Abstract
Background:
Tranexamic acid (TXA) is used to reduce bleeding in body contouring procedures; however, there are no studies that show the effectiveness of TXA when it is also used in the immediate postoperative period.
Methods:
A controlled, randomized, parallel, and open-label clinical trial was carried out in adult patients undergoing liposculpture and/or abdominoplasty. A control group administering presurgical TXA and a study group with presurgical and postsurgical TXA were formed. The decrease in hemoglobin and the incidence of blood transfusions between both groups were compared as well as the possible adverse effects of TXA.
Results:
Four hundred twenty-seven subjects were included, 208 (48.7%) in the control group and 219 (51.3%) in the study group. The median age was 34 years (interquartile range 28–42). Median postoperative hemoglobin levels at 24 hours were similar in both groups (study 11.3 g/dL versus control 11.1 g/dL, P = 0.07); however, at 72 hours, postoperative hemoglobin was higher in the study group versus control (10.8 versus 10.0 g/dL, P ≤ 0.001). The incidence of transfusions at 72 hours was 1.8% in the study group and 8.6% in the control group, for a risk ratio of 0.21 (95% confidence interval 0.07–0.61). There were no adverse or thromboembolic events.
Conclusion:
TXA proved to be more effective in reducing intra- and postsurgical bleeding and the need for transfusions, when used preoperatively and continued for 48 hours after surgery, than when used only preoperatively, without reporting adverse or thromboembolic effects.
Takeaways
Question: What is the effectiveness of tranexamic acid (TXA) in reducing bleeding and the need for transfusions in body contouring surgery when it is administered preoperatively and during the immediate postoperative period?
Findings: A controlled, randomized, parallel, and open clinical trial was carried out in adult patients undergoing liposculpture and/or abdominoplasty, where it was verified that TXA administered intravenously before surgery and continuing its oral administration after surgery reduced postoperative bleeding and the need for transfusions with statistical significance, compared with the group where it was only used in the preoperative period.
Meaning: Using TXA preoperatively and continuing to use it after surgery significantly reduces postoperative bleeding and the need for transfusions in patients undergoing body contouring surgery.
INTRODUCTION
Body contouring surgery procedures continue increasing year after year.1 Advances in surgical techniques, the improvement of results, and the use of new technologies are factors that have led to this great boom in recent times.2 But just as the procedures and surgical techniques to obtain better results have been modified and perfected, the processes to provide safety within these surgical procedures have also evolved in an important way. One of the processes that has been implemented to increase surgical safety is to include protocols to reduce blood loss and thus avoid blood transfusions as much as possible.3,4 Within these processes, the use of tranexamic acid (TXA) as an antifibrinolytic drug has emerged as a highly recommended management.5 Although TXA is a product widely used in various areas of medicine and surgery,6–12 only recently has it been used in different surgical procedures in the field of plastic surgery,13–16 including body contouring surgery.17–20 The usefulness of TXA has already been determined during liposuction, in different forms and processes with very good results.17–20 However, there is no comparative study that evaluates the effect and possible benefits of continuing its administration for 48 hours after surgery. That is why we developed a comparative protocol using it in the transoperative period and continuing its use during the immediate postsurgical period to find out the benefits provided by this scheme and to evaluate possible complications, with the aim of reducing bleeding and the amount of blood transfusions. To our knowledge, this is the first comparative study carried out in body contouring surgery showing the usefulness of TXA in the immediate postsurgical period.
