Abstract
The effect of bacterial secretion of an exopolysaccharide (EPS) on rhizosphere soil physical properties was investigated by inoculating strain NAS206, which was isolated from the rhizosphere of wheat (Triticum durum L.) growing in a Moroccan vertisol and was identified as Pantoea aglomerans. Phenotypic identification of this strain with the Biotype-100 system was confirmed by amplified ribosomal DNA restriction analysis. After inoculation of wheat seedlings with strain NAS206, colonization increased at the rhizoplane and in root-adhering soil (RAS) but not in bulk soil. Colonization further increased under relatively dry conditions (20% soil water content; matric potential, −0.55 MPa). By means of genetic fingerprinting using enterobacterial repetitive intergenic consensus PCR, we were able to verify that colonies counted as strain NAS206 on agar plates descended from inoculated strain NAS206. The intense colonization of the wheat rhizosphere by these EPS-producing bacteria was associated with significant soil aggregation, as shown by increased ratios of RAS dry mass to root tissue (RT) dry mass (RAS/RT) and the improved water stability of adhering soil aggregates. The maximum effect of strain NAS206 on both the RAS/RT ratio and aggregate stability was measured at 24% average soil water content (matric potential, −0.20 MPa). Inoculated strain NAS206 improved RAS macroporosity (pore diameter, 10 to 30 μm) compared to the noninoculated control, particularly when the soil was nearly water saturated (matric potential, −0.05 MPa). Our results suggest that P. agglomerans NAS206 can play an important role in the regulation of the water content (excess or deficit) of the rhizosphere of wheat by improving soil aggregation.
The rhizosphere is a highly important source of organic materials in soils. It contributes to soil organic matter directly through the release of soil root material and its decomposition and indirectly through root exudation, which stimulates microbial activity and biomass (22). The complex and dynamic interactions among microorganisms, roots, soil, and water in the rhizosphere induce changes in soil physicochemical and structural properties. For example, long-term pasture soils which were permanently and densely rooted had greater aggregate water stability than did their long-term arable counterparts (15, 39). Aggregate stability of ryegrass and alfalfa rhizosphere soil has been shown to increase with root growth, and the stabilizing effect apparently originated from polysaccharide production in the rhizosphere (29). The efficiency with which ryegrass increases the size of soil aggregates has been attributed to the activation and growth of mycorrhizal and saprophytic fungal hyphae (23, 38). Inoculation of wheat with Paenibacillus polymyxa in a silty topsoil resulted in an increase in the amount of root-adhering soil (RAS) per unit of root tissue (RT) dry mass (dm) and enhanced the frequency of the aggregate size class of 0.2 to 2 mm (13). The soil water potential determines (i) the availability of water, oxygen, and substrates for plants and microorganisms, (ii) the survival and activity of microorganisms, and (iii) competition and predation relationships among microorganisms (3, 26, 31, 40). Bacterial exopolysaccharides (EPS) can protect bacteria from various stresses. A Pseudomonas sp. strain increased its EPS production during desiccation (30). The production of EPS possibly enhances water retention in the microbial environment and seems to regulate the diffusion of carbon sources such as glucose (5, 6). In Mediterranean agriculture, hydric stress in soil is a major factor limiting crop production. Therefore, we isolated an EPS-producing bacterium from wheat roots growing in a Moroccan clayey soil and characterized, by a combined microbiological and physical approach, the effects of inoculation of EPS-producing bacteria and of soil water content on colonization of the wheat rhizosphere and on rhizosphere soil aggregation.
MATERIALS AND METHODS
Soil.
Soil was collected in September from the homogeneous A1 horizon (0 to 20 cm) in western Morocco (Institut National de la Recherche Agronomique experimental station, Settate). It was previously under a wheat-legume crop rotation. Due to the following characteristics, it was classified as a vertisol (Food and Agriculture Organization classification): clay, 55.1%; fine silt (2 to 20 μm), 21.5%; coarse silt (20 to 50 μm), 9.7%; fine sand (50 to 200 μm), 10.2%; coarse sand (>200 μm), 1.7%; total organic carbon, 1.47%; total nitrogen, 0.10%; pH (H2O) 8.2. As clay minerals, we determined smectite (80%), kaolinite (10%), and chlorite (10%) after clay ultrafractionation (33) and X-ray diffraction analysis. This soil contained 22% carbonates and was saturated with calcium. The soil sample was air dried, sieved to 2 mm, and stored in plastic containers at 4°C (7% soil water content) until use.
Soil water retention (drainage) was determined by drying the initially saturated soil at different matric potentials by using Richard’s pressure membranes. By using this curve, the three values of soil water content determined were: 20% (−0.55 MPa), 24% (−0.20 MPa), and 35% (−0.05 MPa), corresponding to dry, drained, and nearly water-saturated soil hydric conditions, respectively.
Selection of a bacterial strain.
