Supplemental Digital Content is Available in the Text.
Real-world evidence suggests that higher educational level and employment are associated with a better outcome in patients treated with spinal cord stimulation.
Keywords: Neuromodulation, Administrative register, Chronic pain, Effectiveness, Education, Real-world evidence
Abstract
Introduction:
Despite advancements in implanted hardware and development of novel stimulation paradigms in Spinal Cord Stimulation (SCS), real world evidence suggests a large variation in patient reported outcomes and a proportion of patients are later explanted due to loss of analgesia. Possible predictors for outcome have been explored in smaller short-term evaluations, but few clinically applicable robust measures for long term outcome have emerged.
Methods:
We performed a comprehensive retrospective study based on an assembled patient-level aggregated database from multiple local and national registries in Sweden. Variables associated with risk of explantation (due to insufficient analgesia) and analgesic effect was analyzed using a Cox regression analysis and an ordered logit regression model, respectively.
Results:
We found the accumulated risk of explantation due to loss of analgesia to be 10% and 21% at two and ten years follow up, respectively. The use of 10 kHz spinal cord stimulation (compared with Tonic waveform; p = 0.003), and being 60 years or older (reference 18-40 years; p = 0.003) were associated with an increased risk of explantation.
At a mean follow up at 1 year, 48% of patients reported a pain intensity reduction from baseline of at least 30%. Secondary (p = 0.030) and post-secondary (p = 0.001) education (compared with primary education) was associated with an increased probability of successful patient reported outcomes.
Conclusion:
This study suggests that a higher educational level and being employed are associated with successful treatment outcome in patients with chronic pain treated with SCS in Sweden.
1. Introduction
Pain is the most commonly perceived symptom in surveyed adult populations and the primary reason for seeking medical attention in Europe and in the United States of America.31,46 Chronic pain is defined by the International Association for the Study of Pain (IASP) as a pain condition lasting or recurring for more than 3 to 6 months,35 and several chronic pain conditions are among the diagnoses resulting in the most years lived with disability.13
“Real-world data” (RWD) and “real-world evidence” (RWE) are terms increasingly used and discussed in the medical literature, referring to analysis of data collected in routine health care.3 Although analyses of complex unstructured linked data have limitations, they may offer unique possibilities over traditional trials. First, RWD may further clarify treatment outcome in populations or time frames beyond those assessed in most investigational studies. Moreover, RWD could help in the design of future clinical trials, as well as monitor dissemination, effectiveness, and safety of novel technologies and treatments.
Implanted neurostimulation devices, such as spinal cord stimulation (SCS) systems, have been used for decades to alleviate pain from refractory primarily neuropathic pain states.48 The most well-established indication is chronic back and leg pain following spine surgery, known as persistent spinal pain syndrome (PSPS) and formerly as failed back surgery syndrome (FBSS).12 Seminal randomized clinical trials have documented the efficacy of SCS on this indication for both traditional tonic stimulation patterns28,38 and the more novel higher-frequency subsensory stimulation patterns, such as burst stimulation15 and 10 kHz stimulation.24 However, because of their nature, such trials frequently include a selected patient group and a limited follow-up time. Publications on RWD with longer follow-up indicate that exit of therapy is common, and patient benefit varies among individuals over time.22,30,49,56 Several studies have tried to appraise the frequency and cause of explantations and found loss of analgesic effect to be the most common cause for exit from therapy.9,23,40 A number of studies have investigated the association between psychometric variables and SCS treatment outcome, sometimes with conflicting results.41,44,52 Considering the heterogenous RWE for invasive neurostimulation treatments, there is a need for evidence beyond expert opinion to better guide patient selection and inform design of future clinical trials.
To investigate factors associated with SCS outcome and to better understand the population treated with implanted neurostimulation devices, we conducted a study on RWD available in Sweden. The overarching aim of this project was to identify the association of key clinical, patient-reported, and economic outcomes of SCS treatment and potential predictive factors for each outcome. The health economic assessment of this project is published elsewhere.50
The specific study objectives for this publication were (1) to analyze the rate of explantation of SCS systems because of insufficient analgesic effect, (2) to identify possible predictors for explantation of SCS systems because of insufficient analgesic effect, (3) to assess patient-reported analgesic and global effect of therapy, and (4) to identify possible predictors of efficacy in patients treated with SCS.
2. Methods
This is an observational, retrospective, cohort study on patients treated with SCS for chronic pain in Sweden. This study was conducted on data from an extensive research database designed and assembled to manage linked pseudoanonymized data from local and national quality registries and administrative registries.
2.1. Data sources and data collection
Data were collected from 8 Swedish local or national registries. Table 1 provides an overview of data sources.
Table 1.
Overview of data sources.
| Name of register | Type of data | National/Local | Holder of register |
|---|---|---|---|
| RAY | Indications, outcome, and therapy-specific data on invasive neurostimulation treatments | Local | Uppsala University Hospital |
| The national patient register (NPR) | Diagnoses and procedures in inpatient and outpatient care in Sweden | National | The Swedish Board of Health and Welfare |
| The register of the total population (RTP) | Demographic and socioeconomic data | National | Statistics Sweden |
| The cause of death register | Cause of all deaths | National | The Swedish Board of Health and Welfare |
| Prescribed drug register (PDR) | Prescribed pharmaceutical products | National | The Swedish Board of Health and Welfare |
| The LISA Register | Labor market and educational level data | National | Statistics Sweden |
| Data from the Swedish Social insurance Agency | Work status and sick leave | National | The Swedish Social insurance Agency |
| SWESPINE | Outcome and procedural data on spine surgery | National | The Swedish Association of Spine surgeons |
See supplemental data, available at http://links.lww.com/PR9/A210 for a further description of individual data sources.
LISA, national register on labor market and educational level data; RAY, a local registry on invasive neurostimulation treatments for pain; SWESPINE, the national register on spine surgery.
RAY is a local cohort registry of prospectively collected outcome and procedural data on consenting patients implanted with a permanent neurostimulation system at the Multidisciplinary Pain Centre at Uppsala University Hospital, Sweden. All patients included had completed a successful 7-day or 14-day trial of stimulation before permanent implantation. Data on adverse events, reoperations, and explantations were entered into the database prospectively during the study. Baseline Patient-Reported Outcome Measures (PROMs), including Brief Pain Inventory (BPI)53 and a 11-graded numerical rating scale (NRS) for pain intensity, were collected before implantation of a permanent stimulation device. Follow-up PROMs, including a 6-level categorical global assessment of effect of stimulation (EoS), were collected annually at a fixed time point (in January) for up to 5 years by questionnaires mailed to study participants. Returned data were transferred from paper to a structured query language (SQL) database. Before the end of data collection and data lock, procedural data were verified against medical records and PROMs against questionnaires to assure completeness of data.
From the National Patient Register, a second SCS population covering all implants in Sweden was identified, based on surgical procedural codes according to the NOMESCO classification. From this register, the Elixhauser comorbidity index, indicating the degree of comorbidities in an individual, was calculated based on diagnostic codes for admissions and outpatient visits.19 Detailed patient-level demographic data were collected from the Register of the Total Population. Data from the Cause of Death Register, comprising all deaths in Sweden were added to account for deaths in the study population. Information on medication was supplied by the Prescribed Drug Register, which covers all pharmaceutical products dispensed at any pharmacy in Sweden since 2005. Data were extracted as defined daily doses (DDD), which is the assumed average maintenance dose for a certain drug on its main indication in adults.57 Further data on education, income, work status, and sick leave were extracted from the Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA) and the Swedish Social Insurance Agency, covering all individuals who work and live in Sweden. Data from SWESPINE, the National Swedish Spine Register, containing data on 95% of all back surgery performed in Sweden were added.
The research unit at Statistics Sweden, a governmental agency, collected data sets from all sources and linked data on a patient level using the unique social security number of individuals, a variable present in all registers in the study. The final data set was then pseudoanonymized and made available to the study group.
The study period was defined as the start of data availability to the end of data availability (January 1, 2000 to December 31, 2018). The index date was defined as the date of implantation of a permanent SCS system. Follow-up period was from the index date until the occurrence of an outcome event that ends the follow-up, death, or end of data availability (December 31, 2018). For further details and specifics on data sources, see appendix (available at http://links.lww.com/PR9/A210).
