.89, P < .01). In post hoc tests, SW-FW from neural to funny stimulation in the control group was increased, when compared to the results from the dementia group (F = 11.2, P < .01). The SW-FW from neutral to fear stimulation was decreased in the dementia group compared to that of the control group (F = 4.49, P = .04; Figure 2).
Discussion
In explicit affect, patients with dementia showed decreased sensitivity to positive emotion and increased sensitivity to negative emotion, compared to the findings for the control group. However, in implicit affect, patients with dementia showed higher sensitivity to both positive and negative emotions, compared to the scores observed in the control group.
Explicit and Implicit Affects in Patients With Dementia
In explicit affect assessment (PANAS and 5-point Likert visual analogue scale) in the current study, patients with dementia showed decreased positive affect scores of PANAS and increased negative affect scores of PANAS when compared to those of the control group. The scores from the 5-point Likert visual analogue scale with regard to emotion video assessment showed that patients with dementia felt less fun as well as more fear, compared those in the control group. These results are in line with previous studies regarding old age, dementia, and alexithymia. 3,31,32 Usually, older people are more sensitive to negative, compared to positive, emotions. 3 Apathy or alexithymia is regarded as one of the symptoms in patients with dementia that is due to an impairment of emotional processing within frontotemporal areas. 31 Moreover, patients with alexithymia are sensitive to negative emotional stimulation but blunted to positive emotional stimulation. 32
In the implicit affect assessment (HRV and EEG) in the current study, changes in HF in response to funny, as well as fear, stimulation in patients with dementia was greater than those in the control group. Of several physiological variables, HRV, including HF and HF/LF, is known to represent emotional responses including fear and joy. 20,22 In another implicit affect assessment in the current study, changes in SW-FW ratio in response to funny stimuli in patients with dementia was reduced, and the changes in the SW-FW ratio in response to fear in patients with dementia increased, compared to that of the control group. Putman et al 33 reported that SW-FW ratio is negatively correlated with fear modulation as well as with self-reported attentional control. Further, Schutter and van Honk 14 reported that patients with higher SW-FW ratio would predict less risk-aversive situations in response to disadvantageous versus advantageous decision-making strategies. Taken together, patients with dementia with increased HF response as well as decreased SW-FW were more responsive to and attentive to both funny and fear emotions.
Clinical Implications
Compared to participants in the control group, patients with dementia showed an enhanced imbalance between positive affect and negative affect in explicit affect assessment. However, patients with dementia were more responsive and attentive to both positive and negative stimulations, compared to those in the control group. This indicates that patients with dementia may have emotional feelings, but they cannot properly express positive emotions. These results may be associated with emotional rehabilitation and treatment for patients with dementia. Notably, art therapies including music and drawing used for improving anxiety and depression, in patients with dementia, have led patients to express positive emotions. 34 -36 Our results support the idea that emotional rehabilitation using positive emotional stimulation can be a useful method of improving clinical symptoms in patients with dementia.
Further, assessing implicit emotion using implicit affect tests in old age or patients with dementia has many limitations including time, space, and complexity of the procedure. To compensate for these limitations, our current study contains pilot results assessing implicit emotion using a simple method such as a single lead of a headset on the frontal lobe, short-time course, and reduced complexity for patients with dementia.
Limitations
There are several limitations in the current study. First, the number of participants was too small to extrapolate generalized conclusions. Because of this, we cannot classify patients with dementia by degree of clinical symptom severity. Second, the participants’ cognition and function are moderately impaired with mean K-MMSE score of 12.8 and mean CDR score of 1.6, and it would be useful to assess more people with mild dementia in a larger population. Third, due to complexity and time limitations in patients with dementia, we did not assess implicit affect with verified methods such as IPANAT and IAT; we instead used single lead EEG. Future studies should use verified assessments for implicit affect and multichannel EEG in a larger number of patients.
Conclusion
Although patients with dementia were more sensitive to implicit affect, they also showed an enhanced imbalance between positive affect and negative affect in explicit affect assessment, compared to those in the control group. We suggest that single channel EEG and HRV may represent implicit emotional change in patients with dementia.
Footnotes
Authors’ Note: Incheon Eun-Hye Hospital institutional review board approved this study (reference number: EH2017-02).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by The Korean Ministry of Science and ICT.
