Hepatorenal syndrome (HRS) is a well-described decompensating event in patients with cirrhosis and is associated with high mortality.1 Although widely recognized, the clinical diagnostic criteria for HRS have evolved over the years. The most recent Revised Consensus Recommendations from the International Club of Ascites proposed the diagnostic criteria for HRS, which include diagnosis of cirrhosis with ascites, no response after 2 days of diuretic withdrawal and administration of albumin (to rule out prerenal azotemia), absence of shock (which would be more associated with acute tubular necrosis), and absence of macroscopic signs of structural kidney injury (such as hematuria and proteinuria).2 Urine biomarkers may be helpful in distinguishing HRS from other etiologies of renal injury. In particular, fractional excretion of urea <21% is more predictive of HRS than prerenal azotemia, whereas values <28% are more predictive of HRS than nonHRS.3 Additionally, high fractional excretion of sodium may help rule out HRS,4 and urinary neutrophil gelatinase–associated lipocalin >220 ng/mL is predictive of acute tubular necrosis and nonresponse to terlipressin.5 Figure 1 proposes a diagnostic framework for diagnosing HRS in patients with cirrhosis and ascites. For decades, terlipressin has been used in the European Union and other regions across the world for HRS, but it did not obtain the US Food and Drug Administration (FDA) approval until 2022. In light of the recent FDA approval, clinical treatment algorithms for HRS are currently being developed by hospital systems across the country to facilitate the safe incorporation of terlipressin into clinical applications. The purpose of this review is to explore the efficacy of terlipressin in the context of other treatment modalities currently used for HRS and review guidelines and recommendations for its clinical use.
FIGURE 1.
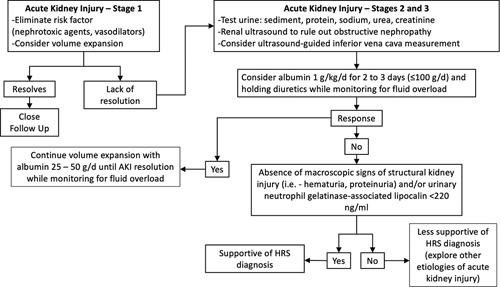
Proposed framework for initial management of acute kidney injury in patients with cirrhosis. Abbreviations: AKI, acute kidney injury; HRS, hepatorenal syndrome.
Mechanistically, terlipressin is a prohormone that is cleaved to its active form, lysine vasopressin. This conversion step allows for prolongation of half-life, allowing for intermittent infusions outside of an intensive care setting rather than continuous infusions that typically require closer monitoring.6 In its active form, terlipressin has a greater affinity for V1 than V2 receptors, which are selectively located in the splanchnic circulation, resulting in decreased portal pressures.7 However, its main pharmacologic effect most likely relies on an increase in systemic (mean) arterial blood pressure and resetting of renal perfusion pressures back to their autoregulatory range.8
The efficacy of terlipressin in treating HRS has been evaluated in multiple placebo-controlled trials. The primary treatment endpoints usually assessed in these trials include treatment success, defined as a serum creatinine <1.6 mg/dL without recurrence or dialysis, and the less stringent endpoint of HRS reversal, defined as a serum creatinine <1.6 mg/dL without dialysis regardless of recurrence in 14 days. The first modest-sized placebo-controlled trial comparing terlipressin to placebo was published in 2008 and included 112 HRS subjects from 35 medical centers. Compared to the placebo group, the terlipressin group had higher HRS reversal rates (33.9% vs. 12.5%, respectively) and double the rate of treatment success at 14 days (25% vs. 12.5%, respectively), but only the former finding was statistically significant. There was no statistically significant difference in 6-month overall survival or transplant-free survival.9 The CONFIRM trial was a larger phase 3 clinical trial with 300 patients, which also demonstrated HRS reversal rates that were significantly higher in patients receiving terlipressin relative to placebo (39% vs. 18%, respectively) with similar findings when a more stringent endpoint of verified reversal of HRS (serum creatinine <1.6 for 2 consecutive measurements up to 14 d without dialysis for additional 10 d) was used. Though no significant difference in overall 90-day survival was seen between the 2 groups, the trial was not specifically powered to detect a survival difference. There were, however, higher rates of 90-day mortality from respiratory causes in the terlipressin group (11% vs. 2%).10 Notably, ad hoc analyses of pooled data from HRS clinical trials found that patients with a creatinine <5 mg/dL benefited the most from terlipressin and had potential improvements in transplant-free survival.11
Compared to other treatments for HRS, terlipressin has been found to be equivocal or superior and has unique clinical advantages. In the absence of terlipressin, the primary treatment used for HRS in the United States in nonintensive care settings includes the combination of 3 different medications—escalating doses of midodrine together with octreotide and albumin (MOA). Terlipressin has been shown to be more efficacious than MOA in a randomized control trial comparing 27 HRS patients receiving terlipressin with albumin to 22 patients receiving MOA. Patients in the terlipressin + albumin group had significantly higher rates of HRS reversal (55% vs. 4.8%), but no significant difference in survival at 1 and 3 months relative to the MOA group.12 Terlipressin has also been shown to be as effective or more effective than other vasopressors13–15 and has the unique clinical advantage of not being restricted to patients with central lines in intensive care settings like most other vasopressors.
