Abstract
The light intensity-dependent transition to state 1 of dark-adapted anaerobic state 2 Chlamydomonas reinhardtii cells is stimulated by oxygen and by other electron acceptors for photosystem I, such as oxaloacetate and methylviologen. This suggests that the transition to state 1 requires the oxidation of the intersystem chain by photosystem I photochemistry. On the other hand, the mere oxidation in the dark of the chain—by addition of O2—leads only to a slow and incomplete transition. The light-driven stimulation by O2 of the state 1 transition is saturated at an O2 concentration of 15 to 20 μm, definitely higher than that of respiration. We suggest that this may represent the affinity for oxygen of the Mehler reaction, a conclusion that is confirmed by the observations that mitochondrial respiration is apparently not involved in modulating state 2-to-state 1 transition. The catalysis of the state 2-to-state 1 transition upon illumination of anaerobically adapted algae might represent, therefore, a relevant physiological role of this process in C. reinhardtii.
The phenomenon of state transitions in the photosynthetic apparatus, discovered by Bonaventura and Myers (1969; Murata, 1969), involves the reversible transfer of a fraction of the PSII outer antenna to PSI, and is understood as a mechanism for balancing the absorption cross-section size of both photosystems under natural illumination conditions and therefore their photochemical activities. The transition from state 1-to-state 2 decreases the PSII antenna to the advantage of that of PSI, and the opposite is true for the reverse process (see the reviews by Allen, 1992; Horton et al., 1996; Gal et al., 1997; Wollman, 2001). The mechanism of state transitions involves the phosphorylation of the light-harvesting chlorophyll a/b proteins of PSII (LHCII) by a membrane-bound protein kinase (state 1-to-state 2 transition) and its dephosphorylation by a phosphatase (state 2-to-state 1 transition). Following phosphorylation, a fraction of LHCII migrates to PSI by lateral diffusion in the thylakoids; the opposite process occurs after dephosphorylation. The kinase is activated by the over-reduction of the intersystem electron carriers (reviews by Allen, 1992; Horton et al., 1996; Gal et al., 1997; Wollman, 2001), and specifically by the binding of plastoquinol (PQH2) to its Qo binding site on the cytochrome b6f complex (Vener et al., 1997; Zito et al., 1999).
In higher plants, state transitions are light dependent, involve the transfer of 15% to 20% of the PSII antenna, and are effective in balancing the relative rates of the two photochemical reactions in series (see Allen, 1992). In the green alga Chlamydomonas reinhardtii, state 2 can be reached upon incubation for a few minutes in dark anaerobic conditions (Delepelaire and Wollman, 1985). During this process, a very large fraction of the PSII antenna is transferred to PSI (approximately 80%; Delosme et al., 1994, 1996). State 2-to-state 1 transition requires the reoxidation of the intersystem electron transport chain necessary to inactivate the LHCII kinase and to activate the phosphatase. This results in the dephosphorylation of the antenna chlorophyll protein complexes and their reassociation with PSI (Allen, 1992; Wollman, 2001). In Chlamydomonas, the state 2-to-state 1 transition is observed upon illumination of the dark anaerobic cells after a time lag of a few minutes, depending on the light intensity (Delepelaire and Wollman, 1985; Finazzi et al., 1999). During the lag, the cells are still in state 2 as judged by their Fv/Fm ratio, and no O2 evolution can be detected. On the other hand, the level of ATP rises very rapidly, in agreement with the operation of a rapid cyclic electron transport involving PSI and the cytochrome b6f complex in state 2 (Finazzi et al., 1999, 2002). It was previously reported that both the redox state of the chain and the level of ATP, or the ATP/ADP ratio, control the efficiency of the state 2-to-state 1 transitions in Chlamydomonas (Bulté et al., 1990). Both these events might take place during illumination of dark anaerobic cells during the lag before any net O2 evolution is observed.
To better understand the intimate mechanism of this process, we have performed a systematic analysis of different events and different factors involved in the state 2-to-state 1 transition. We report that O2 stimulates strongly the recovery of photosynthetic O2 evolution upon illumination of anaerobic state 2 cells. However, the transition to state 1 is slow and incomplete if O2 is added in the dark, as indicated by the changes in fluorescence parameters. Oxaloacetate (OAA), which oxidizes NADPH (accumulated in the dark anaerobic conditions; see Forti et al., 2003) through the action of the stromal NADP-specific malate dehydrogenase, accelerates the transition in the light. However, it has no effect in the dark, indicating that the activity of PSI is necessary for the oxidation of the intersystem chain.
We propose that the role of O2 in the modulation of state transitions occurs via its utilization in the Mehler reaction, rather than in chlororespiration or mitochondrial respiration.
RESULTS AND DISCUSSION
The Role of PSI Photochemistry in State 2-to-State 1 Transition
We have measured the rate of the state 2-to-state 1 transition as the time required for anaerobic Chlamydomonas cells adapted to state 2 in the dark to recover steady-state photosynthetic O2 evolution activity upon illumination. This time, which we call the lag (see Fig. 1), is dependent on light intensity at any particular concentration of chlorophyll (Finazzi et al., 1999). Photosynthesis was measured at a light intensity allowing 20% to 25% of the light-saturated rate. Under these conditions, the size of the PSII antenna limits photochemistry so that the changes in antenna size upon state transitions are readily detected.