2.2. Study population
Variables relevant for the objectives in this study were limited to data from RAY, thus the study population was defined by patients in RAY (cohort 1). For descriptive purposes, 3 reference cohorts were identified: cohort 2 was identified from the National Patient Register, using procedural codes to identify patients implanted with an SCS system. Thus, all patients in cohort 1 exist in cohort 2. Cohort 3 was identified using SWESPINE, detailing baseline characteristics of patients scheduled for spine surgery. In addition, a cohort of matched healthy controls (cohort 4) was identified in the Register of the Total Population. Controls were matched in a 5:1 ratio to cohort 1 using exact matching without replacement. Cases and controls were matched based on age in years, sex, and region of residence. Figure 1 provides details on how the final study population was defined.
Figure 1.
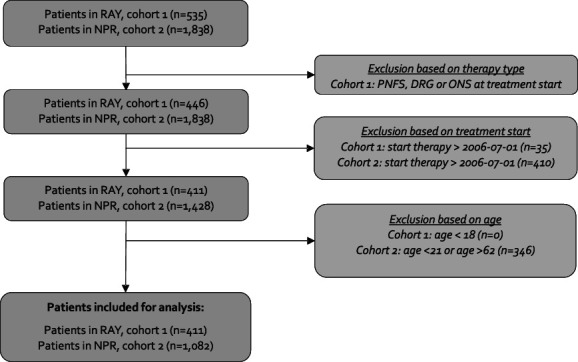
Patient attrition from raw data to study population (cohort 1) and reference (cohort 2). DRG, dorsal root ganglion stimulation; NPR, National Patient Registry in Sweden; ONS, occipital nerve stimulation; PNFS, peripheral nerve field stimulation; RAY, a local registry on invasive neurostimulation treatments for pain.
2.3. Outcome measures
The risk of explantation because of insufficient analgesic effect was measured as the cumulative probability of explantation. To assess the patient global effect of treatment, the variable effect of stimulation (EoS) was used. This is a 6-level categorical patient-reported outcome measurement used at follow-up in RAY as a response to the question “What is the effect of the stimulation?” without any specified recall period. The response possibilities given were as follows: freedom from pain/considerable pain relief/acceptable pain relief/some pain relief/no pain relief/worsened pain. Change in reported pain intensity was measured using the 11-point NRS for global pain (NRS) or BPI item 5 at baseline and follow-up. Pain interference was measured using BPI.
2.4. Statistical methods
All statistical tests are based on two-sided P values. A P-value threshold of 0.05 was used for statistical significance. Data management and statistical analyses were performed using MySQL (Uppsala, Sweden) and Stata16 (StataCorp LLC, College Station, TX).
2.4.1. Objective 1: analysis of risk of explantation because of insufficient analgesic effect
Risk of explantation was analyzed from index date, and continuously over time, until death or end of data availability, using a time-to-event analysis with the first explantation as the failure event. Explantation was a binary variable measured during follow-up for every patient in the RAY register (cohort 1). Patients were censored at death, at the end of data availability, or at replacement of SCS with another neuromodulation therapy (eg, dorsal root ganglion stimulation). No censoring was done for implantable pulse generator (IPG) replacements due to depleted battery. The model used is a single failure model, and thus, multiple explantations for an individual patient will not be accounted for.
2.4.2. Objective 2: analysis of potential risk factors for explantation
The strength of the association of the potential predictors was analyzed using Cox proportional hazard regression. Time-to-event data were presented and visualized by Kaplan–Meier curves.
2.4.3. Objective 3: patient-reported analgesic and global effect
In RAY, depending on the time of patient inclusion, 2 different 11-point NRS scales of pain intensity were used at baseline and follow-up: BPI or NRS for global pain. We hypothesized that the difference from baseline to follow-up could be merged into one outcome variable for pain intensity. A t test for equal means of change in BPI and NRS revealed no significant difference and supported the merger into one outcome variable (see appendix for full analysis, available at http://links.lww.com/PR9/A210).
The EoS levels were categorized into successful or unsuccessful outcome based on the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations on interpreting the clinical importance of treatment outcomes in clinical pain trials, proposing that a clinically meaningful change in pain intensity measures should be >30% decrease in the reported pain intensity from baseline.17 The following EoS levels were thus categorized as successful outcome: freedom of pain, considerable pain relief, and acceptable pain relief; the rest of the EoS levels were categorized as unsuccessful outcome. The EoS levels “no pain relief” and “worsened pain” were merged together due to insufficient number of observations.
Missing data in PROMs increased by time from baseline. Thus, the time point for follow-up was the first available follow-up data after baseline. Mean follow-up time was 384 and 217 days for pain intensity and pain interference, respectively.
2.4.4. Objective 4: potential predictors of analgesic and global effect of therapy
A logistic regression using a binary transformation of EoS as the dependent variable was conducted. The strength of association of potential predictors and EoS levels were analyzed by an ordered logit regression model.
2.5. Ethics
This research project was vetted and approved by the Regional Ethical Board in Stockholm, project number ID 2017/5:3.
3. Results
3.1. Description of study population
Table 2 describes the study population (cohort 1) and 3 reference populations (cohort 2–4) available in the study database. In general, patients included in the RAY cohort (cohort 1) and patients identified in the National Patient Register (cohort 2) were similar in sociodemographic variables and comorbidities. On average, the healthy controls had a higher educational level and employment rates compared with the study population. Comorbidities were on average less frequent in the control group compared with the treatment groups (mean 0.3 vs 1.0 in cohort 1 and 0.8 in cohort 2).
Table 2.
Demographics, comorbidities, and pharmacotherapy in the study and reference populations.
| Study population (n = 411) Cohort 1 |
All SCS implants (n = 1082) Cohort 2 |
SWESPINE (n = 83,786) Cohort 3 |
Healthy controls (n = 2055) Cohort 4 |
Data source(s) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean or percent | SE or count | Mean or percent | SE or count | Mean or percent | SE or count | Mean or percent | SE or count | ||
| Age at index (y) | 52.3 | 0.6 | 47.1 | 0.3 | 57.7 | 16.1 | 52.3 | 0.2 | NPR |
| Follow-up time (y) | 4.7 | 0.7 | 4.4 | 0.4 | 5.1 | 2.9 | N/A | N/A | RAY & SWESPINE |
| Sex | NPR & RTP | ||||||||
| Male | 45% | 186 | 44% | 480 | 49% | 40,738 | 45% | 925 | |
| Female | 55% | 225 | 56% | 602 | 52% | 43,048 | 55% | 1130 | |
| Income (000, €) | 21.1 | 0.6 | 21.6 | 0.6 | 24.8 | 0.2 | 29.4 | 1.2 | LISA |
| Birth country | LISA | ||||||||
| Sweden | 87% | 356 | 86% | 931 | 85% | 71,428 | 81% | 1665 | |
| Europe, except Sweden | 7% | 31 | 9% | 99 | 10% | 8,172 | 8% | 164 | |
| Other | 6% | 24 | 5% | 52 | 5% | 4,186 | 11% | 226 | |
| Education level | LISA | ||||||||
| Primary education | 21% | 85 | 19% | 201 | 23% | 19,538 | 13% | 267 | |
| Secondary education | 55% | 227 | 58% | 629 | 48% | 39,911 | 43% | 884 | |
| Post-secondary/postgraduate education | 22% | 91 | 22% | 242 | 28% | 4,186 | 44% | 904 | |
| Employment status | LISA | ||||||||
| Employed | 42% | 174 | 47% | 508 | 53% | 44,311 | 70% | 1438 | |
| Not employed | 58% | 237 | 53% | 574 | 47% | 39,475 | 30% | 617 | |
| Elixhauser comorbidity index | 1 | 0.1 | 0.8 | 0 | 0.9 | 1.4 | 0.3 | 0.1 | NPR |
| Prior spine surgery | 47% | 191 | 40% | 443 | N/A | N/A | <1% | N/A | SWESPINE & NPR |
| Pharmacotherapy | PDR | ||||||||
| Nonopioid pain medication usage* | 51% | 211 | 45% | 484 | 47% | 39,077 | 5% | 102 | |
| Antidepressant medication usage* | 42% | 173 | 46% | 494 | 17% | 13,972 | 9% | 185 | |
| Any opioid usage* | 60% | 245 | 55% | 594 | 45% | 37,788 | 4% | 82 | |
| Strong opioid usage* | 27% | 110 | 27% | 288 | 19% | 15,695 | 1% | 13 | |
| Weak opioid usage | 40% | 164 | 36% | 394 | 35% | 29,091 | 3% | 66 | |
At least 1 drug dispensation in the prior 3 months before index date.
