Abstract
Plant roots are gravitropic, detecting and responding to changes in orientation via differential growth that results in bending and reestablishment of downward growth. Recent data support the basics of the Cholodny-Went hypothesis, indicating that differential growth is due to redistribution of auxin to the lower sides of gravistimulated roots, but little is known regarding the molecular details of such effects. Here, we investigate auxin and gravity signal transduction by demonstrating that the endogenous signaling molecules nitric oxide (NO) and cGMP mediate responses to gravistimulation in primary roots of soybean (Glycine max). Horizontal orientation of soybean roots caused the accumulation of both NO and cGMP in the primary root tip. Fluorescence confocal microcopy revealed that the accumulation of NO was asymmetric, with NO concentrating in the lower side of the root. Removal of NO with an NO scavenger or inhibition of NO synthesis via NO synthase inhibitors or an inhibitor of nitrate reductase reduced both NO accumulation and gravitropic bending, indicating that NO synthesis was required for the gravitropic responses and that both NO synthase and nitrate reductase may contribute to the synthesis of the NO required. Auxin induced NO accumulation in root protoplasts and asymmetric NO accumulation in root tips. Gravistimulation, NO, and auxin also induced the accumulation of cGMP, a response inhibited by removal of NO or by inhibitors of guanylyl cyclase, compounds that also reduced gravitropic bending. Asymmetric NO accumulation and gravitropic bending were both inhibited by an auxin transport inhibitor, and the inhibition of bending was overcome by treatment with NO or 8-bromo-cGMP, a cell-permeable analog of cGMP. These data indicate that auxin-induced NO and cGMP mediate gravitropic curvature in soybean roots.
Plant growth and development are profoundly influenced by gravity. When roots are gravistimulated by horizontal orientation, they respond by bending via differential growth so as to recover their normal orientation with respect to gravity, the gravitropic set-point angle (Perbal and Driss-Ecole, 2003). Gravity perception and responses probably involve mechanosensing in the root cap and changes in calcium and pH (and other second messengers) that result in relocation of auxin efflux carriers and subsequent lateral (downward) transport of auxin, inducing differential growth and downward bending (Blancaflor and Masson, 2003; Ottenschläger et al., 2003; Perbal and Driss-Ecole, 2003).
Although recent data provide support for the Cholodny-Went hypothesis, one of the oldest and best known in plant science, explaining root gravitropism by downward redistribution of auxin, the precise details of auxin signaling remain somewhat unclear. In this study, we report that gravistimulation induced the asymmetric accumulation of nitric oxide (NO) on the lower side of the apical region of soybean (Glycine max) seedling roots. NO is a diffusible multifunctional molecule involved in numerous processes in bacteria, fungi, animals, and plants. In plants, NO is an endogenous signaling molecule implicated in a growing number of developmental and physiological processes, including plant defense, root initiation, stomatal closure in response to abscisic acid, salt tolerance, seed germination, nutrition, and flowering (Lamattina et al., 2003; Neill et al., 2003; He et al., 2004; Wendehenne et al., 2004). Potential sources of NO in plants include NO synthase (NOS; Guo et al., 2003) and nitrate reductase (NR), in addition to other potential enzymatic and nonenzymatic sources (Neill et al., 2003; Bethke et al., 2004). In mammalian cells, it is known that NO activates the enzyme guanylyl cyclase (GC), resulting in the accumulation of cGMP. NO was reported to induce a transient increase in cGMP in tobacco (Nicotiana tabacum) cells, and specific GC inhibitors were able to suppress NO-induced gene expression (Durner et al., 1998). Moreover, stomatal closure and programmed cell death that are both inducible by NO can also both be inhibited by GC inhibitors, such inhibition being reversible by addition of cell-permeable cGMP analogs (Neill et al., 2003).
NO is synthesized in roots (Pagnussat et al., 2002; Guo et al., 2003), and both NO and cGMP mediate auxin-induced root organogenesis (Pagnussat et al., 2003), but the roles of NO and cGMP in gravity responses are not yet known. Here, gravistimulation of soybean roots induced asymmetric NO accumulation, and direct NO application to the lower side of horizontal roots enhanced gravitropic curvature, whereas application to the upper side suppressed it. Auxin induced NO accumulation in root protoplasts, and asymmetric auxin application to root tips resulted in asymmetric NO accumulation. Moreover, treatments that removed NO or inhibited its synthesis also inhibited gravitropic bending. In addition, both gravistimulation and auxin induced the accumulation of cGMP. Together, these data indicate that NO and cGMP mediate auxin-induced gravitropic bending in soybean roots.