MATERIAL AND METHODS
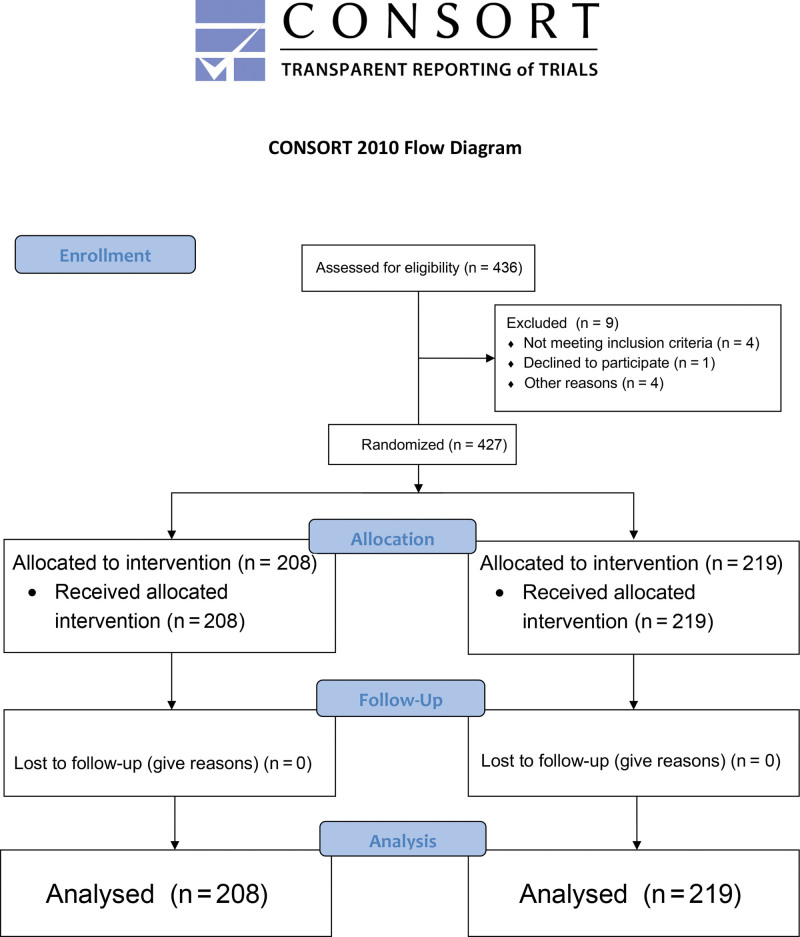
A multicentric, controlled, randomized, parallel, open-label clinical trial was carried out at the El Pinar Clinic in Bucaramanga and in the Dhara Clinic, Total Definer Center, Bogota, Colombia, prepared according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized and parallel clinical trials21 (Fig. 1). Female patients between 18 and 60 years of age were included, with American Society of Anesthesiologists (ASA) scores 1 and 2, who underwent liposuction greater than 3 L and/or abdominoplasty under intravenous general anesthesia in isolation or with additional cosmetic surgical procedures. Patients who did not agree to participate in the study; had liposuctions of less than 3 L, allergy to TXA, history of epilepsy, blood dyscrasias, or history of deep vein thrombosis; and who underwent combined procedures such as facelift, mastopexy, and gluteoplasty were excluded from the study. Two groups were formed, the control group who were administered a single dose of 1 g of intravenous (IV) TXA before surgery, and the study group who were administered the same dose of TXA before surgery but continued with 1 g orally at 12, 24, and 48 hours after surgery. Information related to age, ASA classification, weight, height, body mass index, Caprini score, type of surgical procedure (abdominoplasty, lipoabdominoplasty, liposculpture), infiltration (mL), and liposuction (mL) was collected from all patients. Pronation time (minutes), flap weight (g), preoperative parenteral iron use, normovolemic hemodilution, warm-up time (minutes), esophageal temperature, and preoperative hemoglobin, at 24 and 72 hours, were also determined. All patients were followed up for 30 postoperative days. The assignment of the patients in each group was carried out by simple randomization and with a 1:1 ratio; the sequence was generated using a statistical table of random numbers. Taking into account that the study group required oral administration of TXA, no blinding of patients or physicians was performed; therefore, this study is considered open-label. With a difference in the proportion of transfusions of 5% between the groups (study 1% versus control 6%), a confidence level of 95%, and a power of 80%, with a 1:1 ratio, a sample size of at least 425 patients was calculated using Epidat 4.2 (Epidat; Dirección Xeral de Saúde Pública de la Consellería de Sanidade, Xunta de Galicia, Spain).22 All patients underwent a patient blood management strategy according to a previous publication.4 This strategy has three moments: in the preoperative period, complete blood count and iron deficiency anemia treatment were performed according to the protocol with parenteral iron and erythropoietin.4 During surgery, normovolemic hemodilution was performed if hemoglobin was greater than 11.5g/dL, 1 g IV TXA, for prevention and management of hypothermia, anesthetic management with permissive hypotension, and surgical technique with the shortest possible time in pronation.4 In the postoperative period, hemoglobin concentrations were evaluated 24 and 72 hours after surgery, and transfusion was indicated for patients with levels below 7 g/dL and for symptomatic patients with levels between 7 and 9 g/dL.4 All patients with hemoglobin below 7 g/dL, and symptomatic patients with hemoglobin between 7 and 9 g/d: were transfused. The incidence of TXA-related side effects, specifically venous thromboembolism in the lower limbs, was assessed by the anesthesiology service on days 1, 3, and 5 after surgery and by the treating surgeon weekly up to 30 days. All patients received 3000 U of fraxiparin for 7–21 days after surgery, depending on each patient’s Caprini risk assessment model,23 beginning 12 hours after surgery, after verification that the patient did not present active bleeding. Patients with Caprini values between 2 and 3 received anticoagulation for 7 days, Caprini values of 4 received it for 14 days, and Caprini values of 5 received it for 21 days. Values of 6 or higher were not candidates for body contouring surgery.
