Abstract
Purpose:
To determine the association of serum insulin-like growth factor 1 (IGF-1) and vitamin D levels with cognition status in patients with type 2 diabetes mellitus (T2DM).
Methods:
A total of 173 patients with T2DM were recruited and divided into mild cognitive impairment (MCI) group (n = 94) and normal cognition (NC) group (n = 79). Levels of IGF-1 and 25(OH)D were measured and compared, and the correlations among IGF-1, 25(OH)D, and cognitive function were analyzed.
Results:
Insulin-like growth factor 1 and 25(OH)D levels significantly decreased in MCI group than those in the NC group (both P < .001). Multiple stepwise regression analysis revealed that IGF-1 (β = .146, P < .001) and 25(OH)D (β = .199, P < .001) independently predicted Montreal Cognitive Assessment (MoCA) scores. Partial least square regression showed that contributions of both 25(OH)D (P < .001) and IGF-1 (P < .001) to MoCA scores were significant, while no cross-effect was observed between them (P = .714).
Conclusions:
Low serum IGF-1 and 25(OH)D levels may separately predict poor cognitive performance in patients with diabetes.
Keywords: diabetes mellitus, type 2, IGF-1, vitamin D, mild cognitive impairment, mental status and dementia tests, Montreal Cognitive Assessment
Introduction
Type 2 diabetes mellitus (T2DM) is a growing epidemic worldwide, with considerable impact on health-care expenditures, morbidity, and mortality. It has been increasingly associated with mild cognitive impairment (MCI) and dementia. 1 –4 Mild cognitive impairment, which is considered a transitional stage between normal aging and dementia, was increasingly concerned because of a high risk of developing to Alzheimer disease (AD) at a rate of 10% to 15% per year. 5 Epidemiological and basic science studies have suggested a possible shared pathophysiology between T2DM and AD. 6 Hence, cognitive dysfunction was also considered a chronic neurological complication of diabetes. However, underlying mechanisms of diabetes-related cognitive dysfunction are complicated and not yet been fully understood.
Insulin-like growth factor (IGF)-1, which is structurally related to insulin, is a polypeptide hormone exerting effects in the differentiation, proliferation, and regulation of various central and peripheral tissues. 7, 8 (p1) Previous studies have revealed that serum IGF-1 levels change in patients with AD, 9 that low levels of IGF-1 can be associated with worse cognitive function in older adults, 10 and also predict cognitive decline in AD. 11 However, data on this topic are inconsistent. Results of a systematic review 12 indicated that IGF-1 concentrations do not confer additional diagnostic information for those with cognitive decline.
Increasing evidence suggests that vitamin D may affect the synthesis and/or activity of IGF-1. 13 –15 The interplay between vitamin D and IGF-1 is complicated and occurs at both endocrine and paracrine/autocrine levels. Several studies have also acknowledged the association between vitamin D deficiency with higher risk of MCI and dementia. 16,17 However, the levels of IGF-1 and vitamin D and their correlations with the cognitive dysfunction due to diabetes have been little investigated. This study was designed to assess the association between IGF-1, 25(OH)D levels, and cognitive function in patients with T2DM.
Material and Methods
Patients
Data collection started from November 1, 2015, to April 30, 2017 (we suspended patients enrollment from May to September to avoid 25(OH)D migration induced by changes of sunlight intensity). According to the criteria of 2006 European Alzheimer’s Disease Consortium, 18 173 patients with diabetes (64 men and 109 women, aged 50-73 years) in Shanghai (latitude 31°north) were consecutively enrolled in this study and were divided into 2 groups using the Montreal Cognitive Assessment (MoCA) scoring system. Ninety-four (30 males and 64 females) patients with MoCA scores less than 25 were in the MCI group, and 79 (34 males and 45 females) patients with MoCA scores of 26 or more were in the control group. All of the patients with MCI met the diagnostic criteria for MCI by the European Association for Alzheimer’s Disease MCI Working Group: (1) complaints of cognition from patients or their families; (2) as reported, the decline in cognitive function relative to the previous capacity has declined in the past year (Clinical Dementia Rating score of 0.5); (3) through clinical evaluation, cognitive disorder were evidenced (impairment in memory or some other cognitive field); (4) short of major repercussions in daily life; and (5) the absence of dementia.