LISA, national register on labor market and educational level data; NPR, the national patient register; PDR, the prescribed drug register; RAY, a local therapy specific register on invasive neurostimulation; RTP, the register of the total population; SE, standard error; SWESPINE, the national register on spine surgery.
The average use of opioids, nonopioid pain medications, and antidepressant medications were substantially higher in the treatment groups compared with healthy controls and patients scheduled for spine surgery. Drug usage (opioids, nonopioids, and depression) was fairly similar in cohort 1 and cohort 2.
Patients treated with SCS reported a mean pain duration of 9.3 years before implantation of a permanent system. In 50% of patients, chronic back and leg pain was the main indication for SCS therapy. Conventional tonic simulation was the most commonly used waveform, followed by burst stimulation and 10 kHz stimulation. Nonrechargeable batteries were more commonly used than rechargeable ones. Table 3 provides further details.
Table 3.
Treatment indications, implanted hardware and waveforms in cohort 1 (N = 411).
| Mean or percent | SE or count | |
|---|---|---|
| Mean pain duration in y before implantation | 9.3 | 0.4 |
| Main indications for spinal cord stimulation | ||
| Persistent spinal pain syndrome, type 1 or 2 | 50% | 207 |
| Persistent postsurgical pain | 11% | 47 |
| Neuropathic pain in extremity | 7% | 30 |
| Primary waveform | ||
| Tonic waveform | 55% | 225 |
| Burst waveform | 28% | 114 |
| 10-kHz waveform | 18% | 72 |
| Impulse generator type | ||
| Rechargeable | 26% | 107 |
| Nonrechargeable | 74% | 304 |
| Lead type | ||
| Percutaneous lead | 95% | 392 |
| Surgical lead | 5% | 19 |
Burst, a 5-pulse train paresthesia-free waveform with internal frequency of 500 Hz delivered at 40 Hz using a passive recharge pattern. 10 kHz, a paresthesia-free, 10-kHz, continuous, spinal cord stimulation waveform. Tonic, refers to a 30- to 80-Hz continuous spinal cord stimulation waveform producing paresthesia.
SE, standard error.
3.2. Risk of explantation because of insufficient analgesic effect (objective 1)
The cumulative risk of explantation of SCS system because of insufficient analgesic effect was found to be 9.8%, 15.8%, and 21.3% at 2, 5, and 10 years, respectively. Figure 2 provides a visual representation of the time to explantation (Kaplan–Meier plot).
Figure 2.
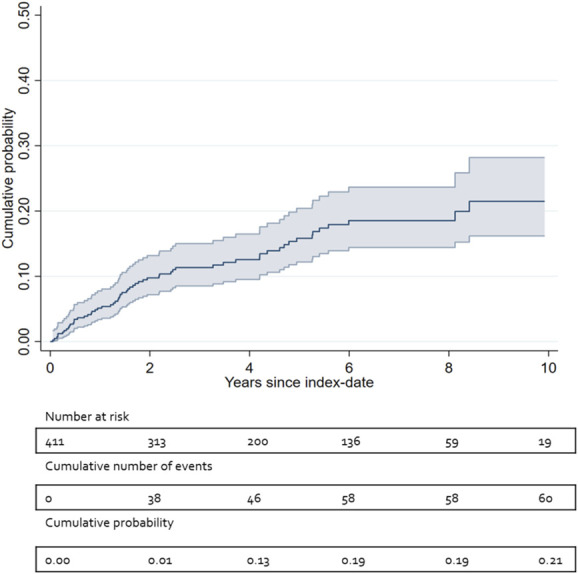
Kaplan–Meier curve: cumulative number of events and probability of explantation because of insufficient analgesic effect, with 95% confidence intervals.
3.3. Predictors for explantation because of insufficient analgesia (objective 2)
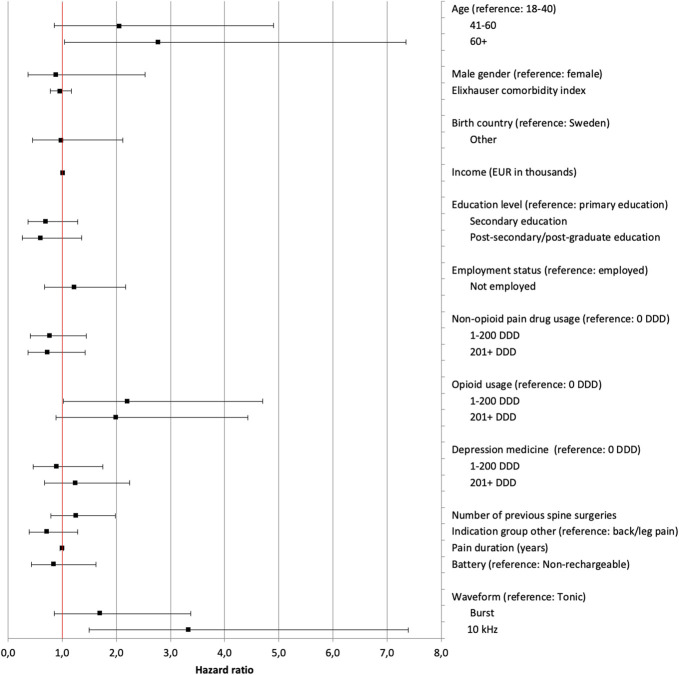
Higher age was associated with higher risk of ExIA (41–60 year old; P = 0.108 and 60+ years old; P = 0.041). Higher opioid consumption was associated with a higher risk of ExIA (1–200 DDD; P = 0.044 and 200+ DDD; P = 0.095). A 10 kHz stimulation was significantly associated with a higher risk of ExIA (P = 0.003). Having a postsecondary/postgraduate education was associated, although insignificantly (P = 0.213), with a lower risk of explantation because of insufficient analgesic effect (ExIA). Figure 3 provides further details.
Figure 3.
Variables analyzed for association with explants because of insufficient analgesic effect. Each point is the hazard ratio, and lines indicate 95% confidence intervals. Higher hazard ratio indicates a stronger association to the risk of explantation. DDD, the total number of defined daily doses dispensed to a patient in the 12 months before implantation. Indication group “Other” refers to a group of indications made up of all treatment indications except “persistent spinal pain syndrome, type 1 or 2” as defined by indication groups in RAY, a local quality registry for implantable neurostimulation therapies for pain at the Uppsala University Hospital, Sweden. Burst: A 5-pulse train paresthesia-free waveform with internal frequency of 500 Hz delivered at 40 Hz using a passive recharge pattern. 10 kHz, a paresthesia-free, 10-kHz, continuous, spinal cord stimulation waveform. Tonic: refers to a 30- to 80-Hz continuous spinal cord stimulation waveform producing paresthesia. See appendix for data in table format, available at http://links.lww.com/PR9/A210.
In a subanalysis (N = 99), having a normal body mass index (BMI) was associated with a lower risk of ExIA compared with overweight patients (HR 0.193; P = 0.019) (see appendix for details, available at http://links.lww.com/PR9/A210).
3.4. Patient-reported analgesic and global effect (objective 3)
As can be observed in Figures 4,5, the difference in pain perception between baseline and follow-up is greater among patients answering “freedom from pain,” with decreasing amount towards “no pain relief,” demonstrating a strong consistency between global perceived effect of stimulation and reduction in pain relief and pain interference. Mean difference in pain intensity across all EoS categories was −21.3% (95% CI −32.9% to −9.6%) and for pain interference, it was −29.4% (95% CI −33.0 to −25.9). Tables 4–6 provide further details.
Figure 4.