RESULTS
Gravistimulation and Auxin Induce NOAccumulation in Soybean Root Tips
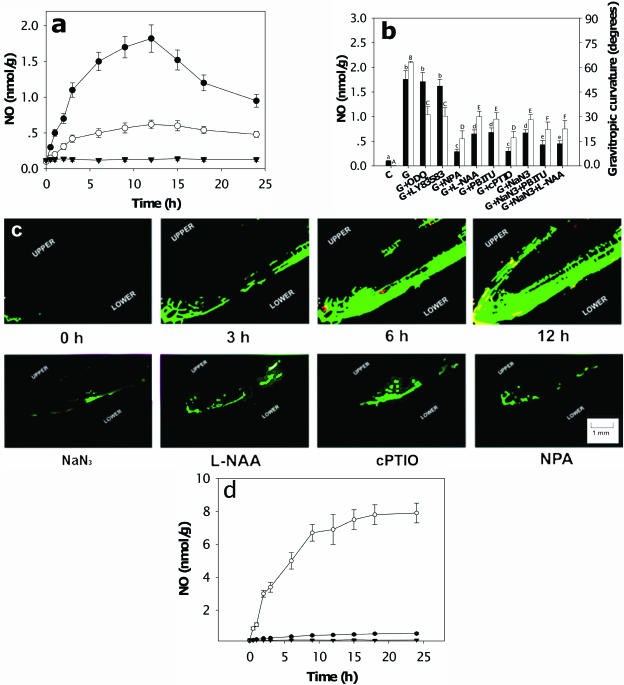
Gravistimulation induced rapid and substantial NO accumulation in the apical regions (Zone 1) of soybean primary roots. NO also accumulated in more basal root tissue (Zone 2) but to a lesser extent (Fig. 1a). To determine a potential role for NO in gravitropic bending, we used the NO scavenger 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), the NOS inhibitors Nω-nitro-l-Arg (l-NNA) and S,S′-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea (PBITU), and the putative NR inhibitor NaN3. To assess any requirement for lateral auxin transport, we used N-1-naphthylphthalamic acid (NPA). Dose-response data for these compounds are shown in Supplemental Figure 1. Figure 1b shows that pretreatment with all these compounds (cPTIO at 50 μm, l-NAA and NaN3 at 20 μm, PBITU and NPA at 30 μm) significantly reduced both gravitropic bending and NO accumulation (P < 0.05; one-way ANOVA followed by a Tukey's test). Treatment with both a NOS and NR inhibitor did reduce both NO content and gravicurvature more than when each compound was applied singly, although inhibition was still not total (Fig. 1b). We also tested the effects of these compounds alone at the above concentrations on soybean root growth: there were no negative effects (P < 0.05). Confocal microscopy using the NO indicator dye diaminofluorescein diacetate (DAF-2DA) demonstrated that NO accumulation was asymmetric, with NO being detected predominantly in the lower cells (Fig. 1c). Moreover, NO accumulation appeared to begin in the most apical region of the root (Fig. 1). Gravity-induced increases in fluorescence were not observed when 4-aminofluorescein diacetate (4-AF DA, the negative control for DAF-2DA) was used (data not shown). Pretreatment with NaN3, l-NNA, cPTIO, or NPA all reduced NO fluorescence (Fig. 1c). At low concentrations (1 and 5 μm), NPA had a greater effect on NO content than it did on gravibending (Supplemental Fig. 1). At these concentrations of NPA, NO distribution in the roots was still asymmetric (data not shown), whereas NO asymmetry was disrupted at higher concentrations, offering a potential explanation for this discrepancy. We also determined the effects of gravistimulation on asymmetric NO accumulation using the hemoglobin assay. Although there were some differences in the absolute amount of NO (for example, Fig. 1a, 1.7 ± 0.18 nmol/g; and Fig. 1d, 3.1 ± 0.23 nmol/g [averaged between upper and lower halves], both after 12 h of gravity treatment; note that these data were obtained at different times using different batches of plant material and assay components), the trends in [NO] were reproducible and confirmed that the NO content of the lower half of horizontal roots increased considerably following gravistimulation. Taken together, these data indicate that NO is required for the gravitropic response and suggest that auxin redistribution activates NO synthesis, potentially via both NOS- and NR-like enzymes.