Fig. 1.
Flowchart in patient selection according to the CONSORT guidelines for randomized and parallel clinical trials.
Statistical Analysis
Qualitative variables are reported as absolute and relative frequencies. Quantitative variables are presented as median and interquartile range given their statistical distribution. Additionally, missing data are reported for each variable collected. The normality of the quantitative variables was evaluated using the Shapiro–Wilks test. To establish differences in the demographic and clinical variables between both groups, the χ2 test or Fisher exact test was used, as appropriate for the qualitative variables, and the Wilcoxon test for the quantitative variables. Differences with a P value less than 0.05 were interpreted as statistically significant, with a two-tailed hypothesis test.
An intention-to-treat analysis was performed. The incidence of transfusions is reported as a proportion accompanied by 95% confidence interval (CI). To establish the effect of intravenous TXA in combination with oral (independent variable) on the need for postoperative transfusion (dependent variable), a binomial generalized linear regression model was applied and reported as risk ratio and 95% CI. Likewise, the absolute risk reduction and the number needed to treat are reported. No interim analyses were performed. The analysis was performed using Stata 13.0 software (StataCorp LLC, College Station, Tex.).
RESULTS
A total of 427 young patients with a low frequency of obesity were included: 208 (48.7%) in the control group with only intravenous TXA administration, and 219 (51.3%) in the study group with intravenous and oral TXA (Fig. 1). Lipoabdominoplasty and liposuction were the most performed procedures. Preoperative parenteral iron use and surgical findings were similar in both groups. Table 1 describes the general characteristics of the population.
Table 1.
Population Characteristics
| Variable | Total, N = 427 | TXA IV, n = 208 | TXA IV and Oral, n = 219 | P |
|---|---|---|---|---|
| Age (y) | 34 (28–42) | 36 (29–43.5) | 33 (27–41) | 0.019 |
| Weight (kg) No data, n (%) |
65 (60–71) 4 |
65 (60–72) 1 |
64 (60–70) 3 |
0.187 |
| BMI | 0.069 | |||
| Normal | 239 (56.5) | 106 (51.2) | 133 (61.6) | |
| Overweight | 167 (39.5) | 90 (43.5) | 77 (35.6) | |
| Obese | 17 (4.0) | 11 (5.3) | 6 (2.8) | |
| ASA, n (%)* | 0.151 | |||
| I | 327 (76.6) | 155 (74.5) | 172 (78.5) | |
| II | 91 (21.3) | 51 (24.5) | 40 (18.3) | |
| III | 1 (0.2) | — | 1 (0.5) | |
| No data, n (%) | 8 (1.9) | 2 (1.0) | 6 (2.7) | |
| Caprini score* | 0.193 | |||
| 2 | 20 (4.6) | 5 (2.4) | 15 (6.9) | |
| 3 | 240 (56.2) | 121 (58.2) | 119 (54.3) | |
| 4 | 95 (22.2) | 49 (23.5) | 46 (21.0) | |
| 5 | 4 (0.9) | 1 (0.5) | 3 (1.4) | |
| No data, n (%) | 68 (15.9) | 32 (15.4) | 36 (16.4) | |
| Medications* | 0.749 | |||
| Yes | 2 (0.5) | 207 (99.5) | 216 (98.6) | |
| No | 423 (99.0) | 1 (0.5) | 1 (0.5) | |
| No data, n (%) | 2 (0.5) | — | 2 (0.9) | |
| Surgical procedure* | 0.198 | |||
| Abdominoplasty | 9 (2.1) | 6 (2.9) | 3 (1.4) | |