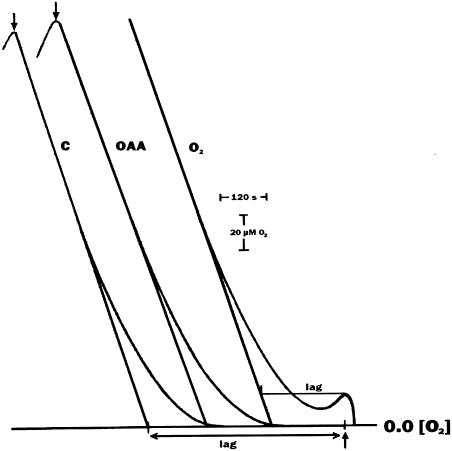
Figure 1.
Effect of O2 and OAA on the lag of O2 evolution upon starting low light intensity illumination of state 2, anaerobic C. reinhardtii cells. Light was turned on at the upward arrow and off at the downward arrow. O2 was added where indicated by the rising trace, upon injection of H2O2 to the catalase-containing oxygen electrode cell. OAA was 2 mm and added O2 was 20 μm. Chlorophyll was 150 μg mL−1 and light intensity was 54 μmol m−2 s−1 Other conditions as in Table I. C, Control. The lag time is defined as the intercept of the straight line drawn from the steady-state O2 evolution trace with the straight line on the time scale, starting at the beginning of illumination (see also Finazzi et al., 1999).
Figure 1 shows that the lag is reduced if O2 or OAA is added at the beginning of illumination. This indicates that the addition of an electron acceptor for PSI accelerates the transition to state 1 in the light. In the absence of any addition, the presence in the cells of endogenous electron acceptors for PSI explains the triggering of the transition, which then proceeds faster when some O2 produced by PSII photochemical activity becomes available. It can indeed be seen (Fig. 1) that the photosynthetic O2 evolution displays initially autocatalytic kinetics, in agreement with the idea that oxygen is the electron acceptor acting at the reducing side of PSI, as it is the product of the photochemical activity of PSII. The electron transfer to O2 on the reducing side of PSI is, by definition, the well-known Mehler reaction, which is therefore suggested by our observations to be involved in the transition from state 2 to state 1.
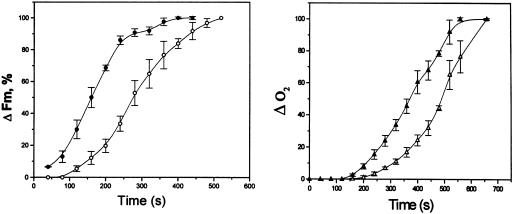
The initial kinetics of the state 2-to-state 1 transition were further studied by monitoring the increase of both O2 and Fm until steady-state photosynthesis was reached. The increase of Fm provides a complementary measure of the transition to state 1, as it monitors the increase of the antenna size of PSII. Both the Fm and O2 changes appeared to be autocatalytic (Fig. 2). As previously observed (Finazzi et al., 1999), the increase of Fm preceded the onset of O2 evolution. Both the O2 evolution and ΔFm were displaced to shorter illumination time when O2 was added at the beginning of illumination, in agreement with the idea that oxygen is required to initiate the state transition.
Figure 2.
Influence of O2 on the kinetics of Fm and O2 changes upon illumination of anaerobic cells in state 2. Chlorophyll was 50 μg mL−1, and light intensity was 24 μmol m−2 s−1. Other conditions and procedures as in Table I. Fluorescence in arbitrary units. White symbols, Control; black symbols, O2 added at the onset of illumination, at the concentration of 75 μm; triangles, O2 changes; circles, Fm increase. Light was turned on at time 0. The bars indicate se of six experiments. All the curves are perfectly fitted by sigmoids (data not shown).
We found (Table I) that the effects of O2 and OAA on the decrease of the lag (at low light intensity) were not significantly additive. This observation therefore suggests that O2 and OAA (which is indirectly an acceptor for PSI because it oxidizes NADPH) are electron acceptors from the same donor, i.e. the reducing side of PSI. The larger variation of the OAA effect (see Table I), as compared to that of the O2 effect, has not been further investigated: it may be due to the changes of endogenous OAA and/or other acceptors during the incubation of the cells in the minimal medium under agitation at low light intensity.
Table I.