Figure 1.
Gravistimulation induces the rapid accumulation of NO required for gravitropism in soybean roots. a, Kinetics of NO accumulation in Zone 1 (•) or Zone 2 (○) from horizontal roots. ▾, Vertical roots. b, Correlation between NO accumulation in Zone 1 (black bars) and gravitropic curvature (white bars). Roots were pretreated as indicated for 12 h and then placed horizontally for 12 h (50 μm cPTIO; 20 μm l-NAA and NaN3; 30 μm PBITU and NPA; 100 μm ODQ and LY83583). On x axis: C, Control; G, gravistimulated. Values are the mean ± se for five independent experiments. Data analyzed by one-way ANOVA followed by Tukey's test. Different symbols indicate significant differences between treatments (P < 0.05). c, Gravistimulation induces asymmetric NO accumulation. Soybean roots were loaded with DAF-2DA and gravistimulated by orientating horizontally. Fluorescence intensity of dissected root tips was observed at the indicated times by confocal fluorescence microscopy. Effects of pretreatment with 20 μm NaN3, 20 μm l-NNA, 50 μm cPTIO, and 10 μm NPA are shown. The pictures were taken after 6 h treatment. Scale bar = 1 mm. Experiments were repeated at least five times with similar results. d, Asymmetric accumulation of NO in root Zone 1. Soybean roots were gravistimulated for different times, bisected horizontally with a razor blade, and NO content assayed. ▾, Vertical roots; ○, lower half of horizontal root; •, upper half of horizontal root.
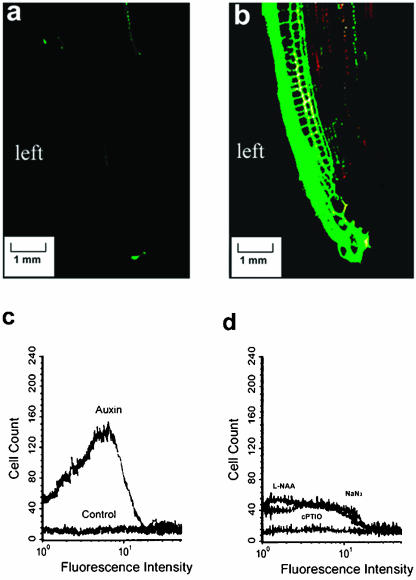
We next examined the effect of auxin on NO accumulation in both intact roots and root protoplasts. The dose-response data for auxin-induced NO accumulation are presented in Supplemental Figure 2. Auxin at 5 μm significantly induced NO accumulation in the root tip. Furthermore, when the auxin was applied unilaterally to roots via agar blocks, NO accumulation was restricted to the side of the root in contact with the auxin-containing agar (Fig. 2, a and b). Auxin also induced rapid NO generation in root protoplasts (Fig. 2c). Replacing DAF-2DA with 4-AF DA did not give rise to fluorescence in the auxin-treated protoplasts (data not shown). NaN3 and l-NAA treatment suppressed auxin-induced NO generation but not completely, whereas treatment with cPTIO strongly suppressed it (Fig. 2d).
Figure 2.
Auxin induces NO accumulation. a and b, Soybean roots were loaded with 10 μm DAF-2DA for 30 min and then control buffer (A) or 5 μm IAA (B) applied via agar block to the left-hand side of the vertically orientated root. NO was imaged after 30 min. Scale bar = 1 mm. Experiments were repeated five times with similar results. c, Protoplasts were loaded with DAF-2DA and then incubated in 5 μm IAA for 30 min prior to flow cytometry. Experiments were repeated three times with similar results. d, Protoplasts were loaded with DAF-2DA and then incubated with 5 μm IAA in combination with 20 μm l-NAA, 20 μm NaN3, or 50 μm cPTIO. Experiments were repeated three times with similar results.