Bacteria were isolated from the wheat rhizosphere 3 weeks after wheat cultivation in plastic pots (5 by 18 cm) containing a soil sampled in Settate with an average soil water content of 24%. This soil sample was not air dried. Bacteria were isolated from root macerates, RAS, and nonrhizosphere soil. Suspensions were serially diluted in 0.85% KCl and plated on agar containing the ingredients of modified Weaver’s medium (yeast extract, 0.1 g liter−1; supersalts solution, 50 ml liter−1; phosphate buffer, 15 ml liter−1) (17) and also sucrose, fructose, sorbitol, glucose, or mannitol (20 g liter−1). Rhizoplane strain NAS206 was chosen for its extensive EPS production on the sucrose-supplemented medium. This strain was phenotypically characterized by using the Biolog system (Biolog Inc., Hayward, Calif.). Inoculated Biolog GN microplates were incubated at 30°C and examined for 4 and 24 h, and bacteria were identified by means of the substrate utilization profile by using Biolog Microlog software. Strain NAS206 was also identified by using Biotype-100 strips (bioMérieux), which is more suitable for the identification of members of the family Enterobacteriaceae. Data were analyzed by P. A. D. Grimont (Institut Pasteur, Paris, France), who used the Recognizer program. Amplified ribosomal DNA (rDNA) restriction analysis of strain NAS206 and the type strains of Pantoea dispersa (LMG 2603T), P. agglomerans (E20T; kindly provided by P.D.A. Grimont), and Rahnella aquatilis (ATCC 33071T) was performed to verify the phenotypic identification. The total 16S rDNAs of the four strains were amplified by PCR and digested with 13 restriction endonucleases (AluI, CfoI, DdeI, HaeIII, HinfI, TaqI, MspI, RasI, NciI, Sau961, ScrfI, NdeII, and Tru91) (21, 42).
Inoculation with strain NAS206 and wheat growth conditions.
Wheat seeds (Triticum durum L. cv. Acsad 65) were surface sterilized with saturated calcium hypochlorite (2 h) and 10% H2O2 (30 min) under a partial vacuum. The seeds were rinsed in sterile water and incubated on nutrient agar plates (Difco) at room temperature to check for sterility. Prior to inoculation, strain NAS206 was grown in Luria-Bertani broth (34) at 30°C for 24 h. Cells were harvested by centrifugation (12,000 × g, 10 min), washed twice (0.85% KCl), and then resuspended in sterile distilled water to a density of 108 bacteria ml−1. Sterilized wheat seeds were coated with 4 g of peat containing 1 ml of a bacterial inoculum (giving 4 × 107 bacteria per seed) and 4 ml of EPS from strain NAS206 (35%, wt/vol). The EPS was extracted as described by Hebbar et al. (16), from a solid medium (24) containing 2% sucrose which was inoculated with strain NAS206 and incubated at 30°C for 16 h and then at 4°C for 24 h. As the control, seeds were coated with the peat mixed with EPS, but with no bacterial inoculum.
Coated seeds (two per pot) were sown 1 cm below the soil surface in plastic pots (5 by 18 cm) filled with 200 g of air-dried, sieved soil (see the earlier paragraphs about soil). Wheat was grown for 20 days in a growth chamber under a 16-h day–8-h night cycle and respective temperatures of 25 and 18°C. Two sets of 10 replicates for the inoculated and control treatments and for each soil water content value (see the earlier paragraphs about soil) were conducted. The soil water content of each pot was adjusted daily with sterile water, which was added by spraying onto the soil surface and by capillary action from the bottom. This combination leads to a comparatively homogeneous water content within the soil column (data not shown). Sampling of RAS and bulk soil for microbiological and physical analyses was carried out 14 h after the last water addition.
Colonization of soil and roots by strain NAS206.
Colonization of wheat roots, rhizosphere, and nonrhizosphere soil by strain NAS206 was studied with 20-day-old plantlets (five replicates per treatment). The entire soil-root system was taken from the pot and agitated gently, giving the bulk soil fraction. The roots were washed by being dipped in sterile water to separate RAS from root tissue RT. To localize strain NAS206 at different wheat root parts, the washed root system was aseptically dissected into three segments: the first 5 cm below the seed (basal region), 4 cm of the middle of the root system, and 2 cm at the root tip. The different root macerates, rhizosphere soil, and bulk soil were serially diluted in 0.85% KCl and plated on petri dishes containing nutrient agar. After incubation at 30°C for 24 h, yellowish pigmented colonies (similar to strain NAS206) and total microflora CFU were counted from appropriate dilutions. To verify that the yellow colonies represented the strain NAS206 inoculum, 24 colonies were randomly selected and checked for their genetic fingerprint by PCR with enterobacterial repetitive intergenic consensus (ERIC) sequences as primers (19). The protocol was adapted from that of de Bruijn (8, 35) and recently described in detail (10).
Characteristics of RAS.
Six plantlets per treatment were randomly chosen 20 days after sowing. Roots with adhering soil were carefully separated from bulk soil by gentle mechanical agitation (Agitest; Bioblock) for 1 min. RAS was removed from RT by washing in distilled water. Soil dm and root fraction dm were measured after 24 h at 105°C, and the RAS/RT ratio was calculated. Water stability of RAS was determined by using the wet-sieving method (2). Each root system with adhering soil was placed onto a sieve (height, 70 mm; diameter, 60 mm; mesh size, 200 μm), immersed in 200 ml of distilled water, and oscillated. The horizontal oscillations applied for 1 h were sinusoidal (2-cm amplitude, 98 oscillations min−1). Amounts of water-stable (>200 μm) aggregates were calculated by substracting coarse sand (1.7%) and root fragments remaining on the sieve. After 1 h of disaggregation, Na-Amberlite resin IR-120 (500 μm) was added to the soil suspension (<200 μm). To remove clay particles from microaggregates, the suspensions were agitated for 16 h with an end-over-end shaker at 40 rpm. Sand, silt, and clay particles were separated by sieving and sedimentation by using the Robinson pipette method (32).
Ultrathin sections 80 nm thick were prepared by the method of Villemin and Toutain (41). Agar cubes containing 1 mm3 of the soil fraction were dehydrated by progressive acetone exchange at 4°C, embedded in Epon 812 resin, and stained with lead citrate and uranyl acetate solutions. The narrower parts of the molded, impregnated aggregates were pyramidally shaped with a Reichert TM60 ultramill and finally cut with a diamond knife (OM U2; Reichert ultramicrotome). Ultrathin sections of RAS were covered with ultrathin carbon layers and examined in a Zeiss EM9 S2 transmission electron microscope.