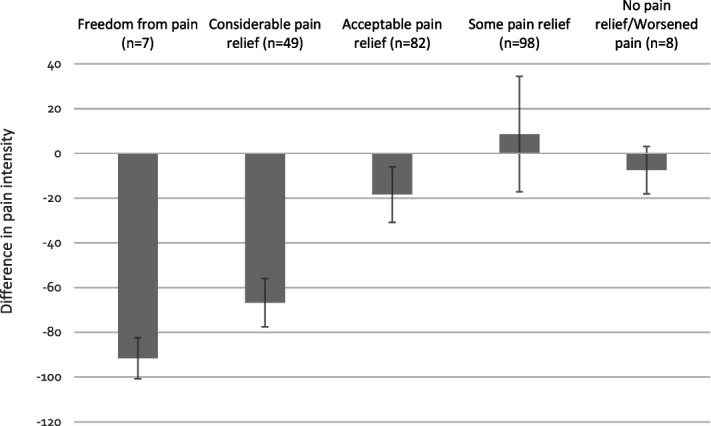
Mean difference in pain intensity between baseline and follow-up, by category of effect of stimulation. Error bars represent 95% confidence interval.
Figure 5.
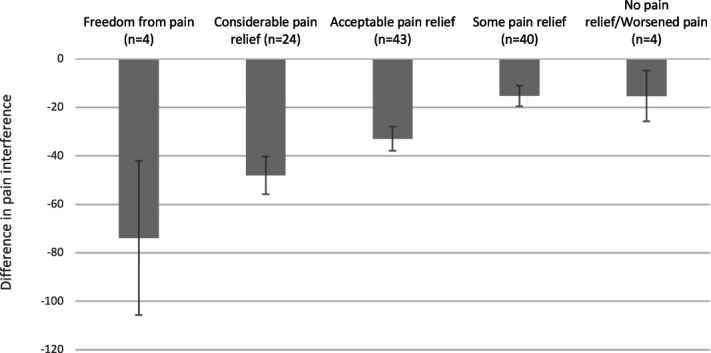
Mean difference in pain interference between baseline and follow-up, by category of effect of stimulation. Error bars represent 95% confidence interval.
Table 4.
Mean pain intensity and pain interference at baseline and follow-up.
| Baseline (BL) | Follow-up (FU) | Mean percent difference from BL to FU (CI) | Proportion of patients reporting > −30% change from BL to FU | Proportion of patients reporting > −50% change from BL to FU | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Pain intensity (N = 244) | 8.0 | 1.8 | 5.4 | 2.2 | −30.6 (−27.2 to −34.0) | 48.3% | 20.1% |
| Pain interference (N = 115) | 6.5 | 2.0 | 4.7 | 2.8 | −21.4 (−9.7 to −33.2) | 43.9% | 31.6% |
Pain intensity and pain interference measured using the Brief Pain Inventory.
CI, 95% confidence interval; SD, standard deviation.
Table 6.
Mean difference in pain interference between baseline and follow-up, by category of the effect of stimulation.
| Pain interference | ||||
|---|---|---|---|---|
| EoS category | N | Mean difference | 95% CI lower | 95% CI upper |
| Freedom from pain | 4 | −73.8 | −105.7 | −42.0 |
| Considerable pain relief | 24 | −48.0 | −55.8 | −40.3 |
| Acceptable pain relief | 43 | −32.9 | −37.9 | −27.9 |
| Some pain relief | 40 | −15.3 | −19.5 | −11.0 |
| No pain relief/worsened pain | 4 | −15.3 | −25.8 | −4.8 |
| All groups | 115 | −29.4 | −33.0 | −25.9 |
Data available for analysis in 115 of 411 patients.
CI, confidence interval; Eos, effect of stimulation, a 6-level, categorical, patient-reported, outcome measurement to assess the global effect of the implanted neurostimulation system at follow-up.
Table 5.
Mean difference in pain intensity between baseline and follow-up, by category of the effect of stimulation.
| Pain intensity | ||||
|---|---|---|---|---|
| EoS category | N | Mean difference (%) | 95% CI lower (%) | 95% CI upper (%) |
| Freedom from pain | 7 | −91.5 | −100.7 | −82.4 |
| Considerable pain relief | 49 | −66.7 | −77.5 | −55.9 |
| Acceptable pain relief | 82 | −18.4 | −30.8 | −6.0 |
| Some pain relief | 98 | 8.6 | −17.2 | 34.4 |
| No pain relief/Worsened pain | 8 | −7.5 | −18.1 | 3.1 |
| All groups | 244 | −21.3 | −32.9 | −9.6 |
Data available for analysis in 244 of 411 patients.
CI, confidence interval; Eos, effect of stimulation, a 6-level, categorical, patient-reported, outcome measurement to assess the global effect of the implanted neurostimulation system at follow-up.
3.5. Predictors for patient-reported effect of stimulation (objective 4)
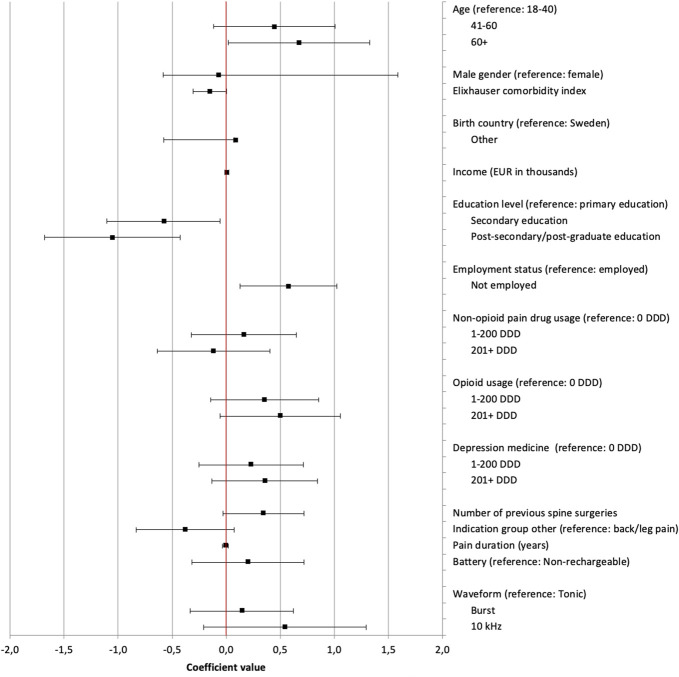
Figure 6 details the results of the ordered logit regression model. Increased probability of successful SCS treatment was significantly associated with secondary education (95% CI −1.10 to −0,06; P = 0.030) and postsecondary/postgraduate education (95% CI −1.68 to −0.43; P = 0.001). Older than 60 years (95% CI 0.02–1.33; P = 0.044) and unemployment (95% CI 0.13–1.02; P = 0.001) were significantly associated with decreased probability of successful outcome. Higher opioid consumption was insignificantly associated with decreased probability of successful outcome (P = 0.078–0.164).
Figure 6.
Variables analyzed for association with successful or unsuccessful outcome of SCS treatment, based on a binary transformation of EoS levels. Each point is the coefficient value from ordered logit regression; lines indicate 95% confidence intervals. Positive coefficient values indicate a stronger association to an unsuccessful outcome. DDD, the total number of defined daily doses dispensed to a patient in the 12 months before implantation. Group “Other” refers to a group of indications made up of all treatment indications except “persistent spinal pain syndrome, type 1 or 2” as defined by indication groups in RAY, a local quality registry for implantable neurostimulation therapies for pain at the Uppsala University Hospital, Sweden. Burst, a 5-pulse train paresthesia-free waveform with internal frequency of 500 Hz delivered at 40 Hz using a passive recharge pattern. 10 kHz, a paresthesia-free, 10-kHz, continuous, spinal cord stimulation waveform. Tonic refers to a 30- to 80-Hz, continuous, spinal cord stimulation waveform producing paresthesia. See appendix for data in table format, available at http://links.lww.com/PR9/A210. SCS, spinal cord stimulation.
4. Discussion
In this study, we chose study objectives we judged as the most relevant to evaluate the clinical effectiveness of implanted neurostimulation devices for chronic pain: patient-reported global assessment of the treatment and explantation and termination of stimulation systems due to inadequate pain relief.