NO Modulates Root Extension and Gravitropic Bending
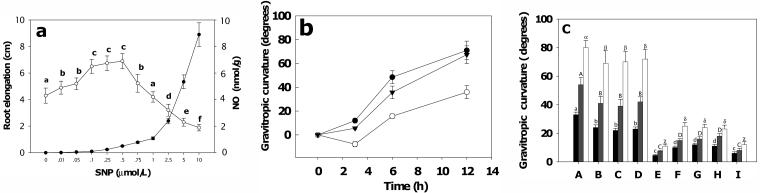
The data in Figure 1d show that the average NO concentration in the upper side of roots was about 0.25 nmol/g, whereas that in the lower side was about 8 nmol/g. To determine the effects of exogenous NO on gravitropic responses, we first assessed its effects on root extension. Guo et al. (2003) reported that NOS-deficient Arabidopsis (Arabidopsis thaliana) mutant plants do not synthesize NO in the roots and have a short root phenotype that can be reversed by treatment with the NO donor sodium nitroprusside (SNP). In our experiments with soybean roots, incubation in solutions of SNP at very low concentrations ([NO] ranging from 0.1–0.75 nmol/g in the root) actually promoted growth (Fig. 3a). However, the root elongation response to NO was positive or negative depending on NO concentration: at concentrations greater than 1 nmol/g, growth was increasingly inhibited (Fig. 3a, P < 0.01). He et al. (2004) have recently reported a similar biphasic response of Arabidopsis roots to NO. Treatment with the SNP analog sodium ferrocyanide that does not release NO had no effect (data not shown). These results suggest that the low concentration of NO in the upper side could promote root elongation, whereas the high concentration of NO in the lower side would suppress elongation, thus effecting gravitropic bending. To demonstrate that NO alone can modulate gravitropic responses, we applied NO via agar blocks containing SNP at a high concentration (5 μm). Bending was enhanced when NO was applied to the lower side of graviresponding roots and, strikingly, reduced when the NO was applied to the upper side (Fig. 3b). We also determined the effects of asymmetric NO application on the inhibition of gravitropism brought about by NPA: supplying NO from 0.5 μm SNP or from 5 μm SNP in agar to the upper or lower side, respectively, of NPA-treated roots overcame the effects of NPA (Fig. 3c). Applying PBTIU, cPTIO, or NaN3 via agar blocks to the lower side of horizontal roots suppressed root bending, suggesting that reducing NO accumulation in the lower side of horizontal roots reduces root bending. Application of these inhibitors to the upper side of horizontal roots had no significant effects, either positive or negative (data not shown).
Figure 3.
Effect of NO on root extension and of asymmetric NO and cGMP application on root curvature of graviresponding soybean roots. a, Effects of incubation in SNP on root extension. Roots were incubated in SNP at various concentrations and then root [NO] (•) and extension (○) measured after 48 h. b, Effects of NO at high concentration on root bending. Agar blocks containing 5 μm SNP (resulting in approximately 5 nmol/g NO in roots after 48 h) were placed on the lower (•) or upper side (○) of horizontally orientated soybean roots and gravitropic curvature measured at the indicated times. ▾, Control root showing normal gravitropism. c, Asymmetric applications of NO and inhibitors. Roots were pretreated with NPA and then gravistimulated (E). Agar blocks containing 5 μm (B) or 0.5 μm (C) SNP were placed on the lower (B) or upper (C) side, or blocks containing 50 pm 8-Br-cGMP (D) were placed on the lower side of NPA-pretreated horizontal roots. For inhibitor experiments, agar blocks containing 20 μm PBTIU (F), 20 μm l-NAA (G), 20 μm NaN3 (H), and 50 μm cPTIO (I), were placed on the lower side of gravistimulated roots. A, Control with no pretreatment. Black bars, 3-h treatment; gray bars, 6-h treatment; and white bars, 12-h treatment. Root curvature was measured at the indicated times. Values are the means ± se for five independent experiments.