Effect of O2 and OAA on the transition state 2-to-state 1 in C. reinhardtii, wild type
Chlamydomonas cells containing 150 μg of chlorophyll mL−1 were incubated at 25°C in the oxygen electrode cell in the minimal medium (see “Materials and Methods”) supplemented with 5 mm NaHCO3 and catalase. The cells were allowed to consume O2 in the dark until anaerobiosis was reached (several minutes were required, during which F0 and Fm were monitored by the PAM fluorimeter). After 5 min in the dark anaerobic condition, state 2 was established, as indicated by the stable fluorescence parameters. The initial (state 1) Fv/Fm ratio was 0.72 ± 0.0 (30 measurements, with several cell cultures), and 0.41 in the dark anaerobic condition (state 2). No influence of OAA on these parameters was ever observed. Light was then turned on and O2 was simultaneously generated (by catalase) as required upon the injection of H2O2. Light was of an intensity producing 20% to 25% of the maximal photosynthetic rate measured under light saturation. The lag time (see Fig. 1) in the control (no additions) is dependent upon light intensity at any particular chlorophyll concentration. Under the conditions indicated here (light intensity was 58 μE m−2 s−1), it was of 489 ± 86 s, and in each experiment the values observed in the treated samples were normalized to their control. Photosynthetic O2 evolutions at steady state were, respectively, 35.1, 35.4, 35 μmol mg chlorophyll h−1 in the control, with O2, OAA, or O2 + OAA added. The light-saturated rate was 146. All figures are averages ± se. The figures in parentheses indicate the number of experiments.
| Additions
| ||||
|---|---|---|---|---|
| None | O2 30 μm | OAA 2 mm | OAA + O2 | |
| Lag (s) | 100 | 64.9 ± 3.2 | 79 ± 9.8 | 61 ± 10 |
| (4) | (9) | (6) | ||
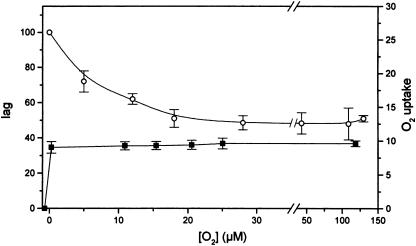
We measured the O2 requirement of the recovery of photosynthetic activity in terms of the concentration needed to saturate the process and compared it with the O2 dependence of respiration. Figure 3 shows that the stimulation of the recovery of photosynthetic activity (i.e. the state 2-to-state 1 transition) is saturated at 15 to 20 μm O2, while respiration is saturated at 3 μm or below, a value in agreement with the well-known high affinity of the mitochondrial cytochrome oxidase for oxygen (for review, see Yonetani, 1963). It seems, therefore, that the light-requiring reaction stimulated by O2 and active in the transition to state 1 must be different from the cytochrome aa3 mitochondrial respiration and is presumably the Mehler reaction. Our data therefore establish the affinity for O2 of this reaction, which is in the range of 15 to 20 μm for saturation.
Figure 3.
Influence of the concentration of O2 on the lag of O2 evolution and on respiratory O2 uptake. The cells contained 150 μg chlorophyll mL−1. The lag was measured as in Figure 1. The bars indicate the se of nine experiments. White circles, Lag; black squares, respiratory O2 uptake in μm mg−1 chlorophyll h−1. Conditions and procedures as in Table I.
The experiments reported so far strongly indicate that the photochemical activity of PSI is required for the state 2-to-state 1 transition of anaerobic cells, with either O2 or OAA as the electron acceptors at the reducing side of PSI. To rule out the possibility that the state transition could also be linked to some step of the photosynthetic process subsequent to the oxidation of the intersystem chain, we used methylviologen (MV) as an electron acceptor for PSI alternative to OAA or any other endogenous acceptor. MV is known to be a very efficient electron acceptor, and MVH2 is reoxidized by O2 in a very fast reaction that produces the radical 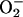 . This is then disproportionated by superoxide dismutase yielding H2O2 and O2. H2O2 is finally disproportionated by catalase, and the resulting O2 exchange of photosynthetic electron transport is zero when electron transport has reached the steady state. In the presence of MV, then, no O2 evolution can be measured, but rather light-dependent O2 uptake if the maximal activity of endogenous superoxide dismutase and catalase is not sufficient to disproportionate the
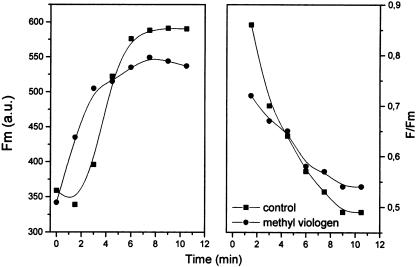
. This is then disproportionated by superoxide dismutase yielding H2O2 and O2. H2O2 is finally disproportionated by catalase, and the resulting O2 exchange of photosynthetic electron transport is zero when electron transport has reached the steady state. In the presence of MV, then, no O2 evolution can be measured, but rather light-dependent O2 uptake if the maximal activity of endogenous superoxide dismutase and catalase is not sufficient to disproportionate the 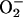 radical and H2O2 at the rate at which they are formed. The use of this reagent then provides a fast linear electron transport dependent on the activity of both photosystems, but prevents the subsequent photosynthetic metabolism. When no O2 evolution can be measured, as is the case in the presence of MV, the transition to state 1 upon illumination is best monitored by both the increase of PSII antenna (i.e. the rise of Fm) and the activation of the steady-state electron transport in the intersystem chain, seen as a decrease of F, the fluorescence actually measured at the low light intensity applied. When no oxidation of Qa− occurs, i.e. when the electron transport is blocked at this level and PSII is unable to inject electrons into the chain, as is the case in state 2 C. reinhardtii (Finazzi et al., 1999), F is equal to Fm. During illumination, F becomes progressively lower than Fm, because Qa− is being oxidized by the chain until a steady state of their ratio is attained (Fig. 4). As shown in Figure 4, the initial increase of Fm and decrease of F/Fm ratio is faster in the MV-treated sample than in the control. This is expected because MV is known to accelerate electron transport to O2, and this process is not limited by any subsequent physiological process, such as the activation of the Calvin cycle enzymes, nor does it require that the appropriate assimilatory power in terms of the ATP/NADPH ratio be reached. The steady-state F/Fm ratio finally attained is, however, similar in the presence or absence of MV, indicating that the steady-state rates of electron transport are comparable as is the extent of the antennae changes associated with the state transition (Fig. 4). The small decrease of Fm in the presence of MV might be due to the fact that PQ concentration is raised by the faster turnover of PSI PQ is known to be a Stern-Volmer quencher of chlorophyll fluorescence (Jennings et al., 1983).