4.1. Patient population
The demographics and indications for SCS of the study population are largely similar to comparable reports previously published.20,34,45,55 Baseline low-dose (1–200 DDD) opioid consumption for implanted patients was 5 times more common and high-dose (>200 DDD) was 20 times more common, compared with the matched healthy control group; 43% of patients in cohort 2 did not consume opioids in the 3 months before implant, which is considerably lower compared with published US national claims data.47
4.2. Socioeconomic status and outcomes of spinal cord stimulation treatment
The main novel findings in this study are that higher education level and employment are associated with better outcome in patients treated with invasive neurostimulation. These associations are in the expected direction and fits well into the extensive literature on the relationship between socioeconomic factors and perceived health, impact, and prevalence of chronic pain and outcome of treatments aimed at treating pain.8,11,25,36,39
The biopsychosocial model, introduced by Engel 1977, has been widely adopted and is implemented clinically in the management of pain through the development and practice of multidisciplinary pain management clinics and programs.21 In research aimed at improving our understanding of factors predicting the outcome of implanted neurostimulation devices, the focus has been mainly directed towards biological10,18,27 and psychological predictors.2,4,6,7,51 To the best of our knowledge, the impact of social aspects on outcome, such as income, education level, or employment status, have not been investigated previously. This study is important because it adds information on the “social” aspects to previous “bio” and “psycho” research on SCS.
4.3. Explantations of spinal cord stimulation systems because of insufficient analgesia and potential predictors
The first objective of this study was to calculate the incidence of explantation due to insufficient analgesic effect (ExIA), which we found to be at 2% per year in the first 10 years after implantation of a permanent SCS system. This is lower compared with other studies.23,40,49 In comparison, a retrospective chart review of 955 SCS implants in 4 European centers found ExIA to be 4.2% per year of follow-up.9 The therapeutic armamentarium in the neuromodulation field has grown over the past years, and the option to switch from one neurostimulation therapy to another has increased.42 Patient switching from spinal cord stimulation to another invasive neurostimulation therapy (eg, dorsal root ganglion stimulation) was censored in our study and not counted as failure of therapy, which may have contributed to the relatively low rate of explants.
The second objective was to investigate potential predictors of ExIA. In contrast to Van Buyten et al.,9 we found higher age, but not sex, to be associated with a higher risk of ExIA. The aforementioned study found rechargeable systems, both high-frequency (10 kHz) and tonic low-frequency systems, to be associated with a higher frequency of ExIA compared with nonrechargeable systems, speculating that the burden of frequent recharging might affect patient experience and risk of explantation. In our study, waveform and type of IPG were treated as separate variables, and 10 kHz was significantly associated with ExIA, whereas type of IPG (rechargeable or nonrechargeable) was not. Moreover, we found opioid consumption in the 12 months before a permanent implant to be significantly associated with a higher risk of ExIA, which is in line with work by Sharan et al.47
4.4. Patient-reported global effect, analgesia, and potential predictors
Objective 3 was to assess the patient-reported global effect and analgesia in SCS-treated patients. We dichotomized outcome as successful or unsuccessful using a 6-level categorical scale constructed to reflect the effect of stimulation (EoS), representing the patient-reported global assessment of invasive neurostimulation. The reasons for this were several. First, pain intensity was reported on an 11-grade NRS scale or using the BPI in the RAY cohort, whereas all patients with available outcome data reported EoS. Second, this approach gives the opportunity to define responders and nonresponders and dichotomize outcome as successful or not successful, which again allows for regression analysis and exploring the association between EoS and other variables. Third, using a categorical primary outcome parameter rather than a continuous variable (eg, VAS pain intensity) is repeatedly emphasized in methodological articles and recommendation on research standards on treatments of pain.16,26 In the studied population, close to half of the patients reported a successful outcome, meaning “acceptable pain relief” or better, with a corresponding mean 30.6% pain intensity reduction. A strong close to linear correlation was found between EoS and mean reduction in pain intensity and pain interference.
Objective 4 was to investigate whether potential predictors of reported EoS could be identified. Of all variables examined, the strongest association was found between Eos and education level, where both secondary and post/secondary education were associated with a higher chance of successful reported outcome. Inversely, unemployment was associated with lower risk of successful outcome, compared with being employed. However, the possible covariance between employment and education level cannot be assessed with the analysis used in this study. Nonetheless, income level, a variable often associated with both education level and degree of employment, was not predictive of reported outcome.
The mean pain duration before implantation of a permanent stimulation system is longer in our study population than reported in most other real-world cohorts studied.14 The relationship between pain duration and outcome of SCS is conflicting, and different reports vary in their definition of a successful outcome and are difficult to compare. A systematic review and meta-analysis using multivariate analysis could not confirm the prior finding that long-term outcome is better in patients with a shorter pain duration.54 Our findings support the fact that pain duration alone does not predict long-term pain relief in the study population.43
Pharmacotherapy data were provided in the DDD format. The DDD concept makes comparison of our findings regarding the effect of opioid dose on outcome difficult, and our study were not able to contribute to the current ongoing discussion on the importance of preimplant opioid tapering and to whether there is a maximum daily average morphine equivalent dose important for long-term outcome.1,20,37 We could not identify any significant association of the level of opioid consumption on reported outcome, which mirrors the findings of Maher et al.29
Two studies in the United States have explored the relationship of body mass index (BMI) and outcome of SCS.32,33 Our data were limited by the fact that only individuals with data from the SWESPINE registry had data on BMI (N = 99). The association between BMI and the risk of ExIA was explored in a separate Cox regression analysis, and normal BMI was associated with a lower risk of ExIA compared with overweight patients, indicating that obesity may be related to a higher risk of explantation (see appendix, available at http://links.lww.com/PR9/A210).
4.5. Limitations
The limitations to this study are multiple and important to consider. The reported outcome should not be interpreted as a measure of efficacy of implanted neurostimulation devices because no control group (ie, sham stimulation) other than healthy controls was used. Moreover, in a retrospective association study with a limited study population, it is difficult to rule out possible covariance between potential explanatory variables in the regression models, eg, between the variables employment and education levels. Our findings would be enhanced by future similar studies in different settings, preferably in a larger, prospective, multicenter trial.
Cohort 1, which provides patient-reported outcome data in this database, is from a tertiary care, high-volume, single center and may reflect practices and patient selection criteria not representative for other populations. The time to follow-up, at which patient-reported outcome criteria were collected, varied, as these data were collected on a fixed time point annually. This fact is not relevant in the time-to event analysis of risk of explants, but should be taken into account when assessing the patient-reported effect of therapy.
A real-world study length of 7 years leads inevitably to the introduction of new technologies and emerging scientific evidence during the study period, adding risk of period effects at different times in the study.
To limit this risk of bias because of data mining, study protocol, and statistical analysis, plan was agreed upon by the study group before analysis. However, retrospective trials with large data sets may be vulnerable to this phenomenon. Recently, Benjamin et al.5 has proposed using a more conservative threshold for statistical significance (P < 0.005 for “statistically significant evidence” and <0.05 for “suggestive evidence”) to lower the risk of false-positive findings. We used the more conventional significance threshold at P < 0.05 but note that several of our findings even hold for thresholds at P < 0.005.
4.6. Conclusion
Socioeconomic factors, especially education and employment, may be associated with outcome in spinal cord stimulation and should be considered both when designing future trials in the area, as well as when interpreting outcome data, both for the individual patient and on an aggregate level.
Disclosures
T.K. has received consulting fees from Abbott Laboratories. E.S. and T.J. was employees of Quantify Research during the study period. F.B. owns stocks in Quantify Research. Other coauthors have no declared conflict of interest. This study was not preregistered.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A210.
Supplementary Material
Acknowledgements
The RAY registry was funded by the Uppsala University Hospital, Sweden. Quantify Research, Stockholm, Sweden, managed and analyzed data from the research database, and the study was funded by Abbott Laboratories, Illinois.
Data availability statement: Data from this study will not be made available to anyone outside the study group.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Emma Söreskog, Email: emma.soreskog@ki.se.