Exclusive criteria are as follows: (1) diabetic ketoacidosis, hypoglycemia coma, hypertonic glucose coma, and other acute complications of diabetes; (2) cerebrovascular accident, infection in central nervous system, stroke, cerebral hemorrhage, or other clinical evidences of central nervous damages; (3) visual and auditory resolution deficiencies; (4) Parkinson disease, systemic disease such as malignancy, severe anemia; thyroid disease, Cushing syndrome, drug abuse or dependence history, and depression (within 3 months); anticoagulants; anti-Parkinson disease drugs, benzodiazepines and barbiturates, antiepileptic drugs, and so on were used in the first 3 months of screening; and (5) patients of long-term bedridden or cannot take care of themselves.
This study was carried out in accordance with the Declaration of Helsinki (2013) of the World Medical Association. The institutional ethical review board approved the study and all participants have given written informed consents.
Clinical Evaluation
Demographic characteristics including age, sex, education levels, smoking history, alcoholism, insomnia, sun exposure status, physical exercise conditions, and history of hypertension and/or dyslipidemia were collected. Physical measurements including body mass index, waist circumference, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were collected using a standard beam balance scale. Patients with SBP ≥140 mm Hg, or DBP ≥90 mm Hg, respectively, or current use of antihypertensive medications were identified as having a history of hypertension. Medication use situations (including antidiabetic medications, antiplatelet medications, lipid-lowering medications, and antihypertensive medications) were also obtained through questionnaires.
Laboratory Methods
Blood samples were obtained from patients by venipuncture after an overnight fast (≥8 hours) and tested within 60 minutes. Serum 25(OH)D, including 25(OH)D2 and 25(OH)D3, thyroid-stimulating hormone (TSH), C-peptide (C-P), and parathyroid hormone (PTH) were tested by electrochemiluminescence immunoassay (Cobas 6000 analyzer; Switzerland, Roche). Insulin-like growth factor 1 was measured by chemiluminescence method (IMMULITE 1000; USA DPC). Fasting blood glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, serum creatinine (1 mg/dL = 88.41 μmoI/L), uric acid, blood urea nitrogen, calcium (Ca), and phosphorus (P) were quantified enzymatically (Cobas 8000 C701 C502 auto chemistry analyzer; Roche); HbA1c was measured by high-performance liquid chromatography (HLC-723G7 analyzer, TOSOH, Japan). The simplified equation (eGFR = 1.86 × Sc−1.154 × age−0.203 × [0.742 if female]) proposed by the Modification of Diet in Renal Disease Study Group was applied to calculate estimated glomerular filtration rate (eGFR).
Ultrasonography of carotid arteries
We located the thickest site of the carotid artery intima–media and to measure the thickness at sites 1 cm downstream using color Doppler ultrasound (Sequoia scanner; SIEMENS, Germany). Mean C-IMT (carotid intima–media thickness) = (C-IMT [left] + C-IMT [right])/2.
Statistical Analyses
Normal test by Kolmogorov-Smirnov test was applied on all continuous data. Since TSH concentrations were not normal distribution, they were log-transformed for analyses. Independent t test was applied to compare measurement data, and qualitative data were analyzed by theχ2 test between 2 groups. Associations of MoCA scores, IGF-1, 25(OH)D, and other variables were analyzed using Pearson/Spearman rank correlation analysis. A prediction model for correlations of variables and MoCA scores was created using multiple linear regressions. Standard least square regression is used to estimate the contribution of dependent variables to the cognitive function (MoCA scores).
JMP 14 (SAS institute Inc) and Statistical Package for the Social Science (SPSS version 19.0, IBM) were applied in this study. Data were expressed in the tables and text as mean (standard deviation) or median (25%-75%). A 2-sided P <.05 was considered to be statistically significant.
Results
As shown in Table 1, the proportion of injection of insulin was higher, and use of oral hypoglycemic drugs was lower in MCI group than those in normal cognition (NC) group. No other differences were observed in medication use between patients in NC group and MCI group.
Table 1.
Comparison of Medication Use Between 2 Groups.a
| MCI | NC | χ2 | P | |
|---|---|---|---|---|
| Number of patients | 94 | 79 | ||
| Insulin (%) | 48 (51.1) | 22 (27.8) | 9.604 | .002 |
| Oral hypoglycemic drugs (%) | 69 (73.4) | 35 (44.3) | 15.161 | <.001 |
| Antihypertensive medications (%) | 46 (48.9) | 38 (48.1) | 0.012 | .913 |
| Antiplatelet medications (%) | 22 (23.4) | 14 (17.7) | 0.841 | .359 |
| Lipid-lowering medications (%) | 20 (21.3) | 12 (15.2) | 1.055 | .304 |
Abbreviations: MCI, mild cognitive impairment; NC, normal cognition.
aχ2 test was used to test for significant differences. All values were >.05.