A Carlo Erba series 2000 pore sizer mercury depression and intrusion porosimeter, linked to a Macropore Unit Series 120/IBM PC computer, was used to determine pore size distribution as a function of either pressure (ranging from 1.25 × 10−3 to 200 MPa) or pore radius (ranging from 3.75 × 105 to 6 × 105 nm). Air-dried millimetric soil aggregates, either rhizosphere soil (plus roots) or bulk soil, degassed at room temperature for 2 h, were tested. Calculations were done with a mercury surface tension of 0.485 N m−1 and a soil contact angle of 141.3° by using the Washburn equation, assuming cylindrical pores. The time for each step was 2 s. A programmable delay system hindered the pump sufficiently long for complete penetration of the pores by mercury, including the ink bottle pores. The relative error of pore volume measurements was 5 to 15%. In addition, to eliminate possible problems associated with damage to the structure of the adhering soil, measurements were made on aggregates attached to the root system. Pore volumes of roots were determined and subtracted from the pore volumes of the corresponding entire system of roots with adhering soil. Root pore volume was negligible.
Statistics.
Analyses of variance were performed by using the Statgraphics software program (version 5.0; STSC software products). The least-significant-difference test (Newman-Keuls test) was used for multiple-range analyses.
RESULTS
Origin, identification, and EPS production of strain NAS206.
Strain NAS206 was isolated from the root surface of wheat which was cultivated in the Settate vertisol studied. On sucrose-supplemented Weaver’s medium, strain NAS206 produced an EPS which was composed of galactose and glucose as monosaccharides and of succinate and pyruvate subunits (17a). Strain NAS206 was identified as P. dispersa by using Biolog microtiter plates, and as P. agglomerans by using the Biotype-100 system. The 13 restriction endonucleases tested revealed a strong polymorphism of the amplified rDNA restriction analysis profiles of NAS206, P. dispersa LMG2603T, P. agglomerans E20T, and R. aquatilis ATCC 33071T. Strain NAS206 was differentiated from R. aquatilis ATCC 33071T by 32 sites and from P. dispersa LMG2603T by 12 sites but from P. agglomerans E20T only 2 sites (obtained by using DdeI). This indicates that the species closest to strain NAS206 is P. agglomerans, which is in agreement with the phenotypic identification by Biotype-100. It was concluded that strain NAS206 belongs to the species P. agglomerans.
Soil hydric conditions.
Under each hydric condition tested, the water content of bulk soil in noninoculated and inoculated treatments was statistically the same: 35% ± 0.7%, 24% ± 0.9%, and 20% ± 0.6% for the nearly water-saturated, drained, and dry soil variants, respectively.
Soil, rhizosphere, and root colonization by P. agglomerans NAS206.
In the noninoculated treatment, after 20 days of wheat growth, the size of the spontaneous P. agglomerans population was about 103 CFU g of dm−1 in bulk soil and 105 CFU g of dm−1 on the root surface (rhizoplane), representing, respectively, less than 0.003 and 3% of the total microflora plate counts. The population size of P. agglomerans in bulk soil was not affected by inoculation of strain NAS206, whereas in the RAS fraction, strain NAS206 appeared to be strongly proliferating and represented about 50% of the total microflora in the dry variant, where its proliferation was most intense (Table 1). The average rhizosphere effect on the P. agglomerans population (number of bacteria in the rhizosphere/number of bacteria in the bulk soil) was 2,540 ± 685. To validate the counts of strain NAS206, 24 yellow and mucoid colonies were randomly selected and submitted to PCR analysis. They provided ERIC-PCR profiles identical to that of strain NAS206 (data not shown).
TABLE 1.
Effect of soil water content on mean densities and percentages of P. agglomerans NAS206 in RAS and bulk soil 20 days after inoculation
| Soil moisture (% dry wt), matric potential (MPa) | Mean log CFU/g of dm (% of total microflora) ± SDa
|
|
|---|---|---|
| Bulk soil | RAS | |
| 20, −0.55 | 4.5 ± 1.4* (0.15 ± 0.08)* | 8.0 ± 0.5* (50.5 ± 5.9)* |
| 24, −0.20 | 3.9 ± 0.9* (0.03 ± 0.02)† | 7.2 ± 0.4*† (25.4 ± 5.1)† |
| 35, −0.05 | 3.5 ± 0.6* (0.06 ± 0.04)*† | 6.8 ± 0.3† (25.1 ± 2.6)† |
Values with different symbols are significantly different at P < 0.05 by the Newman-Keuls multirange test.
Spatial root colonization by strain NAS206 was investigated at the basal, middle, and tip sections of the root system. The highest levels of colonization were observed on the first 5 cm of the root system (basal section), where strain NAS206 represented 80% of the total microflora plate counts (Table 2). At each soil water content tested, this percentage of strain NAS206 decreased from the basal region to the root tip. A negative effect of increasing soil water content on root colonization by strain NAS206 was found at the root tip. The final counts of strain NAS206 ranged from 8.0 ± 0.4 to 9.1 ± 0.4 log CFU g of root dm−1 (from root tip to root base) at a 20% average soil water content and from 6.4 ± 0.4 to 9.2 ± 0.3 log CFU g of root dm−1 at a 35% average soil water content. The percentage of strain NAS206 diminished ninefold in the root tip fraction but only less than twofold in the basal root fraction (Table 2).
TABLE 2.