Trolle Jacobson, Email: Trolle.Jacobson@sironagroup.se.
Rolf Karlsten, Email: rolf.karlsten@akademiska.se.
Niklas Zethraeus, Email: niklas.zethraeus@ki.se.
Fredrik Borgström, Email: fredrik.borgstrom@quantifyresearch.com.
References
- [1].Adil SM, Charalambous LT, Spears CA, Kiyani M, Hodges SE, Yang Z, Lee H-J, Rahimpour S, Parente B, Greene KA, McClellan M, Lad SP. Impact of spinal cord stimulation on opioid dose reduction: a nationwide analysis. Neurosurgery 2020;88:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Al-Shaiji TF, Malallah MA, Yaiesh SM, Al-Terki AE, Hassouna MM. Does psychological disturbance predict explantation in successful pelvic neuromodulation treatment for bladder dysfunction? A short series. Neuromodulation 2018;21:805–8. [DOI] [PubMed] [Google Scholar]
- [3].Basch E, Schrag D. The evolving uses of “real-world” data. JAMA 2019;321:1359–60. [DOI] [PubMed] [Google Scholar]
- [4].Bendinger T, Plunkett N, Poole D, Turnbull D. Psychological factors as outcome predictors for spinal cord stimulation. Neuromodulation 2015;18:465–71; discussion 471. [DOI] [PubMed] [Google Scholar]
- [5].Benjamin DJ, Berger JO, Johannesson M, Nosek BA, Wagenmakers E-J, Berk R, Bollen KA, Brembs B, Brown L, Camerer C, Cesarini D, Chambers CD, Clyde M, Cook TD, Boeck PD, Dienes Z, Dreber A, Easwaran K, Efferson C, Fehr E, Fidler F, Field AP, Forster M, George EI, Gonzalez R, Goodman S, Green E, Green DP, Greenwald AG, Hadfield JD, Hedges LV, Held L, Ho TH, Hoijtink H, Hruschka DJ, Imai K, Imbens G, Ioannidis JPA, Jeon M, Jones JH, Kirchler M, Laibson D, List J, Little R, Lupia A, Machery E, Maxwell SE, McCarthy M, Moore DA, Morgan SL, Munafó M, Nakagawa S, Nyhan B, Parker TH, Pericchi L, Perugini M, Rouder J, Rousseau J, Savalei V, Schönbrodt FD, Sellke T, Sinclair B, Tingley D, Zandt TV, Vazire S, Watts DJ, Winship C, Wolpert RL, Xie Y, Young C, Zinman J, Johnson VE. Redefine statistical significance. Nat Hum Behav 2018;2:6–10. [DOI] [PubMed] [Google Scholar]
- [6].Blackburn DR, Romers CC, Copeland LA, Lynch W, Nguyen DD, Zeber JE, Hoffman MR. Presurgical psychological assessments as correlates of effectiveness of spinal cord stimulation for chronic pain reduction. Neuromodulation 2016;19:422–8. [DOI] [PubMed] [Google Scholar]
- [7].Block AR, Marek RJ, Ben-Porath YS, Kukal D. Associations between pre-implant psychosocial factors and spinal cord stimulation outcome. Assessment 2015;24:60–70. [DOI] [PubMed] [Google Scholar]
- [8].Borsook D, Youssef AM, Simons L, Elman I, Eccleston C. When pain gets stuck: the evolution of pain chronification and treatment resistance. PAIN 2018;159:2421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buyten J-PV, Wille F, Smet I, Wensing C, Breel J, Karst E, Devos M, Pöggel-Krämer K, Vesper J. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation 2017;20:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Campbell CM, Buenaver LF, Raja SN, Kiley KB, Swedberg LJ, Wacnik PW, Cohen SP, Erdek MA, Williams KA, Christo PJ. Dynamic pain phenotypes are associated with spinal cord stimulation-induced reduction in pain: a repeated measures observational pilot study. Pain Med 2015;16:1349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carr JL, Moffett JAK. Review Paper: the impact of social deprivation on chronic back pain outcomes. Chronic Illn 2005;1:121–9. [DOI] [PubMed] [Google Scholar]
- [12].Christelis N, Simpson B, Russo M, Stanton-Hicks M, Barolat G, Thomson S, Schug S, Baron R, Buchser E, Carr DB, Deer TR, Dones I, Eldabe S, Gallagher R, Huygen F, Kloth D, Levy R, North R, Perruchoud C, Petersen E, Rigoard P, Slavin K, Turk D, Wetzel T, Loeser J. Persistent spinal pain syndrome: a proposal for failed back surgery syndrome and ICD-11. Pain Med 2021;22:807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Collaborators G 2015 D and II and P, Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZ, Coggeshall M, Cornaby L, Dandona L, Dicker DJ, Dilegge T, Erskine HE, Ferrari AJ, Fitzmaurice C, Fleming T, Forouzanfar MH, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Hay SI, Johnson CO, Kassebaum NJ, Kawashima T, Kemmer L, Khalil IA, Kinfu Y, Kyu HH, Leung J, Liang X, Lim SS, Lopez AD, Lozano R, Marczak L, Mensah GA, Mokdad AH, Naghavi M, Nguyen G, Nsoesie E, Olsen H, Pigott DM, Pinho C, Rankin Z, Reinig N, Salomon JA, Sandar L, Smith A, Stanaway J, Steiner C, Teeple S, Thomas BA, Troeger C, Wagner JA, Wang H, Wanga V, Whiteford HA, Zoeckler L, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abraham B, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NME, Ackerman IN, Adebiyi AO, Ademi Z, Adou AK, Afanvi KA, Agardh EE, Agarwal A, Kiadaliri AA, Ahmadieh H, Ajala ON, Akinyemi RO, Akseer N, Al-Aly Z, Alam K, Alam NKM, Aldhahri SF, Alegretti MA, Alemu ZA, Alexander LT, Alhabib S, Ali R, Alkerwi A, Alla F, Allebeck P, Al-Raddadi R, Alsharif U, Altirkawi KA, Alvis-Guzman N, Amare AT, Amberbir A, Amini H, Ammar W, Amrock SM, Andersen HH, Anderson GM, Anderson BO, Antonio CAT, Aregay AF, Ärnlöv J, Artaman A, Asayesh H, Assadi R, Atique S, Avokpaho EFGA, Awasthi A, Quintanilla BPA, Azzopardi P, Bacha U, Badawi A, Balakrishnan K, Banerjee A, Barac A, Barker-Collo SL, Bärnighausen T, Barregard L, Barrero LH, Basu A, Bazargan-Hejazi S, Beghi E, Bell B, Bell ML, Bennett DA, Bensenor IM, Benzian H, Berhane A, Bernabé E, Betsu BD, Beyene AS, Bhala N, Bhatt S, Biadgilign S, Bienhoff K, Bikbov B, Biryukov S, Bisanzio D, Bjertness E, Blore J, Borschmann R, Boufous S, Brainin M, Brazinova A, Breitborde NJK, Brown J, Buchbinder R, Buckle GC, Butt ZA, Calabria B, Campos-Nonato IR, Campuzano JC, Carabin H, Cárdenas R, Carpenter DO, Carrero JJ, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Chang J-C, Chiang PP-C, Chibueze CE, Chisumpa VH, Choi J-YJ, Chowdhury R, Christensen H, Christopher DJ, Ciobanu LG, Cirillo M, Coates MM, Colquhoun SM, Cooper C, Cortinovis M, Crump JA, Damtew SA, Dandona R, Daoud F, Dargan PI, das Neves J, Davey G, Davis AC, Leo DD, Degenhardt L, Gobbo LCD, Dellavalle RP, Deribe K, Deribew A, Derrett S, Jarlais DCD, Dharmaratne SD, Dhillon PK, Diaz-Torné C, Ding EL, Driscoll TR, Duan L, Dubey M, Duncan BB, Ebrahimi H, Ellenbogen RG, Elyazar I, Endres M, Endries AY, Ermakov SP, Eshrati B, Estep K, Farid TA, Farinha CSeS, Faro A, Farvid MS, Farzadfar F, Feigin VL, Felson DT, Fereshtehnejad S-M, Fernandes JG, Fernandes JC, Fischer F, Fitchett JRA, Foreman K, Fowkes FGR, Fox J, Franklin RC, Friedman J, Frostad J, Fürst T, Futran ND, Gabbe B, Ganguly P, Gankpé FG, Gebre T, Gebrehiwot TT, Gebremedhin AT, Geleijnse JM, Gessner BD, Gibney KB, Ginawi IAM, Giref AZ, Giroud M, Gishu MD, Giussani G, Glaser E, Godwin WW, Gomez-Dantes H, Gona P, Goodridge A, Gopalani