The clinical characteristics of both groups are shown in Table 2. Comparing with the NC group, patients with MCI had a longer duration of diabetes, fewer education years, less sun exposure, and their SBP, HbA1c, TC, HDL-C, PTH, calcium, and mC-IMT were elevated, and C-P, TG, eGFR, 25(OH)D, IGF-1, and MoCA scores were decreased (P < .05). All the components in MoCA scales significantly decreased in MCI group than in NC group (P < .05).
Table 2.
Clinical Characteristics of Type 2 Diabetic Patients With and Without MCI.a
| MCI | NC | t/χ2 | P | |
|---|---|---|---|---|
| Patients number (M/F) | 94 (30/64) | 79 (34/45) | 2.278 | .131 |
| Age (years) | 63.13 (5.60) | 61.87 (5.83) | 1.560 | .121 |
| Duration of DM (years) | 9.02 (5.72) | 6.81 (4.70) | 2.791 | .006 |
| Education (years) | 8.30 (2.13) | 10.33 (2.56) | −5.697 | <.001 |
| History of hypertension (%) | 60 (63.8) | 39 (49.4) | 3.668 | .055 |
| Ever smoked | 11 (11.7) | 17 (21.5) | 3.049 | .081 |
| Alcoholism | 4 (4.3) | 7 (8.9) | 1.529 | .216 |
| Sun exposure (%) | 25 (36.6) | 36 (45.6) | 6.770 | .009 |
| Exercise (n) | (5, 13, 76) | (4, 14, 60) | 0.547 | .761 |
| Insomnia | 24 (25.5) | 15 (19.0) | 1.053 | .305 |
| BMI (kg/m2) | 25.29 (3.12) | 24.97 (2.88) | 0.696 | .487 |
| Waist circumference (cm) | 86.53 (8.24) | 85.35 (8.25) | 0.936 | .351 |
| SBP (mm Hg) | 145.05 (15.55) | 136.92 (15.61) | 3.419 | .001 |
| DBP (mm Hg) | 82.53 (8.32) | 82.04 (8.56) | 0.384 | .702 |
| FBG (mmol/L) | 9.06 (2.98) | 9.01 (5.65) | 0.084 | .933 |
| C-P (ng/mL) | 1.40 (0.65) | 2.24 (1.20) | −5.29 | <.001 |
| HbA1c (%) | 8.23 (1.69) | 7.37 (1.76) | 3.253 | .001 |
| TC (mmol/L) | 4.95 (1.21) | 3.21 (1.80) | 7.287 | <.001 |
| TG (mmol/L) | 1.75 (1.50) | 3.49 (1.95) | 6.439 | <.001 |
| HDL-C (mmol/L) | 1.45 (0.40) | 1.25 (0.41) | 3.209 | .002 |
| LDL-C (mmol/L) | 3.10 (1.03) | 2.99 (0.97) | 0.754 | .452 |
| UA (mmol/L) | 0.294 (0.08) | 0.312 (0.08) | −1.483 | .140 |
| BUN (mmol/L) | 5.87 (1.53) | 5.78 (1.58) | 0.374 | .709 |
| eGFR (mL/min/1.73 m2) | 96.47 (25.64) | 104.58 (27.51) | −2.004 | .047 |
| 25(OH)D (ng/mL) | 15.75 (6.04) | 23.04 (5.88) | −8.007 | <.001 |
| PTH (pg/ml) | 50.25 (19.21) | 35.00 (15.81) | 5.632 | <.001 |
| TSH (mIU/L) | (1.77, 3.18) | (1.45, 2.50) | 1.404 | .162 |
| IGF-1 (ng/mL) | 114.42 (36.14) | 143.24 (35.03) | −5.296 | <.001 |
| Ca++ (mmol/L) | 2.35 (0.11) | 2.28 (0.28) | 2.218 | .028 |
| P (mmol/L) | 1.16 (0.17) | 1.12 (0.14) | 1.926 | .056 |
| mC-IMT (mm) | 0.84 (0.12) | 0.76 (0.12) | 4.260 | <.001 |
| MoCA | 19.95 (3.19) | 26.68 (0.98) | −19.390 | <.001 |
| Visuospatial/executive | 2.77 (1.02) | 4.25 (0.72) | −10.855 | <.001 |
| Naming | 2.06 (0.87) | 2.68 (0.50) | −5.637 | <.001 |
| Attention | 4.83 (1.07) | 5.72 (0.62) | −6.524 | <.001 |
| Language | 1.73 (0.75) | 2.29 (0.70) | −5.013 | <.001 |
| Abstraction | 0.66 (0.77) | 1.57 (0.61) | −8.650 | <.001 |
| Delayed recall | 2.17 (1.27) | 3.89 (0.95) | −10.176 | <.001 |
| Orientation | 5.69 (0.55) | 5.94 (0.24) | −3.892 | <.001 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IGF-1, insulin-like growth factor 1; LDL-C, low-density lipoprotein cholesterol; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; mC-IMT, mean carotid intima–media thickness; NC, normal cognition; PTH, parathyroid hormone; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; TSH, thyroid-stimulating hormone; WHR, waist-to-hip ratio.