radical and H2O2 at the rate at which they are formed. The use of this reagent then provides a fast linear electron transport dependent on the activity of both photosystems, but prevents the subsequent photosynthetic metabolism. When no O2 evolution can be measured, as is the case in the presence of MV, the transition to state 1 upon illumination is best monitored by both the increase of PSII antenna (i.e. the rise of Fm) and the activation of the steady-state electron transport in the intersystem chain, seen as a decrease of F, the fluorescence actually measured at the low light intensity applied. When no oxidation of Qa− occurs, i.e. when the electron transport is blocked at this level and PSII is unable to inject electrons into the chain, as is the case in state 2 C. reinhardtii (Finazzi et al., 1999), F is equal to Fm. During illumination, F becomes progressively lower than Fm, because Qa− is being oxidized by the chain until a steady state of their ratio is attained (Fig. 4). As shown in Figure 4, the initial increase of Fm and decrease of F/Fm ratio is faster in the MV-treated sample than in the control. This is expected because MV is known to accelerate electron transport to O2, and this process is not limited by any subsequent physiological process, such as the activation of the Calvin cycle enzymes, nor does it require that the appropriate assimilatory power in terms of the ATP/NADPH ratio be reached. The steady-state F/Fm ratio finally attained is, however, similar in the presence or absence of MV, indicating that the steady-state rates of electron transport are comparable as is the extent of the antennae changes associated with the state transition (Fig. 4). The small decrease of Fm in the presence of MV might be due to the fact that PQ concentration is raised by the faster turnover of PSI PQ is known to be a Stern-Volmer quencher of chlorophyll fluorescence (Jennings et al., 1983).
Figure 4.
Influence of MV on the state 2-to-state 1 transition. Chlorophyll was 50 μg mL−1 and light intensity was 24 μmol m−2 s−1. MV was 100 μm, added during the dark anaerobic treatment. Other conditions and procedures as in Table I. Fluorescence in arbitrary units. F indicates the fluorescence actually measured (see text), and Fm has the usual meaning of maximal fluorescence. The data are the average of three experiments, differing by less than 10%.
The Role of Respiration
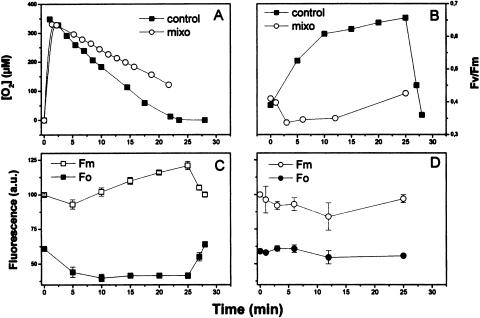
The addition of O2 in the dark produces a partial transition to state 1, although at a much slower rate (Fig. 5), while OAA is completely inactive in the absence of illumination, although it oxidizes NADPH (data not shown). The role of O2 per se, in the absence of light, was investigated measuring the state 2-to-state 1 transition as the increase of the Fv/Fm ratio. Oxygen was added in the dark to state 2 cells incubated 5 min in the usual dark anaerobic conditions (Fig. 5), in the absence (black symbols) or in the presence (white symbols) of mixothiazole (MX), a specific inhibitor of the mitochondrial cytochrome bc1 complex (Von Jagow and Link, 1986). In the control, Fv/Fm ratio increased in 6 min to the value of 0.5 from its state 2 value of 0.37, then in 20 min it reached the value of 0.62, well below the initial state 1 value of 0.75, thus indicating that a nonnegligible fraction of the chains was still in state 2 (Fig. 5B).The initial increase of Fv/Fm is in part due to the decrease of F0, which is faster than the rise of Fm in the control (Fig. 5C), as expected because the reoxidation of Qa− by O2 is certainly faster than the migration of the bulky chlorophyll protein complexes from the stromatic thylakoids to the grana. In the MX-treated samples, both Fm and F0 changes are very small (Fig. 5D). When respiration consumed all the available O2 (Fig. 5A), the Fv/Fm dropped again to the initial state 2 value (Fig. 5B).