SV, Gotay CC, Goto A, Gouda HN, Grainger R, Greaves F, Guillemin F, Guo Y, Gupta R, Gupta R, Gupta V, Gutiérrez RA, Haile D, Hailu AD, Hailu GB, Halasa YA, Hamadeh RR, Hamidi S, Hammami M, Hancock J, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Havmoeller R, Hay RJ, Heredia-Pi IB, Heydarpour P, Hoek HW, Horino M, Horita N, Hosgood HD, Hoy DG, Htet AS, Huang H, Huang JJ, Huynh C, Iannarone M, Iburg KM, Innos K, Inoue M, Iyer VJ, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Javanbakht M, Jayaraman SP, Jayatilleke AU, Jee SH, Jeemon P, Jensen PN, Jiang Y, Jibat T, Jimenez-Corona A, Jin Y, Jonas JB, Kabir Z, Kalkonde Y, Kamal R, Kan H, Karch A, Karema CK, Karimkhani C, Kasaeian A, Kaul A, Kawakami N, Keiyoro PN, Kemp AH, Keren A, Kesavachandran CN, Khader YS, Khan AR, Khan EA, Khang Y-H, Khera S, Khoja TAM, Khubchandani J, Kieling C, Kim P, Kim C, Kim D, Kim YJ, Kissoon N, Knibbs LD, Knudsen AK, Kokubo Y, Kolte D, Kopec JA, Kosen S, Kotsakis GA, Koul PA, Koyanagi A, Kravchenko M, Defo BK, Bicer BK, Kudom AA, Kuipers EJ, Kumar GA, Kutz M, Kwan GF, Lal A, Lalloo R, Lallukka T, Lam H, Lam JO, Langan SM, Larsson A, Lavados PM, Leasher JL, Leigh J, Leung R, Levi M, Li Y, Li Y, Liang J, Liu S, Liu Y, Lloyd BK, Lo WD, Logroscino G, Looker KJ, Lotufo PA, Lunevicius R, Lyons RA, Mackay MT, Magdy M, Razek AE, Mahdavi M, Majdan M, Majeed A, Malekzadeh R, Marcenes W, Margolis DJ, Martinez-Raga J, Masiye F, Massano J, McGarvey ST, McGrath JJ, McKee M, McMahon BJ, Meaney PA, Mehari A, Mejia-Rodriguez F, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Millear A, Miller TR, Mills EJ, Mirarefin M, Mitchell PB, Mock CN, Mohammadi A, Mohammed S, Monasta L, Hernandez JCM, Montico M, Mooney MD, Moradi-Lakeh M, Morawska L, Mueller UO, Mullany E, Mumford JE, Murdoch ME, Nachega JB, Nagel G, Naheed A, Naldi L, Nangia V, Newton JN, Ng M, Ngalesoni FN, Nguyen QL, Nisar MI, Pete PMN, Nolla JM, Norheim OF, Norman RE, Norrving B, Nunes BP, Ogbo FA, Oh I-H, Ohkubo T, Olivares PR, Olusanya BO, Olusanya JO, Ortiz A, Osman M, Ota E, PA M, Park E-K, Parsaeian M, Passos VM de A, Caicedo AJP, Patten SB, Patton GC, Pereira DM, Perez-Padilla R, Perico N, Pesudovs K, Petzold M, Phillips MR, Piel FB, Pillay JD, Pishgar F, Plass D, Platts-Mills JA, Polinder S, Pond CD, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Qorbani M, Rabiee RHS, Radfar A, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman SU, Rai RK, Rajsic S, Ram U, Rao P, Refaat AH, Reitsma MB, Remuzzi G, Resnikoff S, Reynolds A, Ribeiro AL, Blancas MJR, Roba HS, Rojas-Rueda D, Ronfani L, Roshandel G, Roth GA, Rothenbacher D, Roy A, Sagar R, Sahathevan R, Sanabria JR, Sanchez-Niño MD, Santos IS, Santos JV, Sarmiento-Suarez R, Sartorius B, Satpathy M, Savic M, Sawhney M, Schaub MP, Schmidt MI, Schneider IJC, Schöttker B, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Servan-Mori EE, Shackelford KA, Shaheen A, Shaikh MA, Sharma R, Sharma U, Shen J, Shepard DS, Sheth KN, Shibuya K, Shin M-J, Shiri R, Shiue I, Shrime MG, Sigfusdottir ID, Silva DAS, Silveira DGA, Singh A, Singh JA, Singh OP, Singh PK, Sivonda A, Skirbekk V, Skogen JC, Sligar A, Sliwa K, Soljak M, Søreide K, Sorensen RJD, Soriano JB, Sposato LA, Sreeramareddy CT, Stathopoulou V, Steel N, Stein DJ, Steiner TJ, Steinke S, Stovner L, Stroumpoulis K, Sunguya BF, Sur P, Swaminathan S, Sykes BL, Szoeke CEI, Tabarés-Seisdedos R, Takala JS, Tandon N, Tanne D, Tavakkoli M, Taye B, Taylor HR, Ao BJT, Tedla BA, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Thrift AG, Thurston GD, Tobe-Gai R, Tonelli M, Topor-Madry R, Topouzis F, Tran BX, Truelsen T, Dimbuene ZT, Tsilimbaris M, Tura AK, Tuzcu EM, Tyrovolas S, Ukwaja KN, Undurraga EA, Uneke CJ, Uthman OA, van Gool CH, Varakin YY, Vasankari T, Venketasubramanian N, Verma RK, Violante FS, Vladimirov SK, Vlassov VV, Vollset SE, Wagner GR, Waller SG, Wang L, Watkins DA, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, White RA, Williams HC, Wiysonge CS, Wolfe CDA, Won S, Woodbrook R, Wubshet M, Xavier D, Xu G, Yadav AK, Yan LL, Yano Y, Yaseri M, Ye P, Yebyo HG, Yip P, Yonemoto N, Yoon S-J, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zeeb H, Zhou M, Zodpey S, Zuhlke LJ, Murray CJL. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deer T, Skaribas I, McJunkin T, Nelson C, Salmon J, Darnule A, Braswell J, Russo M, Gomezese OF. Results from the partnership for advancement in neuromodulation registry: a 24-month follow-up. Neuromodulation 2016;19:179–87. [DOI] [PubMed] [Google Scholar]
- [15].Deer T, Slavin KV, Amirdelfan K, North RB, Burton AW, Yearwood TL, Tavel E, Staats P, Falowski S, Pope J, Justiz R, Fabi AY, Taghva A, Paicius R, Houden T, Wilson D. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018;21:56–66. [DOI] [PubMed] [Google Scholar]
- [16].Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Korff MV, Weiner DK. Report of the NIH task force on research standards for chronic low back pain. J Pain 2014;15:569–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. [DOI] [PubMed] [Google Scholar]
- [18].Eijs F, Smits H, Geurts JW, Kessels AGH, Kemler MA, Kleef M, Joosten EAJ, Faber CG. Brush‐evoked allodynia predicts outcome of spinal cord stimulation in Complex Regional Pain Syndrome type 1. Eur J Pain 2010;14:164–9. [DOI] [PubMed] [Google Scholar]
- [19].Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- [20].Falowski SM, Moore GA, Cornidez EG, Hutcheson JK, Candido K, Peña I, Blomme B, Capobianco RA. Improved psychosocial and functional outcomes and reduced opioid usage following burst spinal cord stimulation. Neuromodulation 2021;24:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- [22].Han JL, Murphy KR, Hussaini SMQ, Yang S, Parente B, Xie J, Pagadala P, Lad SP. Explantation rates and healthcare resource utilization in spinal cord stimulation. Neuromodulation 2017;20:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation 2015;18:603–9. [DOI] [PubMed] [Google Scholar]
- [24].Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, Amirdelfan K, Morgan DM, Brown LL, Yearwood TL, Bundschu R, Burton AW, Yang T, Benyamin R, Burgher AH. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology 2015;123:851–60. [DOI] [PubMed] [Google Scholar]
- [25].Katz JN. Lumbar disc disorders and low-back pain. J Bone Joint Surg 2006;88:21–4. [DOI] [PubMed] [Google Scholar]
- [26].Kennedy DJ, Schneider B. Lies, damn lies, and statistic: a commentary. Pain Med 2020;21:2052–4. [DOI] [PubMed] [Google Scholar]
- [27].Kinfe TM, Muhammad S, Link C, Roeske S, Chaudhry SR, Yearwood TL. Burst spinal cord stimulation increases peripheral antineuroinflammatory interleukin 10 levels in failed back surgery syndrome patients with predominant back pain. Neuromodulation 2017;263:C1. [DOI] [PubMed] [Google Scholar]