aData were expressed as n (%), mean (SD), or median (25%, 75%) as appropriate. TSH was log-transformed for statistical analyses and then back-transformed to their natural units for presentation. Student t test for comparison of normally distributed quantitative variables between MCI group and control group. χ2 test for comparison of qualitative variables between MCI group and control group. Exercise: 1 = hardly exercise, 2 = exercise <30 min/d, 3 = exercise >30 min/d; sun exposure:1 = expose to sunlight <30 minutes, 2 = expose to sunlight ≥30 minutes.
Univariate analysis of MoCA scores, 25(OH)D, and IGF-1 with other characteristics were listed in Table 3. Montreal Cognitive Assessment scores were positively associated with education years, smoking history, insomnia, TG, eGFR, and C-P and inversely associated with duration of DM, hypertension history, insulin use, oral hypoglycemic drugs use, SBP, HbA1c, TC, HDL-C, 25(OH)D, IGF-1, PTH, and mC-IMT. 25(OH)D levels were positively associated with education, sun exposure, alcoholism, TG, and IGF-1 and inversely associated with sex, duration of DM, insulin use, oral hypoglycemic drugs use, HbA1c, TC, and PTH levels. The levels of IGF-1 were positively associated with education, 25(OH)D, and TG and inversely associated with age, duration of DM, hypertension history, insomnia, oral hypoglycemic drugs use, HbA1c, TC, and PTH.
Table 3.
Univariate Analyses of the Relationship Among MoCA Scores, IGF-1, 25(OH)D, and Other Clinical Characteristics.
| MoCA Scores | 25(OH)D | IGF-1 | |
|---|---|---|---|
| Age | −0.117 | −0.128 | −0.204a |
| Sex b | −0.129 | −0.234a | −0.140 |
| Education | 0.426a | 0.318a | 0.231a |
| Duration of DM | −0.276a | −0.271a | −0.223a |
| Hypertension b | −0.173c | −0.124 | −0.155c |
| Sun exposureb | 0.124 | 0.492a | 0.061 |
| Exerciseb | −0.083 | 0.051 | 0.019 |
| BMI | 0.007 | 0.005 | −0.076 |
| Waist circumference | −0.039 | −0.026 | −0.095 |
| Insulin useb | −0.223a | −0.269a | −0.122 |
| Oral hypoglycemic drugsb | −0.265a | −0.357a | −0.295a |
| Alcoholismb | 0.096 | 0.193c | 0.047 |
| Ever smokedb | 0.172c | 0.102 | 0.119 |
| Insomniab,d | −0.203a | −0.075 | −0.225a |
| SBP | −0.203a | −0.068 | −0.089 |
| DBP | −0.029 | −0.005 | −0.065 |
| FBG | −0.072 | −0.149 | −0.067 |
| HbA1c | −0.245a | −0.183c | −0.302a |
| TC | −0.391a | −0.364a | −0.291a |
| TG | 0.376a | 0.268a | 0.306a |
| HDL-C | −0.202a | −0.095 | 0.016 |
| LDL-C | −0.018 | −0.057 | −0.021 |
| eGFR | −0.155c | 0.117 | −0.060 |
| 25(OH)D | 0.482a | / | 0.419a |
| IGF-1 | 0.438a | 0.419a | / |
| PTH | −0.339a | −0.497a | −0.353a |
| TSH | −0.088 | −0.075 | −0.075 |
| Ca | −0.104c | 0.091 | −0.001 |
| P | 0.075 | −0.122 | −0.083 |
| C-P | 0.359a | 0.300a | 0.336a |
| mC-IMT | −0.438a | −0.202a | −0.233a |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IGF-1, insulin-like growth factor 1; LDL-C, low-density lipoprotein cholesterol; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; mC-IMT, mean carotid intima–media thickness; NC, normal cognition; PTH, parathyroid hormone; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; TSH, thyroid-stimulating hormone.
aP < .01.
bSpearman rank correlation.
cP < .05.
dNo insomnia = 1, insomnia = 2.