NO Effects Are Mediated by cGMP
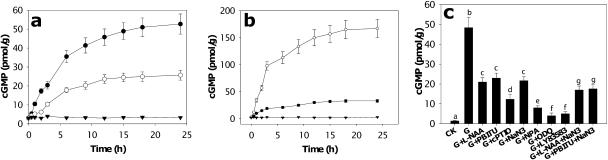
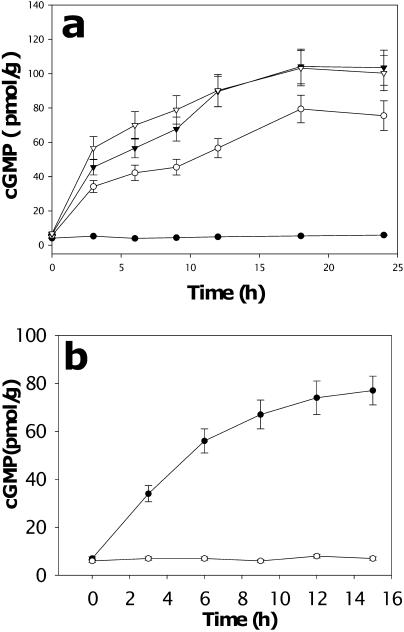
cGMP is an intracellular signaling molecule often synthesized in response to increasing NO concentrations (Neill et al., 2003). Consequently, we assessed the potential involvement of cGMP in gravitropism. Gravistimulation induced a rapid and substantial increase in the endogenous root cGMP content, particularly in apical tissue (Fig. 4a). This accumulation was asymmetric, similar to that seen for NO (Fig. 4b). Again, and in a similar manner, there were some differences in the absolute amounts of cGMP, e.g. average [cGMP] total root in Figure 4b is approximately 80 pmol/g and approximately 45 pmol/g in Figure 4a. Potential reasons for these differences relate to the use of different plants and materials, as mentioned above. Treatments that inhibited NO accumulation and gravitropic bending (Fig. 1b) also inhibited the increase in endogenous cGMP (Fig. 4c). Treatment with both inhibitors of NOS and NR had a slightly greater effect than treatment with either alone (Fig. 4c). In addition, 1H-[1,2,4]-oxadiazole[4,3-a]quinoxalin-1-one (ODQ) and 6-anilino-5,8-quinolinedione (LY83583), inhibitors of the cGMP synthesizing enzyme GC (Neill et al., 2003), prevented increases in cGMP (Fig. 4c) and inhibited bending (Fig. 1b), although they had little effect on NO accumulation (Fig. 1b). Incubation of roots in either SNP or auxin induced a rapid and substantial increase in endogenous cGMP content (Fig. 5, a and b; see Supplemental Fig. 3 for auxin dose response). Application of cGMP (as the cell-permeable analog 8-bromo-cGMP) to roots pretreated with NPA had the same effect as did exogenous NO and overcame the inhibition of gravitropic bending by NPA (Fig. 3c).
Figure 4.
Gravistimulation induces the rapid accumulation of cGMP in soybean roots. a, Kinetics of cGMP accumulation in Zone 1 (•) or Zone 2 (○) from horizontal roots following gravistimulation. ▾, Vertical roots. Values are the means ± se for five independent experiments. b, Asymmetric distribution of cGMP in root Zone 1. Soybean roots were gravistimulated for different times, bisected horizontally with a razor blade, and cGMP content assayed. ▾, Vertical roots; ○, lower half of horizontal root; •, upper half of horizontal root. c, Roots were pretreated as indicated for 12 h and then placed horizontally for 12 h. On x axis: CK, Control; G, gravistimulated. Data analyzed by one-way ANOVA followed by Tukey's test. Different symbols indicate significant differences between treatments (P < 0.05).
Figure 5.
NO and auxin stimulate cGMP accumulation in soybean roots. a, Roots were treated with SNP (•, control; ○, 50 nm; ∇, 100 nm; ▾, 500 nm). b, Roots were treated with 5 μm auxin (•, IAA; ○, control) and cGMP content assayed at different times. Values are the means ± se for five independent experiments.