| Lipoabdominoplasty | 91 (21.3) | 51 (24.5) | 40 (18.2) | |
| Liposuction | 114 (26.7) | 49 (23.6) | 65 (29.7) | |
| Combined surgery, blepharoplasty, breast augmentation, breast lifting | 213 (49.8) | 102 (49.0) | 111 (50.7) | |
| Preoperative parenteral iron* | 0.510 | |||
| Yes | 17 (3.9) | 8 (3.8) | 9 (4.1) | |
| No | 408 (95.6) | 198 (95.2) | 210 (95.9) | |
| No data, n (%) | 2 (0.5) | 2 (1.0) | — | |
| Surgical values | ||||
| Infiltration (mL) | 4000 (3300–5000) | 4000 (3300–4900) | 4000 (3400–5000) | 0.889 |
| No data (n) | 2 | 2 | ||
| Liposuctioned (mL) | 3600 (2800–4700) | 3650 (2700–4600) | 3600 (2950–4800) | 0.621 |
| No data (n) | 5 | 2 | 3 | |
| Time (min) | 225 (180–266) | 223.5 (173–269) | 225 (181–264) | 0.858 |
| No data (n) | 62 | 30 | 32 | |
| 10-min wait | 0.530 | |||
| Yes | 275 (64.4) | 139 (66.8) | 136 (62.1) | |
| No | 76 (17.8) | 33 (15.9) | 43 (19.6) | |
| No data (n) | 76 (17.8) | 36 (17.3) | 40 (18.3) | |
| Pronation (min) | 72 (57.5) | 70 (55–90) | 75 (60–90) | 0.223 |
| No data (n) | 47 | 20 | 27 | |
| Preoperative levels | ||||
| Hemoglobin (mg/dL) | 13.7 (13.0–14.3) | 13.6 (13.0–14.3) | 13.7 (13.0–14.3) | 0.913 |
Estimated difference with the Fisher exact test. Quantitative variables are reported as medians and interquartile range.
BMI, body mass index.
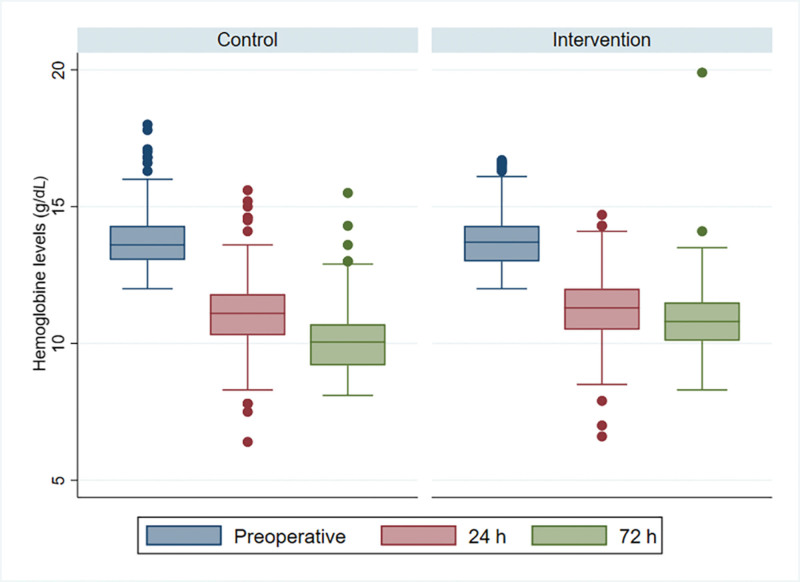
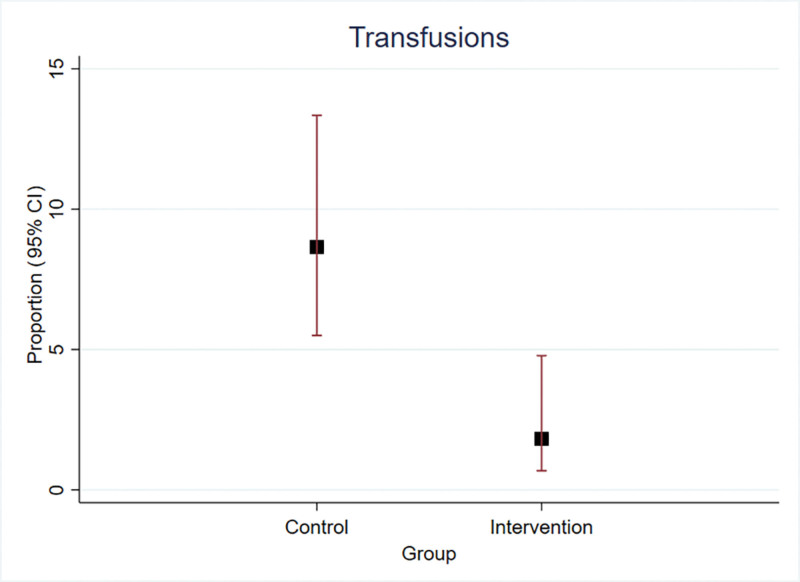
Median postoperative hemoglobin levels at 24 hours were similar in both groups [24 h: study 11.3 g/dL (95% CI 10.5–12.0) versus control 11.1 g/dL (95% CI 10.3–11.8) (P = 0.07)]; however, at 72 hours, postoperative hemoglobin was higher in the study group [10.8 g/dL (95% CI 10.1–11.5)] versus control [10.0 g/dL (95% CI 9.2–10.7), P ≤ 0.001] (Fig. 2). The overall incidence of transfusions was 5.15% (95% CI 3.41–7.71), being higher in the control group [8.65% (95% CI 5.50–13.34)] compared with the study group [1.82% (95% CI 0.68–4.78)] (Fig. 3); this corresponds to an absolute risk reduction of −6.8% (95% CI −11.0 to −2.6) and a number needed to treat of 14.6 (95% CI 8.6–37.6). The effect of TXA in the study group decreased the incidence of transfusions compared with the control group (Table 2). Taking into account that the age was statistically different between the study group and the control group, an adjustment was made in the estimate of the effect; however, the variation of the coefficient was not significant (Table 2). There were no thromboembolic adverse events in either group or adverse effects related to the use of TXA.
Fig. 2.
Hemoglobin levels in the group with only oral TXA (control) and in the group with oral and IV TXA (intervention). Levels were measured preoperatively and 24 and 72 hours after surgery.
Fig. 3.
Incidence of transfusions in the group with only oral TXA (control) and in the group with oral and IV TXA (intervention).
Table 2.
Estimation of the Effect of IV and Oral TXA on the Need for Transfusions
| Model | Coefficient (95% CI) | Relative Risk (95% CI) |
|---|---|---|
| Without adjustment | −1.62 (−2.72 to −0.52) | 0.21 (0.07–0.61) |