Correlations among MoCA scores, IGF-1, 25(OH)D, and cognitive domain scores are presented in Table 4. Levels of IGF-1 and 25(OH)D were positively correlated with all the domains of the cognitive function (using MoCA scales; P < .05).
Table 4.
Univariate Analyses of the Relationship Among MoCA Scores, IGF-1, 25(OH)D, and Cognitive Domain Scores (Using Spearman Rank Correlation).
| MoCA Scores | 25(OH)D | IGF-1 | |
|---|---|---|---|
| Visuospatial/executive | 0.736a | 0.429a | 0.301a |
| Naming | 0.473a | 0.252a | 0.235a |
| Attention | 0.667a | 0.376a | 0.298a |
| Language | 0.488a | 0.167b | 0.241a |
| Abstraction | 0.642a | 0.412a | 0.260a |
| Delayed recall | 0.684a | 0.295a | 0.337a |
| Orientation | 0.386a | 0.201a | 0.301a |
Abbreviations: IGF-1, insulin-like growth factor 1; MoCA, Montreal Cognitive Assessment.
aP < .01.
bP < .05.
After adjusting for age, sex, duration of DM, and education, the regression model included hypertension history, smoking history, insulin use, oral hypoglycemic drugs use, insomnia, SBP, HbA1c, TC, TG, HDL-C, eGFR, 25(OH)D, IGF-1, PTH, Ca, C-P, and mC-IMT as covariates and revealed that 25(OH)D, IGF-1, SBP, C-P, and mC-IMT were independent predictors of MoCA scores (R2 = 0.491; Table 5).
Table 5.
Multiple Linear Regression Analysis Adjusted for Age, Sex, and Education.
| Dependent Variable | Independent Variables | Unstandardized Coefficients | Standardized Coefficients | T | P Value | |
|---|---|---|---|---|---|---|
| B | Standard Error | β | ||||
| MoCA score | Constant | 27.203 | 3.14 | 8.664 | <.001 | |
| 25(OH)D | 0.119 | 0.040 | .199 | 2.991 | .003 | |
| IGF-1 | 0.016 | 0.007 | .146 | 2.238 | .027 | |
| SBP | −0.039 | 0.015 | −.150 | −2.612 | .010 | |
| mC-IMT | −10.000 | 2.000 | −.306 | −5.000 | <.001 | |
| C-P | 0.973 | 0.274 | .223 | 3.553 | .001 | |
Abbreviations: C-P, C-peptide; IGF-1, insulin-like growth factor 1; mC-IMT, mean carotid intima–media thickness; MoCA, Montreal Cognitive Assessment; SBP, systolic blood pressure.
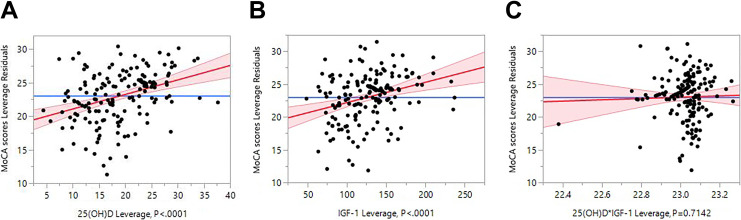
Since 25(OH)D was positively associated with IGF-1 in this study, standard least square regression was applied to estimate the contributions of IGF-1 and 25(OH)D to MoCA score. The results showed that the main effects of both 25(OH)D and IGF-1 were significant, while no cross-effect was observed between them (Table 6 and Figure 1A–C).
Table 6.
Contribution of 25(OH)D and IGF-1 to MoCA Score.
| MoCA Score | Dependent Variables | Estimate | SE | T Ratio | P Value |
|---|---|---|---|---|---|
| Intercept | 14.97 | 1.02 | 14.66 | <.001 | |
| 25(OH)D | 0.21 | 0.04 | 5.13 | <.001 | |
| IGF-1 | 0.03 | 0.01 | 4.04 | <.001 | |
| 25 (OH)D*IGF-1 | <−0.01 | <0.01 | −0.37 | .714 |
Abbreviations: IGF-1, insulin-like growth factor 1; MoCA, Montreal Cognitive Assessment; SE, standard error.
Figure 1.