DISCUSSION
Our data demonstrate that gravistimulation of soybean primary roots induces asymmetric NO generation, NO accumulating in the lower half of horizontally orientated roots. That such NO accumulation is required for a full gravitropic response is indicated by the reduction in gravitropic bending effected by treatments that reduce NO production. Several potential sources of NO exist in plants, including NR and a NOS, AtNOS1 (Guo et al., 2003; Neill et al., 2003). Inhibition of NO accumulation by NOS inhibitors suggests that one source of NO is a NOS-like enzyme, but whether or not the enzyme activity in soybean roots is similar to AtNOS1 is not yet known, although AtNOS1 is expressed in Arabidopsis roots (Guo et al., 2003). It is likely that NR also contributes to NO production in roots, as treatment with the putative NR inhibitor NaN3 reduced both the increases in [NO] and bending. NO generation via NR is known to occur in roots (Dordas et al., 2003), and it may be that NR is responsible for basal NO production that in some way is required for NO generation in addition to elevated NOS activity. It is interesting that NO was visible in the root tip of control roots and still present after l-NAA treatment but not after treatment with NaN3 (Fig. 1b). Nonenzymatic NO generation via reduction of apoplastic nitrite has been reported recently, with functions for apoplastic NO in seed germination and root development being suggested (Bethke et al., 2004). Some apoplastic NO production is a possibility in our study because the inhibitors of NR or NOS did not completely prevent root bending and NO generation. The pH of the root cap in Arabidopsis can decrease from 5.5 to 4.5 within 2 min of gravitropic stimulation (Fasano et al., 2001), potentially providing the necessary acidification for apoplastic NO formation.
The involvement of NO in gravitropism is also indicated by the modulation by exogenous NO of gravitropic bending. At concentrations greater than 500 nm, exogenous NO (approximately 5 nmol/g internal [NO]) inhibits soybean primary root extension, and application to the lower or upper sides of graviresponding roots enhances or reduces bending, respectively. Arabidopsis Atnos1 mutants defective in NO synthesis have shorter roots, a defect restored by exogenous NO (Guo et al., 2003). Our data are consistent with this and with those of He at al. (2004), suggesting that NO can exert positive or negative effects on root growth depending on its concentration and interactions with other signaling molecules, similar to auxin itself (Fu and Harberd, 2003).
Our data also show clearly that auxin stimulates NO generation in soybean roots. Although the effects of auxin on NO generation were not reported for Arabidopsis roots (Guo et al., 2003), auxin has previously been shown to stimulate NO production in cucumber (Cucumis sativus) hypocotyls, and this NO was required for adventitious rooting (Pagnussat et al., 2003). Auxin did not stimulate NO production in tobacco suspension cultures (Tun et al., 2001), suggesting that the effects on NO production may be cell specific or signal specific. Graviperception occurs in the columella cells of the root cap, whereas differential growth occurs in the extension zone (Blancaflor and Masson, 2003). Recent data show that the auxin efflux regulator protein PIN3 relocalizes laterally following gravistimulation (Friml et al., 2002) and that auxin is transported differentially from columella to lateral root cap cells (Ottenschläger et al., 2003). Inhibition of NO accumulation by NPA, an auxin efflux inhibitor (Ottenschläger et al., 2003), indicates that lateral auxin transport is required for asymmetric NO generation. Moreover, the location of NO accumulation is consistent with the site of maximum endogenous auxin accumulation. Subsequent basipetal transport of auxin to cells of the extension zone results in a much lower auxin content there than in the lateral root cap (Ottenschläger et al., 2003); thus, lower amounts of NO also would be expected. Stimulation of NO production by auxin potentially involves some cooperation between NOS and NR, as NPA and both NOS and NR inhibitors all blocked NO accumulation. Moreover, the inhibitory effects of NPA on gravitropic bending and NO accumulation were somewhat greater than those of the NO synthesis inhibitors, suggesting that auxin-induced acidification may also contribute to NO production during graviresponses.
cGMP is a second messenger generated in response to NO (Neill et al., 2003), and its presence in plants has been established unequivocally (Newton et al., 1999). Here, gravistimulation induced asymmetric cGMP similar to the increases in [NO]. In addition, treatment with either NO or auxin also stimulated cGMP accumulation in soybean roots. Inhibition of increased cGMP content by the GC inhibitors ODQ and LY83583 suggests that auxin and NO activate a GC-like enzyme. Such an enzyme has been identified recently in Arabidopsis, but its activation characteristics have not yet been defined fully (Ludidi and Gehring, 2002). The induction of cGMP synthesis in stomatal guard cells by auxin has already been indicated via pharmacological studies (Cousson and Vavasseur, 1998). Inhibition of NO synthesis by NPA, coupled with the lack of effect of ODQ and LY83583 on NO accumulation and the restoration of gravitropic bending by 8-Br-cGMP to NPA-treated roots, indicate that auxin induces NO production that in turn leads to cGMP synthesis.