Spatial resolution of colonization of wheat roots by P. agglomerans NAS206 at different soil water contents
| Soil moisture (% dry wt), matric potential (MPa) | Mean % of P. agglomerans NAS206 in total microflora ± SDa
|
|||
|---|---|---|---|---|
| Root | Basal section | Middle section | Tip section | |
| 20, −0.55 | 71.0 ± 9.2* | 80.7 ± 9.9* | 45.2 ± 16.1* | 44.1 ± 6.4* |
| 24, −0.20 | 44.1 ± 6.9*† | 63.2 ± 9.9* | 23.6 ± 4.7† | 15.7 ± 0.9† |
| 35, −0.05 | 34.4 ± 8.7† | 49.5 ± 11.6† | 8.6 ± 2.9‡ | 5.1 ± 0.6‡ |
Values with different symbols are significantly different at P < 0.05 by the Newman-Keuls multirange test.
Effects of soil water content and inoculation on plant growth.
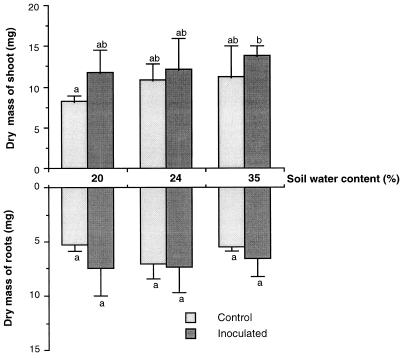
After 20 days of wheat growth, there was a slight positive effect (not significant) of increasing soil water content on wheat shoot production (Fig. 1) and on shoot/root ratios, which increased from 1.6 to 2.0. Root growth seemed to be inhibited at a soil water content of 35% (Fig. 1). For each soil water content tested, there was no significant effect of inoculation on plant growth parameters. However, there was a significantly positive overall effect of inoculation on shoot growth, as shown by variance analysis.
FIG. 1.
Effect of inoculation with P. agglomerans NAS206 on wheat at various levels of soil water content. Values with different letters are significantly different at P < 0.05 by the Newman-Keuls test. Error bars show standard errors of mean values.
Effect of soil water content and inoculation on rhizosphere soil aggregation.
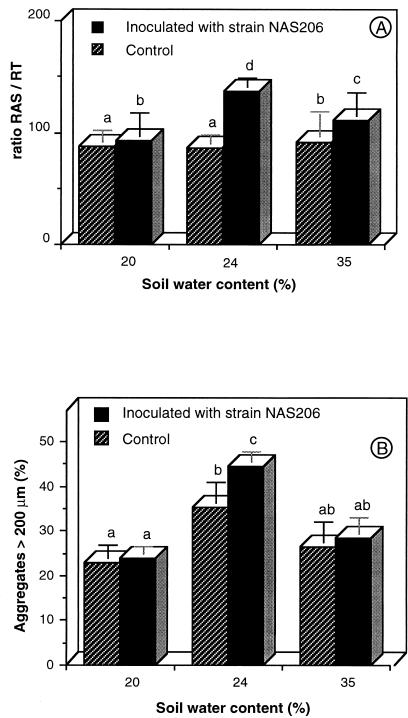
To evaluate the quantitative effect of inoculation and soil water content on soil aggregation, the biomasses of RAS and RT were measured for each wheat plantlet. In control treatments, the RAS/RT ratio was only increased at a soil water content of 35%, compared to a soil water content of 20% (Fig. 2A). There was a positive effect of inoculation on the RAS/RT ratio at each soil water content tested (Fig. 2A). At a 24% soil water content (matric potential of −0.20 MPa), 140 mg of soil was attached to 1 mg of root tissue in the inoculated treatment, compared to the control value of 90 mg of attached soil. Maximum water stability of rhizosphere aggregates was measured for the drained variant of both control and inoculated treatments (24% soil water content, Fig. 2B). Under these conditions, the effect of inoculation was significantly positive, and there was an insignificant trend toward more water-stable aggregates due to inoculation in the other two soil water regimens (Fig. 2B).
FIG. 2.
Cross effects of inoculation with P. agglomerans NAS206 and soil water content on the RAS/RT ratio (A) and the water stability of RAS (B). Values with different letters are significantly different at P < 0.05 by the Newman-Keuls test. Error bars show standard errors of mean values.
Effect of soil water content and inoculation on soil porosity.