Contributions of IGF-1 and 25(OH)D to MoCA score. A, 25(OH)d leverage plot. B, IGF-1 leverage plot. C, 25(OH)D*IGF-1 leverage plot. IGF-1 indicates insulin-like growth factor 1; MoCA, Montreal Cognitive Assessment.
Discussion
The prevalence of CI and its correlation with diabetes has been greatly underestimated. 19 –21 Screen tools and interventions that can prevent, slow, or reverse the progressive of these neurodegenerative illnesses are needed. Mild cognitive impairment is a screening tool with high sensitivity which can be applied to divide patients into MCI and normal-cognition groups in the diabetic population. 22 The current study shows that levels of IGF-1 decreased in MCI group and positively associated with cognitive function according to the MoCA scales in patients with T2DM. Insulin-like growth factor 1 is considered an essential neurotrophic factor and plays a critical role in nervous system homeostasis, 23 which can be correlated with cognitive function in older population. 24 Nevertheless, whether its levels are positively or negatively correlated with cognitive function is a debatable point. A study by Tumati et al 10 revealed that both increased and decreased concentrations of IGF-1 may be associated with cognitive dysfunction in middle-aged and older men. Our data are consistent with studies in people without diabetes 11,25,26 that those with higher levels of IGF-1 gain higher scores in multiple domains and they independently predict better cognitive function.
Vitamin D deficiency is very common in T2DM. 27 Vitamin D exerts some effect in neuroprotection and regulation in the central nervous system. 28 –31 It has been reported 13,32,33 that IGF-1 plays a role through changes in vitamin D activation, and 1,25(OH)2D can regulate IGF-1 axis genes the other way round. However, it is unknown whether they play joint effects on cognitive protection. In the current study, results of partial least square regression showed that IGF-1 and 25(OH)D significantly contributed to MoCA scores, which means they separately influenced the cognitive function. However, although IGF-1 and 25(OH)D correlated with each other, they had no joint effect on MoCA scores (P = .714). Yet, interventional studies haven’t shown a clear benefit from vitamin D supplementation. 34 There might be likely a therapeutic age/time window relevant to the development of disease and therefore vitamin D therapy.
Abnormal PTH levels exert some effect in neuronal calcium dysregulation, hypoperfusion and disrupted neuronal signaling. However, whether PTH levels predict a poor cognitive function in patients with diabetes is controversial. A study by Kalaitzidis et al 35 suggested that increased serum PTH levels are associated with cognitive dysfunction in patients with chronic kidney disease and hypertension. But previous evidence offers weak support for a link between cognition and dementia due to the paucity of high-quality research in this area. 36 Additionally, a recent Atherosclerosis Risk in Communities cohort study of a 20 years’ follow-up has found no association of higher mid-age PTH levels with old-age prevalent diagnosed dementia, 37 which did not support an independent influence of PTH on cognitive decline. Our study indicated that PTH increased in MCI group and was inversely associated with cognitive function in patients with diabetes. But it was not independently correlated with MoCA scores. Since 25(OH)D levels independently predicted MoCA scores, we speculated that the strong inverse association between PTH and 25(OH)D indirectly resulted in the inverse correlation between PTH and MoCA scores, but the observation deserves further cohort study.
Limitations
(1) Self-reported data including history of hypertension, dyslipidemia, and duration of DM may cause recall bias. (2) All results of this study were from a small sample aged from 51 to 73 years, which cannot represent all patients with diabetes. (3) The diagnostic criteria in this study were only MoCA scores, which might lead to bias, while some other investigators carried out their studies with several cognitive scales including Digit Span Test, Verbal Fluency Test, activity of daily living scale, and so on. 4. This is a cross-sectional study, which limits causal inference. In addition, some potential confounders (ie, diabetes duration, C-P, carotid IMT, other diabetic complications, etc) may cause bias of the results. Further investigation with well-matched case–control cohort, more cognitive scales, and the MCI progression follow-up are warranted to verify these preliminary results and explore their clinical significance.
In summary, our current study showed that low levels of IGF-1 and 25(OH)D were associated with MCI in patients with diabetes, and IGF-1 levels were positively associated with serum levels of 25(OH)D. The decreasing of both IGF-1 and 25(OH)D may independently predict MCI. Although this study was not designed to explore the precise mechanism by which vitamin D and IGF-1 modulate cognitive dysfunction, we preliminary studied the associations between these 2 hormones on MCI in patients with diabetes. This study suggested that both serum IGF-1 and 25(OH)D can be serological markers of MCI of diabetes.