While the data reported herein do show that NO and cGMP are required for full gravitropic responses, the lack of complete inhibition by inhibitors of NO and cGMP synthesis indicate that additional mechanisms may mediate auxin effects in root cells. In fact, NO is often generated at the same time as reactive oxygen species (ROS) such as hydrogen peroxide, for example, during pathogen responses or abscisic acid-induced stomatal closure (Neill et al., 2003). Root gravitropism appears to be another example of a physiological process in which both NO and ROS play key roles, as ROS were reported recently to be required for auxin-mediated gravitropic responses in maize (Zea mays; Joo et al., 2001). Preliminary data indicate that ROS scavengers do indeed reduce gravitropic bending in our system (data not shown).
In summary, our data implicate NO as another signal mediating root responses to gravity and auxin, with several potential sources of NO. The recent cloning of NO biosynthetic enzymes from Arabidopsis will no doubt facilitate further experiments to discern the roles of NO signaling in roots.
MATERIALS AND METHODS
Plant Materials
Soybean seeds (Glycine max cv Williams 82) were soaked in distilled water for 6 h, placed between wet paper towels held between plastic sheets mounted vertically in trays, and germinated for 2 d (at this point there was one primary and some secondary roots).
Root Treatments
To induce gravitropism, the trays were turned so that the roots were orientated horizontally. For subsequent analyses, the root was excised at the indicated times and then divided into two parts, Zone 1 and Zone 2, as described by Joo et al. (2001), that were immediately frozen in liquid nitrogen. Zone 1 represented the apical 4 mm of the root, including the root cap, meristem, and sometimes part of the elongation zone. Zone 2 (4–8 mm) represented the rest of the elongation zone.
To pretreat with various chemicals, attached roots (vertically orientated) were dipped in solutions of the appropriate chemicals (l-NNA, PBITU, cPTIO, NPA, ODQ, LY83583) for 12 h. For direct application to roots, agar blocks (0.8% [w/v], 5 mm × 5 mm × 20 mm) were used. Filter-sterilized solutions were added to cooled, molten agar in MES/KCl buffer (10 mm MES, pH 6.15, 10 mm KCl, 50 μm CaCl2) to give the required final concentrations. All manipulations were done in a dark room under dim green light at 24°C ± 1°C. Chemicals were dissolved in distilled water (SNP, indole-3-acetic acid [IAA]) or 1% dimethyl sulfoxide (l-NAA, PBITU, cPTIO, NPA, ODQ, LY83583, 8-Br-cGMP). Control treatments involved addition of equivalent amounts of 1% dimethyl sulfoxide or distilled water.
NO Assay
Primary root tissue (approximately 100 mg; Zone 1 or Zone 2) was homogenized in 1 mL of extraction buffer (10 mm Tris-Cl, pH 8.0, 1 mm phenylmethylsulfonyl fluoride, 10 mm MgSO4, 5 mm KCl, 5 mm NaCl containing 10 μm oxyhemoglobin and 10 units/mL of catalase). The extracts were then centrifuged (15,000g, 10 min) at 4°C and the supernatants used for NO determination using the hemoglobin assay (Delledonne et al., 1998). To assess the asymmetric distribution of NO (and cGMP) in the root tip, the roots were bisected into upper and lower halves with a razor blade prior to freezing and analysis.
cGMP Assay
cGMP was assayed using a 125I-cGMP radioimmunoassay kit (Amersham Biosciences, Chalfont St. Giles, UK). Primary root tissue (approximately 100 mg; Zone 1 or Zone 2) was homogenized at 4°C in 1 mL of 6% trichloroacetic acid. Cellular debris was removed by centrifugation (2,000g, 15 min) and the supernatant extracted 5 times with an equal volume of water-saturated diethyl ether. The aqueous layer was passed through a Dowex-50 column (10 cm × 1 cm), eluted with 5 volumes of water, lyophilized, and redissolved in 500 μL 500 mm acetate, pH 5.8, prior to radioimmunoassay.