ORCID iD: Hyuk Ga  https://orcid.org/0000-0002-8475-2423
https://orcid.org/0000-0002-8475-2423
References
- 1. Ekman P, Oster H. Facial expressions of emotions. Annu Rev Psychol. 1979;30:527–554. [Google Scholar]
- 2. Elamin M, Pender N, Hardiman O, Abrahams SJ. Social cognition in neurodegenerative disorders: a systematic review. J Neurol Neurosurg Psychiatry. 2012;83(11):1071–1079. [DOI] [PubMed] [Google Scholar]
- 3. Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: implications for neuropsychological models of aging. Neurosci Biobehav Rev. 2008;32(4):863–881. [DOI] [PubMed] [Google Scholar]
- 4. Grühn D, Smith J, Baltes PB. No aging bias favoring memory for positive material: evidence from a heterogeneity-homogeneity list paradigm using emotionally toned words. Psychol Aging. 2005;20(4):579–588. [DOI] [PubMed] [Google Scholar]
- 5. Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychol Sci. 2003;14(5):409–415. [DOI] [PubMed] [Google Scholar]
- 6. Hummert ML, Garstka TA, O’Brien LT, Greenwald AG, Mellott DS. Using the implicit association test to measure age differences in implicit social cognitions. Psychol Aging. 2002;17(3):482–495. [DOI] [PubMed] [Google Scholar]
- 7. Quirin M, Kazén M, Kuhl JJ. When nonsense sounds happy or helpless: the Implicit Positive and Negative Affect Test (IPANAT). J Pers Soc Psychol. 2009;97(3):500–516. [DOI] [PubMed] [Google Scholar]
- 8. Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Pers Soc Psychol Rev. 2004;8(3):220–247. [DOI] [PubMed] [Google Scholar]
- 9. Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74(6):1464–1480. [DOI] [PubMed] [Google Scholar]
- 10. Bocharov AV, Knyazev GG, Savostyanov AN. Depression and implicit emotion processing: an EEG study. Neurophysiol Clin. 2017;47(3):225–230. [DOI] [PubMed] [Google Scholar]
- 11. van der Ploeg MM, Brosschot JF, Thayer JF, Verkuil B. The Implicit Positive and Negative Affect Test: validity and relationship with cardiovascular stress-responses. Front Psychol. 2016:7;425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christie GJ, Tata MS. Right frontal cortex generates reward-related theta-band oscillatory activity. Neuroimage. 2009;48(2):415–422. [DOI] [PubMed] [Google Scholar]
- 13. Mulert C, Juckel G, Brunnmeier M, et al. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98(3):215–225. [DOI] [PubMed] [Google Scholar]
- 14. Schutter DJ, van Honk J. Electrophysiological ratio markers for the balance between reward and punishment. Brain Res. Cogn Brain Res. 2005;24(3):685–690. [DOI] [PubMed] [Google Scholar]
- 15. Knyazev GG, Schutter DJ, van Honk J. Anxious apprehension increases coupling of delta and beta oscillations. Int J Psychophysiol. 2006;61(2):283–287. [DOI] [PubMed] [Google Scholar]
- 16. Schutter DJ, van Honk J. Decoupling of midfrontal delta-beta oscillations after testosterone administration. Int J Psychophysiol. 2004;53(1):71–73. [DOI] [PubMed] [Google Scholar]
- 17. Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26(9):1245–1260. [DOI] [PubMed] [Google Scholar]
- 18. Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60(3):393–398. [DOI] [PubMed] [Google Scholar]
- 19. Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25(4):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Couyoumdjian A, Ottaviani C, Petrocchi N, et al. Reducing the meta-emotional problem decreases physiological fear response during exposure in phobics. Front. Psychol. 2016;7:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarlo M, Palomba D, Angrilli A, Stegagno L. Blood phobia and spider phobia: two specific phobias with different autonomic cardiac modulations. Biol Psychol. 2002;60(2):91–108. [DOI] [PubMed] [Google Scholar]
- 22. Quintana DS, Guastella AJ, Outhred T, Hickie IB, Kemp AH. Heart rate variability is associated with emotion recognition: direct evidence for a relationship between the autonomic nervous system and social cognition. Int J Psychophysiol. 2012;86(2):168–172. [DOI] [PubMed] [Google Scholar]
- 23. Ga H. Ten years of long-term care insurance in Korea: light and shade from a geriatric point of view. Ann Geriatr Med Res. 2017;21:147–148. [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 25. Han C, Jo SA, Jo I, Kim E., Park MH, Kang Y. An adaptation of the Korean Mini-Mental State Examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47(3):302–310. [DOI] [PubMed] [Google Scholar]
- 26. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 27. Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96(3):465–490. [PubMed] [Google Scholar]
- 28. Lee HH, Kim EJ, Lee MK. A validation study of Korea Positive and Negative Affect Schedule: the PANAS scales. Kor J Clin Psychol. 2003;22:935–946. [Google Scholar]
- 29. NeuroSky. MindWave Mobile: User Guide. San Jose, CA, USA: NueroSky; 2012. [Google Scholar]
- 30. Zammouri A, Moussa AA, Chevallier S, Monacelli E. Intelligent ocular artifacts removal in a non-invasive single channel EEG recording. In: International Conference on Intelligent Systems and Computer Vision, IEEE ISCV. Fez, Morocco: ISCV; 2015. https://www.scimagojr.com/journalsearch.php?q=21100397733&tip=sid&clean=0 [Google Scholar]
- 31. Ducharme S, Price BH, Dickerson BC. Apathy: a neurocircuitry model based on frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2018;89(4):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyvers M, Kohlsdorf SM, Edwards MS, Thorberg FA. Alexithymia and mood: recognition of emotion in self and others. Am J Psychol. 2017;130(1):83–92. [DOI] [PubMed] [Google Scholar]
- 33. Putman P, van Peer J, Maimari I, van der Werff S. EEG theta/beta ratio in relation to fear-modulated response-inhibition, attentional control, and affective traits. Biol Psychol. 2010;83(2):73–78. [DOI] [PubMed] [Google Scholar]
- 34. de la Rubia Orti JE, Garcia-Pardo MP, Iranzo CC, et al. Does music therapy improve anxiety and depression in Alzheimer’s patients? J Altern Complement Med. 2018; 24(1):33–36. [DOI] [PubMed] [Google Scholar]
- 35. Ozdemir L, Akdemir N. Effects of multisensory stimulation on cognition, depression and anxiety levels of mildly-affected Alzheimer’s patients. J Neurol Sci. 2009;283(1-2):211–213. [DOI] [PubMed] [Google Scholar]
- 36. Särkämö T. Cognitive, emotional, and neural benefits of musical leisure activities in aging and neurological rehabilitation: A critical review. Ann Phys Rehabil Med. 2018;61(6):414–418. [DOI] [PubMed] [Google Scholar]