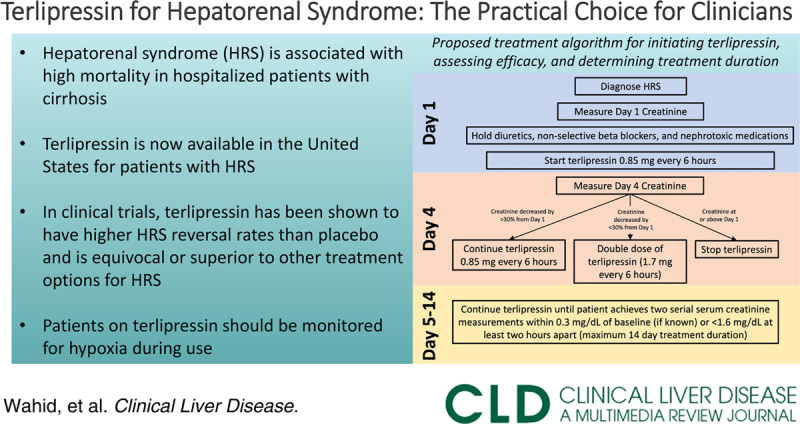
With the recent FDA approval of terlipressin for HRS, hospital systems are currently developing clinical algorithms for terlipressin. Though terlipressin is currently only FDA-approved for intermittent dosing in the United States, a recent randomized control trial comparing intermittent bolus versus continuous infusion dosing found lower rates of adverse events in the continuous infusion group (35.3% vs. 62.2%, respectively) and no significant difference in treatment response.16 The primary contraindications and common adverse reactions of terlipressin are summarized in Table 1. Patients with a baseline serum creatinine of >5 mg/dL are also unlikely to experience benefit from the use of terlipressin. Considering the acute-on-chronic liver failure grade17 in patients with HRS is important prior to initiating treatment because patients with grade 3 (or higher) acute-on-chronic liver failure had reduced survival with terlipressin, mostly due to higher rates of respiratory failure.18 Given that higher rates of respiratory failure were seen in patients receiving terlipressin compared with placebo in the CONFIRM trial,10 it is recommended that patients with hypoxia should not initiate terlipressin, and continuous pulse oximetry should be used for the duration of terlipressin treatment with discontinuation of treatment if hypoxia (ie, SpO2 <90%) develops. The median onset of respiratory failure was 5 days from initiation of terlipressin. Factors associated with respiratory failure in patients treated with terlipressin include acute-on-chronic liver failure grade 3 and those with intravascular volume overload.10 Judicious use of albumin and i.v. fluids is recommended to avoid volume overload. Figure 2 proposes a treatment algorithm for initiating terlipressin, assessing efficacy, and determining treatment duration.
TABLE 1.
Contraindications and common adverse reactions of terlipressin
| Contraindications | Adverse reactions |
|---|---|
| Coronary ischemia | Abdominal pain |
| Peripheral ischemia | Diarrhea |
| Mesenteric ischemia | Nausea |
| Hypoxia | Dyspnea |
| ≥Grade 3 acute-on-chronic liver failure | Respiratory failure |
FIGURE 2.