Footnotes
Authors’ Note: P.Y. and C.R. designed the study and edited the text; J.X. preceded the questionnaires; B.Z. collected the data; J.C. analyzed the data; and P.Y. reviewed the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a joint research project of Shanghai Municipal Health Bureau (201640207 to C.R.) and Pudong Municipal Health Bureau (PW2016A-22).
ORCID iD: Peng Yong-de  https://orcid.org/0000-0001-5483-7007
https://orcid.org/0000-0001-5483-7007
References
- 1. Ritchie K, Carrière I, Ritchie CW, Berr C, Artero S, Ancelin M-L. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ. 2010;341:c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehrabian S, Raycheva M, Gateva A, et al. Cognitive dysfunction profile and arterial stiffness in type 2 diabetes. J Neurol Sci. 2012;322(1-2):152–156. doi:10.1016/j.jns.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 3. Ebady SA, Arami MA, Shafigh MH. Investigation on the relationship between diabetes mellitus type 2 and cognitive impairment. Diabetes Res Clin Pract. 2008;82(3):305–309. doi:0.1016/j.diabres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 4. Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–491. doi:10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 5. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 6. Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71(3):365–376. doi:10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vélez EJ, Lutfi E, Jiménez-Amilburu V, et al. IGF-I and amino acids effects through TOR signaling on proliferation and differentiation of gilthead sea bream cultured myocytes. Gen Comp Endocrinol. 2014;205:296–304. doi:10.1016/j.ygcen.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 8. Bianchi VE, Locatelli V, Rizzi L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int J Mol Sci. 2017;18(11). doi:10.3390/ijms18112441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu X, Yang Y, Gong D. Circulating insulin-like growth factor 1 and insulin-like growth factor binding protein-3 level in Alzheimer’s disease: a meta-analysis. Neurol Sci. 2016;37(10):1671–1677. doi:10.1007/s10072-016-2655 -1. [DOI] [PubMed] [Google Scholar]
- 10. Tumati S, Burger H, Martens S, van der Schouw YT, Aleman A. Association between cognition and serum insulin-like growth factor-1 in middle-aged & older men: an 8 year follow-up study. PLoS One. 2016;11(4). doi:10.1371/journal.pone.0154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vidal J-S, Hanon O, Funalot B, et al. Low serum insulin-like growth factor-i predicts cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2016;52(2):641–649. doi:10.3233/JAD-151162. [DOI] [PubMed] [Google Scholar]
- 12. Frater J, Lie D, Bartlett P, McGrath JJ. Insulin-like growth factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: a review. Ageing Res Rev. 2018;42:14–27. doi:10.1016/j.arr.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 13. Ameri P, Giusti A, Boschetti M, et al. Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency. Eur J Endocrinol. 2013;169(6):767–772. doi:10.1530/EJE-13-0510. [DOI] [PubMed] [Google Scholar]
- 14. Ciresi A, Giordano C. Vitamin D across growth hormone (GH) disorders: from GH deficiency to GH excess. Growth Horm IGF Res. 2017;33:35–42. doi:10.1016/j.ghir.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 15. Darr RL, Savage KJ, Baker M, et al. Vitamin D supplementation affects the IGF system in men after acute exercise. Growth Horm IGF Res. 2016;30-31:45–51. doi:10.1016/j.ghir.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 16. Moon JH, Lim S, Han JW, et al. Serum 25-hydroxyvitamin D level and the risk of mild cognitive impairment and dementia: the Korean Longitudinal Study on Health and Aging (KLoSHA). Clin Endocrinol (Oxf). 2015;83(1):36–42. doi:10.1111/cen.12733. [DOI] [PubMed] [Google Scholar]
- 17. Etgen T, Sander D, Bickel H, Sander K, Förstl H. Vitamin D deficiency, cognitive impairment and dementia: a systematic review and meta-analysis. Dement Geriatr Cogn Disord. 2012;33(5):297–305. doi:10.1159/000339702. [DOI] [PubMed] [Google Scholar]
- 18. Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European consortium on Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(6):714–718. doi:10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DenBoer JW. Cognitive intervention for early stage dementia: research and techniques. Appl Neuropsychol Adult. 2018;25(6):562–571. doi:10.1080/23279095.2017.1330748. [DOI] [PubMed] [Google Scholar]