| Age adjusted | −1.66 (−2.76 to −0.55) | 0.20 (0.07–0.59) |
DISCUSSION
The blood coagulation process is a complex mechanism that involves different factors and stages, beginning when there is a trauma that triggers this mechanism, with thrombin playing a central role in this process.24 Thrombin is derived from the degradation of prothrombin, and one of its main functions is in the conversion of fibrinogen to fibrin. Fibrinogen is a soluble protein present in the blood, and when acted on by thrombin, it breaks specific peptide bonds and forms a three-dimensional network of fibrin filaments. These filaments intertwine to form a stable network that constitutes a blood clot. After the formation of a blood clot, its lysis or disintegration is also necessary. Fibrinolysis is the natural process that dissolves clots formed during coagulation, and in this process, plasminogen plays the main role. Plasminogen is a protein present in the blood that is produced in the liver and is the inactive precursor of the enzyme plasmin. It is secreted as an inactive form and is activated when a blood vessel is injured or ruptured. Plasmin is a serine protease that breaks down fibrin, the main component of blood clots. The balance between the activation of prothrombin and later of plasminogen with the formation of plasmin, to disintegrate fibrin, is crucial to maintain adequate coagulation and fibrinolysis in the body.24
TXA is a synthetic antifibrinolytic derived from the amino acid lysine that decreases bleeding in three ways.5 It competitively binds to the active site of plasmin, preventing its proteolytic action on the fibrin peptide bonds that are forming the clot. In addition to competing with plasmin for the active site, TXA has the ability to bind directly to fibrin, forming a stable complex with the blood clot. This binding strengthens and stabilizes the fibrin structure, preventing its breakdown and further delaying fibrinolysis. Finally, by blocking the binding and action of plasminogen activators, such as tissue plasminogen activator, TXA reduces the conversion of plasminogen to active plasmin and thus also decreases clot lysis capacity.5 Although plasmin can still be formed under these circumstances, it cannot bind to or degrade fibrin.24 Suppression of fibrinolysis by TXA is manifested in surgical patients by reductions in blood D-dimer levels, but the drug has no effect on blood coagulation parameters. Co-administration of heparin does not influence TXA activity.24
The activity of the trans-isomer of TXA was first described in 1964 by Okamoto et al.25 Since then, the drug has been used as an antifibrinolytic in a wide variety of clinical settings, with multiple meta-analyses indicating its effectiveness in different areas of medicine. It is used to decrease bleeding from the gastrointestinal tract,7 in orthopedics in knee and hip surgery,8,26 to decrease menstrual bleeding,9 to perform endoscopies in patients predisposing to bleeding,10 in hereditary bleeding disorders,12 rhinoplasty,27 postpartum bleeding,28 and many other situations.