Figure 5.
Effect of oxygen and MX on the transition state 2-to-state 1 measured as changes of fluorescence in the dark, and on respiration of C. reinhardtii. The cells (50 μg chlorophyll mL−1) were incubated 5 min in anaerobic conditions, then 350 μm O2 were added at time 0. The initial value of Fv/Fm (before anaerobiosis) was 0.75. A, Respiration. B, Fv/Fm. C, Fm and F0, control. D, Fm and F0, MX (abbreviated as mixo in the figure). The data reported are the average of three experiments, where the deviation was no more than 8%. The bars indicate se. MX, when added, was 12 μm. ATP concentration, at steady state in air, was of 90 ± 4 and 69.7 ± 5 nmol mg−1 chlorophyll (average of seven experiments ± se), respectively, in the control and MX-treated samples.
These observations show that the transition to state 1 upon oxygenation of the anaerobic cells in darkness was incomplete and much slower than that observed upon illumination (compare Figs. 1 and 2 with Fig. 5). In the presence of MX, respiration was inhibited by about 30% (Fig. 5A). The initial rate of state transition was inhibited approximately to the same extent, while the final value of Fv/Fm reached was only 0.44, indicating a very partial transition to state 1 and therefore the persistence of a large excess of the PSI antenna size over that of PSII. The simultaneous existence in the same cell, and therefore in the same chloroplast, of chains in state 2 and in state 1 has been reported previously (Forti et al., 2003).
MX decreased the concentration of cellular ATP in the dark (see legend, Fig. 5) by about 23%. This is easily explained because it inhibits mitochondrial respiration at the level of cytochrome bc1, so eliminating two of the three sites of ΔμH+ generation and leaving only the first site utilized by the alternative pathway respiration. The lower rate and the limited extent of the state 2-to-state 1 transition observed (Fig. 5) might therefore be explained on the basis of the lower ATP level, in agreement with Bulté et al. (1990). On the other hand, the inhibition of mitochondrial respiration would also increase the reducing power in the cytoplasm. The latter is known to be transferred into the chloroplast (Bennoun, 1994; for review, see Peltier and Cournac, 2002) so as to maintain the intersystem chain of the thylakoids reduced. This would prevent the inactivation of the LHCII kinase and the activation of the phosphatase, i.e. the enzymatic activities required for the state 2-to-state 1 transition.
The possible role of respiration on the light-driven state 2-to-state 1 transition was also investigated measuring the effects of respiratory inhibitors (MX and salycilhydroxamic acid [SHAM]) and mutations affecting the mitochondrial respiratory chain on fluorescence and O2 evolution parameters. MX, in spite of a large variation of the figures in different experiments, seemed to increase the lag of O2 evolution, although steady-state photosynthesis was also inhibited (Table II). These results therefore suggest that respiration plays a minor role, as compared to the Mehler reaction, in the state 2-to-state 1 transition in Chlamydomonas, both in the dark or upon illumination. The possible role of the alternative oxidase was also tested by measuring fluorescence and O2 evolution parameters in the wild type, using a mixture of MX and SHAM, or using the DUM-1 mutant (Matagne et al., 1989; Cardol et al., 2003), which is lacking cytochrome bc1, the site inhibited by MX in the wild type. In both cases, we found that the lag was unaffected by SHAM, in the absence or presence of added oxygen (data not shown), suggesting that the SHAM-inhibited alternative respiration is not involved in state 2-to-state 1 transition. This inhibitor, however, inhibits photosynthesis more severely than MX, at any light intensity. At a concentration that inhibits O2 uptake by 75%, steady-state O2 evolution was also inhibited by 63%. Therefore, the significance of the effect of inhibitors on the state transition measured as the activation of O2 evolution is rendered uncertain when they also affect steady-state photosynthesis. Indeed, it is difficult to assess whether a given change of Fv/Fm, of Fm, or of the lag of O2 evolution is the result of the state transition, i.e. of the change of antennae relative size or of changes of activity of other factors of photosynthesis such as the activity of Calvin cycle enzymes or the transport of CO2, or any other factor involved in photosynthetic activity.
Table II.
Effect of MX on the lag of O2 evolution
Conditions and procedures as in Figure 1.Chlorophyll was 150 μg mL−1 and MX was 20 μm. Data of five experiments with sd. Cellular ATP, normalized to the control in each experiment, was 0.71 ± 0.15 in the MX-treated, illuminated samples (average ± se of five experiments), and 0.70 ± 5 in the dark-treated samples. The ATP content was not affected by MX at high light intensity.
| Addition | O2 Evolution | Lag |
|---|---|---|
| s | ||
| None | 31.1 ± 0.5 | 456 ± 28 |
| MX | 26.0 ± 0.8 | 747 ± 110 |
The requirement for O2 as the acceptor at the reducing side of PSI was therefore investigated using a different approach, i.e. creating a competition for O2 between the Mehler reaction and an appropriate different process. The respiratory rate of Chlamydomonas is known to be strongly stimulated by acetate; in our conditions, we observed a stimulation of 3-fold (Table III). We therefore tested the effect of this metabolite on the transition to state 1 of anaerobic cells and found that, while acetate has no effect on the length of the lag, it decreases strongly the stimulatory effect of O2 (Table III). This would be expected on the basis of a competition for the externally added O2 between the Mehler reaction in the chloroplasts and the acetate-stimulated respiration. No competition would be expected for the O2 produced initially within the chloroplast, which is immediately available to the Mehler reaction, but has to diffuse out of this organelle to become available in the cytoplasm and in the mitochondria. The observations (Table III) are consistent with these expectations.