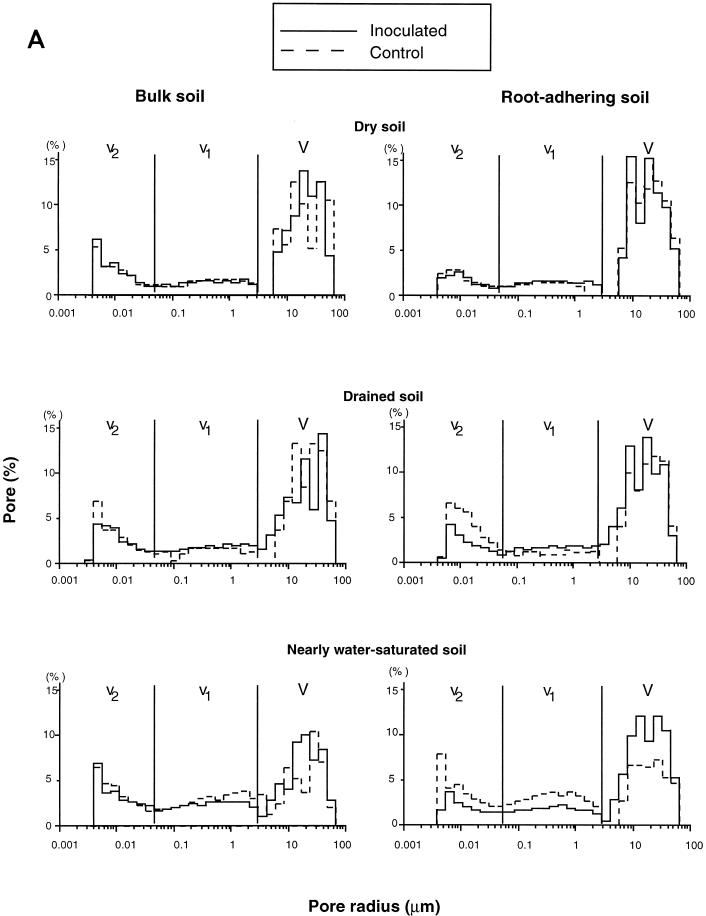
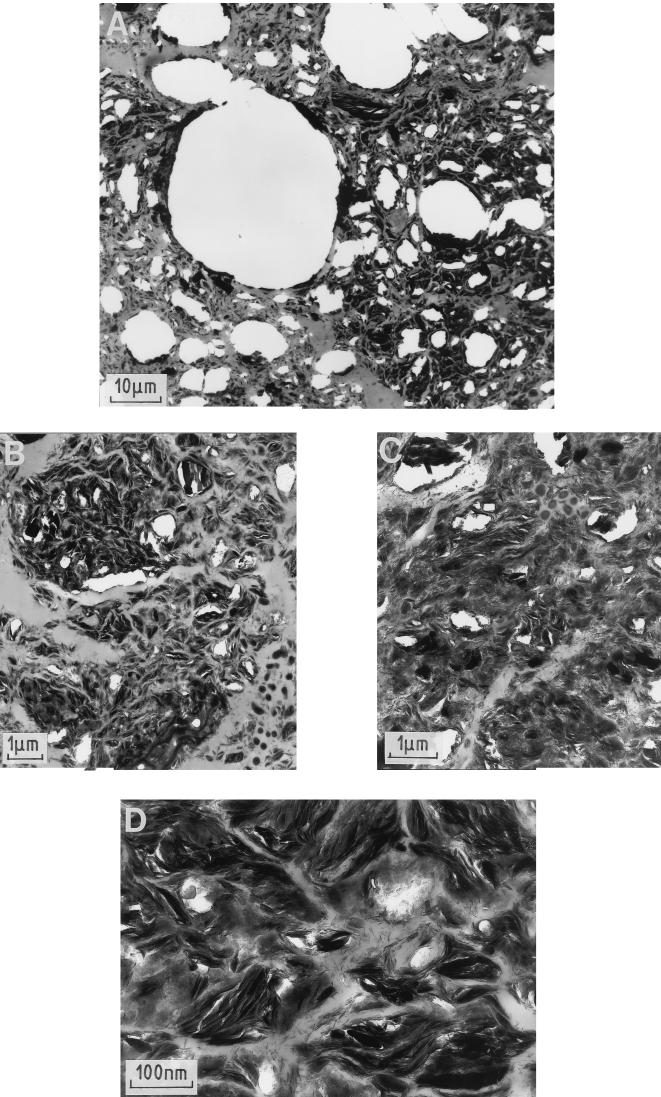
Pore and throat volume distribution curves were always trimodal, with a main mercury macropore volume (V) occurring at throat radii between 3 and 50 μm and two mostly minor mercury micropore volumes at throat radii between 0.05 and 3 μm (v1) and at throat radii of <0.04 μm (v2) (Fig. 3A). Such a distribution could possibly be attributed to pores located between silt-clay assemblages for V, i.e., with throat widths ranging between 5 and 20 μm, as measured on the whole set of pores which were observed by transmission electron microscopy (TEM) (Fig. 4A). Intermicroaggregate porosity (Fig. 4B and C) and clay domain porosity (Fig. 4D), respectively, corresponded to v1 and v2. For these <2-mm aggregates, this could be envisaged as filling of large pores, followed by filling of small pores of sizes similar to those of clay microcracks (clay fabric porosity).
FIG. 3.
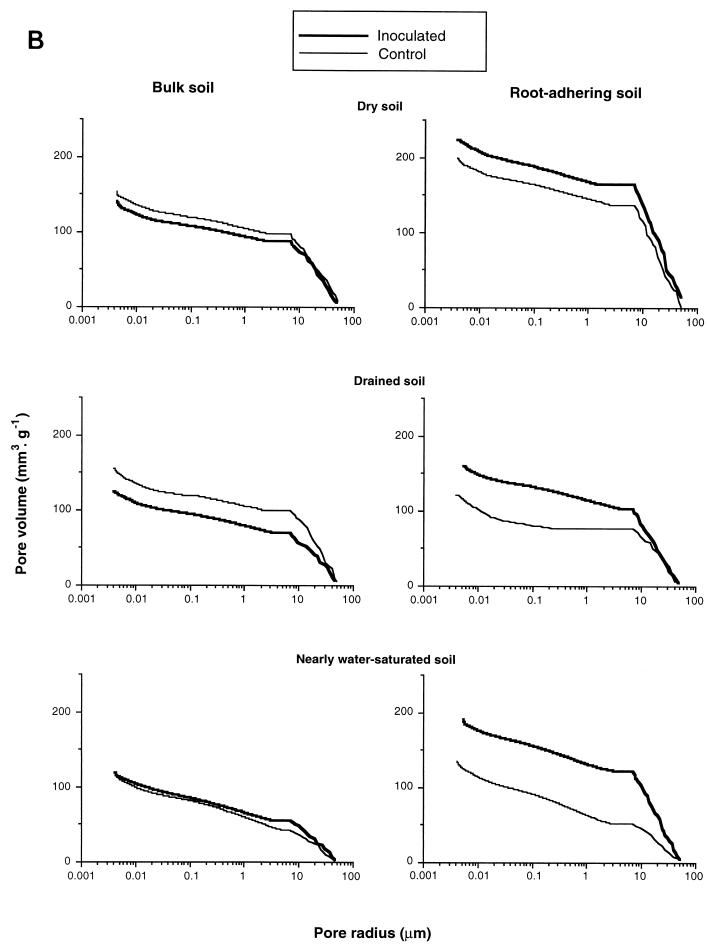
(A) Effect of bacterial inoculation and soil hydric treatment on the mercury pore volume of RAS and bulk soil aggregates. (B) Effect of inoculation with P. agglomerans NAS206 and soil hydric treatment on the cumulative mercury pore volume of RAS and bulk soil aggregates.