Despite the fact that its use has been widely accepted for several years in other branches of medicine, its use in plastic surgery is very recent. Rohrich et al found in a search carried out in 2017 that there were only four articles on its use: two in nasal surgery where it was used orally and another two used topically, one in face lift and another in reduction mammoplasty.13 In the same article, they use it in rhinoplasty, breast augmentation, blepharoplasty, face lift, and abdominoplasty, applying it exclusively in the form of irrigation on the wound or in gauze impregnated with TXA.13 Its use in body contouring is even more recent and continues to be very limited. In 2021, in a systematic search of the literature by Laikhter et al, they found only three articles indicating the benefit of TXA in liposuction, reducing bleeding secondary to surgery.29 One of the first published studies on body contouring surgery was in 2018 by Cansancao et al,18 which, making a comparative study between two groups undergoing liposuction, found that patients who were administered a dose of TXA 30 minutes preoperatively and another dose 30 minutes postoperatively had up to 52.6% less bleeding and 48% less decrease in hematocrit at 7 postoperative days compared with patients who were administered placebo. El Minawi et al also found significantly less bleeding and a lower incidence and severity of ecchymosis in patients who were administered TXA by subcutaneous infiltration for tumescence or IV half an hour before starting surgery compared with a control group.19 In this study, bleeding was less with subcutaneous administration than with IV administration. Hoyos et al found, in a multicenter, randomized, double-blind study, greater effectiveness when using IV TXA half an hour before the start of liposuction, than when using it in the tumescent subcutaneous infiltration than in the placebo group.17 Rodriguez-Garcia and Sanchez-Peña also found less bleeding in the group in which TXA was used in the tumescent infusion before liposuction than in the group in which it was not used.20
In previous studies, we had found the efficiency of the use of 1 g of IV TXA half an hour before the start of body contouring surgery, where blood loss had been less in the group where TXA was used compared with the control group where TXA was not used.17 However, considering that bleeding persists after body contouring surgical procedures, because the area worked on is wide, and wide detachments, it was decided to carry out this study, the main premise of which was to extend the use of TXA up to 48 hours after surgery. The doses used in the study group, 1 g at 12, 24, and 48 hours after surgery, do not exceed the pharmacological values of their indications,16 which correspond to 10–15 mg/kg dose. In both groups, the decrease in hemoglobin values at 24 hours was similar. However, in the group where we continued the use of TXA for 48 hours, the decrease in hemoglobin was less than in the group without postsurgical TXA. Similarly, the need for transfusions in the group without postoperative TXA was greater. Both results reached statistical significance.
Something that is very important to point out is that there were no undesirable or secondary effects in either of the two TXA use schemes, especially the absence of deep vein thrombosis (DVT) data using TXA for 48 hours. There are already scientific reports that there is no higher incidence of DVT with the use of TXA in patients undergoing any surgical procedure. Through a meta-analysis carried out in 2017 by Guo et al, they investigated the beneficial effects and undesirable effects of the use of oral TXA used up to 24 hours after surgery in patients undergoing knee arthroplasty, compared with control groups.8 In this study, a lower reduction in hemoglobin, hematocrit, and bleeding was found in the TXA groups, without finding statistically significant differences in thromboembolic events between the two groups.8 Reale et al in 2021 carried out another meta-analysis in 140 randomized controlled studies on the complications of the use of oral, IV, or intra-articular TXA in patients undergoing orthopedic surgery of the lower extremities. No differences were found in thromboembolic complications between the groups to which TXA was administered in any of its routes of administration, versus the control groups.11 The incidence of these complications is 2.4% and 2.8%, respectively. Murao et al also carried out a systematic review and meta-analysis in 2021 including 234 studies with 102,681 patients to find out complications from the IV use of TXA in patients with bleeding. The presence of thromboembolic events, acute coronary syndrome, heart attack, and seizures was sought. No evidence of an increased risk of venous thromboembolism was found in patients receiving TXA. What they did find was an increased risk of seizures in patients who were administered more than 2 g/d.30 In a similar meta-analysis carried out in 2021 by Taeuber et al on the association of TXA with thromboembolic events and mortality, in 216 studies including 125,550 patients, they found 2.1% of thromboembolic events in patients in whom TXA was administered versus 2.0% in those who were not administered TXA; therefore, there was no association between the use of TXA and the risk of presenting deep vein thrombosis, pulmonary embolism, cardiac infarction or ischemia, and cerebral infarction or ischemia.31 In our study, no case of deep vein thrombosis was detected in either of the two groups, although here it should be noted that we used chemical thromboprophylaxis in patients for whom its application was indicated.32,33 We also had no cases of bleeding secondary to this management or any other side effect from the use of TXA.