Table III.
Effect of acetate and O2 on the lag of O2 evolution
Chlorophyll, 150 μg mL−1; light, 77 μE m−2 s−1. Other conditions as in Table I. The figures for respiration and photosynthesis are in micromoles of O2 mg−1 chlorophyll h−1. Average of four experiments ± se. Acetate had no effect on the level of ATP in the cells. The figures for the lag are normalized to the one of their control.
| Control | Control + O240 μm | Acetate 2 mm | Acetate + O2 | |
|---|---|---|---|---|
| Lag, s | (100) | 54 ± 6.1 | 100 ± 5 | 78 ± 10 |
| Respiration | 6.5 ± 1.87 | 16.3 ± 2.4 | ||
| O2 evolution | 34.1 ± 1.5 | 31.9 ± 1.3 | ||
Chlororespiration
It has been reported that chlororespiration, discovered by Bennoun (1982, 1994), is an enzymatic activity linked to the thylakoids, which catalyzes the oxidation by O2 of the intersystem chain, and of PQH2 in particular, although with a low affinity for O2 (Bennoun, 1994). Although chlororespiration is a process that does not require light, its occurrence during illumination cannot be excluded, although it should be very unlikely because the oxidation of PQH2 by PSI occurs at a rate several orders of magnitude faster. It has been reported that the rate of this activity is at maximum only about 2% of that of photosynthetic electron transport (Cournac et al., 2002; Peltier and Cournac, 2002).To invoke its participation in the state 1 transition, chlororespiration should be able to oxidize the PQH2 pool; however, this seems unlikely because the pool would be kept in the reduced state by the stromal substrates at a rate faster than that of its reoxidation in the dark.
Nevertheless, we studied the possible effect of chlororespiration of the state 2-to-state 1 transition making use of the inhibitor propylgallate (Cournac et al., 2002; Peltier and Cournac, 2002), reported to inhibit this pathway of respiration. The results are shown in Table IV. Propylgallate seemed indeed to inhibit slightly state 2-to-state 1 transition, as shown by the barely significant prolongation of the lag observed in the presence of the inhibitor, but the results were again obscured by the fact that steady-state photosynthesis was significantly inhibited by propylgallate, at low as well as high light intensity. The inhibition of photosynthesis by propylgallate was continuous and progressive during illumination (data not shown). The Fv/Fm ratio was also decreased during illumination in the presence of the inhibitor. The decrease continued in the dark after illumination while respiration was unaffected.
Table IV.
Effect of propylgallate on state 2-to-state 1 transition
Conditions as in Table I. Respiration and photosynthesis are expressed in μmol of O2 mg chlorophyll h−1. Data from 10 experiments for the control, and five for the propylgallate treatments (±se). The lag has been normalized to the one of the control (−O2). Where added, O2 was 150 μm. Light intensity, 54 μE m−2 s−1.
| Additions
|
State 2
|
|||||
|---|---|---|---|---|---|---|
| Lag
|
State 1
|
|||||
| Fv/Fm | −O2 | +O2 | Fv/Fm | Respiration | Photosynthesis | |
| s | ||||||
| None | 0.38 ± 0.06 | 100 | 51 ± 7.9 | 0.74 ± 0.02 | 10.3 ± 0.98 | 37.9 ± 4.5 |
| Propylgallate 1 mm | 0.43 ± 0.04 | 110 ± 7.4 | 54 ± 4 | 0.70 ± 0.02 | 11 ± 0.85 | 33 ± 3.3 |
| Propylgallate 1.5 mm | 0.36 ± 0.05 | 128 ± 2 | 86 ± 12 | 0.67 ± 0.03 | 11 ± 1.1 | 30.3 ± 3.1 |
CONCLUSIONS
The generally accepted ideas (see Bulté et al., 1990; Allen, 1992; Horton et al., 1996; Wollman, 2001) hold that state 2-to-state 1 transition requires the oxidation of PQH2 to inactivate the LHCII kinase and to activate the protein phosphatase, and that these combined changes of enzyme activities result in the dephosphorylation of LHCII. Dephosphorylation is followed by the diffusion of this chlorophyll-protein complex from the antenna of PSI in the stroma lamellae to that of PSII in the granal ones, i.e. state 2-to-state 1 transition. It has also been reported that ATP (or a high ATP/ADP ratio) is required for state 2-to-state 1 transition in C. reinhardtii (Bulté et al., 1990).