FIG. 4.
TEM of the wheat rhizosphere showing the ultrastructure of RAS at various magnifications. Panels: A, whole set of pores; B and C, intermicroaggregate porosity; D, clay domain porosity.
The V of either the bulk soil or the RAS aggregates of control treatments progressively increased from 35 to 20% average soil water content (−0.05- to −0.55-MPa average matric potential), whereas, correlatively, their two counterparts, v1 and v2, decreased (Fig. 3A and B). For each hydric condition tested, the V in RAS of the control treatment was rather similar to its bulk soil counterpart (Fig. 3B). However, there was an exception in dry soil with a much larger V in RAS than in bulk soil (Fig. 3B).
No effect of inoculation was evidenced in the mercury porosimetry (differential and cumulative) curves of bulk soil for each soil water content tested (Fig. 3A and B). In contrast, at both 24 and 35% soil water contents, a significant inoculation effect on the mercury porosimetry curves of RAS was found, i.e., an increase in V. However, there were only minor changes in v1 and v2 (Fig. 3B). The positive effect of inoculation with strain NAS206 on the macroporosity of RAS, compared to the control treatment, was more pronounced in nearly water-saturated soil than in drained or dry soil. The effect of inoculation was to limit the decrease in macroporosity as a function of soil water content, as observed in the control treatment (Fig. 3A). The RAS aggregates of inoculated treatments were also found to be more compact in the drained variant than in the dry and nearly water-saturated variants (Fig. 3). This fact could explain why both the RAS/RT ratio and the amount of water-stable RAS aggregates of inoculated treatments were higher for drained soil than for dry and nearly water-saturated soils (Fig. 2).
DISCUSSION
Wheat rhizosphere colonization by P. agglomerans NAS206.
Inoculation of wheat seeds with strain NAS206 led to pronounced colonization of the rhizoplane of wheat and of rhizosphere soil by this strain. Expressed as a percentage of the total microflora, colonization by this strain was significantly more intense in the basal root section than in the other root zones tested (Table 2). The ability of this strain to form cell aggregates (symplasmata) at the rhizoplane could explain its heterogeneous distribution along the wheat roots (data not shown). Passive transport by root elongation might also be a mechanism of bacterial wheat root colonization. In the absence of water movement, root elongation has been reported to be the major factor in bacterial colonization of wheat by Pseudomonas fluorescens and Bacillus subtilis (9, 18). The relatively low colonization of root tips by strain NAS206, particularly at high water content, may be attributed to the nature of root exudates. It has been shown that low-molecular-weight compounds are rapidly expelled from the root tip into the surrounding soil, explaining why bacterial population densities are always low at the root tip (25).
Strain NAS206 was able to colonize the wheat rhizophere densely at each soil water content tested. Under relatively dry conditions, the proportion of this EPS-producing bacterium in the total microflora was increased at the rhizoplane and in RAS (Tables 1 and 2). Roberson and Firestone (30) suggested that the increase in EPS production by Pseudomonas during desiccation is required to ensure protection of this strain in sandy soil. The high affinity of EPS for water provides protection for bacteria. An EPS matrix surrounding a bacterial colony may slow down the drying process, thereby increasing the time available for metabolic adjustment. The formation of symplasmata by P. agglomerans NAS206 in the wheat rhizosphere could also explain better root colonization under dry conditions (20% soil water content). Symplasmata, as reported by Achouak et al. (1) for Pantoea (formerly Enterobacter) agglomerans NO30, correspond to a structure made up by a large number of bacteria, tightly packed within a common extracellular sheath. On the other hand, the volume of water may influence the habitable pore space and, consequently, the survival of bacteria (11, 20, 27). Decreasing the water content of a sandy loam soil restricted substrate diffusion in pores for an Escherichia coli strain (28). In contrast, increasing the soil water content in a sandy loam soil induced inadequate oxygenation for Rhizobium leguminosarum (26).
Effect of soil water content and inoculation on rhizosphere soil aggregation.
The enhanced population size of inoculated strain NAS206 in the wheat rhizosphere was associated with an increase in the RAS/RT ratio (Fig. 2A). This is probably due to an aggregating effect of the EPS produced by the inoculated bacteria. EPS can increase soil-root adhesion and/or the mechanical stability of the rhizosphere soil, as previously demonstrated for clay minerals by polysaccharide adsorption (4).
On the other hand, RAS aggregates were relatively unstable in water (25 to 45% water-stable aggregates; Fig. 2B). This can be attributed mainly to clay swelling in water (smectites represented 45% of the soil tested). This strongly decreases soil cohesion and, therefore, both tensile strength (14) and aggregate stability (7).
Effect of soil water content and inoculation on soil porosity.
V, which corresponds to a rapid rise in the mercury injection curve, is interpreted as a connected percolation pore network (12, 37). Such percolation pore networks with neck radii of 5 to 20 μm were directly observed in thin sections studied by optical microscopy (results not shown). The V of either RAS or bulk aggregate soil of control treatments progressively increased from the nearly water-saturated soil to the dry soil, whereas v1 and v2 tended to decrease (Fig. 3). This is attributed to shrinkage and associated growth of microcrack networks (Fig. 4), as reported elsewhere (36). The air-drying and vacuum-drying methods applied for mercury porosimetry could induce a strong increase in these processes.