- 20. Xiao S, Lewis M, Mellor D, et al. The China longitudinal ageing study: overview of the demographic, psychosocial and cognitive data of the Shanghai sample. J Ment Health Abingdon Engl. 2016;25(2):131–136. doi:10.3109/09638237.2015.1124385. [DOI] [PubMed] [Google Scholar]
- 21. Downer B, Kumar A, Mehta H, Al Snih S, Wong R. The effect of undiagnosed diabetes on the association between self-reported diabetes and cognitive impairment among older Mexican adults. Am J Alzheimers Dis Other Demen. 2016;31(7):564–569. doi:10.1177/1533317516653824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alagiakrishnan K, Zhao N, Mereu L, Senior P, Senthilselvan A. Montreal Cognitive Assessment is superior to standardized Mini-Mental Status Exam in detecting mild cognitive impairment in the middle-aged and elderly patients with type 2 diabetes mellitus. BioMed Res Int. 2013. doi:10.1155/2013/186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and Alzheimer’s disease and vascular dementia. J Am Geriatr Soc. 2005;53(10):1748–1753. doi:10.1111/j.1532-5415.2005.53524.x. [DOI] [PubMed] [Google Scholar]
- 24. Morley JE, Kaiser F, Raum WJ, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A. 1997;94(14):7537–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma J, Jiang Q, Xu J, et al. Plasma insulin-like growth factor 1 is associated with cognitive impairment in Parkinson’s disease. Dement Geriatr Cogn Disord. 2015;39(5-6):251–256. doi:10.1159/000371510. [DOI] [PubMed] [Google Scholar]
- 26. Doi T, Shimada H, Makizako H, et al. Association of insulin-like growth factor-1 with mild cognitive impairment and slow gait speed. Neurobiol Aging. 2015;36(2):942–947. doi:10.1016/j.neurobiolaging.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 27. Tahrani AA, Ball A, Shepherd L, Rahim A, Jones AF, Bates A. The prevalence of vitamin D abnormalities in South Asians with type 2 diabetes mellitus in the UK. Int J Clin Pract. 2010;64(3):351–355. doi:10.1111/j.1742-1241.2009.02221.x. [DOI] [PubMed] [Google Scholar]
- 28. Smith MP, Fletcher-Turner A, Yurek DM, Cass WA. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31(4):533–539. doi:10.1007/s11064-006-9048-4. [DOI] [PubMed] [Google Scholar]
- 29. Li L, Prabhakaran K, Zhang X, et al. 1Alpha,25-dihydroxyvitamin D3 attenuates cyanide-induced neurotoxicity by inhibiting uncoupling protein-2 up-regulation. J Neurosci Res. 2008;86(6):1397–1408. doi:10.1002/jnr.21596. [DOI] [PubMed] [Google Scholar]
- 30. Taniura H, Ito M, Sanada N, et al. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res. 2006;83(7):1179–1189. doi:10.1002/jnr.20824. [DOI] [PubMed] [Google Scholar]
- 31. Grimm MOW, Lehmann J, Mett J, et al. Impact of vitamin D on amyloid precursor protein processing and amyloid-β peptide degradation in Alzheimer’s disease. Neurodegener Dis. 2014;13(2-3):75–81. doi:10.1159/000355462. [DOI] [PubMed] [Google Scholar]
- 32. Wong MS, Tembe VA, Favus MJ. Insulin-like growth factor-I stimulates renal 1, 25-dihydroxycholecalciferol synthesis in old rats fed a low calcium diet. J Nutr. 2000;130(5):1147–1152. [DOI] [PubMed] [Google Scholar]
- 33. Ameri P, Giusti A, Boschetti M, Murialdo G, Minuto F, Ferone D. Interactions between vitamin D and IGF-I: from physiology to clinical practice. Clin Endocrinol (Oxf). 2013;79(4):457–463. doi:10.1111/cen.12268. [DOI] [PubMed] [Google Scholar]
- 34. Goodwill AM, Szoeke C. A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc. 2017;65(10):2161–2168. doi:10.1111/jgs.15012. [DOI] [PubMed] [Google Scholar]
- 35. Kalaitzidis RG, Karasavvidou D, Tatsioni A, et al. Risk factors for cognitive dysfunction in CKD and hypertensive subjects. Int Urol Nephrol. 2013;45(6):1637–1646. doi:10.1007/s11255-013-0450-y. [DOI] [PubMed] [Google Scholar]
- 36. Lourida I, Thompson-Coon J, Dickens CM, et al. Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS One. 2015;10(5):e0127574. doi:10.1371/journal.pone.0127574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SM, Zhao D, Schneider ALC, et al. Association of parathyroid hormone with 20-year cognitive decline: the ARIC study. Neurology. 2017;89(9):918–926. doi:10.1212/WNL.0000000000004290. [DOI] [PMC free article] [PubMed] [Google Scholar]