Possible Role of the Mehler Reaction in the State 2-to-State 1 Transition
Our observations indicate that the photochemical activity of PSI is required for the oxidation of PQH2 leading to the state 2-to-state1 transition. The addition of electron acceptors for PSI, such as OAA, O2, and MV (in the presence of O2), enhances the rate of the transition. O2 is very efficient (Fig. 1; Tables I and III), but only upon illumination. Thus, we think that state 1 transition is activated by the Mehler reaction under physiological conditions, where O2 acts as an electron acceptor at the reducing side of PSI. When anaerobic cells in state 2 are illuminated in the absence of O2, the presence of any endogenous electron acceptor, such as OAA or any other metabolite capable of oxidizing NADPH, will generate low concentrations of the electron acceptor of PSI and NADP, and lead to oxidation of PQH2 and evolution of some O2 by PSII. Oxygen will then interrupt the cyclic electron flow (Finazzi et al., 1999), initiating the Mehler reaction, which promotes the transition to state 1. This mechanism implies that initially the process must have autocatalytic kinetics, which is indeed observed (see Figs. 1 and 2). Furthermore, when the rate of electron transfer to O2 at the reducing side of PSI is stimulated by MV, the transition to state 1 is also strongly stimulated, measured both as Fm rise and as linear electron transport across the complete chain, as shown in Figure 4.
On the other hand, the ATP requirement of the state 2-to-state 1 transition reported by Bulté et al. (1990) can be fully satisfied by cyclic phosphorylation, occurring at a high rate as soon as the light is turned on in state 2 cells, i.e. during the lag before any photosynthetic O2 evolution can be observed (Finazzi et al., 1999).
Possible Role of Respiration in the State 2-to-State 1 Transition
Mitochondrial respiration can oxidize stromal substrates capable of reducing PQ, because they can be transferred from the chloroplast to the cytosol, so leading indirectly to PQH2 oxidation (Bennoun, 1982, 1994). In principle, therefore, it could be involved in state 1 transition. However, our results seem to rule out a relevant role of mitochondrial respiration on the basis of several observations: (1) O2 concentration required to saturate the rate of the state transition in the light is substantially higher than that required to saturate respiration of C. reinhardtii (Fig. 3); (2) the rate and efficiency of the state 2-to-state 1 transition in the presence of O2 in the dark are much lower than in the light (compare Fig. 5 with Figs. 1, 2, and 4); and (3) the 3-fold stimulation of respiration by acetate has no influence on the rate of state 1 transition, but does prevent the stimulation by added oxygen, indicating a competition with the Mehler reaction (see Table III). This is interpreted as evidence that the latter utilizes the O2 produced within the chloroplast by PSII, and that extrachloroplastic respiration has little role in the state 1 transition.
Possible Role of Chlororespiration in the State 2-to-State 1 Transition
Chlororespiration (Bennoun, 1982, 1994) is not light dependent, and its involvement in the state 2-to-state 1 transition could only be suggested by our observation that the inhibitor propylgallate, which inhibits chlororespiration but not the mitochondrial terminal oxidases nor the Mehler reaction (Cournac et al., 2002; Peltier and Cournac, 2002), delays very slightly the transition to state 1, measured as the recovery of photosynthetic O2 evolution upon illumination of dark anaerobic cells in state 2. However, this inhibitor also inhibits progressively photosynthetic O2 evolution and affects PSII fluorescence, so the significance of the possible inhibition of state transition by this reagent is obscure. Furthermore, the affinity of chlororespiration for O2 has been shown to be very low (Bennoun, 1994), a fact that, in principle, would limit the possibility of its use for transition to state 1 under natural conditions of low O2 concentration. Furthermore, our observations of Figure 5 indicate that the transition is slow and very partial when the cells are oxygenated in the dark, even in the absence of any inhibitor. Thus, all the evidence available stands against any role of chlororespiration in state 2-to-state 1 transition.
MATERIALS AND METHODS
Strains and Culture Conditions
Chlamydomonas reinhardtii wild type (from strain 137 C) was provided by the Laboratoire de Physiologie Cellulaire et Moléculaire du Chloroplaste, at the Institut de Biologie Physico-Chimique (Paris). Cells were grown at 24°C in acetate-supplemented medium (Harris, 1989) under approximately 60 μE m−2 s−1 of continuous white light. They were harvested during exponential growth (approximately 2 × 106 cells mL−1) and resuspended in minimal medium at pH 7.2 (Harris, 1989).
Measurements of Oxygen Evolution, Uptake, and Fluorescence
Photosynthesis and respiration were measured as O2 exchanges in the presence of 5 mm NaHCO3 (unless otherwise indicated) using a Clark-type electrode (Radiometer, Copenhagen) in a home-made cell at 25°C. Illumination was provided by a halogen lamp, which was filtered through a heat filter. Light intensity was adjusted, as needed, by neutral density filters. Fluorescence emission was simultaneously measured in the same cell employed to measure oxygen exchanges using a pulse amplitude measure (PAM) chlorophyll fluorimeter (Walz, Effeltrich, Germany). For measurements, algae were resuspended at 50 to 150 μg chlorophyll mL−1, as indicated. Chlorophyll concentration was determined by measuring the absorbance of the cell culture at 680 nm in a spectrophotometer equipped with a scatter attachment, on the basis of a calibration curve constructed after extraction of the chlorophyll with 80% acetone (Finazzi et al., 1999).