Apparently, there was also an impact of the inoculated strain on soil porosity. First, the absence of an inoculation effect on mercury porosimetry curves in the bulk soil fraction may be a consequence of the weak bacterial colonization of this fraction. In contrast, a significant bacterial effect was shown on the mercury porosimetry curves of RAS at both 24 and 35% average soil water contents, particularly as an increase in V (Fig. 3). The first possible underlying physical process could be aggregation (cementation) of clay microaggregates due to EPS production by the inoculated strain, probably located in intermicroaggregate pores (accessible pores of <6 μm), which leads to an increase in macroaggregates and V (10- to 60-μm pore radii), as a complement. The second potential mechanism could be an acceleration of the air-drying process. Microbial EPS were shown to increase both water retention at low matric potentials and water drainage from −0.01 to −10 MPa when they have been adsorbed to clay or sand. The effects seem to be less intense with smectites than with kaolinite and sand (5). This modification of water loss kinetics should lead to an increase in crack genesis. Further work is required to confirm that inoculated EPS-producing bacteria have effects within the vertisol-wheat rhizosphere on (i) clayey soil wettability, (ii) the clayey soil water retention curve by drainage, (iii) air-drying kinetics, and (iv) crack genesis. Direct physical measurements and image analysis of the rhizosphere soil microstructure should be applied.
In this study, bacteria have been inoculated to improve wheat growth, as mediated by changes in soil physical properties. For initial relatively wet soil conditions followed by a drying event, we could demonstrate that inoculation of the EPS-producing bacterium P. agglomerans NAS206 had a positive effect on rhizosphere soil aggregation and macroporosity and an overall positive effect on plant growth. Strain NAS206 was able to colonize the wheat rhizosphere and rhizoplane densely at each soil water content tested. Further work is in progress to confirm that this strain has a positive effect on the retention of water in rhizosphere soil. The ultimate goal is to use EPS-producing bacteria as inoculants in Mediterranean wheat fields to better regulate hydric stress and, therefore, improve wheat growth.
ACKNOWLEDGMENTS
We thank the following for valuable technical assistance: G. Villemin (CPB-CNRS Vandœuvre) for ultrathin soil sections and corresponding TEM micrographs and R. Philippy (CPB-CNRS Vandœuvre) for aggregate stability and water retention measurements. We also thank P. A. D. Grimont (Institut Pasteur, Paris, France) for strain identification, Y. M. Cabidoche (INRA, Guadeloupe, France), A. R. Dexter (Silsoe Research Institute), and M. Lebuhn (GSF, Neuherberg, Germany) for useful comments on the manuscript.
REFERENCES
- 1.Achouak W, Heulin T, Villemin G, Balandreau J. Root colonization by symplasmata-forming Enterobacter agglomerans. FEMS Microbiol Ecol. 1994;13:287–294. [Google Scholar]
- 2.Bartoli F, Burtin G, Herbillon A J. Disaggregation and clay dispersion of oxisols: Na resin, a recommended methodology. Geoderma. 1991;49:301–317. [Google Scholar]
- 3.Blum A, Johnson J W. Transfer of water from roots into dry soil and the effect on wheat water relations and growth. Plant Soil. 1992;145:141–149. [Google Scholar]
- 4.Chenu C, Guérif J. Mechanical strength of clay minerals as influenced by an adsorbed polysaccharide. Soil Sci Soc Am J. 1991;55:1076–1080. [Google Scholar]
- 5.Chenu C. Clay or sand polysaccharide associations as models for the interface between micro-organisms and soil: water related properties and microstructure. Geoderma. 1993;56:143–156. [Google Scholar]
- 6.Chenu C, Roberson E B. Diffusion of glucose in microbial extracellular polysaccharide as affected by water potential. Soil Biol Biochem. 1996;28:877–884. [Google Scholar]
- 7.Concaret J. Etude des mécanismes de la destruction des agrégats de terre au contact de solutions aqueuses. Ann Agron. 1967;18:99–144. [Google Scholar]
- 8.de Bruijn F J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergenic consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992;58:2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkstra A F, Scholten G H N, van Veen J A. Colonization of wheat seedling (Triticum aestivum) roots by Pseudomonas fluorescens and Bacillus subtilis. Biol Fertil Soils. 1987;4:41–46. [Google Scholar]
- 10.Frey P, Frey-Klett P, Garbaye J, Berge O, Heulin T. Metabolic and genotypic fingerprinting of fluorescent pseudomonads associated with the Douglas fir-Laccaria biocolor mycorrhizosphere. Appl Environ Microbiol. 1997;63:1852–1860. doi: 10.1128/aem.63.5.1852-1860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gammarck S M, Paterson E, Kemp J S, Cresser M G, Killham K. Factors affecting the movement of microorganisms in soils. Soil Biochem. 1992;7:263–305. [Google Scholar]
- 12.Gouyet J F. Physique et structures fractales. Paris, France: Masson; 1992. [Google Scholar]
- 13.Gouzou L, Burtin G, Philippy R, Bartoli F, Heulin T. Effect of inoculation with Bacillus polymyxa on soil aggregation in the wheat rhizosphere: preliminary examination. Geoderma. 1993;56:476–491. [Google Scholar]
- 14.Guérif J. Détermination de la résistance en traction des agrégats terreux: influence de la texture, de la matière organique et de la teneur en eau. Agronomie. 1988;8:379–386. [Google Scholar]
- 15.Haynes R J, Swift R S. Stability of soil aggregates in relation to organic constituents and soil water content. J Soil Sci. 1990;41:73–83. [Google Scholar]
- 16.Hebbar K P, Davey A G, Merrin J, Dart P J. Rhizobacteria of maize antagonistic to Fusarium moniliforme, a soil-borne fungal pathogen: colonization of rhizosphere and roots. Soil Biol Biochem. 1992;24:989–997. [Google Scholar]
- 17.Heulin T, Berge O, Mavingui P, Gouzou L, Hebbar P, Balandreau J. Bacillus polymyxa and Rahnella aquatilis, the dominant N2-fixing bacteria associated with wheat roots in French soils. Eur J Soil Biol. 1994;30:35–42. [Google Scholar]
- 17a.Heyraud, A. (Centre de Recherche sur les Macromolécules Végétales-Centre National de la Recherche Scientifique, Grenoble, France.) Personal communication.