Our study is currently the randomized clinical trial that includes the largest number of patients investigating the efficacy of TXA in body contouring surgery. We had previously shown the effectiveness of TXA in liposuction by administering it intravenously 30 minutes before surgery.17 However, this new study differs from all previous scientific reports showing that the effectiveness is even greater when the administration of TXA is continued after the surgical procedure has finished. Despite the fact that bleeding was similar in both groups at 24 hours postoperatively, the group that received three additional oral doses of TXA had even less bleeding and therefore required fewer transfusions. Both results reached statistical significance. This shows us that blood loss secondary to liposuction continues to be minimal for the first 48 hours postsurgery, and the use of TXA during this time provides even greater protection against postsurgical anemia and the need for blood transfusions. Subsequent studies should determine if its use greater than 48 hours after surgery extends this protection, further reducing bleeding.
CONCLUSIONS
The use of TXA in body contouring surgery proved to be more effective in reducing trans- and postsurgical bleeding and reducing the need for blood transfusions, when used preoperatively and continued for 48 postsurgical hours, than when used only preoperatively. Extending its use for 48 postsurgical hours did not imply an increased risk of adverse reactions or thromboembolic effects.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online 15 November 2023.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.International I, On S. International Society of Aesthetic Plastic Surgery. ISAPS international survey on aesthetic/cosmetic procedures performed in 2021. 2021. Available at https://www.isaps.org/media/vdpdanke/isaps-global-survey_2021.pdf. Accessed May 1, 2023.
- 2.Cook J, Pozner JN, Turer DM, et al. Roundtable discussion: making sense of current liposuction technologies. Aesthetic Surg J Open Forum. 2022;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayter-Marin JE, Cárdenas-Camarena L, Durán H, et al. Effects of thermal protection in patients undergoing body contouring procedures: a controlled clinical trial. Aesthetic Surg J. 2018;38:448–456. [DOI] [PubMed] [Google Scholar]
- 4.Bayter-Marin JE, Cárdenas-Camarena L, Peña WE, et al. Patient blood management strategies to avoid transfusions in body contouring operations: controlled clinical trial. Plast Reconstr Surg. 2021;147:355–363. [DOI] [PubMed] [Google Scholar]
- 5.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011:CD001886. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776–781. [DOI] [PubMed] [Google Scholar]
- 7.Lee PL, Yang KS, Tsai HW, et al. Tranexamic acid for gastrointestinal bleeding: a systematic review with meta-analysis of randomized clinical trials. Am J Emerg Med. 2021;45:269–279. 10.1016/j.ajem.2020.08.062. [DOI] [PubMed] [Google Scholar]
- 8.Olldashi F, Kerçi M, Zhurda T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 9.Basrai Z. Tranexamic acid treatment for heavy menstrual bleeding. J Emerg Med. 2011;40:479. [Google Scholar]
- 10.Davis A, Walsh M, Mccarthy P, et al. Tranexamic acid without prophylactic factor replacement for prevention of bleeding in hereditary bleeding disorder patients undergoing endoscopy: a pilot study. Haemophilia. 2013;19:583–589. [DOI] [PubMed] [Google Scholar]
- 11.Reale D, Andriolo L, Gursoy S, et al. Complications of tranexamic acid in orthopedic lower limb surgery: a meta-analysis of randomized controlled trials. Biomed Res Int. 2021;2021:6961540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisthoff UW, Seyfert UT, Kübler M, et al. Treatment of epistaxis in hereditary hemorrhagic telangiectasia with tranexamic acid—a double-blind placebo-controlled cross-over phase IIIB study. Thromb Res. 2014;134:565–571. [DOI] [PubMed] [Google Scholar]