- [28].Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O'Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. PAIN 2007;132:179–88. [DOI] [PubMed] [Google Scholar]
- [29].Maher DP, Martins YC, Doshi T, Bicket M, Zhang K, Hanna G, Ahmed S. Neuropathic pain medication use does not alter outcomes of spinal cord stimulation for lower extremity pain. Neuromodulation 2018;21:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mann SA, Sparkes E, Duarte RV, Raphael JH. Attrition with spinal cord stimulation. Br J Neurosurg 2015;29:823–8. [DOI] [PubMed] [Google Scholar]
- [31].Mäntyselkä P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamäki H, Halonen P, Takala J. Pain as a reason to visit the doctor: a study in Finnish primary health care. PAIN 2001;89:175–80. [DOI] [PubMed] [Google Scholar]
- [32].Marola O, Cherala R, Prusik J, Kumar V, Fama C, Wilock M, Crimmins J, Pilitsis JG. BMI as a predictor of spinal cord stimulation success in chronic pain patients. Neuromodulation 2017;20:269–73. [DOI] [PubMed] [Google Scholar]
- [33].Mekhail N, Mehanny D, Armanyous S, Saweris Y, Costandi S. The impact of obesity on the effectiveness of spinal cord stimulation in chronic spine-related pain patients. Spine J 2018;19:476–86. [DOI] [PubMed] [Google Scholar]
- [34].Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract 2010;11:148–53. [DOI] [PubMed] [Google Scholar]
- [35].Merskey H, Bogduk N, editors. Classification of chronic pain. 2nd ed. Seattle: IASP Press, 1994. [Google Scholar]
- [36].Moffett JAK, Underwood MR, Gardiner ED. Socioeconomic status predicts functional disability in patients participating in a back pain trial. Disabil Rehabil 2009;31:783–90. [DOI] [PubMed] [Google Scholar]
- [37].Nissen M, Ikäheimo T, Huttunen J, Leinonen V, Jyrkkänen H-K, von Und Zu Fraunberg M. Higher preimplantation opioid doses associated with long‐term spinal cord stimulation failure in 211 patients with failed back surgery syndrome. Neuromodulation 2021;24:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].North RB, Kumar K, Wallace MS, Henderson JM, Shipley J, Hernandez J, Mekel-Bobrov N, Jaax KN. Spinal cord stimulation versus re-operation in patients with failed back surgery syndrome: an international multicenter randomized controlled trial (EVIDENCE study). Neuromodulation 2011;14:330–6. [DOI] [PubMed] [Google Scholar]
- [39].Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. PAIN 2008;136:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pope JE, Deer TR, Falowski S, Provenzano D, Hanes M, Hayek SM, Amrani J, Carlson J, Skaribas I, Parchuri K, McRoberts WP, Bolash R, Haider N, Hamza M, Amirdelfan K, Graham S, Hunter C, Lee E, Li S, Yang M, Campos L, Costandi S, Levy R, Mekhail N. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation 2017;20:543–52. [DOI] [PubMed] [Google Scholar]
- [41].Poulsen DM, Sørensen JCH, Blichfeldt‐Eckhardt MR, Gulisano HA, Knudsen ALH, Nikolajsen L, Meier K. Pain catastrophizing does not predict spinal cord stimulation outcomes: a cohort study of 259 patients with long‐term follow‐up. Neuromodulation 2021;24:76–85. [DOI] [PubMed] [Google Scholar]
- [42].Reddy RD, Moheimani R, Yu GG, Chakravarthy KV. A review of clinical data on salvage therapy in spinal cord stimulation. Neuromodulation 2020;23:562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ridder DD, Vancamp T, Lenders MWPM, de Vos CC, Vanneste S. Is preoperative pain duration important in spinal cord stimulation? A comparison between tonic and burst stimulation. Neuromodulation 2015;18:13–7. [DOI] [PubMed] [Google Scholar]
- [44].Rosenberg JC, Schultz DM, Duarte LE, Rosen SM, Raza A. Increased pain catastrophizing associated with lower pain relief during spinal cord stimulation: results from a large post-market study. Neuromodulation 2015;18:277–84. [DOI] [PubMed] [Google Scholar]
- [45].Sanders RA, Moeschler SM, Gazelka HM, Lamer TJ, Wang Z, Qu W, Hoelzer BC. Patient outcomes and spinal cord stimulation: a retrospective case series evaluating patient satisfaction, pain scores, and opioid requirements. Pain Pract 2015;16:899–904. [DOI] [PubMed] [Google Scholar]
- [46].Weiss AJ, Jiang HJ. Most frequent reasons for emergency department visits, 2018. HCUP Statistical Brief #286. December 2021. Rockville, MD: Agency for Healthcare Research and Quality, www.hcup-us.ahrq.gov/reports/statbriefs/sb286-ED-Frequent-Conditions-2018.pdf. [Google Scholar]
- [47].Sharan AD, Riley J, Falowski S, Pope JE, Connolly AT, Karst E, Dalal N, Provenzano DA. Association of opioid usage with spinal cord stimulation outcomes. Pain Med 2017;19:699–707. [DOI] [PubMed] [Google Scholar]
- [48].Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns. Anesth Analgesia 1967;46:489–91. [PubMed] [Google Scholar]
- [49].Slyer J, Scott S, Sheldon B, Hancu M, Bridger C, Pilitsis JG. Less pain relief, more depression, and female sex correlate with spinal cord stimulation explants. Neuromodulation 2020;23:673–9. [DOI] [PubMed] [Google Scholar]
- [50].Söreskog E, Jacobson T, Kirketeig T, Fritzell P, Karlsten R, Zethraeus N, Borgström F. Impact of spinal cord stimulation on sick leave and disability pension in patients with chronic neuropathic pain: a real-world evidence study in Sweden. PAIN 2023;164:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sparkes E, Duarte RV, Mann S, Lawrence TR, Raphael JH. Analysis of psychological characteristics impacting spinal cord stimulation treatment outcomes: a prospective assessment. Pain Physician 2015;18:E369–77. [PubMed] [Google Scholar]
- [52].Sparkes E, Raphael JH, Duarte RV, LeMarchand K, Jackson C, Ashford RL. A systematic literature review of psychological characteristics as determinants of outcome for spinal cord stimulation therapy. PAIN 2010;150:284–9. [DOI] [PubMed] [Google Scholar]
- [53].Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain 2004;5:133–7. [DOI] [PubMed] [Google Scholar]
- [54].Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract 2014;14:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Thomson SJ, Kruglov D, Duarte RV. A spinal cord stimulation service review from a single centre using a single manufacturer over a 7.5 year follow-up period. Neuromodulation 2017;20:589–99. [DOI] [PubMed] [Google Scholar]
- [56].Wang VC, Bounkousohn V, Fields K, Bernstein C, Paicius RM, Gilligan C. Explantation rates of high frequency spinal cord stimulation in two outpatient clinics. Neuromodulation 2021;24:507–11. [DOI] [PubMed] [Google Scholar]
- [57].Wertheimer AI. The defined daily dose system (DDD) for drug utilization review. Hosp Pharm 1986;21:233–4, 239–41, 258. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A210.