Cells were placed in state 2 upon incubation in the dark, until O2 was completely consumed by respiration. After a few minutes of anaerobiosis, state 2 was obtained as indicated by the changes of the fluorescence parameters, which were continuously monitored.
Determination of ATP, ADP, NADP, and NADPH
An aliquot of the cell suspension used to measure oxygen exchanges and fluorescence emission was used to measure ATP and ADP in TCA extracts as previously described (Finazzi et al., 1999). Oxygen contents and fluorescence in the solution were monitored until the moment of quenching the cell sample.
Acknowledgments
We thank Dr. Giovanni Finazzi and Stefano Santabarbara for helpful discussion and suggestions and for reading the manuscript.
This work was supported by a contribution from the Italian Ministry for Universities and Research under the project FIRB RBAU01E3CX.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.048256.
References
- Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098: 275–335 [DOI] [PubMed] [Google Scholar]
- Bennoun P (1982) Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA 79: 4352–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P (1994) Chlororespiration revisited: mitochondrial-plastid interactions in Chlamydomonas reinhardtii. Biochim Biophys Acta 1186: 59–66 [Google Scholar]
- Bonaventura C, Myers J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta 189: 366–383 [DOI] [PubMed] [Google Scholar]
- Bulté L, Gans P, Rebéillé F, Wollman FA (1990) ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim Biophys Acta 1020: 72–80 [Google Scholar]
- Cardol P, Gloire G, Havaux M, Remacle C, Matagne R, Franck F (2003) Photosynthesis and state transitions in mitochondrial mutants of Chlamydomonas reinhardtii affected in respiration. Plant Physiol 133: 2010–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Latouche G, Cerovic Z, Redding K, Ravenel J, Peltier G (2002) In vivo interactions between photosynthesis, mitorespiration, and chlororespiration in Chlamydomonas reinhardtii. Plant Physiol 129(4): 1921–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P, Wollman FA (1985) Correlation between fluorescence and phosphorylation changes in thylakoid membranes of Chlamydomonas reinhardtii in vivo: a kinetic analysis. Biochim Biophys Acta 809: 277–283 [Google Scholar]
- Delosme R, Béal D, Joliot P (1994) Photoacoustic detection of flash-induced charge separation in photosynthetic systems. Spectral dependence of the quantum yield. Biochim Biophys Acta 1185: 56–64 [Google Scholar]
- Delosme R, Olive J, Wollman FA (1996) Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta 1273: 150–158 [Google Scholar]
- Finazzi G, Furia A, Barbagallo RP, Forti G (1999) State transitions, cyclic and linear electron transport and photophosphorylation in Chlamydomonas reinhardtii. Biochim Biophys Acta 1413: 117–129 [DOI] [PubMed] [Google Scholar]
- Finazzi G, Rappaport F, Furia A, Fleischmann M, Rochaix JD, Zito F, Forti G (2002) Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. EMBO Rep 3: 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti G, Furia A, Bombelli P, Finazzi G (2003) In vivo changes of the oxidation-reduction state of NADP and of the ATP/ADP cellular ratio linked to the photosynthetic activity in Chlamydomonas reinhardtii. Plant Physiol 132: 1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Zer H, Ohad I (1997) Redox-controlled thylakoid protein phosphorylation. News and views. Physiol Plant 100: 869–885 [Google Scholar]
- Harris E (1989) The Chlamydomonas Sourcebook. Academic Press, San Diego
- Horton P, Ruban AV, Walters AV (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 655–684 [DOI] [PubMed] [Google Scholar]
- Jennings RC, Garlaschi FM, Gerola PD (1983) A study on the lateral distribution of the plastoquinone pool with respect to photosystem II in stacked and unstacked spinach chloroplasts. Biochim Biophys Acta 722: 144–149 [Google Scholar]
- Matagne RF, Michel-Wolwertz M-R, Munaut C, Duyckaerts C, Sluse F (1989) Induction and characterisation of mitochondrial DNA mutants in Chlamydomonas reinhardtii. J Cell Biol 108: 1221–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172: 242–251 [DOI] [PubMed] [Google Scholar]
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53: 523–550 [DOI] [PubMed] [Google Scholar]
- Vener AV, van Kan PJ, Rich PR, Ohad II, Andersson B (1997) Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single-turnover flash. Proc Natl Acad Sci USA 94: 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Jagow G, Link TA (1986) Use of specific inhibitors on mitochondrial bc1 complex. Methods Enzymol 126: 253–271 [DOI] [PubMed] [Google Scholar]
- Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T (1963) The a-type cytochromes. In PD Boyer, H Lardy, K Myrback, eds, The Enzymes, Vol 8. Academic Press, New York and London, pp 41–79
- Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman FA (1999) The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J 18: 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]