- 13.Rohrich RJ, Cho MJ. The role of tranexamic acid in plastic surgery: review and technical considerations. Plast Reconstr Surg. 2018;141:507–515. [DOI] [PubMed] [Google Scholar]
- 14.Murphy GRF, Glass GE, Jain A. The efficacy and safety of tranexamic acid in cranio-maxillofacial and plastic surgery. J Craniofac Surg. 2016;27:374–379. [DOI] [PubMed] [Google Scholar]
- 15.Ausen K, Fossmark R, Spigset O, et al. Safety and efficacy of local tranexamic acid for the prevention of surgical bleeding in soft-tissue surgery: a review of the literature and recommendations for plastic surgery. Plast Reconstr Surg. 2022;149:774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown S, Yao A, Taub PJ. Antifibrinolytic agents in plastic surgery: current practices and future directions. Plast Reconstr Surg. 2018;141:937e–949e. [DOI] [PubMed] [Google Scholar]
- 17.Hoyos AE, Duran H, Cardenas-Camarena L, et al. Use of tranexamic acid in liposculpture: a double-blind, multicenter, randomized clinical trial. Plast Reconstr Surg. 2022;150:569–577. [DOI] [PubMed] [Google Scholar]
- 18.Cansancao AL, Condé-Green A, David JA, et al. Use of tranexamic acid to reduce blood loss in liposuction. Plast Reconstr Surg. 2018;141:1132–1135. [DOI] [PubMed] [Google Scholar]
- 19.El Minawi HM, Kadry HM, El-Essawy NM, et al. The effect of tranexamic acid on blood loss in liposuction: a randomized controlled study. Eur J Plast Surg. 2023;46:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Garcia F, Sanchez-Peña M. Efficacy and safety of tranexamic acid for the control of surgical bleeding in patients under liposuction. Aesthetic Plast Surg. 2022;46:258–264. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, et al. ; CONSORT. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- 22.EPIDAT—Consellería de Sanidade—Servizo Galego de Saúde. 2022. Available at https://www.sergas.es/Saude-publica/EPIDAT?idioma=es. Accessed May 1, 2023.
- 23.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. [DOI] [PubMed] [Google Scholar]
- 24.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto S, Hijikata-Okunomiya A, Wanaka K, et al. Enzyme-controlling medicines: introduction. Semin Thromb Hemost. 1997;23:493–501. [DOI] [PubMed] [Google Scholar]
- 26.Kayupov E, Fillingham YA, Okroj K, et al. Oral and intravenous tranexamic acid are equivalent at reducing blood loss following total hip arthroplasty. J Bone Joint Surg Am. 2017;99:373–378. [DOI] [PubMed] [Google Scholar]
- 27.Eftekharian HR, Rajabzadehy Z. The efficacy of preoperative oral tranexamic acid on intraoperative bleeding during rhinoplasty. J Craniofac Surg. 2016;27:97–100. [DOI] [PubMed] [Google Scholar]
- 28.Diop A, Abbas D, Ngoc NTN, et al. A double-blind, randomized controlled trial to explore oral tranexamic acid as adjunct for the treatment for postpartum hemorrhage. Reprod Health. 2020;17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laikhter E, Comer CD, Shiah E, et al. A systematic review and meta-analysis evaluating the impact of tranexamic acid administration in aesthetic plastic surgery. Aesthetic Plast Surg. 2022;42:548–558. [DOI] [PubMed] [Google Scholar]
- 30.Murao S, Nakata H, Roberts I, et al. Effect of tranexamic acid on thrombotic events and seizures in bleeding patients: a systematic review and meta-analysis. Crit Care. 2021;25:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taeuber I, Weibel S, Herrmann E, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality. JAMA Surg. 2021;156:e210884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannucci CJ, Macdonald JK, Ariyan S, et al. Benefits and risks of prophylaxis for deep venous thrombosis and pulmonary embolus in plastic surgery: a systematic review and meta-analysis of controlled trials and consensus conference. Plast Reconstr Surg. 2016;137:709–730. [DOI] [PubMed] [Google Scholar]
- 33.Cuenca-Pardo J, Ramos-Gallardo G, Cárdenas-Camarena L, et al. Searching for the best way to assess the risk of thrombosis in aesthetic plastic surgery; the role of the Caprini/Pannucci score. Aesthetic Plast Surg. 2019;43:1387–1395. [DOI] [PubMed] [Google Scholar]