Fluorescence Microscopy
NO was imaged using DAF-2DA and confocal microscopy. Roots were loaded with 10 μm DAF-2DA (Calbiochem, San Diego) in MES/KCl buffer for 30 min, and washed (3 × 3 min) in MES/KCl buffer. Roots were placed horizontally or vertically for various times as indicated in the text, then bisected longitudinally and viewed microscopically (PCM2000 Nikon, excitation 488 nm, emission 515–560 nm; Nikon, Tokyo). Experiments were repeated at least five times and similar results obtained. All manipulations were performed under dim green light at 24°C ± 1°C.
Determination of Curvature and Elongation of Soybean Roots
After various treatments and at various times, images of growing roots were recorded with a cooled CCD camera (CCD512BKS; Princeton Instruments, Trenton, NJ) and analyzed using Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA). Curvature and elongation were measured using a protractor and ruler. All manipulations were done in the darkroom under dim green light at 24°C ± 1°C.
Preparation of Soybean Root Protoplasts and Flow Cytometry
Protoplasts were prepared from 2-d-old soybean roots (1 g) essentially as described by Joo et al. (2001). Protoplasts were separated from the partially digested tissues by passage through a nylon mesh (75 μm) and pelleted at 60g for 5 min. The protoplasts were washed three times with MES/KCl buffer supplemented with 0.45 m mannitol and 1 mm CaCl2 and stored in the dark. Protoplasts were incubated in 10 μm DAF-2DA in MES/KCl buffer supplemented with 0.45 m mannitol and 1 mm CaCl2 for 10 min and then washed in buffer (3 × 5 min) prior to incubation in buffer plus or minus 5 μm IAA. Fluorescence intensity was measured by flow cytometry using a FACScan (Becton-Dickinson, Franklin Lakes, NJ) with excitation and emission settings of 488 and 530 nm, respectively. Counting of cells stopped at 30,000. Gating (gate set at 75 μm) was performed prior to data collection. All experiments were repeated three times with similar results.
Supplementary Material
Acknowledgments
We thank R. Desikan and J. Hancock (University of the West of England, Bristol) for their helpful comments on the manuscript.
This work was supported by the Chinese Academy of Sciences (grant no. KSCX2–SW–322), the Shanghai Institute of Plant Physiology and Ecology, and the National Natural Sciences Foundation of China (grant no. 39770199). Collaboration between the United Kingdom and China was supported by The Royal Society and The Biotechnology and Biological Sciences Research Council.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.054494.
References
- Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16: 332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism: unraveling the ups and downs of a complex process. Plant Physiol 133: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousson A, Vavasseur A (1998) Putative involvement of cytosolic Ca2+ and GTP-binding proteins in cyclic-GMP-mediated induction of stomatal opening by auxin in Commelina communis L. Planta 206: 308–314 [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394: 585–588 [DOI] [PubMed] [Google Scholar]
- Dordas C, Hasinoff BB, Igamberdiev AU, Manac'h N, Rivoal J, Hill RD (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 35: 763–770 [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95: 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13: 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421: 740–743 [DOI] [PubMed] [Google Scholar]
- Guo F-Q, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase involved in hormonal signaling. Science 302: 100–103 [DOI] [PubMed] [Google Scholar]
- He Y, Tang R-H, Hao Y, Steven RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Guo F, et al (2004) Nitric oxide represses the Arabidopsis floral transition. Science 305: 1968–1971 [DOI] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126: 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54: 109–136 [DOI] [PubMed] [Google Scholar]
- Ludidi N, Gehring C (2002) Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J Biol Chem 278: 6490–6494 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159: 11–35 [DOI] [PubMed] [Google Scholar]
- Newton RP, Roef L, Witters E, van Onckelen H (1999) Cyclic nucleotides in plants: the enduring paradox. New Phytol 143: 427–455 [DOI] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalero RP, Sandberg G, Ishikawa H, Evans ML, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132: 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129: 954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal G, Driss-Ecole D (2003) Mechanotransduction in gravisensing cells. Trends Plant Sci 8: 498–504 [DOI] [PubMed] [Google Scholar]
- Tun NN, Hok A, Scherer GFE (2001) Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Lett 509: 174–176 [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol 7: 449–455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.