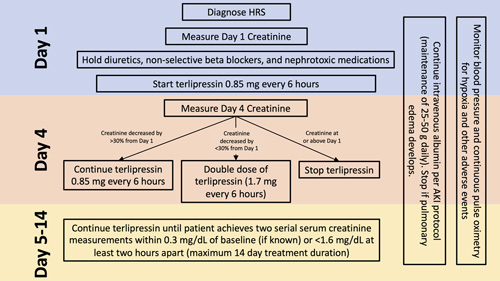
Proposed treatment algorithm for initiating terlipressin, assessing efficacy, and determining treatment duration. Abbreviations: AKI, acute kidney injury; HRS, hepatorenal syndrome.
Terlipressin has extensive trial and real-world empiric data demonstrating its effectiveness and safety for treating HRS. Although no clear survival benefit of terlipressin has been demonstrated compared to preexisting treatments for HRS like MOA and vasopressors, it effectively improves renal function and can be administered through a peripheral line outside of intensive care settings—providing a unique practical advantage. As providers in the United States gain more experience with using terlipressin, it will certainly become the mainstay of the treatment for HRS. Experience from centers outside the United States, where terlipressin has been used for over a decade, will prove to be invaluable in developing clinical algorithms for using terlipressin domestically.
Acknowledgments
CONFLICTS OF INTEREST
Andres Duarte-Rojo consults for and received grants from Axcella. He received grants from Echosens and Mallinkrodt. Justin R. Boike consults for and received grants from W.L. Gore and Associates. He consults for Cook Medical and FUJIFILM. The remaining author has no conflicts to report.
EARN CME FOR THIS ARTICLE
Footnotes
Abbreviations: FDA, US Food and Drug Administration; HRS, hepatorenal syndrome; MOA, midodrine with octreotide and albumin.
Contributor Information
Nabeel Wahid, Email: nabeel.wahid@northwestern.edu.
Andres Duarte-Rojo, Email: andres.duarte@northwestern.edu.
Justin R. Boike, Email: justin.boike@northwestern.edu.
REFERENCES
- 1.Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020;370:m2687. [DOI] [PubMed] [Google Scholar]
- 2.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–7. [DOI] [PubMed] [Google Scholar]
- 3.Patidar KR, Kang L, Bajaj JS, Carl D, Sanyal AJ. Fractional excretion of urea: a simple tool for the differential diagnosis of acute kidney injury in cirrhosis. Hepatology. 2018;68:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambino C, Piano S, Stenico M, Tonon M, Brocca A, Calvino V, et al. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and acute kidney injury. Hepatology. 2023;77:1630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarin SK, Sharma P. Terlipressin: an asset for hepatologists!. Hepatology. 2011;54:724–8. [DOI] [PubMed] [Google Scholar]
- 7.Møller S, Hansen EF, Becker U, Brinch K, Henriksen JH, Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver. 2000;20:51–9. [DOI] [PubMed] [Google Scholar]
- 8.Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818–28. [DOI] [PubMed] [Google Scholar]
- 11.Curry MP, Vargas HE, Befeler AS, Pyrsopoulos NT, Patwardhan VR, Jamil K. Early treatment with terlipressin in patients with hepatorenal syndrome yields improved clinical outcomes in North American studies. Hepatol Commun. 2023;7:e1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62:567–74. [DOI] [PubMed] [Google Scholar]
- 13.Israelsen M, Krag A, Allegretti AS, Jovani M, Goldin AH, Winter RW, et al. Terlipressin versus other vasoactive drugs for hepatorenal syndrome. Cochrane Database Syst Rev. 2017;9:Cd011532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arora V, Maiwall R, Rajan V, Jindal A, Muralikrishna Shasthry S, Kumar G, et al. Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure. Hepatology. 2020;71:600–10. [DOI] [PubMed] [Google Scholar]
- 15.Singal AK, Palmer G, Melick L, Abdallah M, Kwo P. Vasoconstrictor therapy for acute kidney injury hepatorenal syndrome: a meta-analysis of randomized studies. Gastro Hep Advances. 2023;2:p455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63:983–92. [DOI] [PubMed] [Google Scholar]
- 17.Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. [DOI] [PubMed] [Google Scholar]
- 18.Wong F, Pappas SC, Reddy KR, Vargas H, Curry MP, Sanyal A, et al. Terlipressin use and respiratory failure in patients with hepatorenal syndrome type 1 and severe acute-on-chronic liver failure. Aliment Pharmacol Ther. 2022;56:1284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