- 18.Howie W, Cook R J. The effect of motility on wheat root colonization by fluorescent Pseudomonas suppressive antagonistic to take-all of wheat. Phytopathology. 1985;75:1344. [Google Scholar]
- 19.Hulton C S J, Higgins C F, Sharp P M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli and other enterobacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 20.Kilbertus G. Etude des microhabitats contenus dans les agrégats du sol, leur relation avec la biomasse bactérienne et la taille des procaryotes présents. Rev Ecol Biol Sol. 1980;17:543–557. [Google Scholar]
- 21.Laguerre G, Rigottier-Gois L, Lemanceau P. Fluorescent Pseudomonas species categorized by using polymerase chain reaction (PCR)/restriction fragment analysis of 16S rDNA. Mol Ecol. 1994;3:479–487. doi: 10.1111/j.1365-294x.1994.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 22.Lynch J M. The rhizophere. New York, N.Y: John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 23.Miller R M, Jastrow J D. Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol Biochem. 1990;22:579–584. [Google Scholar]
- 24.Morin A, Moresoli C, Rodrigue N, Dumont J, Racine M, Poitras E. Effect of carbon, nitrogen, and agitation on exopolysaccharide production by Enterobacter agglomerans grown on low-grade maple sap. Enzyme Microb Technol. 1992;15:500–507. [Google Scholar]
- 25.Newman E I, Waston A. Microbial abundance in the rhizosphere: a computer model. Plant Soil. 1977;48:17–56. [Google Scholar]
- 26.Postma J, van Veen J A, Walter S. Influence of different initial soil moisture contents on the distribution and population dynamics of introduced Rhizobium leguminosarum biovar trifolii. Soil Biol Biochem. 1989;21:437–442. [Google Scholar]
- 27.Postma J, van Veen J A. Habitable pore space and survival of Rhizobium leguminosarum biovar trifolii introduced into soil. Microb Ecol. 1990;19:149–161. doi: 10.1007/BF02012096. [DOI] [PubMed] [Google Scholar]
- 28.Rattray E A S, Prosser J I, Glover L A, Killham K. Matric potential in relation to survival and activity of a genetically modified microbial inoculum in soil. Soil Biol Biochem. 1992;24:421–425. [Google Scholar]
- 29.Reid J B, Goss M J. Effect of living roots of different plant species on the aggregate stability of two arable soils. J Soil Sci. 1981;32:521–541. [Google Scholar]
- 30.Roberson E B, Firestone M. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol. 1992;58:1284–1291. doi: 10.1128/aem.58.4.1284-1291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert M, Chenu C. Water potential, soil microhabitats, and microbial development. In: Hung P M, Berthelin J, Bollaag J-M, McGill W B, Page A L, editors. Environmental impact of soil component interactions. Vol. 1. New York, N.Y: Lewis Publishers; 1995. pp. 75–87. [Google Scholar]
- 32.Rouiller J, Burtin G, Souchier B. La dispersion des sols dans l’analyse granulométrique, méthode utilisant les résines échangeuses d’ions. Bull Ec Nat Super Agron Ind Aliment. 1972;XIV:193–205. [Google Scholar]
- 33.Rouiller J, Brethes A, Burtin G, Guillet B. Fractionnement des argiles par ultracentrifugation en continu: evolution des illites en milieu podzolique. Sci Geol Bull. 1984;37:319–331. [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Schneider M, de Bruijn F J. Rep-PCR mediated genomic fingerprinting of rhizobacteria and computer-assisted phylogenetic pattern analysis. World J Microbiol Biotechnol. 1996;12:163–174. doi: 10.1007/BF00364681. [DOI] [PubMed] [Google Scholar]
- 36.Tessier D, Beaumont A, Pedro G. Influence of clay mineralogy and rewetting rate on clay microstructure. In: Douglas L A, editor. Soil micromorphology. Amsterdam, The Netherlands: Elsevier Science Publishing Co.; 1990. pp. 115–121. [Google Scholar]
- 37.Thompson A H, Katz A J, Krohn C E. The microgeometry and transport properties of sedimentary rock. Adv Phys. 1987;36:625–694. [Google Scholar]
- 38.Tisdall J M, Oades J M. Stabilization of soil aggregates by the root systems of rye-grass. Aust J Soil Res. 1979;17:429–441. [Google Scholar]
- 39.Tisdall J M, Oades J M. The effects of crop rotation on aggregation in a red-brown earth. Aust J Soil Res. 1980;18:423–434. [Google Scholar]
- 40.Van Gestel M V, Merckx R, Vlassak K. Microbial biomass responses to soil drying and rewetting: the fate of fast- and slow-growing microorganisms in soils from different climates. Soil Biol Biochem. 1993;25:109–123. [Google Scholar]
- 41.Villemin G, Toutain F. Méthode de fixation d’échatillons organo-minéraux de sols pour la microscopie électronique à transmission. In: Fedoroff N, Bresson L M, Courty M A, editors. Micromorphology des sols. Paris, France: AFES-AISS Publications; 1987. pp. 43–48. [Google Scholar]
- 42.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]